Urinalysis in Great Dane Puppies from Birth to 28 Days of Age
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ethics
2.3. Sample Collection and Urinalysis
2.4. Statistical Analysis
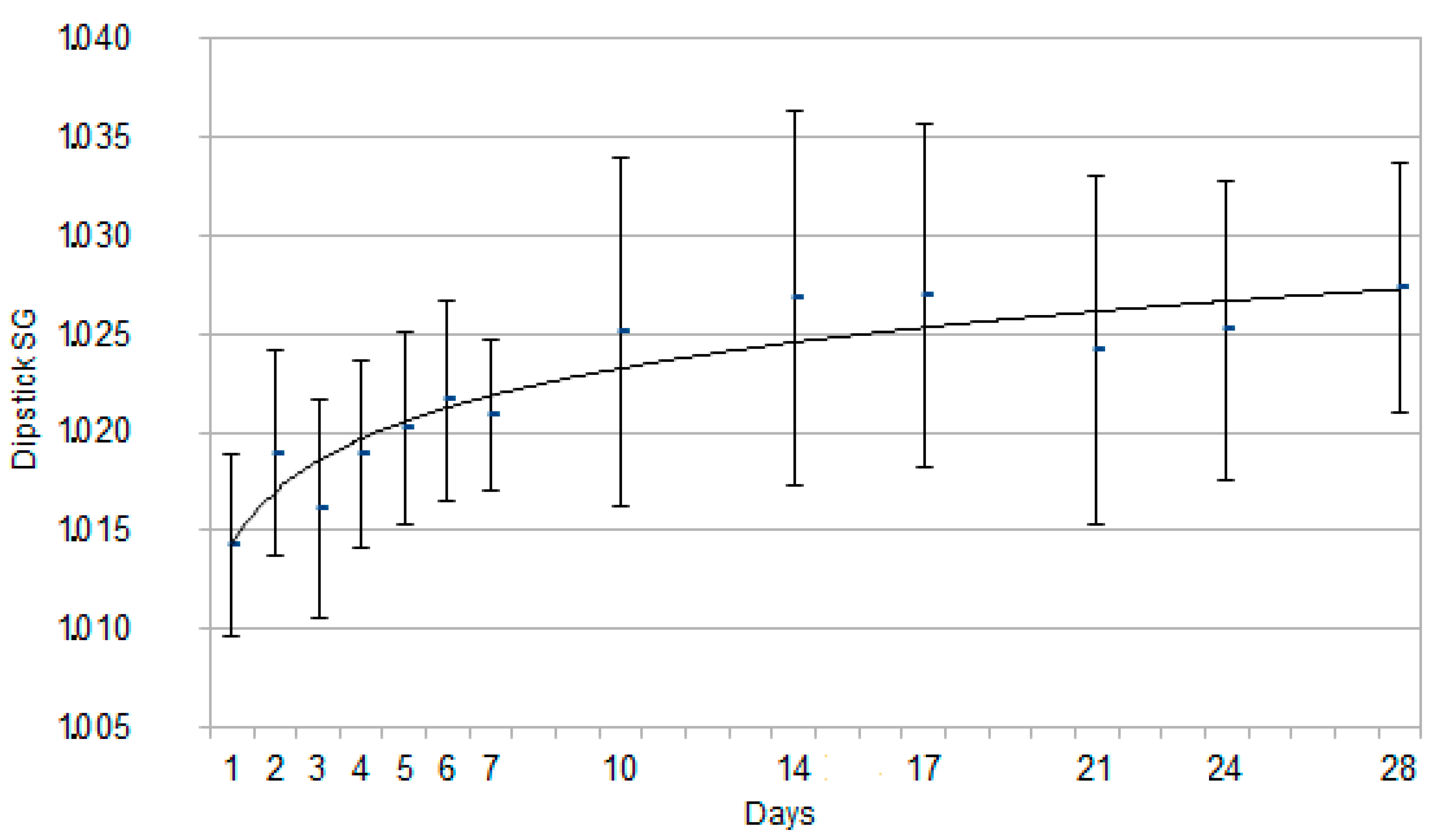
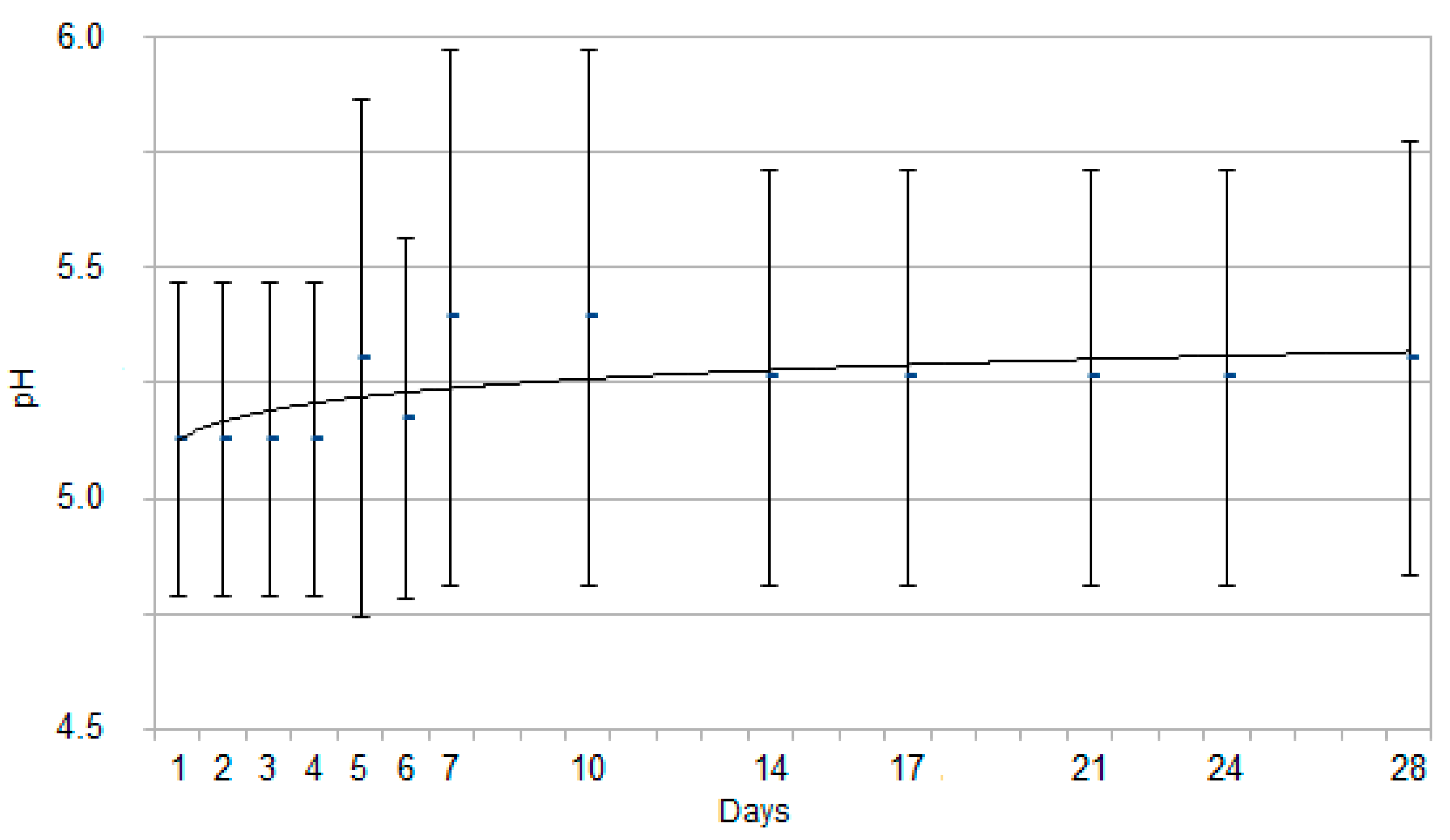
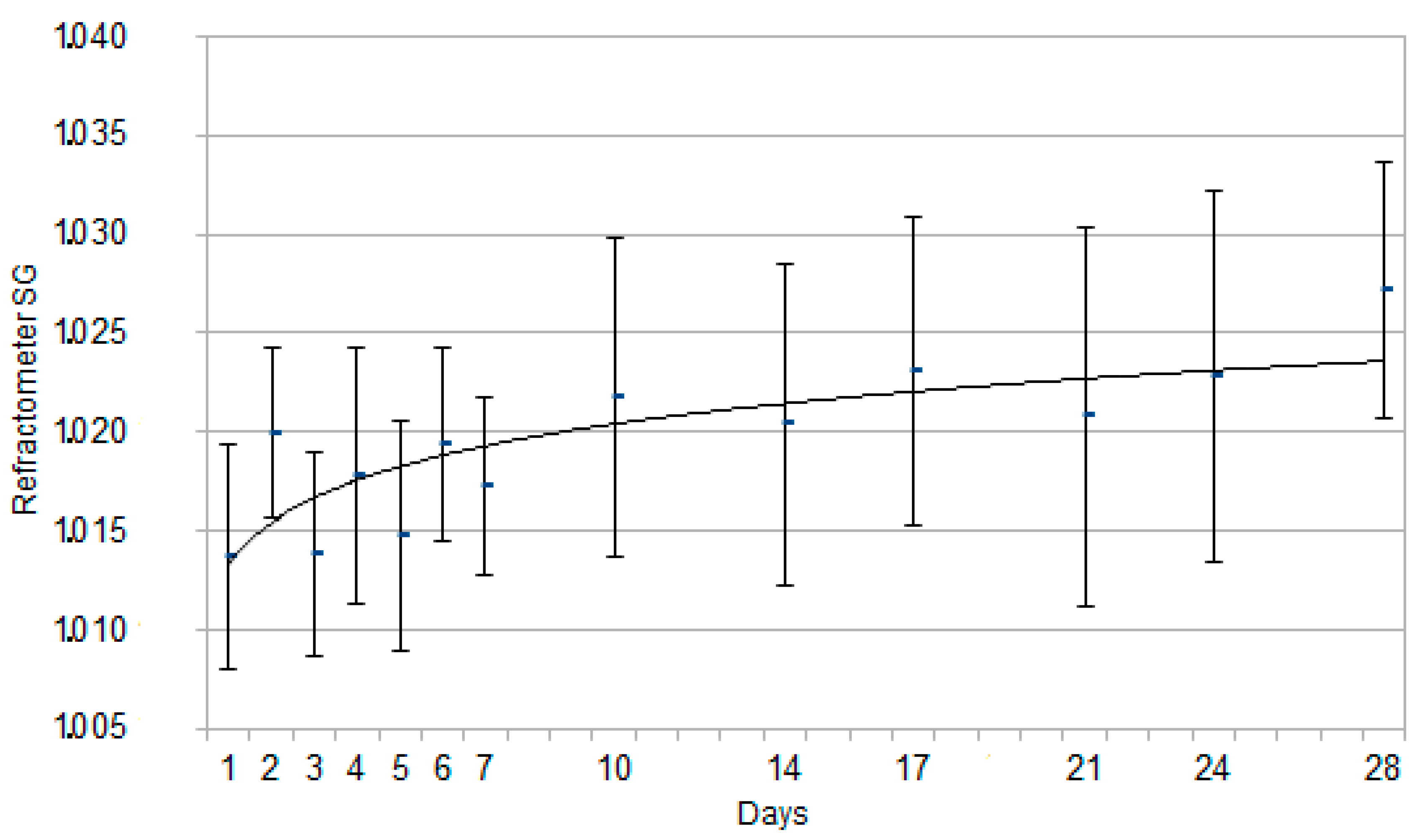
3. Results
3.1. Animals
3.2. Urinalysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Veronesi, M.C. Neonatologia del cane e del gatto: patologie neonatali. In Neonatologia Veterinaria, 1st ed.; Veronesi, M.C., Castagnetti, C., Taverne, M.A.M., Eds.; EdiSES: Naples, Italy, 2013; pp. 93–140. [Google Scholar]
- Veronesi, M.; Panzani, S.; Faustini, M.; Rota, A. An Apgar scoring system for routine assessment of newborn puppy viability and short-term survival prognosis. Theriogenology 2009, 72, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Mila, H.; Grellet, A.; Delebarre, M.; Mariani, C.; Feugier, A.; Chastant-Maillard, S. Monitoring of the newborn dog and prediction of neonatal mortality. Prev. Vet. Med. 2017, 143, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Balogh, O.; Roch, M.; Keller, S.; Michel, E.; Reichler, I. The use of semi-quantitative tests at Cesarean section delivery for the differentiation of canine fetal fluids from maternal urine on the basis of biochemical characteristics. Theriogenology 2017, 88, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, M.; Bolis, B.; Faustini, M.; Rota, A.; Mollo, A. Biochemical composition of fetal fluids in at term, normal developed, healthy, viable dogs and preliminary data from pathologic littermates. Theriogenology 2018, 108, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Utsch, B.; Klaus, G. Urinalysis in Children and Adolescents. Dtsch. Aerzteblatt Online 2014, 111, 617–626. [Google Scholar] [CrossRef]
- Faulks, R.D.; Lane, I.F. Qualitative urinalysis in puppies 0 to 24 weeks of age. J. Am. Anim. Hosp. Assoc. 2003, 39, 369–378. [Google Scholar] [CrossRef]
- Clement, M.G. Minzione. In Fisiologia Degli Animali Domestici con Elementi di Etologia, 2nd ed.; Aguggini, G., Beghelli, V., Clement, M.G., D’Angelo, A., Debenedetti, A., Facello, C., Giulio, L.F., Guglielmino, R., Lucaroni, A., Maffeo, G., et al., Eds.; UTET: Turin, Italy, 2002; pp. 650–652. [Google Scholar]
- Lane, I.F.; Shaw, D.H.; Burton, S.A.; Donald, A.W. Quantitative urinalysis in healthy Beagle puppies from 9 to 27 weeks of age. Am. J. Vet. Res. 2000, 61, 577–581. [Google Scholar] [CrossRef]
- Sterpellone, L. Raccolta dei campioni. In L’esame dell’urina, 1st ed.; Sterpellone, L., Ed.; Società Editrice Universo: Rome, Italy, 1983; pp. 22–24. [Google Scholar]
