Effects of Dietary β-Mannanase Supplementation on Growth Performance, Apparent Total Tract Digestibility, Intestinal Integrity, and Immune Responses in Weaning Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, Housing, and Feeding
2.2. Experimental Procedures and Measurement
2.3. Chemical Analyses
2.4. Statistical Analysis
3. Results and Discussion
3.1. Growth Performance
3.2. Apparent Total Tract Digestibility
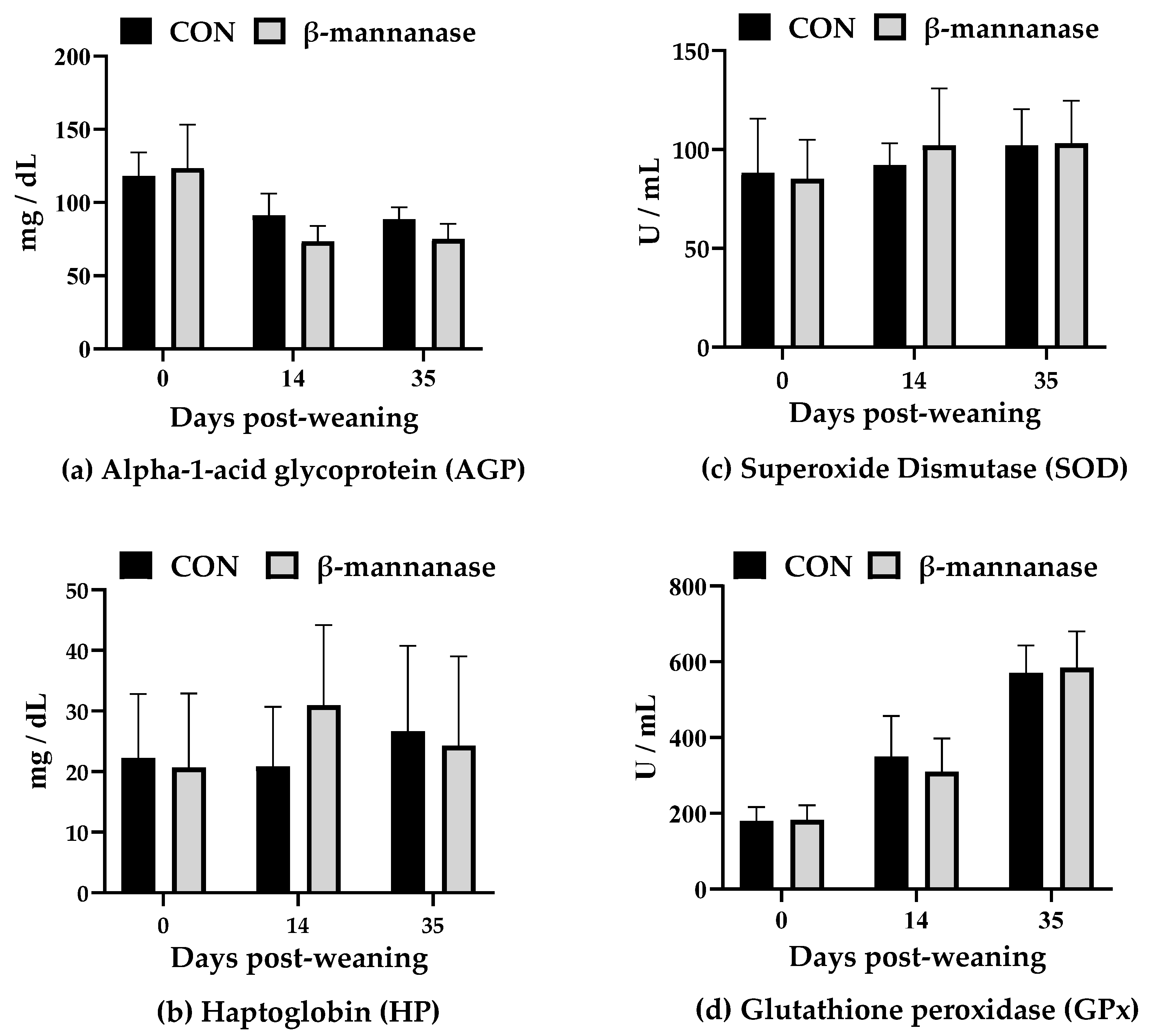
3.3. Serum Oxidative Stress and Immune Parameters
3.4. Intestinal Morphology
3.5. Intestinal Microflora
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choct, M.; Hughes, R.J.; Trimble, R.P.; Angkanaporn, K.; Annison, G. Non-starch polysaccharide-degrading enzymes increase the performance of broiler chickens fed wheat of low apparent metabolizable energy. J. Nutr. 1995, 125, 485–492. [Google Scholar] [CrossRef]
- McCleary, B.V. β-D-mannanase. Methods Enzymol. 1988, 160, 596–610. [Google Scholar] [CrossRef]
- Hsiao, H.Y.; Anderson, D.M.; Dale, N.M. Levels of ß-mannan in soybean meal. Poult. Sci. 2006, 85, 1430–1432. [Google Scholar] [CrossRef] [PubMed]
- Dierick, N.A. Biotechnology aids to improve feed and feed digestion: Enzyme and fermentation. Arch. Anim. Nutr. 1989, 3, 241–261. [Google Scholar] [CrossRef]
- Veum, T.L.; Odle, J. Feeding neonatal pigs. In Swine Nutrition, 2nd ed.; Lewis, A.J., Southern, L.L., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 675–694. [Google Scholar]
- Owusu-Asiedu, A.; Patience, J.F.; Laarveld, B.; Van Kessel, A.G.; Simmins, P.H.; Zijlstra, R.T. Effects of guar gum and cellulose on digesta passage rate, ileal microbial populations, energy and protein digestibility, and performance of grower pigs. J. Anim. Sci. 2006, 84, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Badia, R.; Zanello, G.; Chevaleyre, C.; Lizardo, R.; Meurens, F.; Martínez, P.; Brufau, J.; Salmon, H. Effect of Saccharomyces cerevisiae var. Boulardii and β-galactomannan oligosaccharide on porcine intestinal epithelial and dendritic cells challenged in vitro with Escherichia coli F4 (K88). Vet. Res. 2012, 43, 4. [Google Scholar] [CrossRef]
- Dhawan, S.; Kaur, J. Microbial mannanases: An overview of production and applications. Crit. Rev. Biotechnol. 2007, 27, 197–216. [Google Scholar] [CrossRef]
- Pettey, L.A.; Carter, S.D.; Senne, B.W.; Shriver, J.A. Effects of β-mannanase addition to corn-soybean meal diets on growth performance, carcass traits, and nutrient digestibility of weanling and growing-finishing pigs. J. Anim. Sci. 2002, 80, 1012–1019. [Google Scholar] [CrossRef]
