The Effect of Adding Different Levels of Curcumin and Its Nanoparticles to Extender on Post-Thaw Quality of Cryopreserved Rabbit Sperm
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Curcumin Nanoparticles
2.2. Animals and Semen Collection
2.3. Semen Processing and Experimental Design
2.4. Assessment of Post-Thawed Sperm Quality
2.4.1. Sperm Progressive Motility and Plasma Membrane Integrity
2.4.2. Sperm Viability and Abnormalities
2.4.3. Sperm Apoptosis
2.4.4. Ultrastructure Changes of Spermatozoa
2.4.5. Biochemical Evaluation of Antioxidants Indices
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Curcumin Nanoparticles (CUNPs)
3.2. Effect of CU and CUNPs on Sperm Characteristics in Post-Thawed Rabbit Semen
3.3. Effect of CU and CUNPs on Sperm Apoptosis in Post-Thawed Rabbit Sperm
3.4. Effect on Antioxidants Indices Post-Thawed Rabbit Sperm
3.5. Effect on Sperm Ultrastructure in Post-Thawed Rabbit Sperm
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mocé, E.; Vicente, J.S. Rabbit sperm cryopreservation: A review. Anim. Reprod. Sci. 2009, 110, 1–24. [Google Scholar] [CrossRef]
- López-Gatius, F.; Sances, G.; Sancho, M.; Yániz, J.; Santolaria, P.; Gutiérrez, R.; Núñez, M.; Núñez, J.; Soler, C. Effect of solid storage at 15 °C on the subsequent motility and fertility of rabbit semen. Theriogenology 2005, 64, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Mostek, A.; Dietrich, M.A.; Słowińska, M.; Ciereszko, A. Cryopreservation of bull semen is associated with carbonylation of sperm proteins. Theriogenology 2017, 92, 95–102. [Google Scholar] [CrossRef]
- Watson, P.F. The causes of reduced fertility with cryopreserved semen. Anim. Reprod. Sci. 2000, 60–61, 481–492. [Google Scholar] [CrossRef]
- Ezzati, M.; Shanehbandi, D.; Hamdi, K.; Rahbar, S.; Pashaiasl, M. Influence of cryopreservation on structure and function of mammalian spermatozoa: An overview. Cell Tissue Bank. 2020, 21, 1–15. [Google Scholar] [CrossRef]
