Metabolic and Biomolecular Changes Induced by Incremental Long-Term Training in Young Thoroughbred Racehorses during First Workout Season
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Ethical Animal Research
2.2. Animals
2.3. Sample Collection
2.4. Serum Biochemical Parameters
2.5. RT-qPCR Analyses
2.6. Statistical Analysis
3. Results
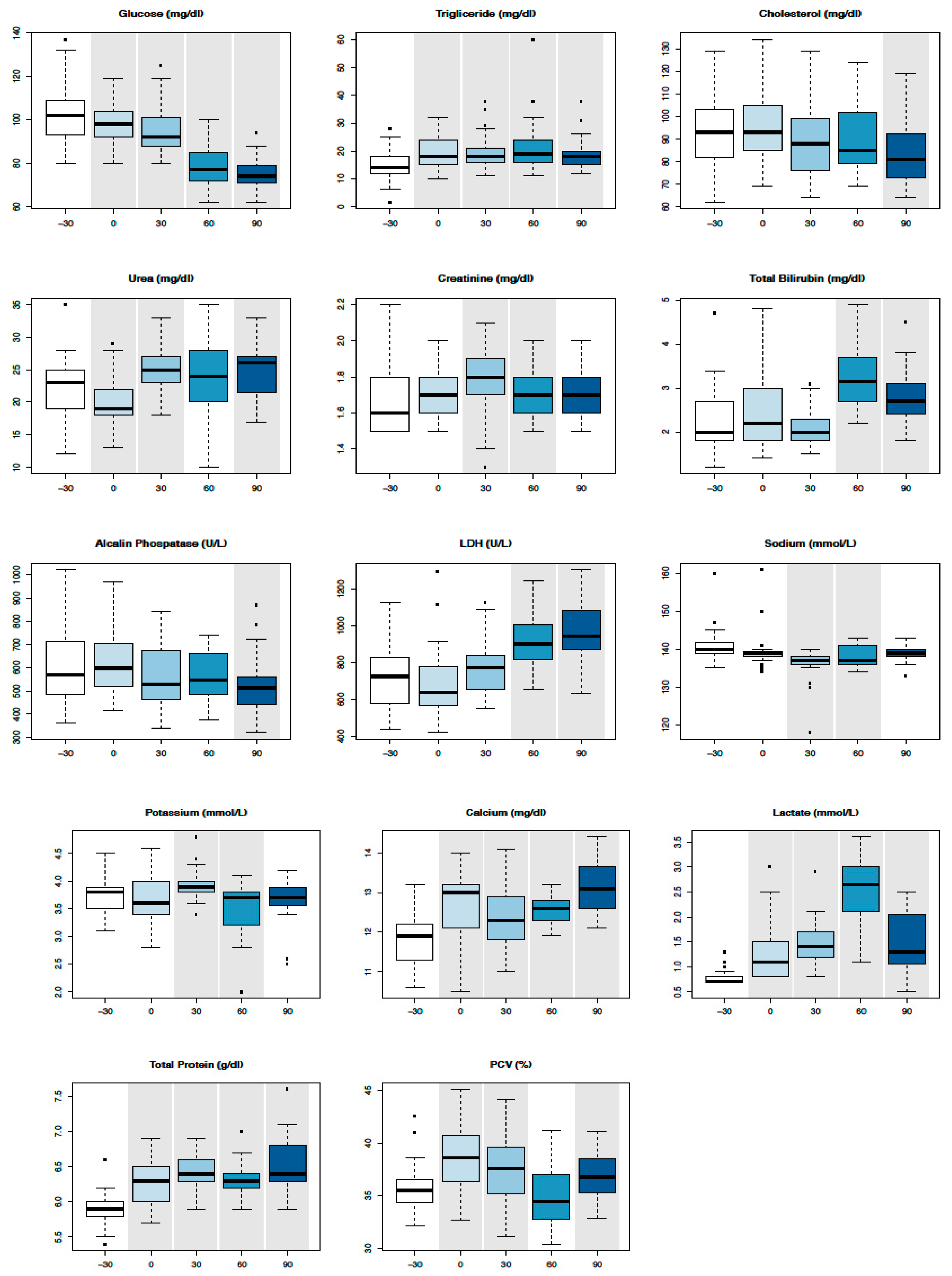
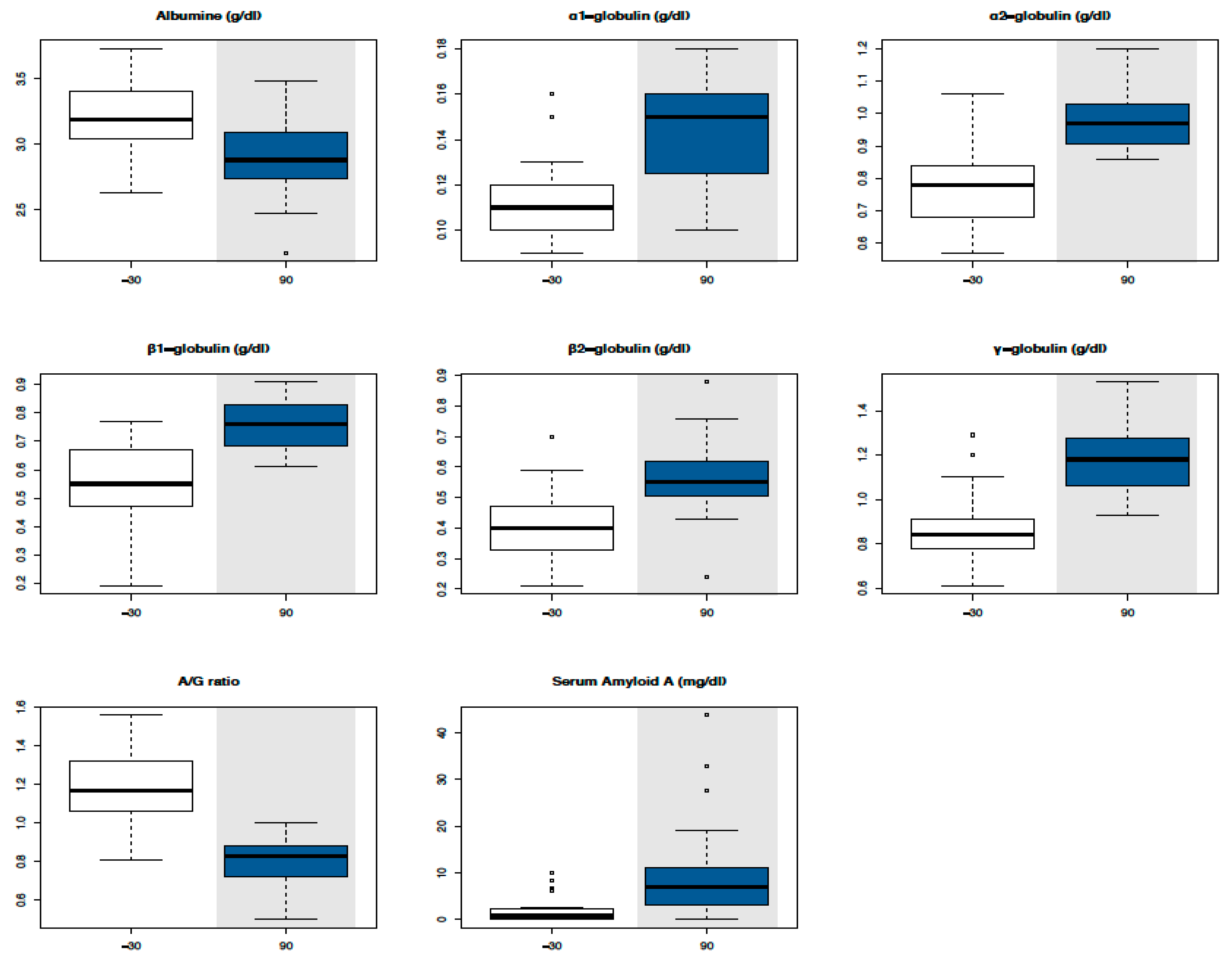
3.1. Serum Biochemical Parameters
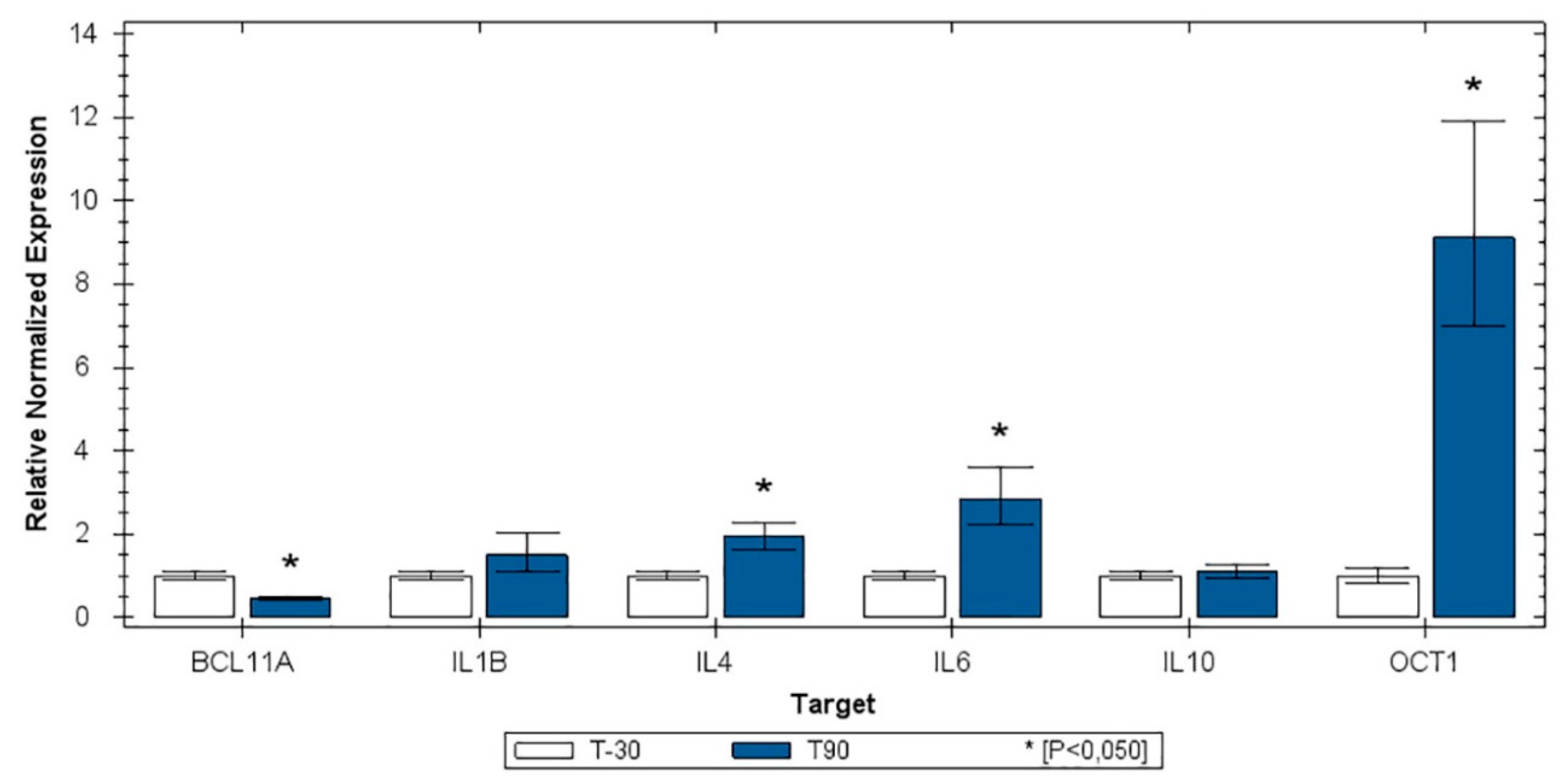
3.2. RT-qPCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PCV | plasma cell volume |
| TG | triglycerides |
| Chol | cholesterol |
| AST | aspartate aminotransferase |
| GGT | γ-glutamyltransferase |
| ALP | alkaline phosphatase |
| Tbil | total bilirubin |
| LDH | lactate dehydrogenase |
| CK | creatine kinase |
| Alb | albumin |
| TPs | total proteins |
| P | phosphorus |
| Ca2+ | calcium |
| Mg | magnesium |
| Na+ | sodium |
| K− | potassium |
| Cl | chloride |
| SPE | serum protein electrophoresis |
| SAA | serum amyloid A |
| RT-qPCR | real-time qPCR |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IL-1β | Interleukin-1β |
| OCT1 | Octamer-Binding Transcription Factor 1, https://www.genecards.org/cgi-bin/carddisp.pl?gene=POU2F1 |