- Carr, S.N.; Alee, G.L.; Rincker, P.J.; Fry, R.S.; Boler, D.D. Effects of endo-1,4-β-d-mannanase enzyme (Hemicell HT 1.5×) on the growth performance of nursery pigs. Prof. Anim. Sci. 2014, 30, 393–399. [Google Scholar] [CrossRef]
- Huntley, N.F.; Nyachoti, C.M.; Patience, J.F. Lipopolysaccharide immune stimulation but not β-mannanase supplementation affects maintenance energy requirements in young weaned pigs. J. Anim. Sci. Biotechnol. 2018, 9, 47. [Google Scholar] [CrossRef]
- National Research Council (NRC). Nutrient Requirements of Swine, 11th ed.; NRC: Washington, DC, USA, 2012. [Google Scholar]
- Pluske, J.R.; Kim, J.C.; McDonald, D.E.; Pethick, D.W.; Hampson, D.J. Non-starch polysaccharides in the diets of young weaned piglets. In The Weaner Pig: Nutrition and Management; Varley, M.A., Wiseman, J., Eds.; CABI Publishing: Wallingford, UK, 2001; pp. 81–112. [Google Scholar] [CrossRef]
- Jaworski, N.M.; Stein, H.H. Disappearance of nutrients and energy in the stomach and small intestine, cecum, and colon of pigs fed corn-soybean meal diets containing distillers dried grains with solubles, wheat middlings, or soybean hulls. J. Anim. Sci. 2017, 95, 727–739. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Nutrient Requirements of Swine, 10th ed.; NRC: Washington, DC, USA, 1998. [Google Scholar]
- Adeola, O. Digestion and balance techniques in pigs. In Swine Nutrition, 2nd ed.; Lewis, A.J., Southern, L.L., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 903–916. [Google Scholar]
- AOAC International. Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Kim, S.W.; Knabe, D.A.; Hong, J.W.; Easter, R.A. Use of carbohydrases in corn soybean meal based nursery diets. J. Anim. Sci. 2003, 81, 2496–2504. [Google Scholar] [CrossRef] [PubMed]
- Omogbenigun, F.O.; Nyachoti, C.M.; Slominski, B.A. Dietary supplementation with multienzyme preparations improves nutrient utilization and growth performance in weaned pigs. J. Anim. Sci. 2004, 82, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Wenk, C. The role of dietary fibre in the digestive physiology of the pig. Anim. Feed Sci. Technol. 2001, 90, 21–33. [Google Scholar] [CrossRef]
- Kusakabe, I.; Takahashi, R. Enzymatic preparation of β-1,4-mannooligosaccharides and β-1,4-glucomannooligosaccharides. Methods Enzymol. 1988, 160, 518–523. [Google Scholar] [CrossRef]
- Kwon, W.B.; Kim, B.G. Effects of Supplemental Beta-mannanase on Digestible Energy and Metabolizable Energy Contents of Copra Expellers and Palm Kernel Expellers Fed to Pigs. Asian-Australas. J. Anim. Sci. 2015, 28, 1014–1019. [Google Scholar] [CrossRef]
- Borel, P.; Lairon, D.; Senft, M.; Chautan, M.; Lafont, H. Wheat bran and wheat germ: Effect on digestion and intestinal absorption of dietary lipids in the rat. Am. J. Clin. Nutr. 1989, 49, 1192–1202. [Google Scholar] [CrossRef]