- Sørensen, T.M.; Jensen, A.; Damborg, P.; Bjornvad, C.; Guardabassi, L.; Jessen, L.R. Evaluation of different sampling methods and criteria for diagnosing canine urinary tract infection by quantitative bacterial culture. Vet. J. 2016, 216, 168–173. [Google Scholar] [CrossRef]
- Lage, A.L. Neonatal clinical nephrology. In Current Veterinary Therapy; Kirk, R.W., Ed.; Saunders Co.: Philadelphia, PA, USA, 1980; Volume 7, pp. 1085–1087. [Google Scholar]
- Kruger, J.M.; Osborne, C.A.; Lulich, J.P.; Polzin, D.P.; Fitzgerald, S.D. The urinary system. In Veterinary Pediatrics: Dogs and Cats from Birth to Six Months, 3rd ed.; Hoskins, J.D., Ed.; WB Saunders: Philadelphia, PA, USA, 2001; pp. 371–401. [Google Scholar]
- Osborne, C.A.; Stevens, J.B.; Lulich, J.P. A clinician’s analysis of urinalysis. In Canine and Feline Nephrology and Urology; Osborne, C.A., Finco, D.R., Eds.; Williams and Wilkins: Baltimore, MD, USA, 1995; pp. 136–205. [Google Scholar]
- Clement, M.G. Urine: pH. In Fisiologia Degli Animali Domestici con Elementi di Etologia, 2nd ed.; Aguggini, G., Beghelli, V., Clement, M.G., D’Angelo, A., Debenedetti, A., Facello, C., Giulio, L.F., Guglielmino, R., Lucaroni, A., Maffeo, G., et al., Eds.; UTET: Turin, Italy, 2002; p. 644. [Google Scholar]
- Van Der Weyden, G.C.; A Taverne, M.; Dieleman, S.J.; Wurth, Y.; Bevers, M.M.; A Van Oord, H. Physiological aspects of pregnancy and parturition in dogs. J. Reprod. Fertil. Suppl. 1989, 39, 211–224. [Google Scholar]