- Westfalewicz, B.; Dietrich, M.A.; Ciereszko, A. Impact of cryopreservation on bull (Bos taurus) semen proteome1. J. Anim. Sci. 2015, 93, 5240–5253. [Google Scholar] [CrossRef]
- Khalil, W.A.; El-Harairy, M.A.; Zeidan, A.E.B.; Hassan, M.A.E. Impact of selenium nano-particles in semen extender on bull sperm quality after cryopreservation. Theriogenology 2019, 126, 121–127. [Google Scholar] [CrossRef]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of oxidative stress on male reproduction. World J. Mens Health 2014, 32, 1–17. [Google Scholar] [CrossRef]
- Safa, S.; Moghaddam, G.; Jozani, R.J.; Daghigh Kia, H.; Janmohammadi, H. Effect of vitamin E and selenium nanoparticles on post-thaw variables and oxidative status of rooster semen. Anim. Reprod. Sci. 2016, 174, 100–106. [Google Scholar] [CrossRef]
- Isaac, A.V.; Kumari, S.; Nair, R.; Urs, D.R.; Salian, S.R.; Kalthur, G.; Adiga, S.K.; Manikkath, J.; Mutalik, S.; Sachdev, D.; et al. Supplementing zinc oxide nanoparticles to cryopreservation medium minimizes the freeze-thaw-induced damage to spermatozoa. Biochem. Biophys. Res. Commun. 2017, 494, 656–662. [Google Scholar] [CrossRef]
- Najafi, D.; Taheri, R.A.; Najafi, A.; Shamsollahi, M.; Alvarez-Rodriguez, M. Effect of astaxanthin nanoparticles in protecting the post-thawing quality of rooster sperm challenged by cadmium administration. Poult. Sci. 2020, 99, 1678–1686. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, X.; Liu, Y.; Wang, X.; Ling, X.; Ge, H.; Zhang, J. Curcumin improves asthenozoospermia by inhibiting reactive oxygen species reproduction through nuclear factor erythroid 2-related factor 2 activation. Andrologia 2020, 52, e13491. [Google Scholar] [CrossRef]
- Ogbuewu, I.P.; Okehi, M.C.; Jiwuba, P.C. Effect of phytobiotic (turmeric) supplementation on semen and blood characteristics of rabbits. Comp. Clin. Path. 2017, 26, 817–822. [Google Scholar] [CrossRef]
- Code of Federal Regulations (CFR). Food and Drug Administration. Food and Drugs, Part 170- Food additives. Subpart B- Food additive safety. In Sec. 170.30 Eligibility for Classification as Generally Recognized as Safe (GRAS); CFR: Silver Spring, MD, USA, 2017. [Google Scholar]