| BCL11A | https://www.uniprot.org/uniprot/Q9H165 |
References
- Allaam, M.A.; Elseady, Y.; Nayel, M.H.; El-sify, A.; Salama, A.; Hassan, H.Y.; Hassan, M.M.; Kamar, A. Physiological and hemato-chemical evaluation of thoroughbred race horse after exercise. IJAVMS 2014, 8, 81–93. [Google Scholar]
- McGowan, C. Clinical pathology in the racing horse; the role of clinical pathology in assessing fitness and performance in the racehorse. Vet. Clin. N. Am. Equine Pract. 2008, 24, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Piccione, G.; Casella, S.; Giannetto, C.; Messina, V.; Monteverde, V.; Caola, G.; Guttadauro, S. Hemathological and haematobiochemical responses to training and competition in standardbred horses. Comp. Cl. Pathol. 2010, 19, 95–101. [Google Scholar] [CrossRef]
- Hassan, H.Y.; Aly, M.A.; ELseady, Y.M.; Nayel, M.A.; Elsify, A.M.; Salama, A.A.; Hassan, M.S.; Elbarody, E.F.; Kamar, A.B. The Effect of Race in the Clinical, Hematological and Biochemical Biomarkers in Thoroughbred Horses. Alexandria J. Vet. Sci. 2015, 46, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Piccione, G.; Casella, S.; Giannetto, C.; Monteverde, V.; Ferrantelli, V. Exercise-induced Modifications on Haematochemical and Electrophoretic Parameters During 1600 and 2000 Meters Trot Races in Standardbred Horses. J. Appl. Anim. Res. 2009, 35, 131–135. [Google Scholar] [CrossRef]
