Vitrification Using Soy Lecithin and Sucrose: A New Way to Store the Sperm for the Preservation of Canine Reproductive Function
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Semen Collection
2.2. Basic Semen Evaluation
2.3. Other Semen Analysis
2.3.1. Acrosome Assessment
2.3.2. Hypoosmotic Swelling Test (HOST)
2.3.3. DNA Fragmentation
2.4. Experimental Setup
2.5. Preparation of Extenders Used in the Study
2.6. Vitrification Process
2.7. Devitrification Procedure
2.8. Statistics
2.8.1. Sample Size
2.8.2. Statistical Analysis
3. Results
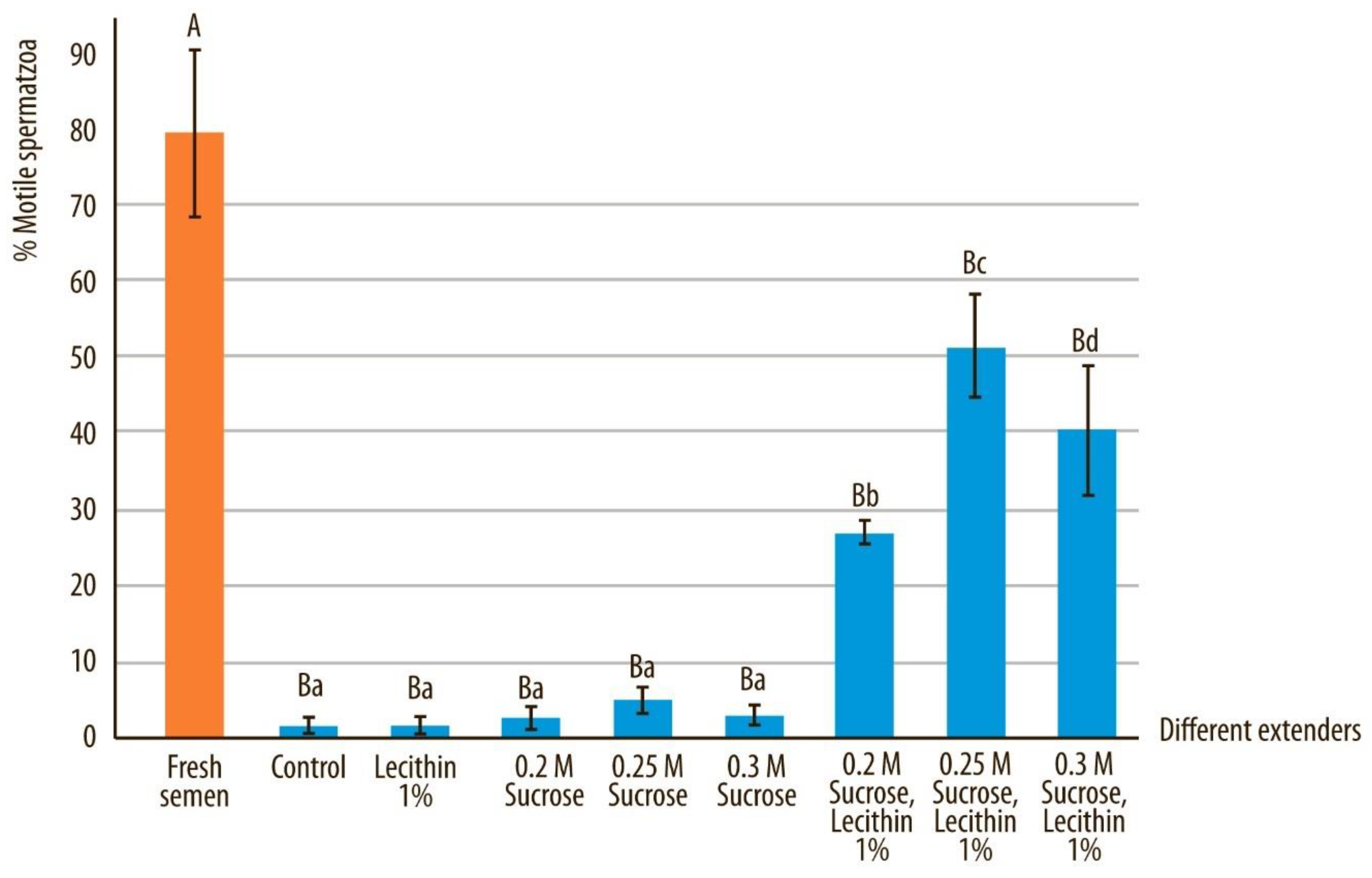
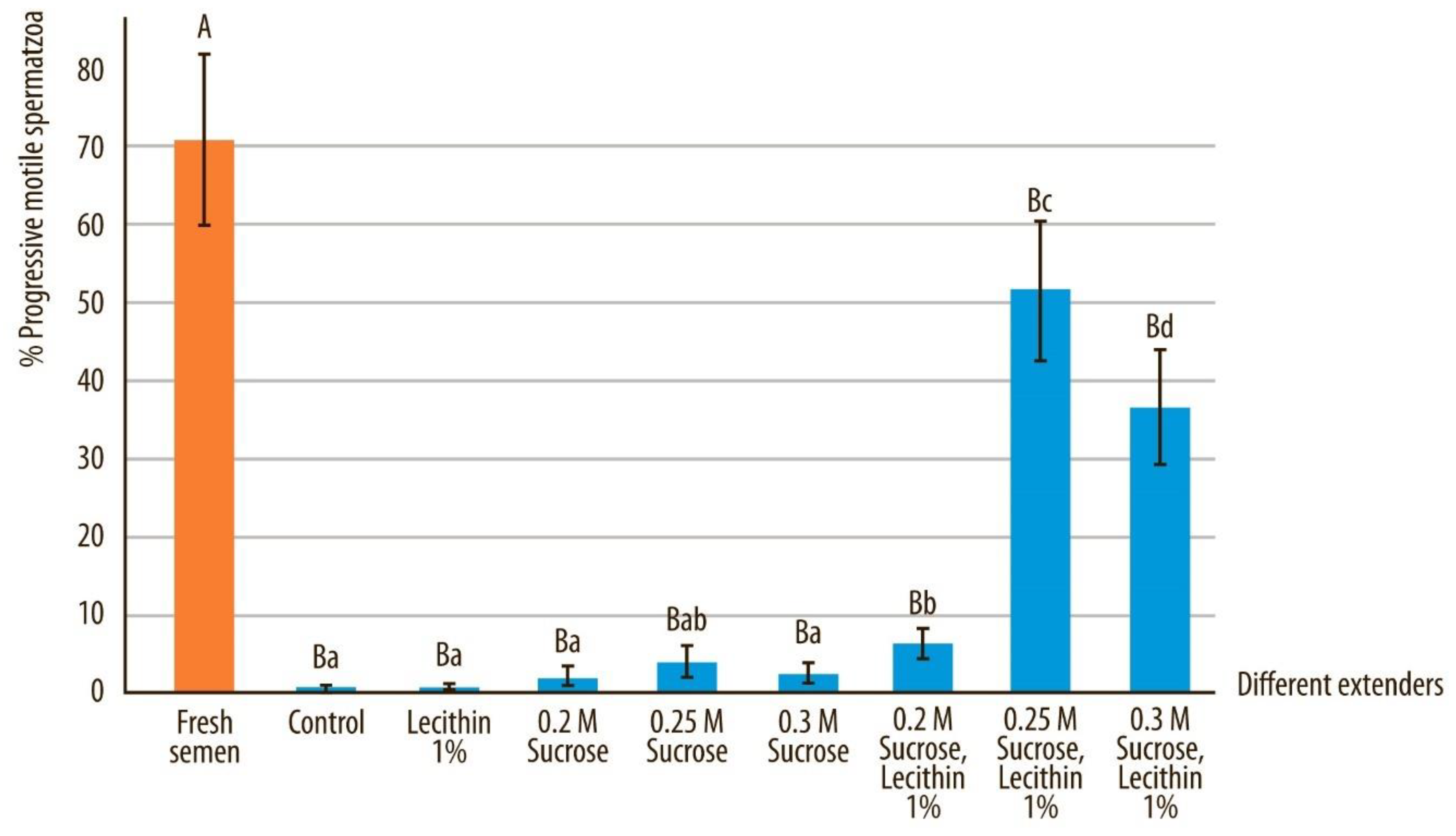
3.1. Semen Motility and Progressive Motility
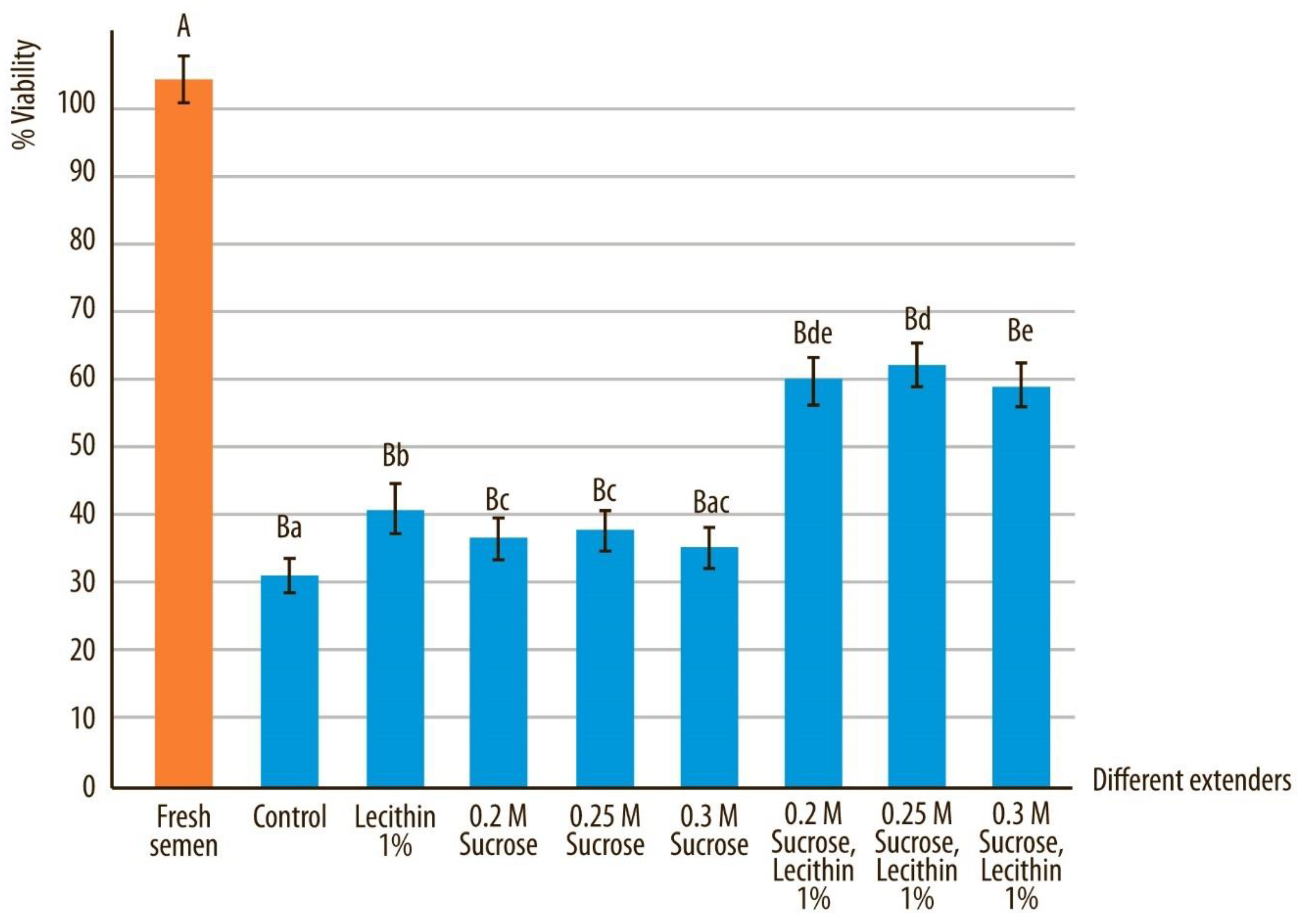
3.2. Viability
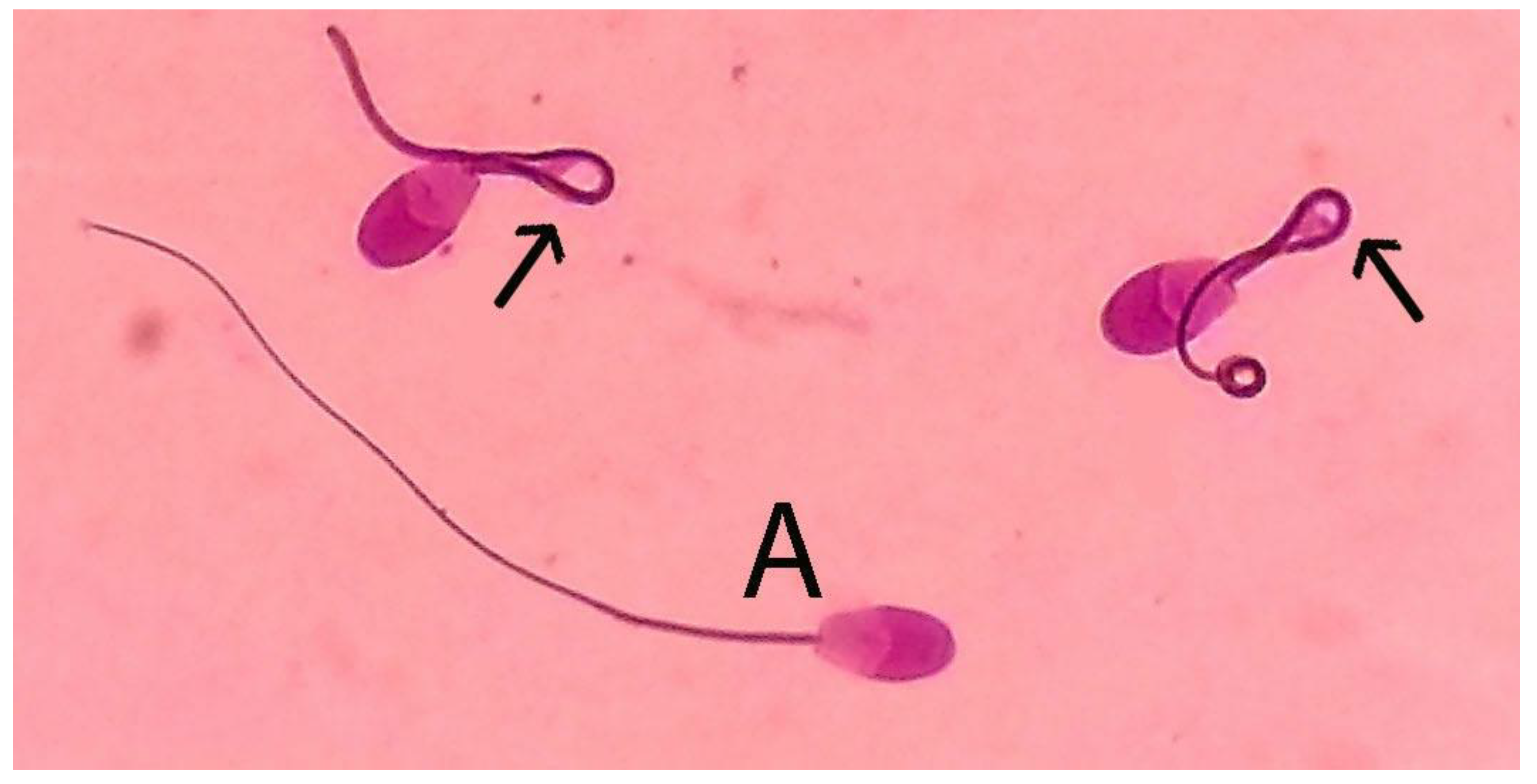
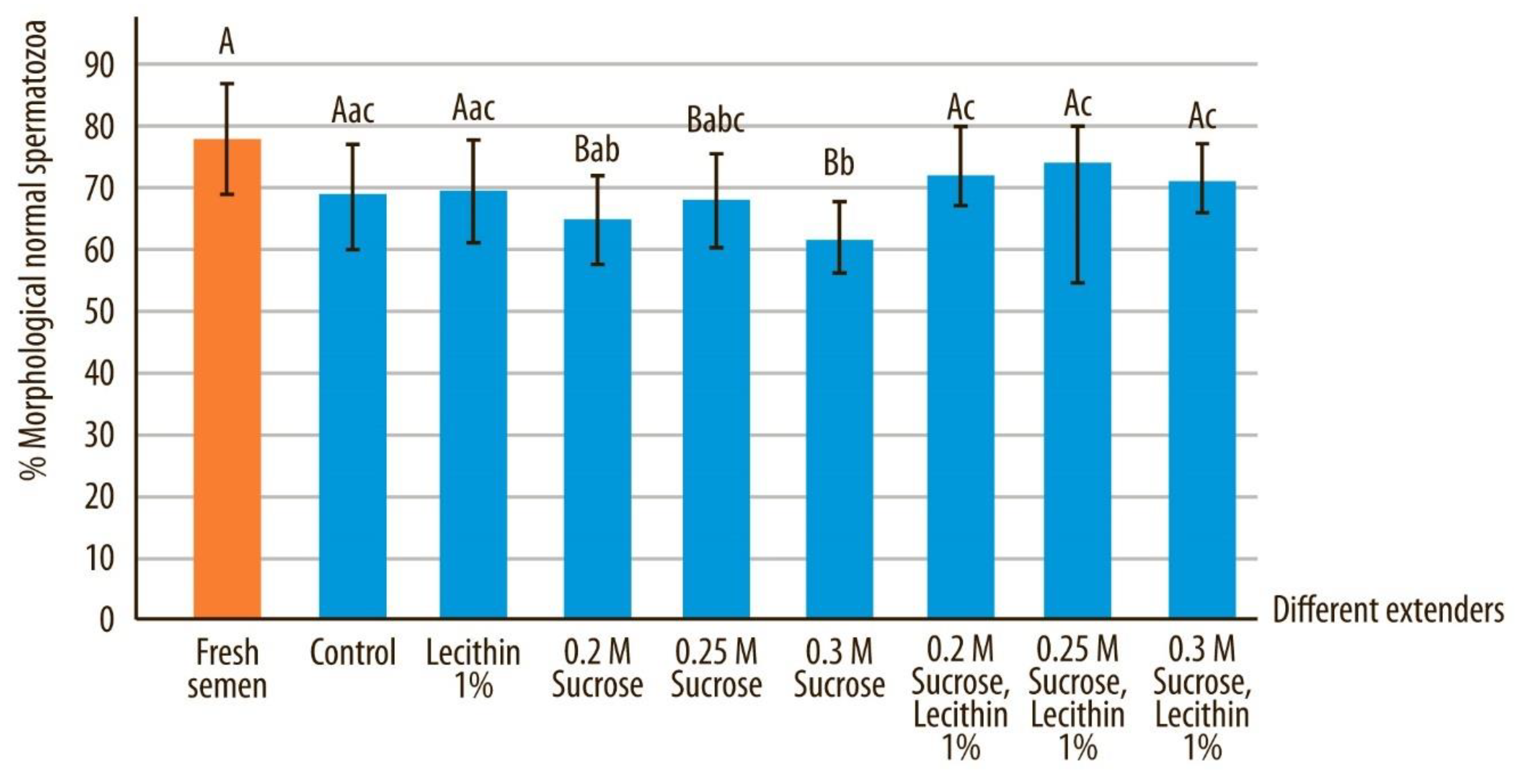
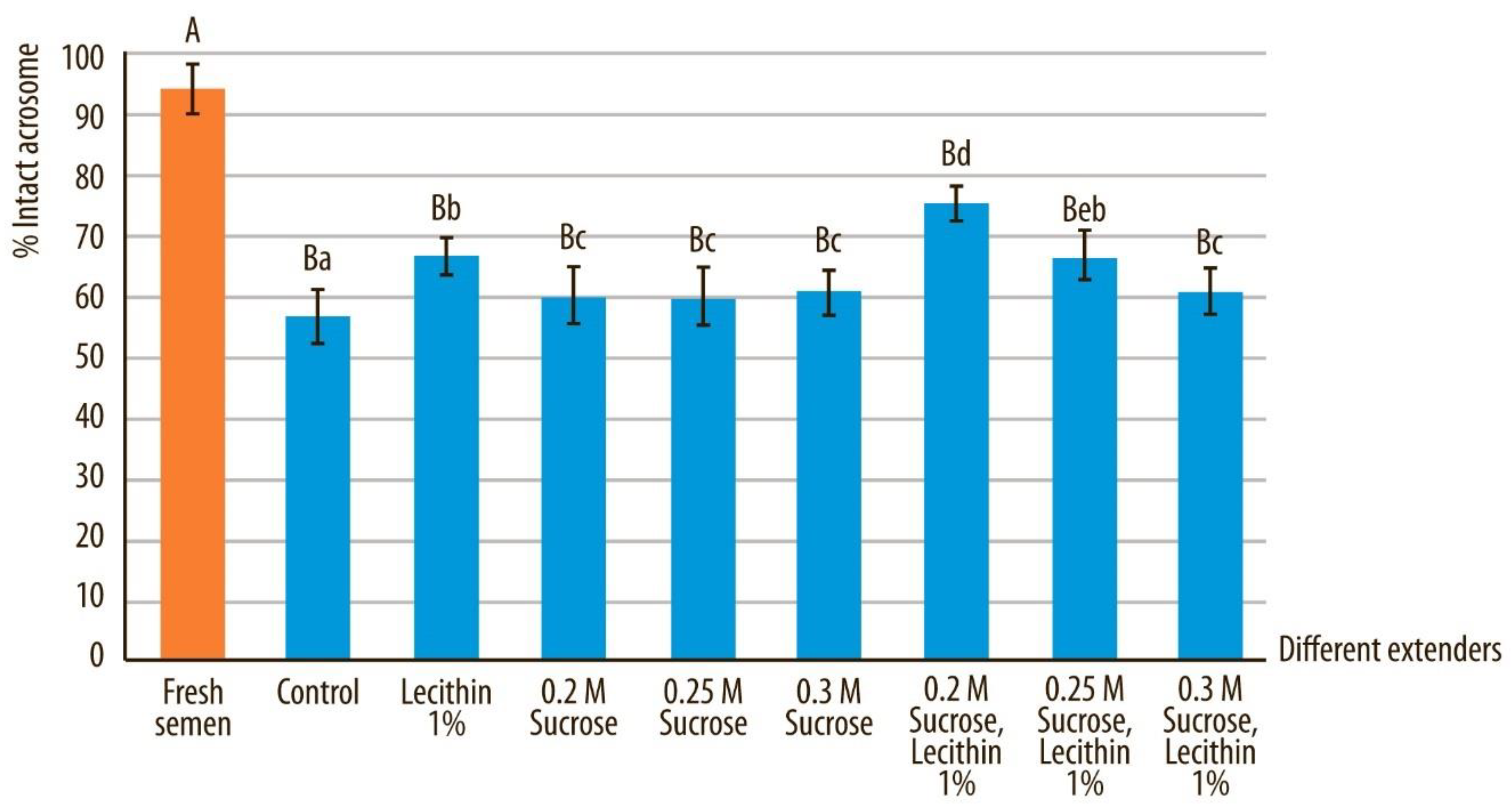
3.3. Normal Morphology and Acrosome-Intact Sperm
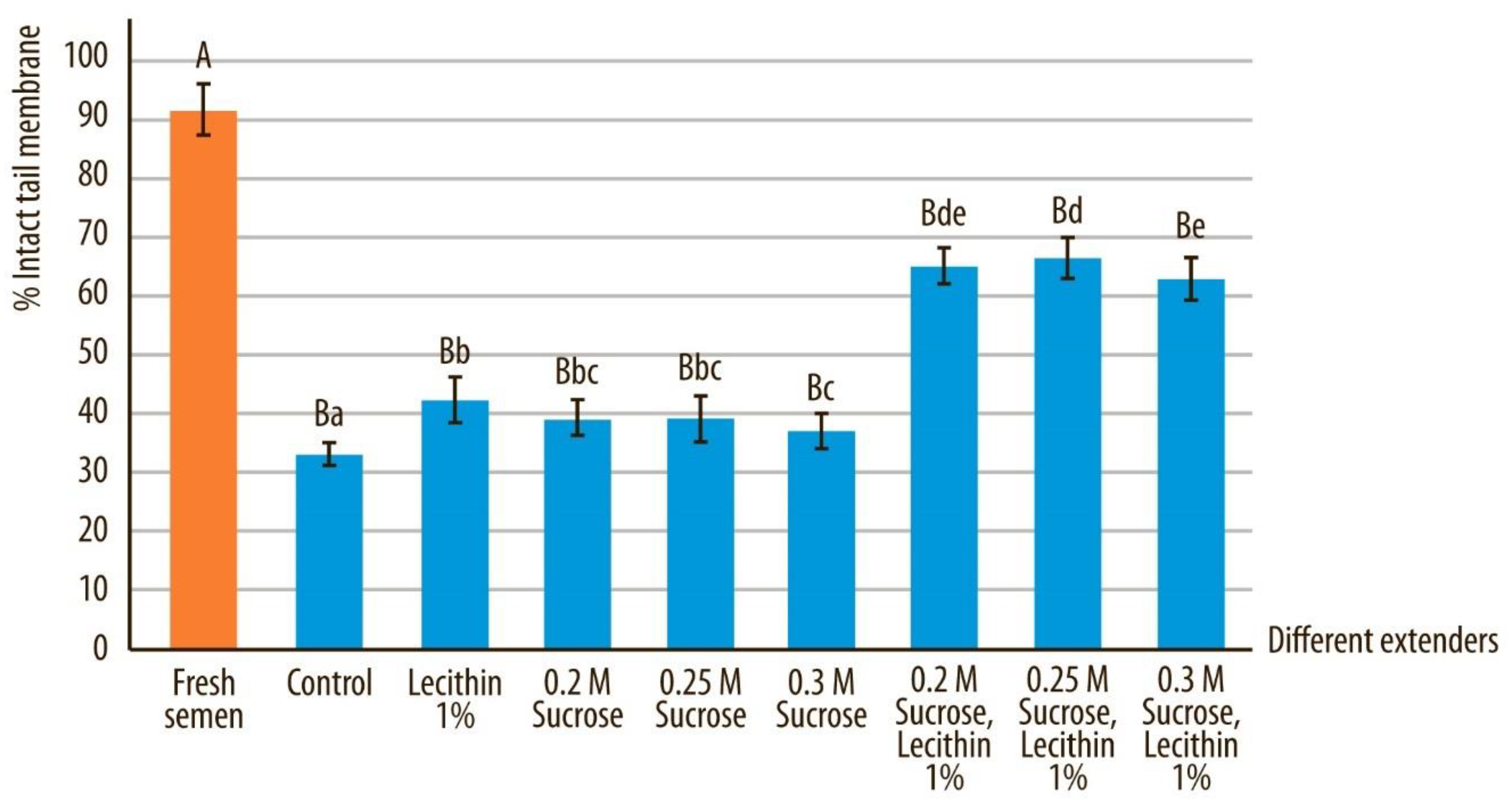
3.4. Tail Membrane Integrity
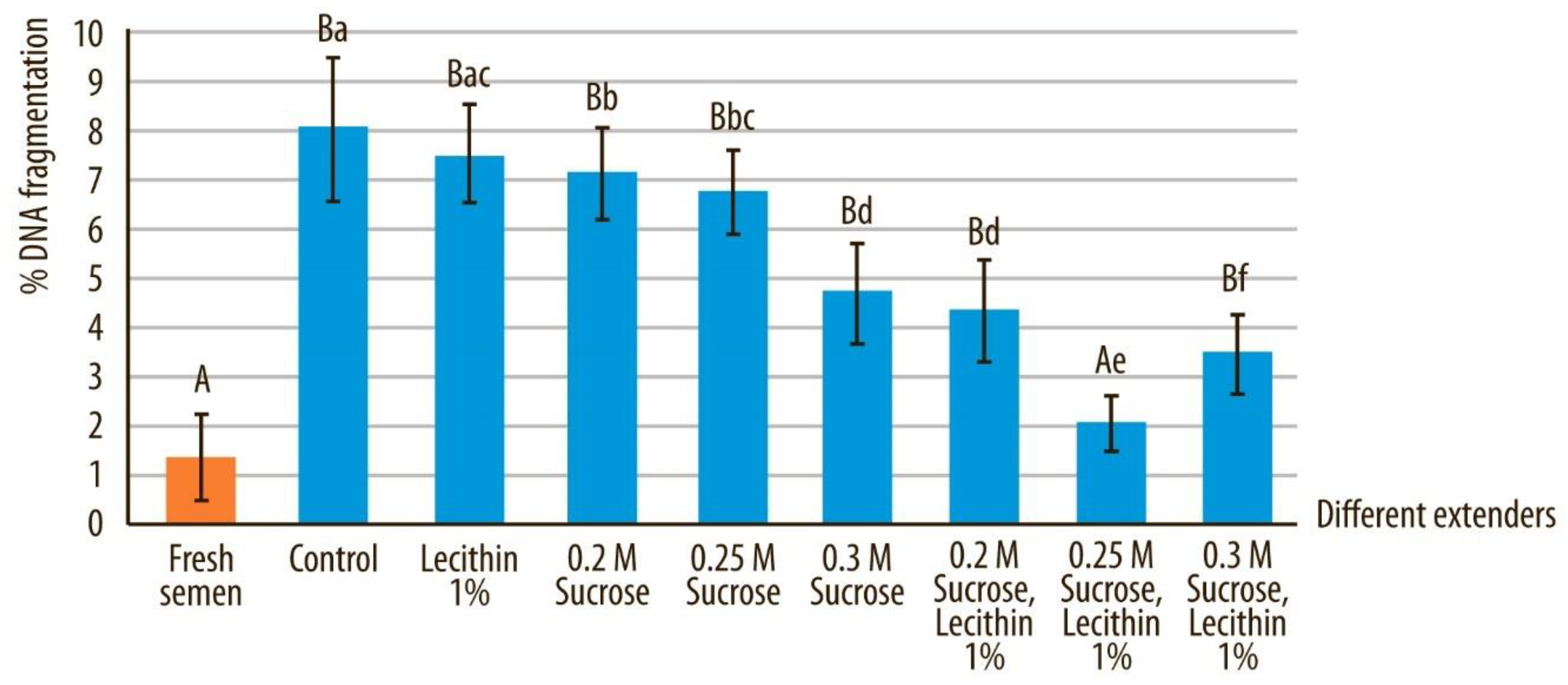
3.5. DNA Integrity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Isachenko, V.; Rahimi, G.; Mallmann, P.; Sanchez, R.; Isachenko, E. Technologies of cryoprotectant-free vitrification of human spermatozoa: asepticity as criterion of effectiveness. Andrology 2017, 5, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Isachenko, E. DNA integrity and motility of human spermatozoa after standard slow freezing versus cryoprotectant-free vitrification. Hum. Reprod. 