- Fettman, M.J.; Allen, T.A. Developmental aspects of fluid and electrolyte metabolism and renal function in neonates. Comp. Cont. Edu. Pract. Vet. 1991, 13, 392–403. [Google Scholar]
- Lee, J.A.; Cohn, L.A. Fluid Therapy for Pediatric Patients. Vet. Clin. North Am. Small Anim. Pr. 2017, 47, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Dial, S.M. Hematology, chemistry profile and urinalysis of pediatric patients. Comp. Cont. Edu. Pract. Vet. 1992, 14, 305–308. [Google Scholar]
- Schäfer-Somi, S.; Bar-Schadler, S.; Aurich, J.E. Proteinuria and immunoglobinuria in neonatal dogs. Vet. Rec. 2005, 157, 378–382. [Google Scholar] [CrossRef]
- Laroute, V.; Chetboul, V.; Roche, L.; Maurey, C.; Costes, G.; Pouchelon, J.-L.; De La Farge, F.; Boussouf, M.; Lefebvre, H. Quantitative evaluation of renal function in healthy Beagle puppies and mature dogs. Res. Vet. Sci. 2005, 79, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Bovee, K.C.; Jezyk, P.F.; Segal, S.C. Postnatal development of renal tubular amino acid reabsorption in canine pups. Am. J. Vet. Res. 1984, 45, 830–832. [Google Scholar]
- Horster, M.; Valtin, H. Postnatal development of renal function: micropuncture and clearance studies in the dog. J. Clin. Investig. 1971, 50, 779–795. [Google Scholar] [CrossRef]