- Snow, D.H.; Mackenzie, G. Effect of Training on some Metabolic Changes associated with Submaximal Endurance Exercise in the Horse. Equine Vet. J. 1977, 9, 226–230. [Google Scholar] [CrossRef]
- Jablonska, E.M.; Ziolkowska, S.M.; Gill, J.; Szykula, R.; Faff, J. Changes in some haematological and metabolic indices in young horses during the first year of jump-training. Equine Vet. J. 1991, 23, 309–311. [Google Scholar] [CrossRef]
- Pedersen, B.K. The anti-inflammatory effect of exercise: Its role in diabetes and cardiovascular disease control. Essays Biochem. 2006, 42, 105–117. [Google Scholar]
- Pedersen, B.K.; Hoffman-Goetz, L. Exercise and the immune system: Regulation, integration, and adaptation. Physiol. Rev. 2000, 80, 1055–1081. [Google Scholar] [CrossRef] [Green Version]
- Cappelli, K.; Felicetti, M.; Capomaccio, S.; Nocelli, C.; Silvestrelli, M.; Verini-Supplizi, A. Effect of training status on immune defence related gene expression in Thoroughbred: Are genes ready for the sprint? Vet. J. 2013, 195, 373–376. [Google Scholar] [CrossRef]
- Horohov, D.W.; Sinatra, S.T.; Chopra, R.K.; Jankowitz, S.; Betancourt, A.; Bloomer, R.J. The Effect of Exercise and Nutritional Supplementation on Proinflammatory Cytokine Expression in Young Racehorses During Training. J. Equine Vet. Sci. 2012, 32, 805–815. [Google Scholar] [CrossRef]
- Miglio, A.; Antognoni, M.T.; Miniscalco, B.; Caivano, D.; Lepri, E.; Birettoni, F.; Mangili, V. Acute undifferentiated leukaemia in a dog. Aust. Vet. J. 2014, 92, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Spada, E.; Antognoni, M.T.; Proverbio, D.; Ferro, E.; Mangili, V.; Miglio, A. Haematological and biochemical reference intervals in adult Maine Coon cat blood donors. J. Feline Med. Surg. 2015, 17, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, K.; Felicetti, M.; Capomaccio, S.; Spinsanti, G.; Silvestrelli, M.; Verini Supplizi, A. Exercise induced stress in horses: Selection of the most stable reference genes for quantitative RT-PCR normalization. BMC Mol. Biol. 2008, 9, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology 2002, 3, research0034.1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–143. 2019. Available online: https://CRAN.R-project.org/package=nlme (accessed on 25 August 2019).