2004, 19, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Mandawala, A.; Harvey, S.C.; Roy, T.; Fowler, K. Cryopreservation of animal oocytes and embryos: Current progress and future prospects. Theriogenology 2016, 86, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Ozkavukcu, S.; Erdemli, E.; Isik, A.; Öztuna, D.; Karahuseyinoglu, S. Effects of cryopreservation on sperm parameters and ultrastructural morphology of human spermatozoa. J. Assist. Reprod. Genet. 2008, 25, 403–411. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, J.; Srivastava, N.; Ghosh, S. Strategies to Minimize Various Stress-Related Freeze-Thaw Damages During Conventional Cryopreservation of Mammalian Spermatozoa. Biopreservation Biobanking 2019, 17, 603–612. [Google Scholar] [CrossRef]
- Vick, M.; Bateman, H.; Lambo, C.; Swanson, W. Improved cryopreservation of domestic cat sperm in a chemically defined medium. Theriogenology 2012, 78, 2120–2128. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Yang, H.; Kim, Y.-J. Rapid freezing without cooling equilibration in canine sperm. Anim. Reprod. Sci. 2012, 130, 111–118. [Google Scholar] [CrossRef]
- Sánchez, R.; Risopatron, J.; Schulz, M.; Villegas, J.; Isachenko, V.; Kreinberg, R.; Isachenko, E. Canine sperm vitrification with sucrose: effect on sperm function. Andrology 2011, 43, 233–241. [Google Scholar] [CrossRef]
- Pradieé, J.; Esteso, M.; López-Sebastián, A.; A, T.-D.; Castaño, C.; Carrizosa, J.; Urrutia, B.; Santiago-Moreno, J. Successful ultrarapid cryopreservation of wild Iberian ibex (Capra pyrenaica) spermatozoa. Theriogenology 2015, 84, 1513–1522. [Google Scholar] [CrossRef]