- Lees, G.; Helman, R.; Homco, L.; Millichamp, N.; Hunter, J.; Frey, M. Early diagnosis of familial nephropathy in English cocker spaniels. J. Am. Anim. Hosp. Assoc. 1998, 34, 189–195. [Google Scholar] [CrossRef]
- Nielsen, L.N.; Jensen, A.L.; Kristensen, A.T.; Kjelgaard-Hansen, M. Breed-specific variation of hematologic and biochemical analytes in healthy adult Bernese Mountain dogs. Vet. Clin. Pathol. 2010, 39, 20–28. [Google Scholar] [CrossRef]
- Chang, Y.-M.; Hadox, E.; Szladovits, B.; Garden, O.A. Serum Biochemical Phenotypes in the Domestic Dog. PLOS ONE 2016, 11, e0149650. [Google Scholar] [CrossRef]
- Cox, M.L.; Lees, G.E.; Kashtan, C.E.; Murphy, K.E. Genetic cause of X-linked Alport syndrome in a family of domestic dogs. Mamm. Genome 2003, 14, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Melandri, M.; Barella, G.; Alonge, S. Assessment of the optimal age for a preventive ultrasonographic screening of the uterine health in bitches. Reprod. Domest. Anim. 2019, 54, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Ahlstrom, O.; Biagi, G.; Dobenecker, B.; Hendricks Hesta, M.; Iben, C.; Nguyen, P.; Paragon, B.; Villaverde, C.; Zentek, J. Nutritional Guidelines for Complete and Complementary Pet Food for Cats and Dogs; FEDIAF: Bruxelles, Belgium. Available online: http://www.fediaf.org (accessed on 1 November 2016).
