Simple Summary
The oxidant stress which piglets suffer from during the weaning period has caused huge losses to the pig farm industry. It is important for scientists to find an effective way to alleviate the oxidant stress in weaned piglets. This study was designed to test the hypothesis that dietary puerarin supplementation alleviates oxidative stress in the small intestine of diquat-challenged piglets. Interestingly, dietary puerarin supplementation improved intestinal morphology, cell proliferation, barrier function, and increased Nrf2 and its downstream enzymes in diquat-challenged piglets, which shows that puerarin has potent protective effects against diquat-induced oxidative stress. These findings will be very beneficial to the pig industry, especially to the development of antibiotic-free diets, new anti-inflammatory drugs and the application of puerarin in piglets.
Abstract
This study was conducted to demonstrate that dietary puerarin supplementation alleviates oxidative stress in the small intestine of diquat-challenged piglets. The results showed that puerarin administration markedly alleviated diquat-induced intestinal injury, which was indicated by the improvement of intestinal morphology, cell proliferation and barrier function. One of the potential mechanisms responsible for this was the decrease in oxidative stress, as evidenced by the increase in activities of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and total antioxidant capacity (T-AOC) in the small intestine. Puerarin increased the protein expression levels of NF-E2-related factor 2 (Nrf2) and its downstream enzymes, including heme oxygenase 1 (HO-1), glutamate–cysteine ligase catalytic and its modifier subunit (GCLc and GCLm) in the jejunal mucosa of diquat-induced piglets. Puerarin administration improved intestinal morphology, cell proliferation, and barrier function, and increased Nrf2 and its downstream enzymes. These findings indicate that the dietary supplementation of puerarin attenuates the oxidative stress involving Nrf2 signaling pathways in diquat-challenged piglets.
1. Introduction
Piglets are prone to oxidative balance disruption and oxidative injury during the weaning period. The previous study demonstrated that weaning is associated with oxidative injury of the lipid, protein, and DNA, as well as declines in the activities of the intestinal antioxidant enzymes under the p65 and NF-E2-related factor 2 (Nrf2) signals [1]. Under normal physiological conditions, the production of oxidants and antioxidants is balanced in biological systems [2]. Oxidative stress occurs when antioxidant systems cannot neutralize the excess reactive oxygen species (ROS) produced in cells and tissues [3]. As the products of mitochondrial metabolism of the eukaryotic cell, ROS are capable of initiating oxidation and play an important role in maintaining cell homeostasis and regulating signal transduction, gene expression, and enzyme activation at low levels [4,5].
The supplementation of exogenous antioxidants may help restore the pro-oxidative–antioxidative balance [6]. Puerarin, a natural isoflavone compound, has been reported to have strong antioxidant activities and exert a wide range of beneficial effects [7,8,9]. Puerarin can scavenge free radicals, inhibit pro-inflammatory cytokines, decrease inflammatory genes, up-regulate antioxidant enzymes, modulate transcription factors, and enhance the Nrf2 signaling pathway [10,11]. It has been reported that puerarin effectively inhibited inflammation in the kidney induced by both lipopolysaccharide and AGEs in mouse mesangial cells [12,13]. Puerarin also exerted renal protective effects in diabetic rats and renovascular hypertensive rats [14,15]. However, the protective effects of puerarin on oxidative stress in the intestines of weanling piglets have rarely been reported.
Therefore, the current study was designed to explore the protective roles of puerarin in piglets suffering from oxidative stress. We hypothesized that puerarin would exert protective effects on oxidative stress in the intestines of diquat-challenged piglets, which may involve Nrf2 signaling pathways.
2. Materials and Methods
2.1. Animals and Experimental Design
The animal experiments were approved by the Institutional Animal Care and Use Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences (2013020).
A total of 48 healthy piglets with body weight (BW) 7.26 ± 0.51 kg weaned at 21 days were randomly assigned to receive one of three treatments, with eight replicate pens per treatment, and two piglets per pen. The three treatments included a basal diet, a basal diet + diquat, and a 0.1‰ puerarin diet + diquat. (The puerarin dose was based on the growth performance of piglets in the preliminary experiment.) Diquat was purchased from Sigma-Aldrich (St. Louis, MO, USA). The basal diets were designed to meet the nutrient requirements for weaned piglets, as shown in Table 1. The piglets were housed in a room with hard plastic-slatted flooring. All piglets had free access to drinking water.

Table 1.
Composition of Basal Diets (as-fed basis).
After an adaptation period of seven days, the piglets were fed their respective diets three times per day for a 14-day experimental period. On day 7 after the initiation of treatment, the piglets on the basal diet + diquat and 0.1‰ puerarin + diquat treatments received an intraperitoneal injection of diquat at 8 mg/kg BW (a dose of 8 mg/kg BW was used according to the results reported by Yin et al) [16], while the piglets on the control treatment received the same volume of sterilized saline. On day 14, 24 piglets (one pig per pen) were randomly selected and slaughtered. The intestinal samples were collected from the center of the jejunum and ileum and were stored at −80 °C or fixed in a formaldehyde solution. Weight gain and feed intake of each treatment were calculated on the basis of the average value of each pen.
2.2. Intestinal Morphology
The fixed samples of the jejunum and ileum in the formaldehyde solution were embedded in paraffin. Using a microtome, cross sections of the samples were cut to an approximate thickness of 5 μm. Then, hematoxylin–eosin staining was performed with the procedures of dehydration, embedding, sectioning, and staining. Villous height and crypt depth were measured with computer-assisted microscopy [17] (Micrometrics TM; Nikon ECLIPSE E200, Tokyo, Japan).
2.3. Intestinal Mucosal Protein, DNA and RNA
The concentrations of protein, DNA and RNA in the jejunal and ileal mucosa were measured by a BCA assay kit, fluorometric assay and spectrophotometry, respectively, according to the methods of Liu et al [18].
2.4. Immunohistochemical Analysis
The expressions of the proliferating cell nuclear antigen (PCNA) in the jejunal and ileal mucosa were determined using immunohistochemical analysis in accordance with the methods of Wang et al. (2015). The anti-PCNA (1:200; Wuhan Boster Biological Technology Co., Ltd., Wuhan, China) and an SV mouse or rabbit hypersensitivity 2-step immunohistochemical kit (Boster Biological Technology) were used. The stained sections were reviewed and scored independently by two investigators using a microscope (Olympus, Tokyo, Japan). The PCNA labeling index was expressed as the ratio of cells that were positively stained for PCNA to all epithelial cells in at least five areas that were randomly selected for counting at less than 200-fold magnification. All data were expressed as the relative values to those of the control piglets.