- Pradiee, J.; Esteso, M.C.; Castaño, C.; A, T.-D.; Lopez-Sebastián, A.; Guerra, R.; Santiago-Moreno, J. Conventional slow freezing cryopreserves mouflon spermatozoa better than vitrification. Andrology 2016, 49, e12629. [Google Scholar] [CrossRef]
- Li, Y.-X.; Zhou, L.; Lv, M.-Q.; Ge, P.; Liu, Y.-C.; Zhou, D.-X. Vitrification and conventional freezing methods in sperm cryopreservation: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Boil. 2019, 233, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Swanson, W.F.; Bateman, H.; VanSandt, L. Urethral catheterization and sperm vitrification for simplified semen banking in felids. Reprod. Domest. Anim. 2016, 52, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Gharajelar, S.N.; Sadrkhanloo, R.A.; Onsori, M.; Saberivand, A. A comparative study on the effects of different cryoprotectants on the quality of canine sperm during vitrification process. Vet. Res. Forum. Int. Q. J. 2016, 7, 235–239. [Google Scholar]
- Caturla-Sánchez, E.; Sánchez-Calabuig, M.; Gutiérrez, J.F.P.-; Cerdeira, J.; Castaño, C.; Santiago-Moreno, J. Vitrification of dog spermatozoa: Effects of two cryoprotectants (sucrose or trehalose) and two warming procedures. Cryobiology 2018, 80, 126–129. [Google Scholar] [CrossRef]
- Isachenko, E.; Isachenko, V.; Katkov, I.I.; Dessole, S.; Nawroth, F. Vitrification of mammalian spermatozoa in the absence of cryoprotectants: from past practical difficulties to present success. Reprod. Biomed. Online 2003, 6, 191–200. [Google Scholar] [CrossRef]