- Alonge, S.; Melandri, M.; Meloni, T.; Morselli, M.G.; Luvoni, G.C. Could a single P4 determination predict the days for successful breedings in bitches? In Proceedings of the 16th European Veterinary Society for Small Animal Reproduction (EVSSAR) Congress, Toulouse, France, 5–6 July 2013; p. 135. [Google Scholar]
- Alonge, S.; Melandri, M.; Leoci, R.; Lacalandra, G.M.; Caira, M.; Aiudi, G.G. The Effect of Dietary Supplementation of Vitamin E, Selenium, Zinc, Folic Acid, and N-3 Polyunsaturated Fatty Acids on Sperm Motility and Membrane Properties in Dogs. Animals 2019, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Alonge, S.; Melandri, M. Effect of delivery management on first-week neonatal outcome: How to improve it in Great Danes. Theriogenology 2019, 125, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Alonge, S.; Beccaglia, M.; Melandri, M.; Luvoni, G.C. Prediction of whelping date in large and giant canine breeds by ultrasonography foetal biometry. J. Small Anim. Pr. 2016, 57, 479–483. [Google Scholar] [CrossRef]
- Beccaglia, M.; Alonge, S.; Trovo’, C.; Luvoni, G.C. Determination of gestational time and prediction of parturition in dogs and cats: An update. Reprod. Domest. Anim. 2016, 51, 12–17. [Google Scholar] [CrossRef]
- Alonge, S.; Mauri, M.; Faustini, M.; Luvoni, G.C. Feto-maternal heart rate ratio in pregnant bitches: Effect of gestational age and maternal size. Reprod. Domest. Anim. 2016, 51, 688–692. [Google Scholar] [CrossRef]
- Melandri, M.; Alonge, S.; Peric, T.; Bolis, B.; Veronesi, M. Effects of Alfaxalone or Propofol on Giant-Breed Dog Neonates Viability During Elective Caesarean Sections. Animals 2019, 9, 962. [Google Scholar] [CrossRef]
- Meloni, T.; Comin, A.; Rota, A.; Peric, T.; Contri, A.; Veronesi, M. IGF-I and NEFA concentrations in fetal fluids of term pregnancy dogs. Theriogenology 2014, 81, 1307–1311. [Google Scholar] [CrossRef]
- Melandri, M.; Alonge, S.; Veronesi, M.C. Factors affecting neonatal bodyweight and growth rate in Great Dane puppies. In Proceedings of the 19th European Veterinary Society for Small Animal Reproduction (EVSSAR) Congress, Paris, France, 22–25 June 2016; p. 115. [Google Scholar]
- Melandri, M.; Barella, G.; Aiudi, G.G.; Lacalandra, G.M.; Alonge, S. Colour Flow Mapping examination: An useful screening test for the early diagnosis of ductus venosus patency in canine newborns. Reprod. Domest. Anim. 2018, 53, 1130–1135. [Google Scholar] [CrossRef]
- Vonderen, I.K.; Kooistra, H.S.; Rijnberk, A. Intra- and Interindividual Variation in Urine Osmolality and Urine Specific Gravity in Healthy Pet Dogs of Various Ages. J. Vet. Intern. Med. 1997, 11, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Fiszdon, K.; Kowalczyk, I. Litter size, puppy weight at birth and growth rates in different breeds of dogs. J. Anim. Sci. 2009, 46, 161–168. [Google Scholar]
