Documenting Aggression, Dominance and the Impacts of Visitor Interaction on Galápagos Tortoises (Chelonoidis nigra) in a Zoo Setting
Simple Summary
Abstract
1. Introduction
2. Methods
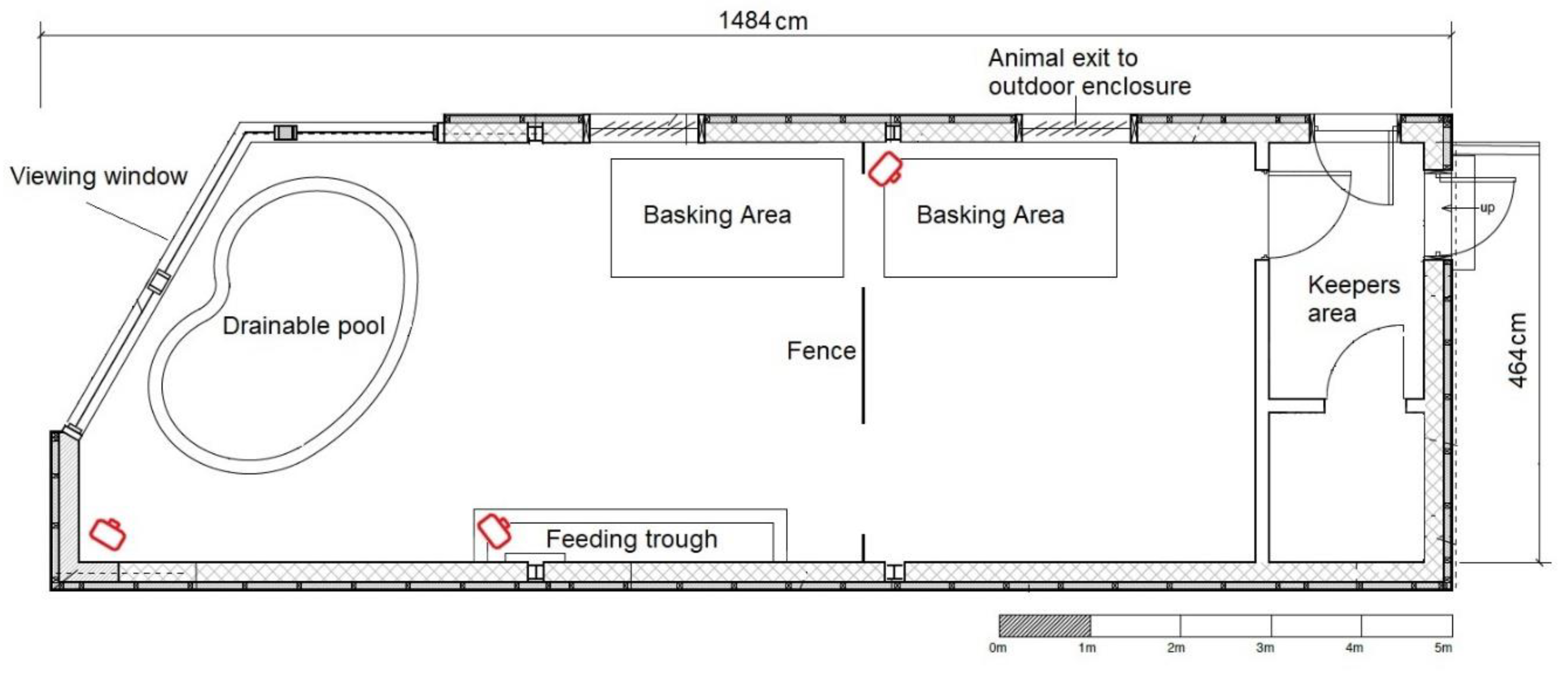
2.1. Study Subjects
2.2. Study Design
2.3. Aggression Levels on Visit and Control Days
2.4. Aggression and Activity before, during and after Visit
2.5. Hierarchy Assessment
2.6. Statistical Analyses
2.7. Post-Study Mitigation
3. Results
3.1. Description of Aggressive Interactions
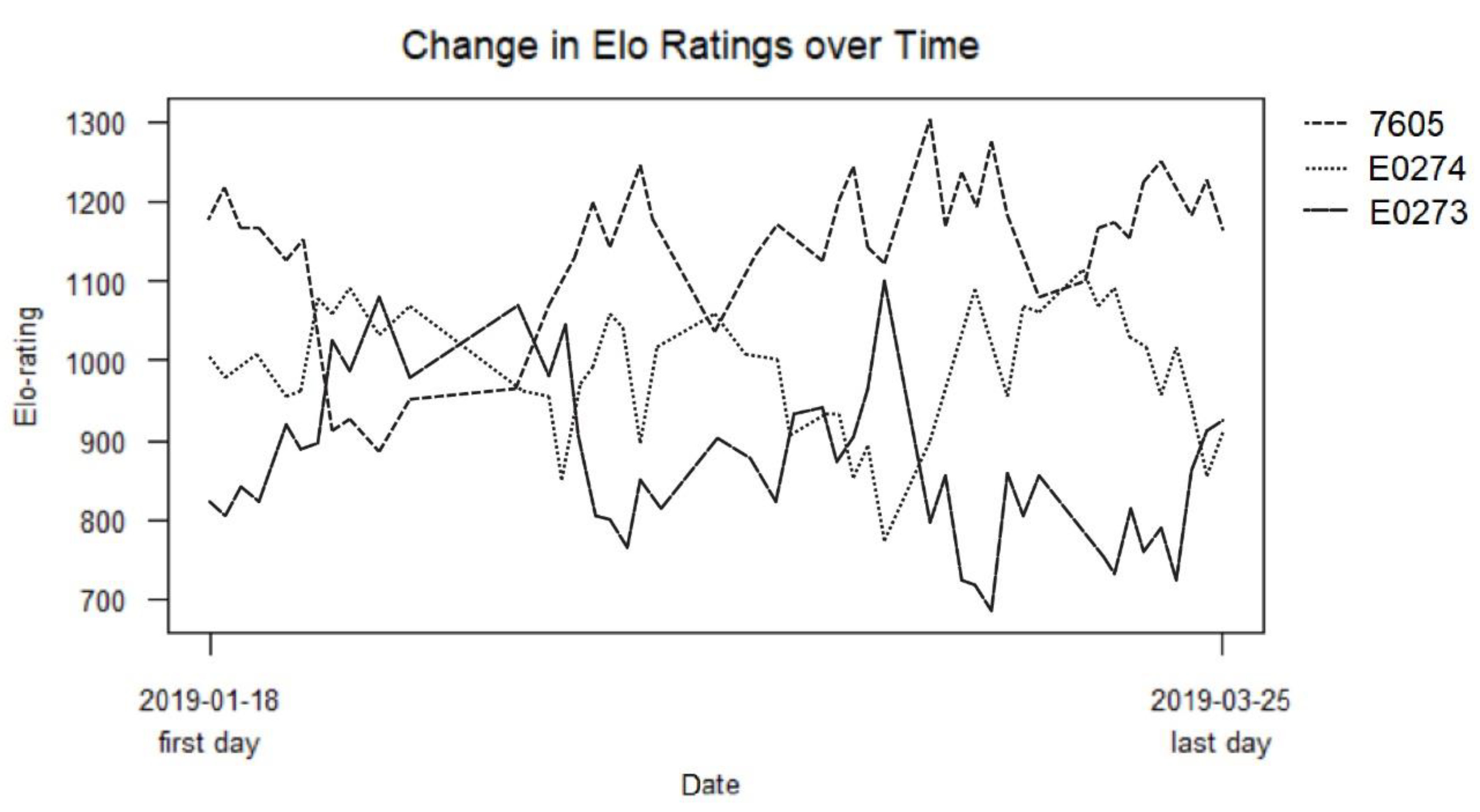
3.2. Hierarchy
3.3. Aggression, Activity and Visitor Interaction
3.4. Post-Study Mitigation Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kreger, M.; Mench, J.A. Visitor-Animal Interaction at the Zoo. Anthrozoos 1995, 8, 143–158. [Google Scholar] [CrossRef]
- Hosey, G.R. Zoo animals and their human audiences: What is the visitor effect? Anim. Welf. 2000, 9, 343–357. [Google Scholar]
- D’Cruze, N.; Khan, S.; Carder, G.; Megson, D.; Coulthard, E.; Norrey, J.; Groves, G. A Global Review of Animal–Visitor Interactions in Modern Zoos and Aquariums and Their Implications for Wild Animal Welfare. Animals 2019, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Mehrkam, L.R.; Dorey, N.R. Is Preference a Predictor of Enrichment Efficacy in Galapagos Tortoises (Chelonoidis nigra)? Zoo Biol. 2014, 33, 275–284. [Google Scholar] [CrossRef]
- Williams, J. Stress in chelonians (tortoises, terrapins and turtles). Vet. Nurse 2017, 8, 264–271. [Google Scholar] [CrossRef]
- Fernandez, E.J.; Tamborski, M.A.; Pickens, S.R.; Timberlake, W. Animal-visitor interactions in the modern zoo: Conflicts and interventions. Appl. Anim. Behav. Sci. 2009, 120, 1–8. [Google Scholar] [CrossRef]
- Lee, H.-S. Measurement of visitors’ satisfaction with public zoos in Korea using importance-performance analysis. Tour. Manag. 2015, 47, 251–260. [Google Scholar] [CrossRef]
- Rodhouse, P.; Barling, R.W.A.; Clark, W.I.C.; Kinmonth, A.-L.; Mark, E.M.; Roberts, D.; Armitage, L.E.; Austin, P.R.; Baldwin, S.P.; Bellairs, A.D.; et al. The feeding and ranging behaviour of Galapagos giant tortoises (Geochelone elephantopus). J. Zool. Lond. 1975, 176, 297–310. [Google Scholar] [CrossRef]
- Deane, K. Training zoo animals for better welfare, better nursing. Vet. Nurse 2017, 8, 116–122. [Google Scholar] [CrossRef]