2.5. Real-Time Quantitative Reverse Transcriptase PCR
The expressions of E-cadherin, occludin and zonula occludens (ZO-1) in the jejunal and ileal mucosa were determined by real-time quantitative reverse transcriptase PCR (real-time qRT-PCR). Primers were designed with Primer 5.0 (PREMIER Biosoft International, Palo Atlo, CA, USA) according to the gene sequence of the pig, to produce an amplification product that was shown in the previous study [19]. Beta-actin was used as a housekeeping gene to normalize target gene transcript levels [20]. The comparative threshold cycle (Ct) value method was employed to the quantitative expression levels of the target genes relative to those of β-Actin. Data are expressed as the relative values to those of the control piglets [19].
2.6. Antioxidative Capacity of Intestinal Mucosa
Jejunal and ileal mucosal concentrations of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), malondialdehyde (MDA), total antioxidant capacity (T-AOC), and glutathione (GSH) were measured using their corresponding assay kits (Nanjing Jiancheng, Nanjing, China) according to manufacturer instructions. SOD, CAT, and GSH-Px were analyzed by the xanthine–oxidase-xanthine reaction method, the CAT-H2O2 reaction method, and the reduced glutathione method, respectively. MDA capacity was assayed by the 2-thiobarbituric acid method and T-AOC was detected by the ferric-reducing antioxidant power reaction method. All samples were measured by UV-visible spectrophotometry (UV-2450, Shimadzu, Kyoto, Japan).
2.7. Western Blot Analysis
Jejunal mucosal samples were homogenized and protein concentrations were measured using the bicinchoninic acid assay method with BSA as the standard (Beyotime Institute of Biotechnology, Shanghai, China). All samples were adjusted to an equal concentration (50µg protein). The antibodies used in this study were as follows: Nrf2 antibody (1:500, Santa Cruz Biotechnology), heme oxygenase 1 (HO-1) antibody (1:500, Bioss Inc.), NAD(P)H:quinone oxidoreductase 1 (NQO-1) antibody (1:10000, Invitrogen), glutamate–cysteine ligase catalytic subunit (GCLc) antibody (1:1000, LifeSpan BioSciences), glutamate–cysteine ligase modifier (GCLm) antibody (1:1000, LifeSpan BioSciences) and β-actin (1:400; Santa Cruz Biotechnology). The expression ratio of the target proteins was normalized against β-actin, and the data are expressed relative to the values of the piglets on the basal diet treatment.
2.8. Statistical Analysis
All data were subjected to analysis of variance (ANOVA) using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). The differences among the treatments were evaluated using Tukey’s test. Probability values < 0.05 were taken to indicate statistical significance.
3. Results
3.1. Growth Performance
The results of the growth performance are shown in Table 2. The injection of diquat significantly decreased (p < 0.05) the final BW, average daily weight gain (ADG), average daily feed intake (ADFI) and gain:feed (G:F) ratio in the piglets. However, the growth performance of the piglets on the puerarin diet + diquat treatment showed no difference compared to those on the basal diet and basal diet + diquat treatments (p > 0.05).

Table 2.
Effects of dietary puerarin supplementation on the growth performance of the piglets.
3.2. Jejunal and Ileal Morphology
In the jejunum, diquat challenge reduced (p < 0.05) villous height and the ratio of villous height to crypt depth. The ratio of villous height to crypt depth in the piglets on the puerarin diet + diquat treatment was greater than those on the basal diet + puerarin treatment (p < 0.05), as shown in Table 3.

Table 3.
Effects of dietary puerarin supplementation on jejunal and ileal morphology in the piglets.
In the ileum, the ratio of villous height to crypt depth in the piglets on the basal diet + diquat treatment was decreased in comparison to those on the basal diet treatment (p < 0.05). However, no differences were observed in villous height, crypt depth and the ratio of villous height to crypt depth between the piglets on the basal diet + diquat treatment and those on the puerarin diet + diquat treatment (p > 0.05), as shown in Table 3.
3.3. Protein, DNA and RNA Contents in the Jejunal and Ileal Mucosa
Exposure to diquat decreased (p < 0.05) the jejunal mucosal protein content, as well as the ileal mucosal protein content and the ratio of RNA to DNA. In diquat-treated piglets, dietary puerarin increased (p < 0.05) the protein content in the jejunal mucosa, as well as the protein content, RNA to DNA ratio and protein to DNA ratio in the ileum mucosa, as displayed in Table 4.

Table 4.
Effects of dietary puerarin supplementation on the protein, DNA and RNA contents in the jejunal and ileal mucosa of the piglets.
3.4. PCNA Positive Cells in the Jejunum and Ileum
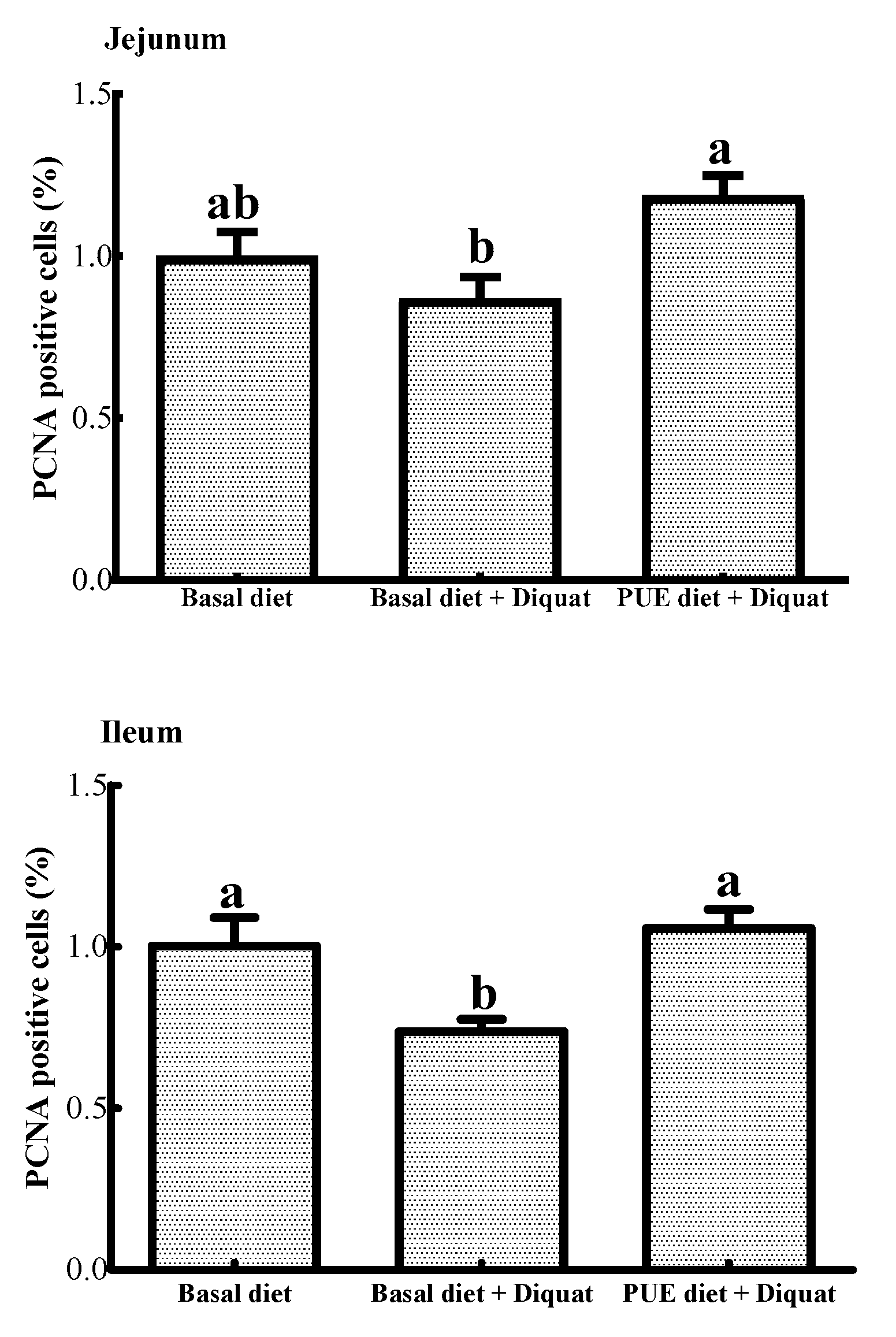
As shown in Figure 1, the percentage of PCNA positive cells in the ileum of the piglets treated with the basal diet + diquat was decreased (p < 0.05) when compared to that of the piglets on the basal diet treatment. The puerarin supplementation restored the inhibitory effect of the jejunal and ileal cell proliferation caused by diquat (p < 0.05).