- Dalmazzo, A.; Losano, J.; Rocha, C.C.; Tsunoda, R.H.; Angrimani, D.D.S.R.; Mendes, C.M.; Assumpção, M.E.O.D.; Nichi, M.; Barnabe, V.H. Effects of Soy Lecithin Extender on Dog Sperm Cryopreservation. Anim. Biotechnol. 2017, 29, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Kustritz, M.V.R. The value of canine semen evaluation for practitioners. Theriogenology 2007, 68, 329–337. [Google Scholar] [CrossRef]
- E Oettlé, E. Sperm morphology and fertility in the dog. J. Reprod. Fertil. Suppl. 1993, 47, 257–260. [Google Scholar]
- Peng, N.; Zou, X.; Li, L. Comparison of different counting chambers using a computer-assisted semen analyzer. Syst. Boil. Reprod. Med. 2015, 61, 307–313. [Google Scholar]
- Watson, P. Use of a Giemsa stain to detect changes in acrosomes of frozen ram spermatozoa. Vet. Rec. 1975, 97, 12–15. [Google Scholar] [CrossRef]
- Hishinuma, M.; Sekine, J. Evaluation of membrane integrity of canine epididymal spermatozoa by short hypoosmotic swelling test with ultrapure water. J. Vet. Med Sci. 2003, 65, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, W.M.Z.W. Sample Size Calculation in Animal Studies Using Resource Equation Approach. Malays. J. Med. Sci. 2017, 24, 101–105. [Google Scholar]
- Isachenko, E.; Isachenko, V.; Weiss, J.M.; Kreienberg, R.; I Katkov, I.; Schulz, M.; I Lulat, A.G.-M.; Risopatron, M.J.; Sanchez, R. Acrosomal status and mitochondrial activity of human spermatozoa vitrified with sucrose. Reproduction 2008, 136, 167–173. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, H.C.; Nikoloska, M.; Ho, H.; Doshi, A.; Maalouf, W. Improved cryopreservation of spermatozoa using vitrification: comparison of cryoprotectants and a novel device for long-term storage. J. Assist. Reprod. Genet. 2019, 36, 1713–1720. [Google Scholar] [CrossRef]