- Dégen, L.; Halas, V.; Babinszky, L. Effect of dietary fibre on protein and fat digestibility and its consequences on diet formulation for growing and fattening pigs: A review. Acta Agric. Scand Sect. 2007, 57, 1–9. [Google Scholar] [CrossRef]
- Pasquier, B.; Armand, M.; Castelain, C.; Guillon, F.; Borel, P.; Lafont, H.; Lairon, D. Emulsification and lipolysis of triacylglycerols are altered by viscous soluble dietary fibres in acidic gastric medium in vitro. Biochem. J. 1996, 314, 269–275. [Google Scholar] [CrossRef]
- McCleary, B.V.; Matheson, N.K. Polysaccharides having a β-D-xylan backbone. Adv. Carbohydr. Chem. Biochem. 1986, 44, 158–164. [Google Scholar]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Pantano, C.; Reynaert, N.L.; van der Vliet, A.; Janssen-Heininger, Y.M. Redox-sensitive kinases of the nuclear factor-kappaB signaling pathway. Antioxid. Redox Signal. 2006, 8, 1791–1806. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, U.P.; Chen, H.; Kim, S.W.; Jha, R. Supplemental effect of xylanase and mannanase on nutrient digestibility and gut health of nursery pigs studied using both in vivo and in vitro models. Anim. Feed Sci. Technol. 2018, 245, 77–90. [Google Scholar] [CrossRef]
- Duarte, M.E.; Zhou, F.X.; Dutra, W.M., Jr.; Kim, S.W. Dietary supplementation of xylanase and protease on growth performance, digesta viscosity, nutrient digestibility, immune and oxidative stress status, and gut health of newly weaned pigs. Anim. Nutr. 2019, 5, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Montagne, L.; Pluske, J.; Hampson, D.A. Review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed Sci. Technol. 2003, 108, 95–117. [Google Scholar] [CrossRef]
- Davidson, M.H.; McDonald, A. Fiber: Forms and functions. Nutr. Res. 1998, 18, 617–624. [Google Scholar] [CrossRef]
- McDonald, D.E.; Pethick, D.W.; Mullan, B.P.; Hampson, D.J. Increasing viscosity of the intestinal contents alters small intestinal structure and intestinal growth, and stimulates proliferation of enterotoxigenic Escherichia coli in newly-weaned pigs. Br. J. Nutr. 2001, 86, 487–498. [Google Scholar] [CrossRef]
- Hedemann, M.S.; Eskildsen, M.; Lærke, H.N.; Pedersen, C.; Lindberg, J.E.; Laurinen, P.; Bach Knudsen, K.E. Intestinal morphology and enzymatic activity in newly weaned pigs fed contrasting fiber concentrations and fiber properties. J. Anim. Sci. 2006, 84, 1375–1386. [Google Scholar] [CrossRef]
- Hopwood, D.E.; Pethick, D.W.; Pluske, J.R.; Hampson, D.J. Addition of pearl barley to a rice-based diet for newly weaned piglets increases the viscosity of the intestinal contents, reduces starch digestibility and exacerbates post-weaning colibacillosis. Br. J. Nutr. 2004, 92, 419–427. [Google Scholar] [CrossRef]