- Xing, L.; Nørregaard, R. Influence of sex on aquaporin1–4 and vasopressin V2 receptor expression in the pig kidney during development. Pediatr. Res. 2016, 80, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Willard, M.D.; Tvedten, H. Nozioni generali di laboratorio. In Diagnostica di Laboratorio Nei Piccoli Animali; Willard, M.D., Tvedten, H., Eds.; Elsevier: Milan, Italy, 2007; pp. 1–13. [Google Scholar]
- Rahill, W.J.; Subramanian, S. The use of fetal animals to investigate renal development. Lab. Anim. Sci. 1973, 23, 92–96. [Google Scholar] [PubMed]
- Kleinman, L.I.; Lubbe, R.J. Factors affecting the maturation of glomerular filtration rate and renal plasma flow in the new-born dog. J. Physiol. 1972, 223, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.A. The urinary system. In Veterinary Pediatrics: Dogs and Cats from Birth to Six Months, 3rd ed.; Hoskins, J.D., Ed.; WB Saunders: Philadelphia, PA, USA, 1990; pp. 271–292. [Google Scholar]
- Vannucchi, C.I.; Kishi, D.; Regazzi, F.; Silva, L.; Veiga, G.; Angrimani, D.D.S.R.; Lúcio, C.; Nichi, M. The Oxidative Stress, Antioxidant Profile and Acid-base Status in Preterm and Term Canine Neonates. Reprod. Domest. Anim. 2015, 50, 240–246. [Google Scholar] [CrossRef]
- Knopp, J.L.; Pretty, C.G.; Alsweiler, J.M.; Lynn, A.; Chase, J.G. Insulin kinetics and the Neonatal Intensive Care Insulin–Nutrition–Glucose (NICING) model. Math. Biosci. 2017, 284, 61–70. [Google Scholar]
- Hays, S.P.; Smith, E.O.; Sunehag, A.L. Hyperglycemia Is a Risk Factor for Early Death and Morbidity in Extremely Low Birth-Weight Infants. Pediatrics 2006, 118, 1811–1818. [Google Scholar] [CrossRef]
- Di Bartola, S.P. Nephropathy: Clinical approach and laboratory evaluation. In Textbook of Veterinary Internal Medicine, 6th ed.; Ettinger, S.J., Feldman, E.C., Eds.; Elsevier Masson: Milan, Italy, 2007; pp. 1756–1770. [Google Scholar]
- Moxey-Mims, M. Hematuria and proteinuria. In Clinical Pediatric Nephrology, 2nd ed.; Informa Healthcare: London, UK, 2006; pp. 129–141. [Google Scholar]
- Roberts, K.B. Urinary Tract Infection: Clinical Practice Guideline for the Diagnosis and Management of the Initial UTI in Febrile Infants and Children 2 to 24 Months. Pediatrics 2011, 128, 595–610. [Google Scholar]
- Armengol, C.E.; Hendley, J.O.; Schlager, T.A. Should we abandon standard microscopy when screening for urinary tract infections in young children? Pediatr. Infect. Dis. J. 2001, 20, 1176–1177. [Google Scholar] [CrossRef]
- Glissmeyer, E.W.; Korgenski, E.K.; Wilkes, J.; Schunk, J.E.; Sheng, X.; Blaschke, A.J.; Byington, C. Dipstick Screening for Urinary Tract Infection in Febrile Infants. Pediatrics 2014, 133, e1121–e1127. [Google Scholar] [CrossRef] [PubMed]