- Hidalgo, M.; Consuegra, C.; Dorado, J.; Diaz-Jimenez, M.; Ortiz, I.; Pereira, B.; Sanchez, R.; Crespo, F. Concentrations of non-permeable cryoprotectants and equilibration temperatures are key factors for stallion sperm vitrification success. Anim. Reprod. Sci. 2018, 196, 91–98. [Google Scholar] [CrossRef]
- Molinia, F.; Evans, G.; Maxwell, C. In vitro evaluation of zwitterion buffers in diluents for freezing ram spermatozoa. Reprod. Nutr. Dev. 1994, 34, 491–500. [Google Scholar] [CrossRef]
- Koshimoto, C.; Gamliel, E.; Mazur, P. Effect of Osmolality and Oxygen Tension on the Survival of Mouse Sperm Frozen to Various Temperatures in Various Concentrations of Glycerol and Raffinose. Cryobiology 2000, 41, 204–231. [Google Scholar] [CrossRef]
- Dalmazzo, A.; Angrimani, D.D.S.R.; Losano, J.; Rocha, C.C.; Sobrinho, C.A.B.; Gurgel, J.R.C.; Pacheco, P.I.M.; Minazaki, C.K.; Crusco, S.E.; Nichi, M.; et al. Insights into soy lecithin and egg yolk-based extenders for chilling canine spermatozoa. Zygote 2018, 27, 17–24. [Google Scholar] [CrossRef]
- Axnér, E.; Lagerson, E. Cryopreservation of Dog Semen in a Tris Extender with 1% or 2% Soya Bean Lecithin as a Replacement of Egg Yolk. Reprod. Domest. Anim. 2016, 51, 262–268. [Google Scholar] [CrossRef]
- Hidalgo, M.; Bayon, J.C.; Galvez, M.J.; Urbano, M.; Ortiz, I.; Dorado, J. Cryopreservation of dog semen using a soybean lecithin-based extender: effect on sperm viability and acrosome integrity. Reprod. Domest. Anim. 2014, 49, 72. [Google Scholar]
- Mertins, O.; Sebben, M.; Schneider, P.H.; Pohlmann, A.R.; Silveira, N. Caracterização da pureza de fosfatidilcolina da soja através de RMN de 1H e de 31 P. Química Nova 2008, 31, 1856–1859. [Google Scholar] [CrossRef][Green Version]