- Sinha, A.K.; Kumar, V.; Makkar, H.P.S.; De Boeck, G.; Becker, K. Non-starch polysaccharides and their role in fish nutrition—A review. Food Chem. 2011, 127, 1409–1426. [Google Scholar] [CrossRef]
- Ofek, I.; Mirelman, D.; Sharon, N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature 1977, 265, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Högberg, A.; Lindberg, J.E. Influence of cereal non-starch polysaccharides and enzyme supplementation on digestion site and gut environment in weaned piglets. Anim. Feed Sci. Technol. 2004, 116, 113–128. [Google Scholar] [CrossRef]
- Kim, J.; Shim, Y.; Ingale, S.L.; Hosseindoust, A.; Lee, S.; Rathi, P.C.; Choi, Y.; Kim, M.; Chae, B. The microbial pH-stable exogenous multienzyme improved growth performance and intestinal morphology of weaned pigs fed a corn–soybean-based diet. J. Appl. Anim. Res. 2018, 46, 559–565. [Google Scholar] [CrossRef]
| Ingredients, % | Phase 1 | Phase 2 |
|---|---|---|
| (Day 0–14 Post-Weaning) | (Day 14–35 Post-Weaning) | |
| Corn | 17.8 | 38.22 |
| Soybean meal, 44 % CP | 22.08 | 24 |
| HP300 2 | 10 | 9 |
| Whey powder | 10 | 0 |
| Lactose | 15 | 5 |
| Barley, dehulled | 20 | 20 |
| Soybean oil | 1 | 1 |
| Monocalcium phosphate | 1.01 | 1.28 |
| Limestone | 0.92 | 0.92 |
| L-Lysine·HCl | 0.13 | 0.14 |
| Vitamin premix 3 | 0.12 | 0.12 |
| Trace mineral premix 4 | 0.12 | 0.12 |
| Salt | 0.1 | 0.1 |
| Choline-Cl (25%) | 0.1 | 0.1 |
| Zinc oxide (ZnO) 5 | 0.1 | 0.1 |
| Calculated chemical composition | ||
| Metabolizable energy, kcal/kg | 3400 | 3350 |
| Crude protein, % | 21.5 | 22.5 |
| SID 6 lysine, % | 1.35 | 1.23 |
| SID methionine, % | 0.39 | 0.36 |
| Total Ca, % | 0.8 | 0.7 |
| STTD 6 P, % | 0.4 | 0.35 |
| Total NSP 7, % | 10.93 | 12.89 |
| Insoluble NSP 7, % | 9.94 | 11.43 |
| β-mannan 8, % | 0.57 | 0.61 |
| Items | Treatment 2 | SEM 3 | p-Value | |
|---|---|---|---|---|
| CON | β-Mannanase | |||
| Body weight, kg | ||||
| day 0 post-weaning (initial) | 6.96 | 6.95 | 0.430 | 0.98 |
| day 14 post-weaning | 9.18 | 9.37 | 0.601 | 0.82 |
| day 35 post-weaning | 16.79 | 17.01 | 1.078 | 0.89 |
| Average daily gain, g/day | ||||
| day 0–14 post-weaning (Phase 1) | 158 | 173 | 15.8 | 0.52 |
| day 14–35 post-weaning (Phase 2) | 354 | 364 | 26.4 | 0.80 |
| day 0–35 post-weaning (Overall) | 256 | 269 | 20.2 | 0.66 |
| Average daily feed intake, g/day | ||||
| day 0–14 post-weaning (Phase 1) | 273 | 295 | 19.0 | 0.43 |
| day 14–35 post-weaning (Phase 2) | 742 | 836 | 52.4 | 0.23 |