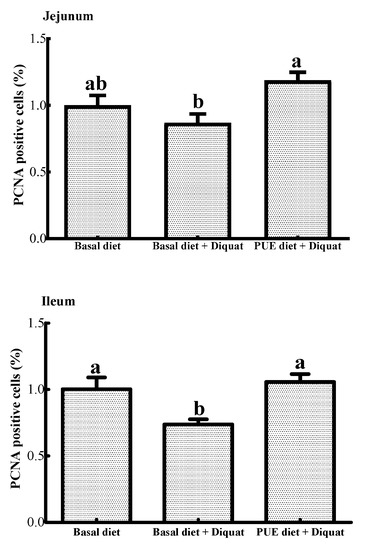
Figure 1.
The percentage of proliferating cell nuclear antigen (PCNA) positive cells in the jejunum and ileum of the piglets. Data are expressed as means ± SEM, n =8. a,b Values with different lowercase letters are different (p < 0.05).
3.5. The Relative mRNA Levels of Intercellular Junction Proteins in the Jejunum and Ileum
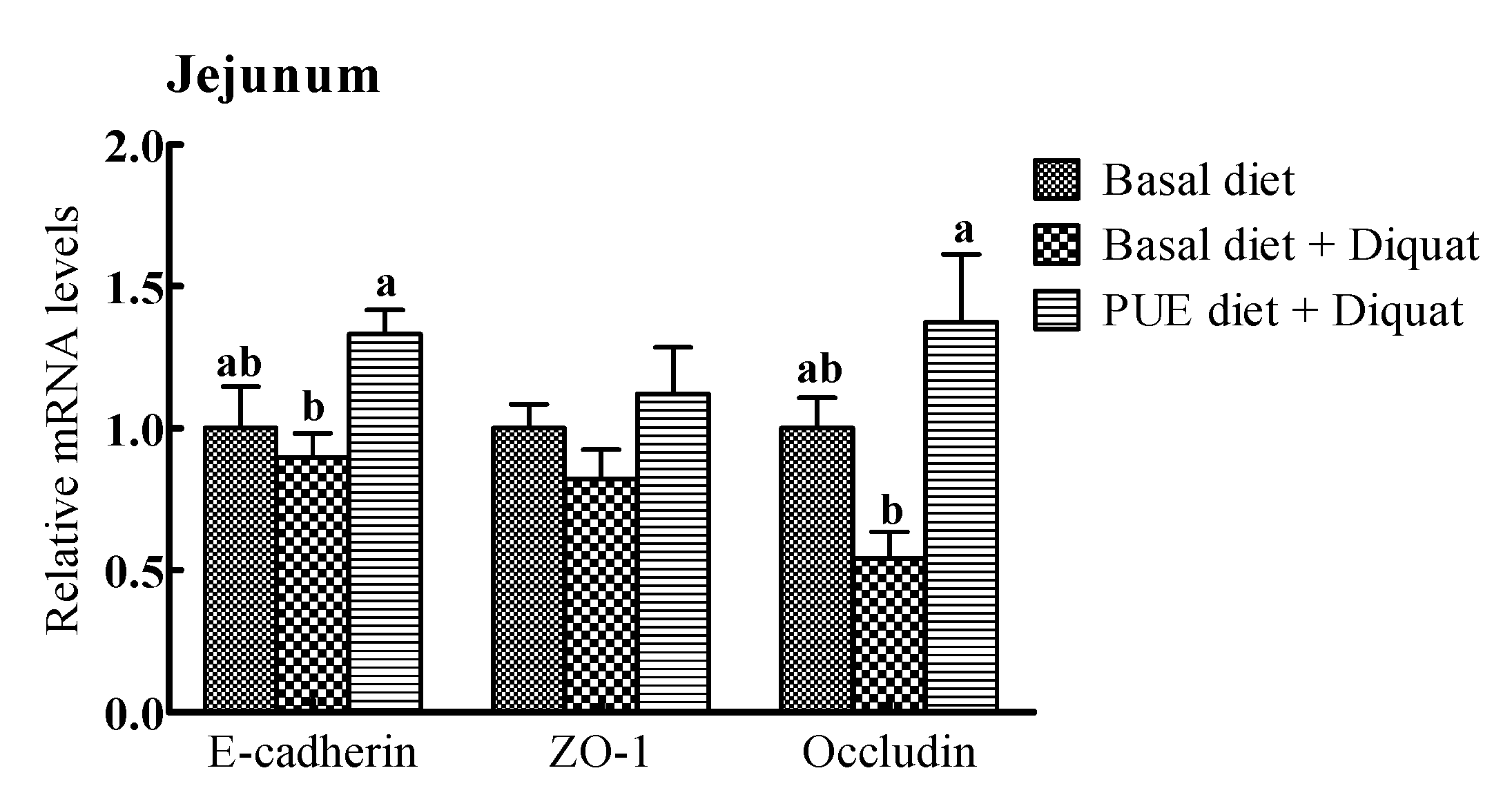
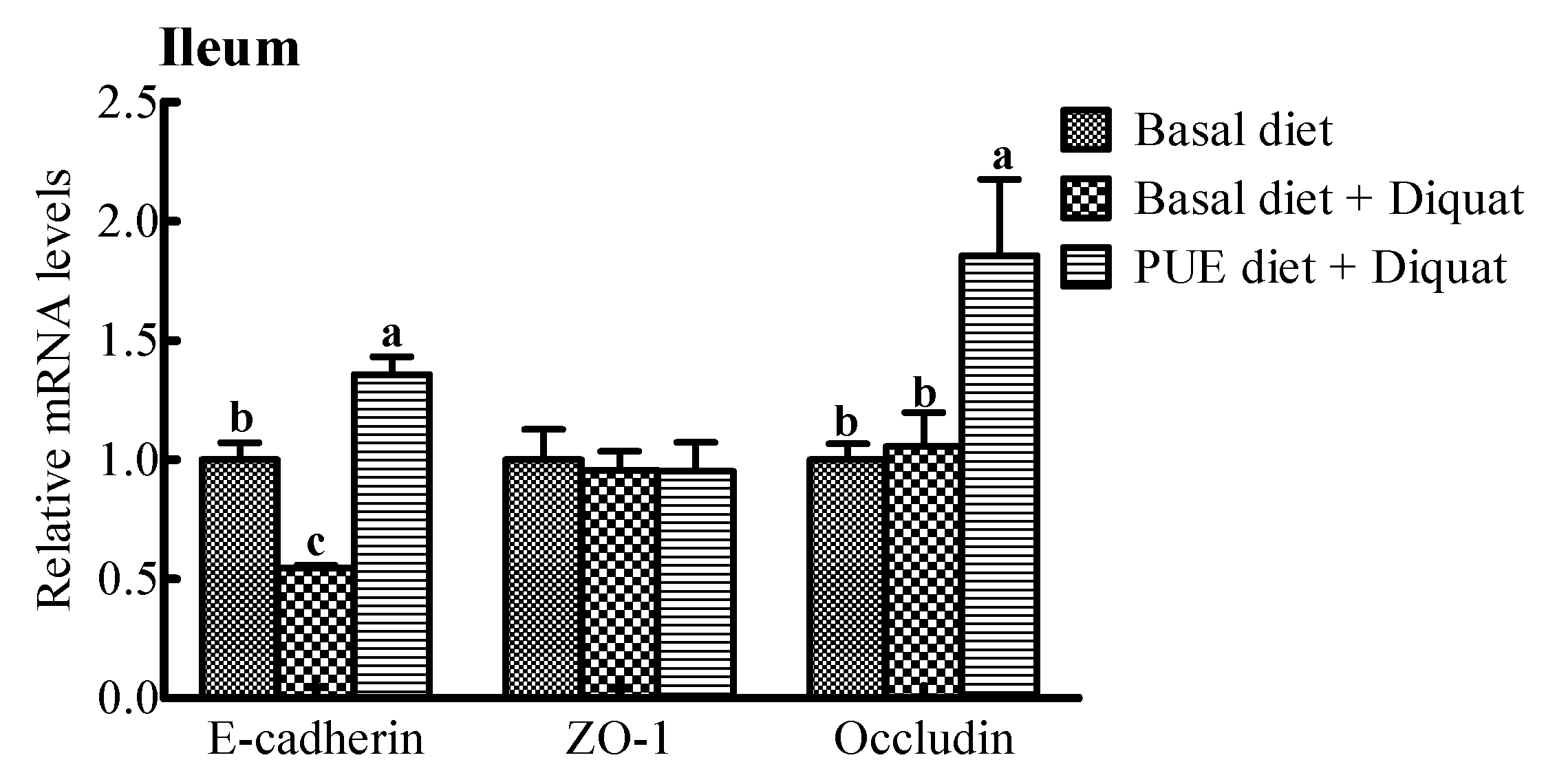
The relative mRNA expressions of intercellular junction proteins (E-cadlherin, ZO-1, occludin) are shown in Figure 2. The E-cadherin and occludin mRNA expressions in the jejunum and ileum of the piglets treated with the puerarin diet were greater (p < 0.05) than those on the basal diet + diquat treatment. However, there were no significant differences in the ZO-1 mRNA levels in the jejunum and ileum among the piglets receiving the three treatments (p > 0.05).
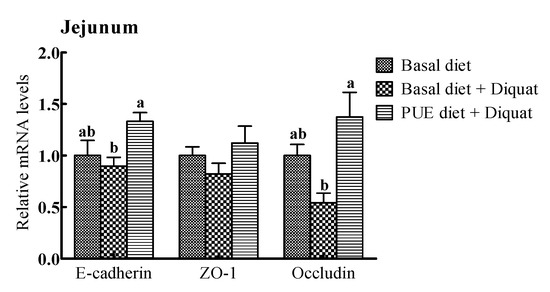
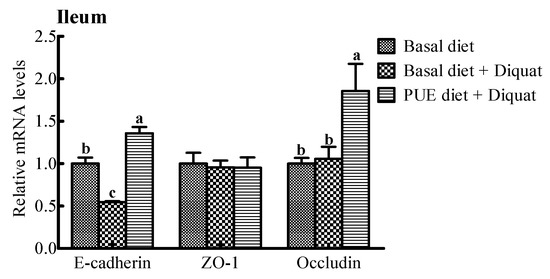
Figure 2.
The relative mRNA levels of E-cadherin, zonula occludens (ZO-1) and occludin in the jejunum and ileum of the piglets. The mRNA expressions were expressed relative to the values of the piglets on the basal diet treatment. Data are expressed as means ± SEM, n = 8. a,b Values with different lowercase letters are different (p < 0.05).
3.6. Antioxidant Parameters in the Jejunum and Ileum
As shown in Table 5, exposure to diquat decreased (p < 0.05) concentrations of GSH-Px in the jejunum, and GSH-Px and T-AOC in the ileum. However, the supplementation of puerarin increased (p < 0.05) jejunal concentrations of SOD, GSH-Px and T-AOC, as well as ileal concentrations of GSH-Px and T-AOC compared with those on the basal diet + diquat treatment. Concentrations of CAT and MDA in the jejunum and ileum showed no difference in the piglets on all three treatments (p > 0.05).

Table 5.
Effects of dietary puerarin supplementation on the jejunal and ileal mucosal concentrations of SOD, GSH-Px, CAT, MDA, T-AOC, GSH in the piglets.