- Bryant, Z.; Harding, L.; Grant, S.; Rendle, M. A method for blood sampling the Galápagos tortoise, Chelonoidis using operant conditioning for voluntary blood draws. Herpetol. Bull. 2016, 135, 7–10. [Google Scholar]
- Benn, A.L.; McLelland, D.J.; Whittaker, A.L. A Review of Welfare Assessment Methods in Reptiles, and Preliminary Application of the Welfare Quality® Protocol to the Pygmy Blue-Tongue Skink, Tiliqua adelaidensis, Using Animal-Based Measures. Animals 2019, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Hatt, J.M. Raising giant tortoises. Zoo Wild Anim. Med. Curr. Ther. 2008, 6, 144–153. [Google Scholar]
- Warwick, C. Reptilian Ethology in Captivity: Observations of Some Problems and an Evaluation of Their Aetiology. Appl. Anim. Behav. Sci. 1990, 26, 1–13. [Google Scholar] [CrossRef]
- Sherwen, S.L.; Hemsworth, P.H. The Visitor Effect on Zoo Animals: Implications and Opportunities for Zoo Animal Welfare. Animal 2019, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Auffenberg, W. Display Behavior in Tortoises. Am. Zool. 1977, 17, 241–250. [Google Scholar] [CrossRef]
- Schafer, S.F.; O’neil Krekorial, C. Agonistic Behavior of the Galapagos Tortoise, Geochelone elephantopus, with Emphasis on Its Relationship to Saddle-Backed Shell Shape. Herpetologica 1983, 39, 448–456. [Google Scholar]
- Alberts, A.C. Dominance hierarchies in male lizards: Implications for zoo management programs. Zoo Biol. 1994, 13, 479–490. [Google Scholar] [CrossRef]
- Woods, J.M.; Ross, S.R.; Cronin, K.A. The Social Rank of Zoo-Housed Japanese Macaques is a Predictor of Visitor-Directed Aggression. Animals 2019, 9, 316. [Google Scholar] [CrossRef]
- Elo, A.E. The Rating of Chess Players, Past and Present; Arco: New York, NY, USA, 1978. [Google Scholar]
- Albers, P.C.H.; De Vries, H. Elo-rating as a tool in the sequential estimation of dominance strengths. Anim. Behav. 2001, 61, 489–495. [Google Scholar] [CrossRef]
- Brattstrom, B.H. The Evolution of Reptilian Social Behavior. Am. Zool. 1974, 14, 35–49. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for ## Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- Martin, P.; Bateson, P.P.G.; Bateson, P. Measuring Behaviour: An Introductory Guide; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Neumann, C.; Duboscq, J.; Dubuc, C.; Ginting, A.; Irwan, A.M.; Agil, M.; Widdig, A.; Engelhardt, A. Assessing dominance hierarchies: Validation and advantages of progressive evaluation with elo-rating. Anim. Behav. 2011, 82, 911–921. [Google Scholar] [CrossRef]