- Aizpurua, J.; Medrano, L.; Enciso, M.; Sarasa, J.; Romero, A.; Fernández, M.; Gómez-Torres, M. New permeable cryoprotectant-free vitrification method for native human sperm. Hum. Reprod. 2017, 32, 2007–2015. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, M.; Arakkal, D.; Mangalaraj, A.M.; Kamath, M.S. Comparison of Conventional Slow Freeze versus Permeable Cryoprotectant-Free Vitrification of Abnormal Semen Sample: A Randomized Controlled Trial. J. Hum. Reprod. Sci. 2019, 12, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Le, M.T.; Nguyen, T.T.T.; Nguyen, T.T.; Nguyen, V.T.; Nguyen, T.T.A.; Nguyen, H.V.Q.; Cao, N.T. Cryopreservation of human spermatozoa by vitrification versus conventional rapid freezing: Effects on motility, viability, morphology and cellular defects. Eur. J. Obstet. Gynecol. Reprod. Boil. 2019, 234, 14–20. [Google Scholar] [CrossRef]
- Agha-Rahimi, A.; Khalili, M.A.; Nabi, A.; Ashourzadeh, S. Vitrification is not superior to rapid freezing of normozoospermic spermatozoa: effects on sperm parameters, DNA fragmentation and hyaluronan binding. Reprod. Biomed. Online 2014, 28, 352–358. [Google Scholar] [CrossRef][Green Version]
- Risopatron, J.; Catalán, S.; Miska, W.; Schill, W.-B.; Sánchez, R. Effect of albumin and polyvinyl alcohol on the vitality, motility and acrosomal integrity of canine spermatozoa incubated in vitro. Reprod. Domest. Anim. 2002, 37, 347–351. [Google Scholar] [CrossRef]
| Control | Ext. A | Ext. B | Ext. C | Ext. D | Ext. E | Ext. F | Ext. G | |