- Ahmed-Farid, O.A.H.; Nasr, M.; Ahmed, R.F.; Bakeer, R.M. Beneficial effects of curcumin nano-emulsion on spermatogenesis and reproductive performance in male rats under protein deficient diet model: Enhancement of sperm motility, conservancy of testicular tissue integrity, cell energy and seminal plasma amino acids content. J. Biomed. Sci. 2017, 24, 66. [Google Scholar]
- Feugang, J.M.; Rhoads, C.E.; Mustapha, P.A.; Tardif, S.; Parrish, J.J.; Willard, S.T.; Ryan, P.L. Treatment of boar sperm with nanoparticles for improved fertility. Theriogenology 2019, 137, 75–81. [Google Scholar] [CrossRef]
- Pinto, S.C.C.; Almeida, D.S.; Alves, M.B.R.; Florez-Rodriguez, S.A.; Abreu Júnior, G.S.; Alves, N.; Celeghini, E.C.C.; Laskoki, L.M.; Souza, F.A. Does supplementation of vitamin C, reduced glutathione or their association in semen extender reduce oxidative stress in bovine frozen semen? Arquivo Brasileiro de Medicina Veterinária e Zootecnia 2020, 72, 9–17. [Google Scholar] [CrossRef]
- Zaki, S.M.; Algaleel, W.A.A.; Imam, R.A.; Soliman, G.F.; Ghoneim, F.M. Nano-curcumin versus curcumin in amelioration of deltamethrin-induced hippocampal damage. Histochem. Cell Biol. 2020, 1–19. [Google Scholar] [CrossRef]
- Alizadeh, F.; Javadi, M.; Karami, A.A.; Gholaminejad, F.; Kavianpour, M.; Haghighian, H.K. Curcumin nanomicelle improves semen parameters, oxidative stress, inflammatory biomarkers, and reproductive hormones in infertile men: A randomized clinical trial. Phytother. Res. 2018, 32, 514–521. [Google Scholar] [CrossRef]
- Romero-Pérez, A.; García-García, E.; Zavaleta-Mancera, A.; Ramírez-Bribiesca, J.E.; Revilla-Vázquez, A.; Hernández-Calva, L.M.; López-Arellano, R.; Cruz-Monterrosa, R.G. Designing and evaluation of sodium selenite nanoparticles in vitro to improve selenium absorption in ruminants. Vet. Res. Commun. 