3.7. Protein Expressions of Nrf2, HO-1, NQO-1, GCLc and GCLm in the Jejunum
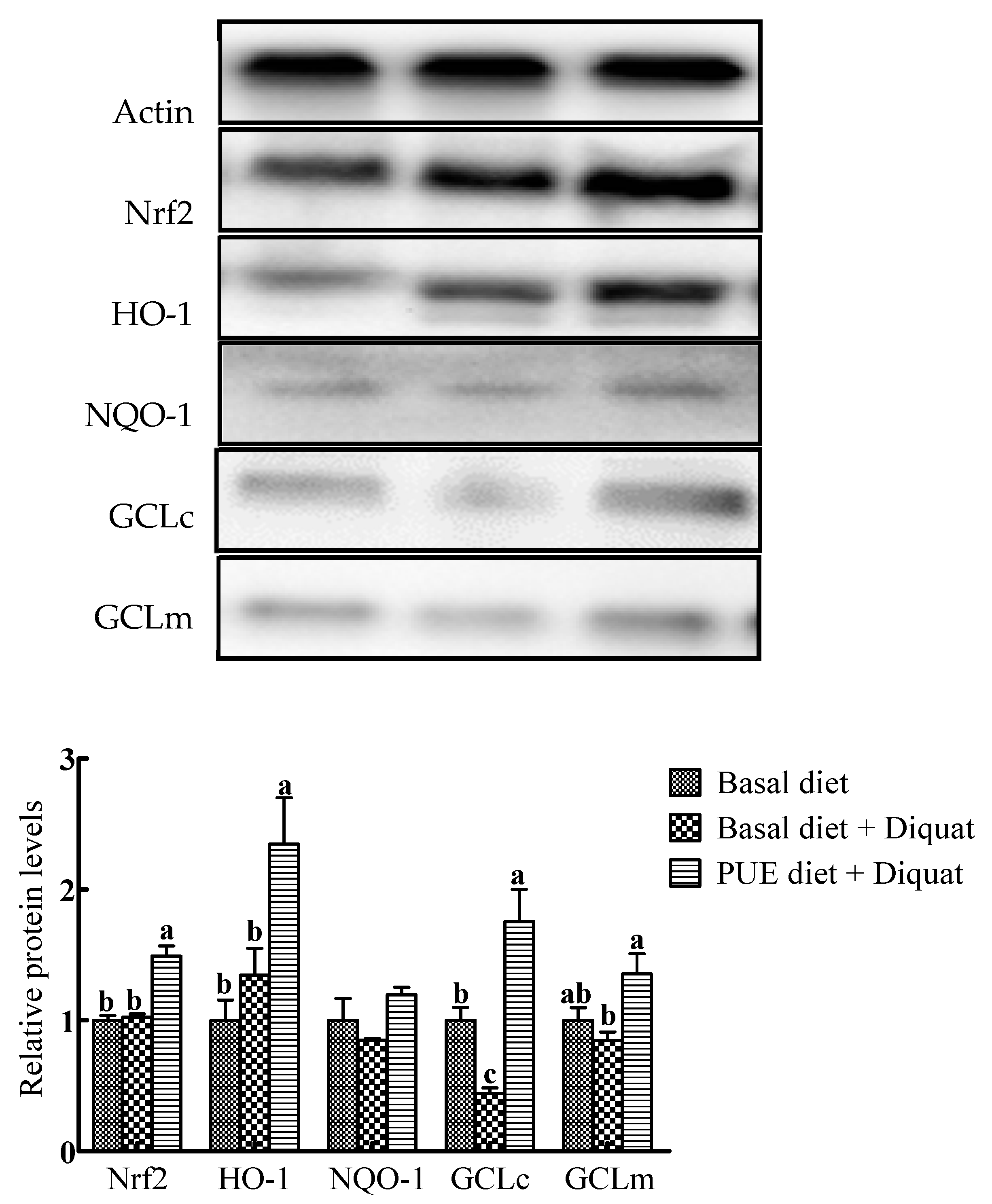
Representative Western blots and relative expressions of Nrf2 and antioxidant enzymes in the jejunum are shown in Figure 3. In comparison with the basal diet treatment, the protein expressions of Nrf2, HO-1 and GCLc in the piglets on the puerarin diet + diquat treatment were increased (p < 0.05), whereas GCLc protein expressions in the jejunum of piglets on the basal diet + diquat treatment were decreased (p < 0.05). The protein expressions of Nrf2, HO-1, GCLc and GCLm in the piglets on the puerarin diet + diquat treatment were greater than those on the basal diet + diquat treatment (p < 0.05).
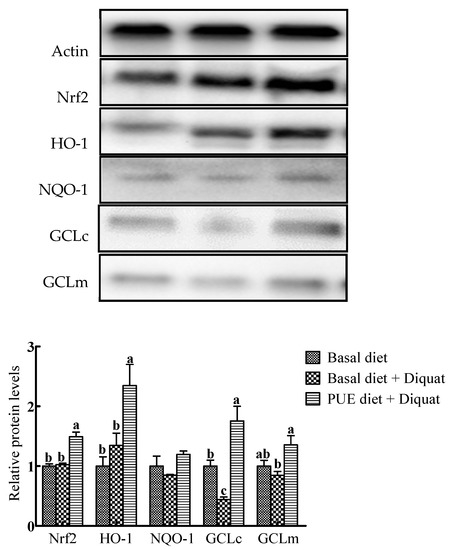
Figure 3.
Representative Western blot images and the relative protein levels of NF-E2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), NADH dehydrogenase quinone 1(NQO-1), glutamate–cysteine ligase catalytic subunit (GCLc) and glutamate–cysteine ligase modifier subunit (GCLm) in the jejunum of piglets. The protein expressions were expressed relative to the values of the piglets on the basal diet treatment. Data are expressed as means ± SEM, n = 8. a-c Values with different lowercase letters are different (p < 0.05).
4. Discussion
Diquat has been widely used to induce oxidative stress, and diquat-challenging oxidative stress has been reported to affect growth performance and nutrient utilization in animals [7,16,21]. In the present study, the injection of diquat reduced growth performance and impaired gut morphology, which is in accordance with the previous report [7]. These negative effects are mainly due to the disruption in the oxidative–antioxidative balance [22]. The main antioxidant enzymes, SOD, GSH-Px and CAT, can scavenge ROS in the biological system [23,24]. The present study showed that intestinal concentrations of GSH-Px and T-AOC were decreased after exposure to diquat in the piglets on the basal diet treatment, which supports previous results in which diquat injection inhabited the activities of GSH-Px in the serum of piglets [7]. Furthermore, diquat treatment inhibited mucosal cell proliferation, and the gut barrier function showed a decreasing percentage of PCNA positive cells, as well as a relative mRNA level of E-cadherin. The present results also showed that the main antioxidant enzyme, GCLc, decreased in percentage in the jejunum after diquat injection. These results indicate that diquat induced oxidative stress in piglets, which is in accordance with the previous research [7,19,25,26].
Puerarin, the main isoflavonoid found in the Chinese herb Pueraria lobate, has been widely used in traditional Chinese medicine for thousands of years [27,28,29]. It has been reported to improve growth performance and health in animals [26,30,31]. The present study showed that there was no difference in growth performance between the piglets on the basal diet treatment and those on the puerarin diet + diquat treatment, although diquat significantly inhibited the growth of the piglets. This result indicates that the dietary supplementation of puerarin alleviated the growth performance impairment induced by diquat.