|---|---|---|---|---|---|---|---|---|
| Tris | 2.4 g | 2.4 g | 2.4 g | 2.4 g | 2.4 g | 2.4 g | 2.4 g | 2.4 g |
| Citric Acid | 1.4 g | 1.4 g | 1.4 g | 1.4 g | 1.4 g | 1.4 g | 1.4 g | 1.4 g |
| Fructose | 1.0 g | 1.0 g | 1.0 g | 1.0 g | 1.0 g | 1.0 g | 1.0 g | 1.0 g |
| Streptomycin | 5 µg | 5 µg | 5 µg | 5 µg | 5 µg | 5 µg | 5 µg | 5 µg |
| Penicillin | 10kIU | 10kIU | 10kIU | 10kIU | 10kIU | 10kIU | 10kIU | 10kIU |
| Sucrose | None | None | 0.2 M | 0.25 M | 0.3 M | 0.2 M | 0.25 M | 0.3 M |
| Soy lecithin | None | 1% | None | None | None | 1% | 1% | 1% |
| Morphological Defects | Fresh Semen | Control | Ext. A | Ext. B | Ext. C | Ext. D | Ext. E | Ext. F | Ext. G |
|---|---|---|---|---|---|---|---|---|---|
| Head | 2.11 ± 2.71A | 2.75 ± 2.12 Aa | 4.85 ± 1.30 Bb | 3.30 ± 2.75 Aa | 2.93 ± 2.35 Aa | 3.95 ± 3.05 Bb | 2.33 ± 2.03 Aa | 2.20 ± 1.96 Aa | 2.45 ± 1.87 Aa |
| Detached acrosome | 0.78 ± 1.23 A | 4.85 ± 1.30 Ba | 4.75 ±1.49 Ba | 5.38 ± 1.30 Bb | 5.20 ± 1.06 Bab | 6.25 ± 1.40 Bc | 4.73 ± 1.26 Ba | 4.43 ± 0.85 Ba | 5.03 ± 1.28 Ba,b |
| Midpiece defects | 2.93 ± 2.91 A | 2.83 ± 1.89 Aa | 3.00 ± 2.99 Aa | 3.25 ± 2.56 Aa | 3.13 ± 2.34 Aa | 3.33 ± 2.68 Aa | 2.58 ± 2.68 Aa | 2.38 ± 2.74 Aa | 3.03 ± 3.28 Aa |
| Proximal droplets | 2.33 ±1.75 A | 4.03 ± 2.38 Aa | 3.43 ± 2.23 Aa | 4.03 ± 2.00 Aa | 3.83 ± 1.87 Aa | 4.63 ± 2.16 a | 3.05 ± 2.17 Aa | 3.13 ± 2.34 Aa | 3.10 ± 2.15 Aa |
| Distal droplets | 4.00 ± 5.60A | 5.73 ± 3.81 Aa | 4.95 ± 3.48 Ab | 5.43 ± 3.18 Aa | 5.28 ± 2.61 Aa | 6.10 ± 2.91 Aa | 4.30 ± 3.35 Ab | 4.10 ± 2.90 Ab | 4.35 ± 3.52 Ab |