2010, 34, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, Y.; Yang, S.; Yue, L.; Jiang, Q.; Xia, W. Synthesis and antioxidant properties of chitosan and carboxymethyl chitosan-stabilized selenium nanoparticles. Carbohydr. Polym. 2015, 132, 574–581. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Rabbits, 9th ed.; The National Academies Press: Washington, DC, USA, 1977. [Google Scholar]
- Di Iorio, M.; Manchisi, A.; Rocco, M.; Chrenek, P.; Iaffaldano, N. Comparison of Different Extenders on the Preservability of Rabbit Semen Stored at 5 °C for 72 Hours. Ital. J. Anim. Sci. 2014, 13, 3444. [Google Scholar] [CrossRef]
- Salisbury, G.W.; VanDemark, N.L.; Lodge, J.R. Physiology of Reproduction and Artificial Insemination of Cattle; W.H. Freeman and Company: New York, NY, USA, 1978. [Google Scholar]
- Caycho, K.; Santolaria, P.; Soler, C.; Yániz, J. Effect of hypoosmotic swelling test and water test on the distribution of sperm subpopulations in bull. Anim. Reprod. Sci. 2016, 169, 101. [Google Scholar] [CrossRef]
- Menon, A.G.; Thundathil, J.C.; Wilde, R.; Kastelic, J.P.; Barkema, H.W. Validating the assessment of bull sperm morphology by veterinary practitioners. Can. Vet. J. 2011, 52, 407–408. [Google Scholar] [PubMed]
- Chaveiro, A.; Santos, P.; Da Silva, F. Assessment of Sperm Apoptosis in Cryopreserved Bull Semen After Swim-up Treatment: A Flow Cytometric Study. Reprod. Domest. Anim. 2007, 42, 17–21. [Google Scholar] [CrossRef]
- Masters, A.; Harrison, P. Platelet counting with the BD AccuriTM C6 flow cytometer. Platelets 2014, 25, 175–180. [Google Scholar] [CrossRef]
- Peña, F.J.; Johannisson, A.; Wallgren, M. Assessment of fresh and frozen–thawed boar semen using an Annexin-V assay: A new method of evaluating sperm membrane integrity. Theriogenology 2003, 60, 677–689. [Google Scholar] [CrossRef]
- Oliveira, L.Z.; Hossepian de Lima, V.F.; Levenhagen, M.A.; Dos Santos, R.M.; Assumpção, T.I.; Jacomini, J.O.; De Andrade, A.F.; De Arruda, R.P.; Beletti, M.E. Transmission electron microscopy for characterization of acrosomal damage after Percoll gradient centrifugation of cryopreserved bovine spermatozoa. J. Vet. Sci. 2011, 12, 267–272. [Google Scholar] [CrossRef]