The previous study has shown that the supplementation of Eucommia ulmoides flavones can restore the intestinal morphological structure and barrier function in piglets [7]. Puerarin has also been demonstrated to improve the structure and barrier function in cells [32]. In the present study, a higher rate of villous height to crypt depth in the jejunum indicates that dietary puerarin improved the jejunal morphological structure to some extent. E-cadherin and occludin are the main transmembrane proteins in the apical junctional complex (AJC) [33], which regulate the paracellular diffusion of ions and small molecules across epithelial barriers [34,35]. It has been reported that disorder of the AJC is a common feature of numerous inflammatory diseases [36]. For example, down-regulations of occludin and E-cadherin are linked with epithelial-mesenchymal transition [37]. The decreased expression of occludin has been considered to be associated with the staging, invasiveness and metastatic potential of epithelial cancers [37,38,39,40]. Thus, the increased expression of E-cadherin and occludin in the present study evidences that the intestinal barrier function was restored in the diquat-induced piglets treated with purarin. The improvement in the mucosal barrier function may indicate the alleviation of oxidative stress [41].
In agreement with the effect of puerarin on the intestinal barrier function, the percentage of PCNA positive cells in the jejunal and ileal mucosa increased in response to the puerarin administration. This result indicates that puerarin promoted mucosal cell proliferation, and this was possible due to the regulation of puerarin on DNA synthesis. The proliferation of eucaryon occurs in the way of mitosis. Cell division begins with intermitosis, where DNA, RNA, and protein are synthesized to prepare for the mitotic cycle [42]. The present study showed that the contents of DNA, RNA and protein in the ileum significantly increased after the administration of dietary puerarin. The increased synthesis of DNA may reflect more activities of cell proliferation. Previous studies have reported that puerarin enhanced the proliferation of human bone marrow stromal cells, and attenuated the proliferation of diabetes-induced vascular smooth muscle cells and bladder cancer cells [43,44,45], all of which is also beneficial to animals.
In this study, significant up-regulations of SOD, GSH-Px, and T-AOC activities in the intestinal mucosa of diquat-induced piglets were observed after puerarin exposure. The up-regulation of antioxidant enzymes indicated a positive effect of puerarin on antioxidant capacity. The mechanisms of the antioxidant capacity of puerarin include the scavenging of ONOONO and total ROS, as well as the inhibition of ONOO-mediated tyrosine nitration [13]. These functions are a consequence of the activities of antioxidant enzymes such as SOD, GSH-Px, and T-AOC. SOD has been considered the first line of defense against the deleterious effects of oxyradicals on the cell, by converting superoxide radicals into hydrogen peroxide and molecular oxygen [46]. GSH-Px converts glutathione from its reduced form into its oxidative form by catalyzing hydrogen peroxide into hydrogen oxide [21,24].
GCL is the main rate-limiting enzyme for the de novo synthesis of intracellular GSH, which is one of the most versatile cellular antioxidants [47]. Interestingly, all enzymes involved in GSH biosynthesis are controlled by Nrf2 [48]. Therefore, Nrf2 plays an important role in the modulation of oxidative stress and homeostasis [49]. When stimulated by inducers, Nrf2 is released from the Kelch-like ECH-associated protein 1 (Keap1), and translocates into the nucleus where it dimerizes with cofactors and binds to ARE [50,51], thus activating downstream enzymes, including NQO1, HO-1, GCLc, and GCLm that protect cells against oxidative stress damage [51,52]. HO-1, a well-characterized cytoprotective gene controlled by Nrf2, is a microsomal enzyme that cleaves heme to produce biliverdin, carbon monoxide, and iron [53]. Mice lacking Nrf2 exhibit lower GSH levels and GCLc expression [54]. The present study showed that puerarin supplementation markedly promoted the protein expressions of Nrf2, HO-1, GCLc and GCLm in diquat-induced piglets. These results suggest that puerarin could alleviate oxidative injury involving the Nrf2 pathway caused by diquat.
5. Conclusions
This study shows that puerarin has potent protective effects against diquat-induced oxidative stress. The results indicate that dietary puerarin supplementation alleviated the intestinal damage, oxidative stress, and Nrf2 pathway induced by diquat in the piglets. These findings will be helpful for the development of antibiotic-free diets, new anti-inflammatory drugs and the application of puerarin in piglets.
Author Contributions
D.Y. and B.T. designed the experiments; D.Y., M.L., H.J. and B.T. conducted the experiments; M.L. and B.T. analyzed the data; M.L. wrote the manuscript; B.T. and Y.L. revised the manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (31960666 and 31872991), the Earmarked Fund for China Agriculture Research System (CARS-35) and Open Fund of National and Local United Engineering Laboratory of Integrative Utilization Technology of Eucommia ulmoides (NLE201703).
Acknowledgments
We thank Changsha Xinqidian Biotechnology Limited Company for providing technical assistance.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Statement of Ethics
Animal use and animal trials in this study have been approved by the Animal Care and Use Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences.
Availability of Data and Materials
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
References
- Yin, J.; Wu, M.M.; Xiao, H.; Ren, W.K.; Duan, J.L.; Yang, G.; Li, T.J.; Yin, Y.L. Development of an antioxidant system after early weaning in piglets. J. Anim. Sci. 2014, 92, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, M.; Skibola, C.; Smith, C. Polymorphisms in the oxidative stress genes, superoxide dismutase, glutathione peroxidase and catalase and risk of non-Hodgkin’s lymphoma. Haematologica 2006, 91, 1819–1828. [Google Scholar]