| day 0–35 post-weaning (Overall) | 558 | 537 | 35.2 | 0.31 |
| Gain to feed ratio | ||||
| day 0–14 post-weaning (Phase 1) | 0.557 | 0.585 | 0.019 | 0.31 |
| day 14–35 post-weaning (Phase 2) | 0.481 | 0.436 | 0.022 | 0.17 |
| day 0–35 post-weaning (Overall) | 0.519 | 0.510 | 0.015 | 0.69 |
| Items | Treatment 2 | SEM 3 | p-Value | |
|---|---|---|---|---|
| CON | β-Mannanase | |||
| Apparent total tract digestibility (%) | ||||
| Dry matter | 87.92 | 87.78 | 1.148 | 0.93 |
| Crude protein | 86.63 | 86.56 | 1.591 | 0.98 |
| Crude ash | 56.14 | 58.30 | 4.371 | 0.74 |
| Crude fat | 70.13 | 76.51 | 4.783 | 0.01 |
| Nitrogen (N) retention (g) | ||||
| N intake | 6.79 | 7.05 | 0.058 | 0.65 |
| Fecal N | 0.91 | 0.95 | 0.109 | 0.81 |
| Urinary N | 1.77 | 1.86 | 0.263 | 0.81 |
| N retention 4 | 4.11 | 4.24 | 0.293 | 0.77 |
| Items | Treatment 2 | SEM 3 | p-Value | |
|---|---|---|---|---|
| CON | β-Mannanase | |||
| Jejunum | ||||
| Villus height (µm) | 369.92 | 428.75 | 15.707 | 0.01 |
| Crypt depth (µm) | 244.67 | 212.50 | 11.182 | 0.05 |
| Villus:Crypt ratio | 1.58 | 2.03 | 0.104 | 0.01 |
| Ileum | ||||
| Villus height (µm) | 340.42 | 378.08 | 17.499 | 0.14 |
| Crypt depth (µm) | 188.83 | 191.58 | 10.862 | 0.86 |
| Villus:Crypt ratio | 1.83 | 2.08 | 0.141 | 0.23 |
| Items | Treatment 2 | SEM 3 | p-Value | |
|---|---|---|---|---|
| CON | β-Mannanase | |||
| E. coil counts (log10 CFU/g fresh digesta) | ||||
| Ileum | 7.40 | 6.68 | 0.509 | 0.33 |
| Cecum | 8.78 | 7.32 | 0.508 | 0.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.-C.; Kim, K.H.; Jang, Y.D.; Kim, Y.Y. Effects of Dietary β-Mannanase Supplementation on Growth Performance, Apparent Total Tract Digestibility, Intestinal Integrity, and Immune Responses in Weaning Pigs. Animals 2020, 10, 703. https://doi.org/10.3390/ani10040703
Jang J-C, Kim KH, Jang YD, Kim YY. Effects of Dietary β-Mannanase Supplementation on Growth Performance, Apparent Total Tract Digestibility, Intestinal Integrity, and Immune Responses in Weaning Pigs. Animals. 2020; 10(4):703. https://doi.org/10.3390/ani10040703
Chicago/Turabian StyleJang, Jae-Cheol, Kwang Ho Kim, Young Dal Jang, and Yoo Yong Kim. 2020. "Effects of Dietary β-Mannanase Supplementation on Growth Performance, Apparent Total Tract Digestibility, Intestinal Integrity, and Immune Responses in Weaning Pigs" Animals 10, no. 4: 703. https://doi.org/10.3390/ani10040703
APA StyleJang, J.-C., Kim, K. H., Jang, Y. D., & Kim, Y. Y. (2020). Effects of Dietary β-Mannanase Supplementation on Growth Performance, Apparent Total Tract Digestibility, Intestinal Integrity, and Immune Responses in Weaning Pigs. Animals, 10(4), 703. https://doi.org/10.3390/ani10040703