- Feyzi, S.; Sharafi, M.; Rahimi, S. Stress preconditioning of rooster semen before cryopreservation improves fertility potential of thawed sperm. Poult. Sci. 2018, 97, 2582–2590. [Google Scholar] [CrossRef]
- SAS. SAS/Stat. User’s Guide Static’s, Version, 6.06, 4th ed.; SAS Institute Inc.: Cary, NC, USA, 2004. [Google Scholar]
- Duncan, B.D. Multiple range and multiple F-test. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Peris-Frau, P.; Martín-Maestro, A.; Iniesta-Cuerda, M.; Sánchez-Ajofrín, I.; Cesari, A.; Garde, J.J.; Villar, M.; Soler, A.J. Cryopreservation of ram sperm alters the dynamic changes associated with in vitro capacitation. Theriogenology 2020, 145, 100–108. [Google Scholar] [CrossRef]
- Raza, H.; John, A.; Brown, E.M.; Benedict, S.; Kambal, A. Alterations in mitochondrial respiratory functions, redox metabolism and apoptosis by oxidant 4-hydroxynonenal and antioxidants curcumin and melatonin in PC12 cells. Toxicol. Appl. Pharmacol. 2008, 226, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Diao, R.Y.; Duan, Y.G.; Yi, T.H.; Cai, Z.M. In vitro antioxidant effect of curcumin on human sperm quality in leucocytospermia. Andrologia 2017, 49, e12760. [Google Scholar] [CrossRef] [PubMed]
- Falchi, L.; Galleri, G.; Dore, G.M.; Zedda, M.T.; Pau, S.; Bogliolo, L.; Ariu, F.; Pinna, A.; Nieddu, S.; Innocenzi, P.; et al. Effect of exposure to CeO(2) nanoparticles on ram spermatozoa during storage at 4 °C for 96 hours. Reprod. Biol. Endocrinol. 2018, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Feugang, J.M.; Youngblood, R.C.; Greene, J.M.; Fahad, A.S.; Monroe, W.A.; Willard, S.T.; Ryan, P.L. Application of quantum dot nanoparticles for potential non-invasive bio-imaging of mammalian spermatozoa. J. Nanobiotechnol. 2012, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sharma, R.K.; Sikka, S.C.; Thomas, A.J., Jr.; Falcone, T.; Agarwal, A. Oxidative stress is associated with increased apoptosis leading to spermatozoa DNA damage in patients with male factor infertility. Fertil. Steril. 2003, 80, 531–535. [Google Scholar] [CrossRef]