- Dryden, G.W.; Deaciuc, I.; Arteel, G. Clinical Implications of oxidative stress and antioxidant therapy. Curr. Gastroenterol. Rep. 2005, 7, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Sosa, V.; Moline, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; ME, L.L. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Bebrevska, L.; Foubert, K.; Hermans, N.; Chatterjee, S.; Van Marck, E.; De Meyer, G.; Vlietinck, A.; Pieters, L.; Apers, S. In vivo antioxidative activity of a quantified Pueraria lobata root extract. J. Ethnopharmacol. 2010, 127, 112–117. [Google Scholar] [CrossRef]
- Yuan, D.; Hussain, T.; Tan, B.; Liu, Y.; Ji, P.; Yin, Y. The evaluation of antioxidant and anti-inflammatory effects of Eucommia ulmoidesflavones using diquat-Cchallengedpiglet models. Oxid. Med. Cell Longev. 2017, 2017, e8140962. [Google Scholar] [CrossRef]
- Jiang, R.W.; Lau, K.M.; Lam, H.M.; Yam, W.S.; Leung, L.K.; Choi, K.L.; Waye, M.M.; Mak, T.C.; Woo, K.S.; Fung, K.P. A comparative study on aqueous root extracts of Pueraria thomsonii and Pueraria lobata by antioxidant assay and HPLC fingerprint analysis. J. Ethnopharmacol. 2005, 96, 133–138. [Google Scholar] [CrossRef]
- Cos, P.; De Bruyne, T.; Apers, S.; Vanden Berghe, D.; Pieters, L.; Vlietinck, A. Phytoestrogens: Recent developments. Planta Med. 2003, 69, 589–599. [Google Scholar]
- Ribeiro, D.; Freitas, M.; Lima, J.L.; Fernandes, E. Proinflammatory Pathways: The Modulation by Flavonoids. Med. Res. Rev. 2015, 35, 877–936. [Google Scholar] [CrossRef]
- Nagle, D.G.; Ferreira, D.; Zhou, Y.D. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Jung, D.H.; Jang, D.S.; Kim, Y.S.; Kim, J.M.; Kim, H.N.; Surh, Y.J.; Kim, J.S. Puerarin suppresses AGEs-induced inflammation in mouse mesangial cells: A possible pathway through the induction of heme oxygenase-1 expression. Toxicol. Appl. Pharmacol. 2010, 244, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.E.; Son, Y.K.; Min, B.S.; Jung, H.A.; Choi, J.S. Anti-inflammatory and antioxidant activities of constituents isolated from Pueraria lobata roots. Arch. Pharm. Res. 2012, 35, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xiao, Y.; Gong, H.; Shen, D.; Zhu, F.; Wu, Q.; Chen, H.; Zhong, H. Effect of puerarin on the expression of extracellular matrix in rats with streptozotocin-induced diabetic nephropathy. Natl. Med. J. India 2009, 22, e9. [Google Scholar]
- Bai, S.; Huang, Z.G.; Chen, L.; Wang, J.T.; Ding, B.P. Effects of felodipine combined with puerarin on ACE2-Ang (1-7)-Mas axis in renovascular hypertensive rat. Regul. Pept. 2013, 184, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Liu, M.; Ren, W.; Duan, J.; Yang, G.; Zhao, Y.; Fang, R.; Chen, L.; Li, T.; Yin, Y. Effects of dietary supplementation with glutamate and aspartate on diquat-induced oxidative stress in piglets. PLoS ONE 2015, 10, e0122893. [Google Scholar] [CrossRef]
- Xiao, H.; Tan, B.E.; Wu, M.M.; Yin, Y.L.; Li, T.J.; Yuan, D.X.; Li, L. Effects of composite antimicrobial peptides in weanling piglets challenged with deoxynivalenol: II. Intestinal morphology and function. J. Anim. Sci. 2013, 91, 4750–4756. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Hou, Y.; Zhu, H.; Zhao, S.; Ding, B.; Yin, Y.; Yi, G.; Shi, J.; Fan, W. Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br. J. Nutr. 2008, 100, 552–560. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.R.; Tan, B.E.; Xiong, X.; Kong, X.F.; Xiao, D.F.; Xu, L.W.; Wu, M.M.; Huang, B.; Kim, S.W.; et al. Oral administration of putrescine and proline during the suckling period improves epithelial restitution after early weaning in piglets. J. Anim. Sci. 2015, 93, 1679–1688. [Google Scholar] [CrossRef]
- Tan, B.; Li, X.G.; Kong, X.; Huang, R.; Ruan, Z.; Yao, K.; Deng, Z.; Xie, M.; Shinzato, I.; Yin, Y.; et al. Dietary L-arginine supplementation enhances the immune status in early-weaned piglets. Amino Acids 2009, 37, 323–331. [Google Scholar] [CrossRef]
- Lv, M.; Yu, B.; Mao, X.B.; Zheng, P.; He, J.; Chen, D.W. Responses of growth performance and tryptophan metabolism to oxidative stress induced by diquat in weaned pigs. Animal 2012, 6, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.B.; Chen, D.W.; Zhang, K.Y.; Yu, B. Effects of oxidative stress on growth performance, nutrient digestibilities and activities of antioxidative enzymes of weanling pigs. Asian-Australas. J. Anim. Sci. 2007, 20, 1600–1605. [Google Scholar] [CrossRef]
- Wozniak, A.; Drewa, G.; Wozniak, B.; Schachtschabel, D.O. Activity of antioxidant enzymes and concentration of lipid peroxidation products in selected tissues of mice of different ages, both healthy and melanoma-bearing. Z. GerontolGeriatr. 2004, 37, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Lestaevel, P.; Romero, E.; Dhieux, B.; Ben Soussan, H.; Berradi, H.; Dublineau, I.; Voisin, P.; Gourmelon, P. Different pattern of brain pro-/anti-oxidant activity between depleted and enriched uranium in chronically exposed rats. Toxicology 2009, 258, 1–9. [Google Scholar] [CrossRef]
- Tossou, M.C.; Liu, H.; Bai, M.; Chen, S.; Cai, Y.; Duraipandiyan, V.; Liu, H.; Adebowale, T.O.; Al-Dhabi, N.A.; Long, L.; et al. Effect of High Dietary Tryptophan on Intestinal Morphology and Tight Junction Protein of Weaned Pig. Biomed. Res. Int. 2016, 2016, e2912418. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, D.; Yang, X.; Fu, C.; Zou, L.; Zhang, J. Traditional Chinese medicine GegenQinlian decoction ameliorates irinotecan chemotherapy-induced gut toxicity in mice. Biomed. Pharmacother. 2019, 109, 2252–2261. [Google Scholar] [CrossRef]
- Fang, Q. Some current study and research approaches relating to the use of plants in the traditional Chinese medicine. J. Ethnopharmacol. 1980, 2, 57–63. [Google Scholar] [CrossRef]
- Wei, S.-Y.; Chen, Y.; Xu, X.-Y. Progress on the pharmacological research of puerarin: A review. Chin. J. Nat. Med. 2014, 12, 407–414. [Google Scholar] [CrossRef]
- Xiong, F.L.; Sun, X.H.; Gan, L.; Yang, X.L.; Xu, H.B. Puerarin protects rat pancreatic islets from damage by hydrogen peroxide. Eur. J. Pharmacol. 2006, 529, 1–7. [Google Scholar]