- Henningsen, J.P.; Irschick, D.J. An experimental test of the effect of signal size and performance capacity on dominance in the green anole lizard. Funct. Ecol. 2012, 26, 3–10. [Google Scholar] [CrossRef]
- Silk, M.J.; Cant, M.A.; Cafazzo, S.; Natoli, E.; McDonald, R.A. Elevated aggression is associated with uncertainty in a network of dog dominance interactions. Proc. R. Soc. B 2019, 286, 20190536. [Google Scholar] [CrossRef] [PubMed]
- Thompson, F.J.; Donaldson, L.; Johnstone, R.A.; Field, J.; Cant, M.A. Dominant aggression as a deterrent signal in paper wasps. Behav. Ecol. 2014, 25, 706–715. [Google Scholar] [CrossRef]
- Gerlach, J. Captive breeding of Dipsochelys giant tortoises. Phelsuma 2003, 11, 8–12. [Google Scholar]
- Larsen, M.J.; Sherwen, S.L.; Rault, J.-L. Number of nearby visitors and noise level affect vigilance in captive koalas. Appl. Anim. Behav. Sci. 2014, 154, 76–82. [Google Scholar] [CrossRef]
- Maia, C.M.; Volpato, G.L.; Santos, E.F. A Case Study: The Effect of Visitors on Two Captive Pumas with Respect to the Time of the Day. Appl. Anim. Welf. Sci. 2012, 15, 3. [Google Scholar] [CrossRef]
- Wickins-Drazilova, D. Zoo Animal Welfare. J. Agric. Environ. Ethics 2006, 19, 27–36. [Google Scholar] [CrossRef]
- Young, R.J. Environmental Enrichment for Captive Animals; Blackwell Publishing: Oxford, UK, 2008; Volume 2. [Google Scholar]
- Appleby, M.; Olsson, A.; Galindo, F. Animal Welfare, 3rd ed.; CABI Publishing: Wallingford, UK, 2018. [Google Scholar]
- Barnett, S.A. Modern Ethology: The Science of Animal Behavior; Oxford University Press: New York, NY, USA, 1981; pp. 548–549. [Google Scholar]
- Alcock, J. Animal Behavior: An Evolutionary Approach, 9th ed.; Sinauer Assocites: Sunderland, MA, USA, 2009; pp. 110–111. [Google Scholar]
- Macfarland, C.; Reeder, W.G. Cleaning Symbiosis Involving Galapagos Tortoises and Two Species of Darwin’s Finches. Z. Für Tierpsychol. 1974, 34, 464–483. [Google Scholar] [CrossRef]
- Bassett, L.; Buchanan-Smith, H.M. Effects and predictability on the welfare of captive animals. Appl. Anim. Behav. Sci. 2007, 102, 223–245. [Google Scholar] [CrossRef]


| Behaviour | Group | Description |
|---|---|---|