- Revelle, W. psych: Procedures for Psychological, Psychometric, and Personality Research. Northwestern University, Evanston, Illinois. R package version 1.9.12. 2019. Available online: https://CRAN.R-project.org/package=psych (accessed on 25 August 2019).
- Rossdales Laboratories, Reference Ranges. Available online: https://www.rossdales.com/laboratories/reference-ranges (accessed on 25 August 2019).
- Miglio, A.; Morelli, C.; Maresca, C.; Felici, A.; Moscati, L.; Maria, T.A. Biochemical reference intervals for the Italian Heavy Draft horse. Comp. Clin. Pathol. 2019, 28, 841–851. [Google Scholar] [CrossRef]
- Miglio, A.; Morelli, C.; Maresca, C.; Felici, A.; Di Gianbattista, A.; Antognoni, M.T. Serum protein concentrations and protein fractions in clinically healthy Italian Heavy Draft Horses using agarose gel electrophoresis. Vet. Clin. Pathol. 2019, 48, 677–682. [Google Scholar] [CrossRef]
- Moghetti, P.; Bacchi, E.; Brangani, C.; Donà, S.; Negri, C. Metabolic Effects of Exercise. Front. Horm. Res. 2016, 47, 44–57. [Google Scholar]
- Geor, R.J.; McCutcheon, L.J.; Hinchcliff, K.W.; Sams, R.A. Training-induced alterations in glucose metabolism during moderate-intensity exercise. Equine Vet. J. 2002, 34, 22–28. [Google Scholar] [CrossRef]
- Fragala, M.S.; Bi, C.; Chaump, M.; Kaufman, H.W.; Kroll, M.K. Associations of aerobic ad strength exercise with clinical laboratory test values. PLoS ONE 2017, 12, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Lee, P.; Mori, N.; Yamamoto, I.; Arai, T. Long term intensive exercise training leads to a higher plasma malate/lactate dehydrogenase (M/L) ratio and increased level of lipid mobilization in horses. Vet. Res. Commun. 2012, 36, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Hargreaves, M. Exercise, Glut4, and skeletal muscle glucose uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pösö, A.R.; Viljanen-Tarifa, E.; Soveri, T.; Oksanen, H.E. Exercise-induced transient hyperlipidemia in the racehorse. J. Vet. Med. Ser. A 1989, 36, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashizume, M.; Mihara, M. IL-6 and lipid metabolism. Inflamm. Regen. 2011, 31, 325–333. [Google Scholar] [CrossRef]
- Capomaccio, S.; Cappelli, K.; Spinsanti, G.; Mencarelli, M.; Muscettola, M.; Felicetti, M.; Supplizi, A.; Bonifazi, M. Athletic humans and horses: Comparative analysis of interleukin-6 (IL-6) and IL-6 receptor (IL-6R) expression in peripheral blood mononuclear cells in trained and untrained subjects at rest. BMC Physiol. 2011, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Kitaoka, Y.; Endo, Y.; Mukai, K.; Aida, H.; Hiraga, A.; Hatta, H. Muscle glycogen breakdown and lactate metabolism during intensive exercise in Thoroughbred horses. J. Phys. Fit. Sports Med. 2014, 3, 451–456. [Google Scholar] [CrossRef]
- Nalbandian, M.; Takeda, M. Lactate as a Signaling Molecule That Regulates Exercise-Induced Adaptations. Biology 2016, 5, 38. [Google Scholar] [CrossRef] [Green Version]