- Guerra, M.C.; Speroni, E.; Broccoli, M.; Cangini, M.; Pasini, P.; Minghett, A.; Crespi-Perellino, N.; Mirasoli, M.; Cantelli-Forti, G.; Paolini, M. Comparison between Chinese medical herb Pueraria lobata crude extract and its main isoflavone puerarin antioxidant properties and effects on rat liver CYP-catalysed drug metabolism. Life Sci. 2000, 67, 2997–3006. [Google Scholar] [CrossRef]
- Li, R.; Xu, L.; Liang, T.; Li, Y.; Zhang, S.; Duan, X. Puerarin mediates hepatoprotection against CCl4-induced hepatic fibrosis rats through attenuation of inflammation response and amelioration of metabolic function. Food Chem. Toxicol. 2013, 52, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Du, S.; Lu, Y.; Liu, C.; Wu, H.; Yang, B.; Bai, J.; Li, P. Influence of puerarin, paeoniflorin, and menthol on structure and barrier function of tight junctions in MDCK and MDCK-MDR1 Cells. J. Trad. Chin. Med. Sci. 2015, 2, 111–119. [Google Scholar] [CrossRef]
- Severson, E.A.; Kwon, M.; Hilgarth, R.S.; Parkos, C.A.; Nusrat, A. Glycogen Synthase Kinase 3 (GSK-3) influences epithelial barrier function by regulating occludin, claudin-1 and E-cadherin expression. Biochem. Biophys. Res. Commun. 2010, 397, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Gumbiner, B. Structure, biochemistry, and assembly of epithelial tight junctions. Am. J. Physiol.-Cell Physiol. 1987, 253, C749–C758. [Google Scholar] [CrossRef]
- Wong, V. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J. Cell Biol. 1997, 136, 399–409. [Google Scholar] [CrossRef]
- Schmitz, H.; Barmeyer, C.; Fromm, M.; Runkel, N.; Foss, H.D.; Bentzel, C.J.; Riechken, E.O.; Schulzke, J.D. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology 1999, 116, 301–309. [Google Scholar] [CrossRef]
- Wang, Z.; Mandell, K.J.; Parkos, C.A.; Mrsny, R.J.; Nusrat, A. The second loop of occludin is required for suppression of Raf1-induced tumor growth. Oncogene 2005, 24, 4412–4420. [Google Scholar] [CrossRef]
- Hoover, K.B.; Liao, S.-Y.; Bryant, P.J. Loss of the tight junction MAGUK ZO-1 in breast cancer. Am. J. Pathol. 1998, 153, 1767–1773. [Google Scholar] [CrossRef]
- Tobioka, H.; Isomura, H.; Kokai, Y.; Tokunaga, Y.; Yamaguchi, J.; Sawada, N. Occludin expression decreases with the progression of human endometrial carcinoma. Hum. Pathol. 2004, 35, 159–164. [Google Scholar] [CrossRef]
- Dhawan, P.; Singh, A.B.; Deane, N.G.; No, Y.; Shiou, S.R.; Schmidt, C.; Neff, J.; Washington, M.K.; Beauchamp, R.D. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J. Clin. Investig. 2005, 115, 1765–1776. [Google Scholar] [CrossRef]
- Forster, C. Tight junctions and the modulation of barrier function in disease. Histochem. Cell Biol. 2008, 130, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Spiegelman, S. Ciba Foundation Symposium on ionizing Radiations and Cell Metabolism; J. & A. Churchill: London, UK, 1956. [Google Scholar] [CrossRef]
- Zhu, L.H.; Wang, L.; Wang, D.; Jiang, H.; Tang, Q.Z.; Yan, L.; Bian, Z.Y.; Wang, X.A.; Li, H. Puerarin attenuates high-glucose-and diabetes-induced vascular smooth muscle cell proliferation by blocking PKCbeta2/Rac1-dependent signaling. Free Radic. Biol. Med. 2010, 48, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, S.; Li, Y.; Cheng, B.; Tan, B.; Wang, G. Puerarininhibits proliferation and induces apoptosis by upregulation of miR-16 in bladder cancer cell line T24. Oncol. Res. 2018, 26, 1227–1234. [Google Scholar] [CrossRef]
- Lv, H.; Che, T.; Tang, X.; Liu, L.; Cheng, J. Puerarin enhances proliferation and osteoblastic differentiation of human bone marrow stromal cells via a nitric oxide/cyclic guanosine monophosphate signaling pathway. Mol. Med. Rep. 2015, 12, 2283–2290. [Google Scholar] [CrossRef] [PubMed]
- Romeu, M.; Mulero, M.; Giralt, M.; Folch, J.; Nogués, M.R.; Torres, A.; Fortuño, A.; Sureda, F.X.; Cabré, M.; Paternáin, J.L. Parameters related to oxygen free radicals in erythrocytes, plasma and epidermis of the hairless rat. Life Sci. 2002, 71, 1739–1749. [Google Scholar] [CrossRef]
- Quintana-Cabrera, R.; Bolanos, J.P. Glutathione and gamma-glutamylcysteine in the antioxidant and survival functions of mitochondria. Biochem. Soc. Trans. 2013, 41, 106–110. [Google Scholar] [CrossRef]
- Escartin, C.; Won, S.J.; Malgorn, C.; Auregan, G.; Berman, A.E.; Chen, P.C.; Deglon, N.; Johnson, J.A.; Suh, S.W.; Swanson, R.A. Nuclear factor erythroid 2-related factor 2 facilitates neuronal glutathione synthesis by upregulating neuronal excitatory amino acid transporter 3 expression. J. Neurosci. 2011, 31, 7392–7401. [Google Scholar] [CrossRef]
- Kang, K.W.; Lee, S.J.; Kim, S.G. Molecular mechanism of nrf2 activation by oxidative stress. Antioxid. Redox Signal. 2005, 7, 1664–1673. [Google Scholar] [CrossRef]
- Leong, P.K.; Chiu, P.Y.; Chen, N.; Leung, H.; Ko, K.M. Schisandrin B elicits a glutathione antioxidant response and protects against apoptosis via the redox-sensitive ERK/Nrf2 pathway in AML12 hepatocytes. Free Radic. Res. 2011, 45, 483–495. [Google Scholar] [CrossRef]
- Yang, Y.C.; Lii, C.K.; Lin, A.H.; Yeh, Y.W.; Yao, H.T.; Li, C.C.; Liu, K.L.; Chen, H.W. Induction of glutathione synthesis and heme oxygenase 1 by the flavonoids butein and phloretin is mediated through the ERK/Nrf2 pathway and protects against oxidative stress. Free Radic. Biol. Med. 2011, 51, 2073–2081. [Google Scholar] [CrossRef]
- Lee, I.C.; Kim, S.H.; Baek, H.S.; Moon, C.; Kang, S.S.; Kim, S.H.; Kim, Y.B.; Shin, I.S.; Kim, J.C. The involvement of Nrf2 in the protective effects of diallyl disulfide on carbon tetrachloride-induced hepatic oxidative damage and inflammatory response in rats. Food Chem. Toxicol. 2014, 63, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Hong, B.; Fan, L.; Zhou, L.; Liu, Y.; Wu, Q.; Zhang, X.; Dong, M. Protective effect of puerarin against beta-amyloid-induced oxidative stress in neuronal cultures from rat hippocampus: Involvement of the GSK-3beta/Nrf2 signaling pathway. Free Radic. Res. 2013, 47, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Emmert, S.W.; Karam, E.B.; Arunangshu, D.; Yuan-Wan, S.; Shantu, A.; Dhimant, D.; Cesar, A.; Richie, J.P. Induction of lung glutathione and glutamylcysteine ligase by 1,4-phenylenebis(methylene)selenocyanate and its glutathione conjugate: Role of nuclear factor-erythroid 2-related factor 2. Free Radic. Biol. Med. 2012, 52, 2064–2071. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).