| Sleeping | Non-active | Flat on floor, still, head down |
| Resting | Non-active | Flat on floor, some movement of head and/or legs |
| Walking | Active | Locomotion, excluding the below behaviours |
| Standing | Active | Raised above the floor, stationary |
| Eating | Active | Consuming food or water |
| Finching | Active | Fully raised head and body, while stationary |
| Aggressive Interaction | Active | Raising head higher than resting level or air biting in close proximity to another tortoise, or biting another tortoise |
| Non- Aggressive Interaction | Active | Movement of head in close proximity to another tortoise, not including actions included within ‘aggressive interaction’ |
| Bathing | Active | Part or all of the tortoise in the pond |
| Other | N/A | Any behaviour not described by other definitions |
| Out of Sight | N/A | The tortoise is not visible from any camera |
| Individual | Final Elo Rating | No. of Times Actor of Aggression | No. of Times Recipient of Aggression | No. of Times Winner of Aggressive Interaction | No. of Times Loser of Aggressive Interaction | Total No. of Aggressive Interactions | Height (cm) |
|---|---|---|---|---|---|---|---|
| 7605 | 1165 | 69 | 119 | 146 | 42 | 188 | 86.5 |
| E0274 | 924 | 104 | 92 | 55 | 141 | 196 | 84 |
| E0273 | 911 | 106 | 68 | 78 | 96 | 174 | 85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freeland, L.; Ellis, C.; Michaels, C.J. Documenting Aggression, Dominance and the Impacts of Visitor Interaction on Galápagos Tortoises (Chelonoidis nigra) in a Zoo Setting. Animals 2020, 10, 699. https://doi.org/10.3390/ani10040699
Freeland L, Ellis C, Michaels CJ. Documenting Aggression, Dominance and the Impacts of Visitor Interaction on Galápagos Tortoises (Chelonoidis nigra) in a Zoo Setting. Animals. 2020; 10(4):699. https://doi.org/10.3390/ani10040699
Chicago/Turabian StyleFreeland, Laura, Charlotte Ellis, and Christopher J. Michaels. 2020. "Documenting Aggression, Dominance and the Impacts of Visitor Interaction on Galápagos Tortoises (Chelonoidis nigra) in a Zoo Setting" Animals 10, no. 4: 699. https://doi.org/10.3390/ani10040699
APA StyleFreeland, L., Ellis, C., & Michaels, C. J. (2020). Documenting Aggression, Dominance and the Impacts of Visitor Interaction on Galápagos Tortoises (Chelonoidis nigra) in a Zoo Setting. Animals, 10(4), 699. https://doi.org/10.3390/ani10040699