- Weaver, C.W.; Heaney, R.H. Influence of physical activity on calcium and bone. In Calcium in Human Health (Nutrition and Health), 1st ed.; Humana Press Inc.: Totowa, NJ, USA, 2005; pp. 227–246. [Google Scholar]
- Makhro, A.; Haider, T.; Wang, J.; Bogdanov, N.; Steffen, P.; Wagner, C.; Meyer, T.; Gassmann, M.; Hecksteden, A.; Kaestner, L.; et al. Comparing the impact of an acute exercise bout on plasma amino acid composition, intraerythrocytic Ca2+ handling, and red cell function in athletes and untrained subjects. Cell. Calcium 2016, 60, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Piccione, G.; Arfuso, F.; Marafioti, S.; Giannetto, C.; Giudice, E.; Fazio, F. Different training schedules influence serum electrophoretic protein profile in the athletic horse. J. Equine Vet. Sci. 2015, 35, 856–859. [Google Scholar] [CrossRef]
- Tothova, C.; Nagy, O.; Kovac, G. Serum proteins and their diagnostic utility in veterinary medicine: A review. Vet. Med. (Praha) 2016, 61, 475–496. [Google Scholar] [CrossRef] [Green Version]
- Witkowska-Piłaszewicz, O.D.; Żmigrodzka, M.; Winnicka, A.; Miśkiewicz, A.; Strzelec, K.; Cywińska, A. Serum amyloid A in equine health and disease. Equine Vet. J. 2019, 51, 293–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witkowska-Piłaszewicz, O.; Bąska, P.; Czopowicz, M.; Żmigrodzka, M.; Szczepaniak, J.; Szarska, E.; Winnicka, A.; Cywińska, A. Changes in Serum Amyloid A (SAA) Concentration in Arabian Endurance Horses During First Training Season. Animals 2019, 9, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karacabey, K.; Saygin, O.; Ozmerdivenli, R.; Zorba, E.; Godekmerdan, A.; Bulut, V. The effects of exercise on the immune system and stress hormones in sportswomen. Neuroendocrinol. Lett. 2005, 26, 361–366. [Google Scholar]
- Shah, P.C.; Bertolino, E.; Singh, H. Using altered specificity Oct-1 and Oct-2 mutants to analyze the regulation of immunoglobulin gene transcription. EMBO J. 1997, 16, 7105–7117. [Google Scholar] [CrossRef] [Green Version]
- Gasparello, J.; Fabbri, E.; Bianchi, N.; Breveglieri, G.; Zuccato, C.; Borgatti, M.; Gambari, R.; Finotti, A. BCL11A mRNA Targeting by miR-210: A Possible Network Regulating γ-Globin Gene Expression. Int. J. Mol. Sci. 2017, 18, 2530. [Google Scholar] [CrossRef] [Green Version]
| February | March (T-30) | April (T0) | May (T30) | June (T60) | July (T90) |
|---|---|---|---|---|---|
| 15 min Walk 10 min Trot Rest 10 min Trot Walk | 15 min Walk 10 min Trot 3 min Canter | 15 min Walk 10 min Trot 6 min Canter every Tuesday: 1 min Gallop | 15 min Walk 10 min Trot 6 min Canter every Tuesday: 2 min Gallop | 15 min Walk 10 min Trot 6 min Canter every Tuesday: 3 min Gallop | 15 min Walk 10 min Trot 6 min Canter every Tuesday: 4 min Gallop |
| Gene Name | Accession ID | Amplicon Length (bp) | Primer Forward | Primer Reverse | Reference |
|---|---|---|---|---|---|