- Shah, S.A.H.; Andrabi, S.M.H.; Qureshi, I.Z. Freezability of water buffalo bull (Bubalus bubalis) spermatozoa is improved with the addition of curcumin (diferuoyl methane) in semen extender. Andrologia 2017, 49, e12713. [Google Scholar] [CrossRef]
- Jiang, Z.; Wan, Y.; Li, P.; Xue, Y.; Cui, W.; Chen, Q.; Chen, J.; Wang, F.; Mao, D. Effect of Curcumin Supplement in Summer Diet on Blood Metabolites, Antioxidant Status, Immune Response, and Testicular Gene Expression in Hu Sheep. Animals 2019, 9, 720. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]

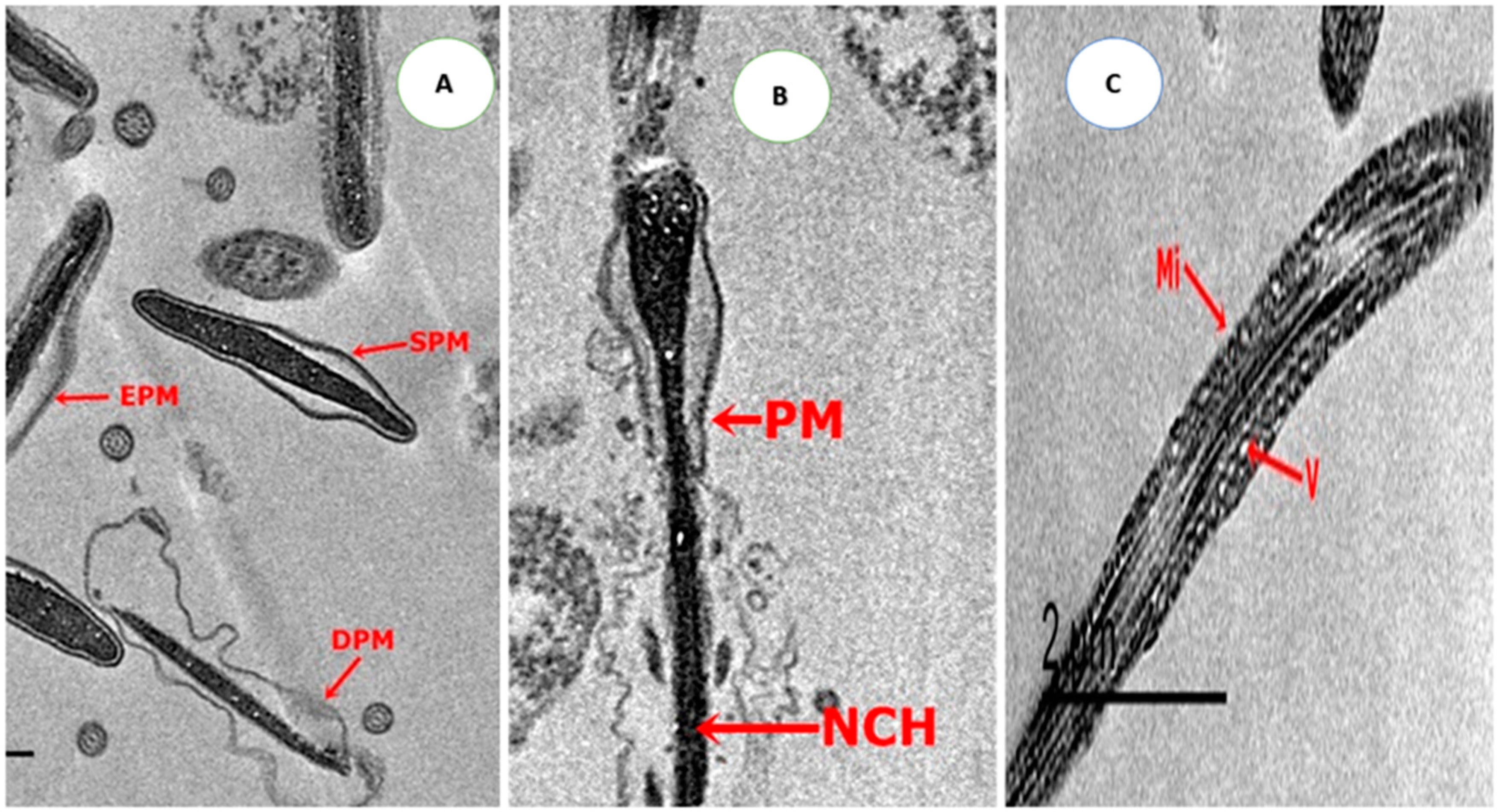
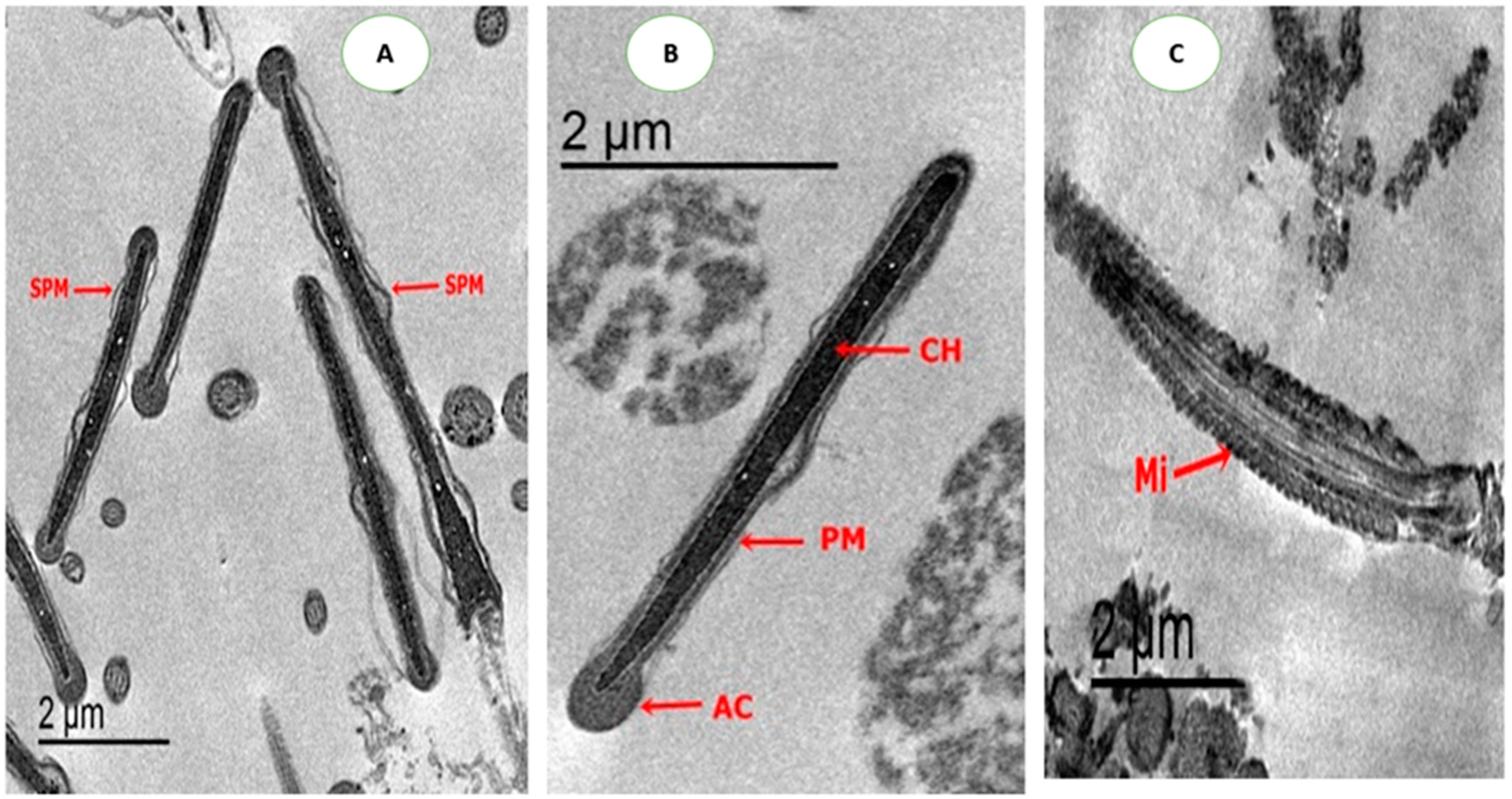
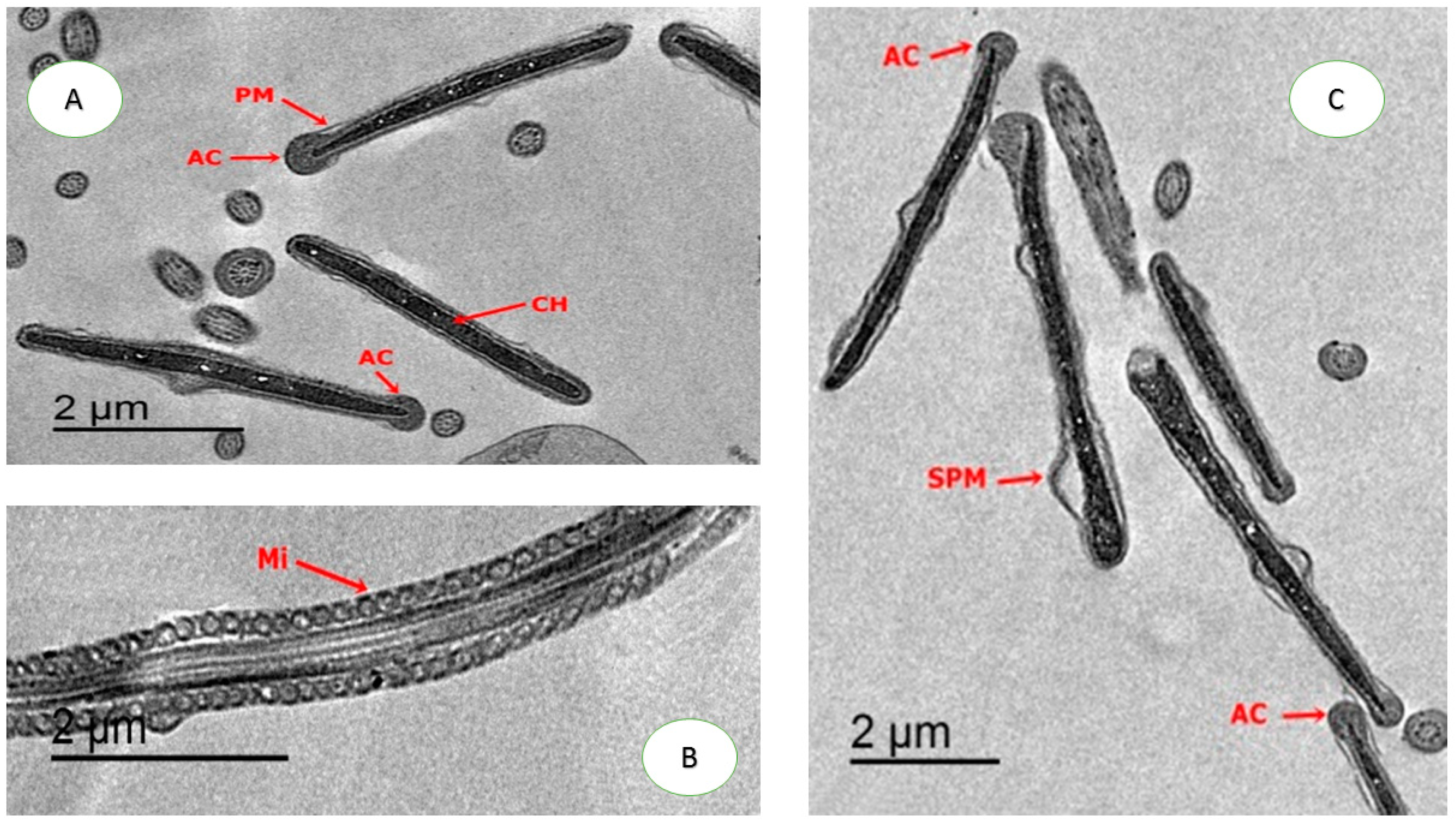
| Parameters | Control | CU0.5 | CU1.0 | CU1.5 | CUNPs0.5 | CUNPs1.0 | CUNPs1.5 | SEM | P-Value |
|---|---|---|---|---|---|---|---|---|---|
| Progressive motility (%) | 38.00 f | 53.00 e | 60.25 d | 63.50 c | 58.00 d | 68.00 b | 75.25 a | 0.966 | <0.001 |
| Membrane integrity (%) | 44.20 f | 51.25 e | 62.50 c | 66.00 b | 55.50 d | 63.50 cb | 73.50 a | 0.985 | <0.001 |
| Viability (%) | 46.00 a | 50.00 b | 45.50 c | 82.75 d | 56.75 c | 83.75 d | 71.75 e | 0.985 | <0.001 |
| Abnormality (%) | 15.25 a | 15.00 a | 15.00 a | 12.75 b | 14.75 a | 12.00 b | 10.00 c | 0.381 | <0.001 |
| Parameters | Control | CU0.5 | CU1.0 | CU1.5 | CUNPs0.5 | CUNPs1.0 | CUNPs1.5 | SEM | P-Value |
|---|---|---|---|---|---|---|---|---|---|
| Viable (%) | 30.40 f | 41.15 e | 46.85 d | 50.85 c | 45.90 d | 53.60 b | 62.30 a | 0.458 | <0.001 |
| Early apoptosis (%) | 23.25 a | 15.00 b | 14.00 cb | 12.85 d | 13.60 cd | 12.75 d | 9.85 e | 0.335 | <0.001 |
| Late apoptosis (%) | 25.65 a | 24.50 b | 20.45 d | 17.90 e | 22.10 c | 16.75 f | 12.30 g | 0.343 | <0.001 |
| Necrosis (%) | 20.70 a | 19.35 b | 18.70 cb | 18.40 c | 18.40 c | 16.90 d | 15.55 e | 0.225 | <0.001 |