- Hogg, R.J. Adolescents with proteinuria and/or the nephrotic syndrome. Adolesc. Med. Clin. 2005, 16, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Barsanti, A.J.; Lees, G.E.; Willard, M.D.; Green, R.A. Urinary alterations. In Laboratory Analysis in Small Animals, 4th ed.; Willard, M.D., Tvedten, H., Eds.; Elsevier Masson: Milan, Italy, 2007; pp. 59–130. [Google Scholar]
| Combur-Test® Parameter | Semi-Quantitative Results Corresponding to the Dipstick Visual Reference Colorimetric Scale | ||||||
|---|---|---|---|---|---|---|---|
| Specific gravity kg/L | 1.000 | 1.005 | 1.010 | 1.015 | 1.020 | 1.025 | 1.030 |
| pH | 5 | 6 | 7 | 8 | 9 | ||
| Leukocytes cells/μL | Negative | 10–25 (1+) | 75 (2+) | 500 (3+) | |||
| Nitrites mg/dL | Negative | >0.05 (1+) | |||||
| Proteins mg/dL | Negative | 30 (1+) | 100 (2+) | 500 (3+) | |||
| Glucose mg/dL | Negative | 50 (1+) | 100 (2+) | 300 (3+) | 1000 (4+) | ||
| Ketones mg/dL | Negative | 10 (1+) | 50 (2+) | 150 (3+) | |||
| Urobilinogen mg/dL | Negative | 1 (1+) | 4 (2+) | 8 (3+) | 12 (4+) | ||
| Bilirubin mg/dL | Negative | 0.5 (1+) | (2+) | 6 (3+) | |||
| Blood erythrocytes/µL | Negative | 5–10 (1+) | 25 (2+) | 50 (3+) | 250 (4+) | ||
| Day | Leukocytes Cells/μL | Proteins mg/dL | Blood ery/μL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervals | 0 | 10–25 | 75 | 500 | 0 | 30 | 100 | 500 | 0 | 5–10 | 25 | 50 | 250 |
| 1 | 48 * | 0 | 0 | 0 | 44 * | 4 | 0 | 0 | 24 | 20 * | 4 | 0 | 0 |
| 2 | 46 * | 2 | 0 | 0 | 42 * | 6 | 0 | 0 | 24 | 14 * | 10 | 0 | 0 |
| 3 | 29 | 6 * | 13 | 0 | 44 * | 4 | 0 | 0 | 20 | 10 * | 6 | 0 | 12 |
| 4 | 4 | 6 | 36 * | 2 | 44 * | 4 | 0 | 0 | 18 | 30 * | 0 | 0 | 0 |
| 5 | 22 | 10 * | 12 | 4 | 44 * | 4 | 0 | 0 | 16 | 18 * | 8 | 4 | 2 |
| 6 | 16 | 8 * | 16 | 8 | 44 * | 4 | 0 | 0 | 10 | 24 * | 6 | 4 | 4 |
| 7 | 16 | 14 * | 14 | 4 | 46 * | 2 | 0 | 0 | 12 | 18 * | 6 | 10 | 2 |
| 10 | 30 | 2 * | 10 | 6 | 18 | 30 * | 0 | 0 | 34 | 4 * | 0 | 6 | 4 |
| 14 | 30 | 2 * | 10 | 6 | 31 * | 15 | 2 | 0 | 28 * | 4 | 2 | 4 | 0 |
| 17 | 29 | 1 * | 8 | 10 | 28 * | 20 | 0 | 0 | 39 | 2 * | 2 | 0 | 5 |
| 21 | 29 | 1 * | 10 | 8 | 38 * | 8 | 2 | 0 | 36 | 6 * | 2 | 0 | 4 |
| 24 | 28 | 2 * | 4 | 14 | 42 * | 6 | 0 | 0 | 38 | 4 * | 2 | 0 | 4 |
| 28 | 25 | 3 * | 6 | 14 | 46 * | 2 | 0 | 0 | 38 * | 6 | 0 | 4 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melandri, M.; Veronesi, M.C.; Alonge, S. Urinalysis in Great Dane Puppies from Birth to 28 Days of Age. Animals 2020, 10, 636. https://doi.org/10.3390/ani10040636
Melandri M, Veronesi MC, Alonge S. Urinalysis in Great Dane Puppies from Birth to 28 Days of Age. Animals. 2020; 10(4):636. https://doi.org/10.3390/ani10040636
Chicago/Turabian StyleMelandri, Monica, Maria Cristina Veronesi, and Salvatore Alonge. 2020. "Urinalysis in Great Dane Puppies from Birth to 28 Days of Age" Animals 10, no. 4: 636. https://doi.org/10.3390/ani10040636
APA StyleMelandri, M., Veronesi, M. C., & Alonge, S. (2020). Urinalysis in Great Dane Puppies from Birth to 28 Days of Age. Animals, 10(4), 636. https://doi.org/10.3390/ani10040636