| Bent tail | 2.78 ± 1.79 A | 5.20 ± 2.73 Ba | 5.45 ± 2.76 Ba | 7.03 ± 3.30 Bb | 5.60 ± 2.90 Ba | 7.70 ± 3.51 Bb | 5.15 ± 2.68 Ba | 4.70 ± 2.78 Ba | 5.95 ± 3.04 Ba |
| Coiled tail | 6.06 ± 6.71 A | 6.95 ± 4.41 Aa | 6.80 ± 4.75 Aa | 7.63 ± 3.98 Aa | 6.95 ± 4.29 Aa | 7.93 ± 3.79 Aa | 6.28 ± 3.94 Aa | 5.68 ± 3.43 Aa | 6.13 ± 3.40 Aa |
| Normal sperm | 77.39 ± 10.51 A | 68.85 ± 8.01 Bac | 69.73 ± 8.38 Bac | 64.7 0 ± 7.22 Bab | 68.20 ± 7.58 Babc | 61.40 ± 6.68 Bb | 72.18 ± 7.51 Ac | 74.15 ± 6.02 Ac | 70.48 ± 6.92 Bc |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakošek Pipan, M.; Casal, M.L.; Šterbenc, N.; Virant Klun, I.; Mrkun, J. Vitrification Using Soy Lecithin and Sucrose: A New Way to Store the Sperm for the Preservation of Canine Reproductive Function. Animals 2020, 10, 653. https://doi.org/10.3390/ani10040653
Zakošek Pipan M, Casal ML, Šterbenc N, Virant Klun I, Mrkun J. Vitrification Using Soy Lecithin and Sucrose: A New Way to Store the Sperm for the Preservation of Canine Reproductive Function. Animals. 2020; 10(4):653. https://doi.org/10.3390/ani10040653
Chicago/Turabian StyleZakošek Pipan, Maja, Margret L. Casal, Nataša Šterbenc, Irma Virant Klun, and Janko Mrkun. 2020. "Vitrification Using Soy Lecithin and Sucrose: A New Way to Store the Sperm for the Preservation of Canine Reproductive Function" Animals 10, no. 4: 653. https://doi.org/10.3390/ani10040653
APA StyleZakošek Pipan, M., Casal, M. L., Šterbenc, N., Virant Klun, I., & Mrkun, J. (2020). Vitrification Using Soy Lecithin and Sucrose: A New Way to Store the Sperm for the Preservation of Canine Reproductive Function. Animals, 10(4), 653. https://doi.org/10.3390/ani10040653