| Parameters | Control | CU0.5 | CU1.0 | CU1.5 | CUNPs0.5 | CUNPs1.0 | CUNPs1.5 | SEM | P-Value |
|---|---|---|---|---|---|---|---|---|---|
| TAC (ng/mg) | 14.85 g | 16.17 e | 19.72 b | 20.13 a | 15.73 f | 17.90 d | 19.00 c | 0.11 | <0.001 |
| SOD (U/mg) | 32.91 e | 34.83 d | 44.75 b | 45.41 b | 38.58 c | 47.25 a | 48.66 a | 0.59 | <0.001 |
| GPX (ng/mg) | 26.90 g | 31.38 e | 35.90 b | 38.62 a | 28.40 f | 33.67 d | 34.78 c | 0.28 | <0.001 |
| MDA (nmol/mg) | 77.62 a | 55.25 c | 51.62 d | 34.25 e | 64.62 b | 55.00 c | 52.25 d | 0.63 | <0.001 |
| POC (ng/mg) | 3.17 a | 2.26 c | 1.94 d | 1.52 e | 2.69 b | 2.28 c | 1.98 d | 0.04 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelnour, S.A.; Hassan, M.A.E.; Mohammed, A.K.; Alhimaidi, A.R.; Al-Gabri, N.; Al-Khaldi, K.O.; Swelum, A.A. The Effect of Adding Different Levels of Curcumin and Its Nanoparticles to Extender on Post-Thaw Quality of Cryopreserved Rabbit Sperm. Animals 2020, 10, 1508. https://doi.org/10.3390/ani10091508
Abdelnour SA, Hassan MAE, Mohammed AK, Alhimaidi AR, Al-Gabri N, Al-Khaldi KO, Swelum AA. The Effect of Adding Different Levels of Curcumin and Its Nanoparticles to Extender on Post-Thaw Quality of Cryopreserved Rabbit Sperm. Animals. 2020; 10(9):1508. https://doi.org/10.3390/ani10091508
Chicago/Turabian StyleAbdelnour, Sameh A., Mahmoud A. E. Hassan, Amer K. Mohammed, Ahmad R. Alhimaidi, Naif Al-Gabri, Khalid O. Al-Khaldi, and Ayman A. Swelum. 2020. "The Effect of Adding Different Levels of Curcumin and Its Nanoparticles to Extender on Post-Thaw Quality of Cryopreserved Rabbit Sperm" Animals 10, no. 9: 1508. https://doi.org/10.3390/ani10091508
APA StyleAbdelnour, S. A., Hassan, M. A. E., Mohammed, A. K., Alhimaidi, A. R., Al-Gabri, N., Al-Khaldi, K. O., & Swelum, A. A. (2020). The Effect of Adding Different Levels of Curcumin and Its Nanoparticles to Extender on Post-Thaw Quality of Cryopreserved Rabbit Sperm. Animals, 10(9), 1508. https://doi.org/10.3390/ani10091508