| IL-4 | NM_001082519.1 | 75 | AAGAATGCCTGAGCGGACTG | TGGCTTCATTCACAGTACAGCA | This work |
| IL-10 | XM_014739408.1 | 107 | TTCAGCAGGGTGAAGACTTTCT | AAGGCTTGGCAACCCAGGTA | This work |
| Oct1 | XM_023640479.1 | 165 | GATTGAGGGCTTGAACCGC | ACCAAACACGAATCACCTCC | This work |
| BCL11A | XM_023619062.1 | 87 | TTTGCCCCAAACAGGAACAC | ATGCACTGGTGAATGGCTGT | This work |
| IL-6 | NM_001082496 | 98 | TCAAGGGTGAAAAGGAAAACATC | GGTGGTTACTTCTGGATTCTTC | [8] |
| IL-1β | NM_001082526 | 135 | AGAACCTGTACCTGTCTTGTG | CGTTGCCCTTGATTTCCATC | [8] |
| HPRT1 | AY372182 | 121 | AATTATGGACAGGACTGAACGG | ATAATCCAGCAGGTCAGCAAAG | [9] |
| SDHA | DQ402987 | 91 | GAGGAATGGTCTGGAATACTG | GCCTCTGCTCCATAAATCG | [9] |
| Parameters | T-30 | T0 | T30 | T60 | T90 | RIs of Two-Year-Old Thoroughbred Horses in Training [17,18,19] | RIs of Adult Thoroughbred Horses at Rest [17,18,19] |
|---|---|---|---|---|---|---|---|
| PCV (%) | 35 ± 2.5 | 38 ± 3.1 | 37 ± 3.3 | 35 ± 3.09 | 36 ± 2.2 | 34–45 | 38–50 |
| Gluc (mg/dL) | 104 ± 14.6 | 98 ± 9.3 | 95 ± 11.6 | 78 ± 9.5 | 75 ± 6.9 | 61–106 | 75–115 |
| TG (mg/dL) | 15 ± 5.5 | 19 ± 5.8 | 19 ± 6.9 | 22 ± 10.6 | 19 ± 5.8 | 23–230 | 4–44 |
| Chol (mg/dL) | 92.2 ± 16.0 | 94.8 ± 17.1 | 90.1 ± 17.2 | 90.5 ± 15.3 | 83.2 ± 14.4 | 77–128 | 75–150 |
| Urea (mg/dL) | 22 ± 4.7 | 19 ± 4.2 | 25 ± 3.9 | 23 ± 5.8 | 24 ± 4.0 | 11–20 | 21.4–51.4 |
| Creat (mg/dL) | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.2–1.8 | 1.2–1.9 |
| Tbil (mg/dL) | 2.2 ± 0.7 | 2.5 ± 0.8 | 2.064 ± 0.4 | 3.2 ± 0.6 | 2.7 ± 0.6 | 0.8–2.3 | 1–2 |
| AST (IU/L) | 501 ± 211 | 477 ± 262 | 474 ± 221 | 489 ± 213 | 453 ± 148 | 308–820 | 243–327 |
| GGT (IU/L) | 22 ± 10.6 | 27 ± 18.0 | 23 ± 10.1 | 23 ± 14.0 | 22 ± 7.8 | 12–40 | 4.3–13.4 |
| ALP (IU/L) | 591 ± 149 | 619 ± 133 | 569 ± 141 | 561 ± 99 | 520 ± 129 | 293–672 | 143–395 |
| CK (IU/L) | 364 ± 176 | 412 ± 387 | 337 ± 137 | 347 ± 156 | 392 ± 196 | 166–572 | 76–154 |
| LDH (IU/L) | 735 ± 183 | 686 ± 195 | 777 ± 146 | 920 ± 146 | 953 ± 168 | 569–917 | 477–813 |
| P (mg/dL) | 4.8 ± 0.7 | 4.893 ± 0.5 | 4.9 ± 0.7 | 4.6 ± 0.8 | 4.9 ± 0.7 | 3.4–5.0 | 3.1–5.6 |
| Mg (mg/dL) | 1.7 ± 1.1 | 1.9 ± 0.0 | 2.0 ± 0.1 | 1.9 ± 0.3 | 1.9 ± 0.1 | 1.5–2.2 | 2.2–2.8 |
| Na (mmol/L) | 140 ± 4.4 | 139 ± 4.9 | 136 ± 4.4 | 137 ± 2.7 | 139 ± 1.9 | 130–142 | 132–146 |
| K (mmol/L) | 3.7 ± 0.3 | 3.6 ± 0.4 | 3.9 ± 0.2 | 3.5 ± 0.5 | 3.6 ± 0.4 | 2.8–4.2 | 2.4–4.7 |
| CL (mmol/L) | 98.7 ± 2.0 | 99.5 ± 8.0 | 98.4 ± 1.2 | 99.6 ± 1.2 | 99.7 ± 1.8 | 99–109 | 99–109 |
| Ca (mg/dL) | 11.8 ± 0.6 | 12.5 ± 0.9 | 12.3 ± 0.8 | 12.5 ± 0.3 | 13.1 ± 0.6 | 11.6–13.2 | 11.2–13.6 |
| Lac (mmol/L) | 0.7 ± 0.1 | 1.2 ± 0.5 | 1.4 ± 0.4 | 2.6 ± 0.6 | 1.4 ± 0.5 | 1.11–1.78 | 1.11–1.78 |
| Alb (g/dL) | 3.8 ± 0.2 | 3.9 ± 0.2 | 3.8 ± 0.2 | 3.8 ± 0.2 | 3.5 ± 0.2 | 3.5–3.9 | 2.6–3.7 |
| TP (g/dL) | 5.9 ± 0.2 | 6.2 ± 0.3 | 6.4 ± 0.2 | 6.3 ± 0.2 | 6.5 ± 0.3 | 5.9–6.6 | 5.2–7.9 |
| α1-glob (g/dL) | 0.11 ± 0.02 | - | - | - | 0.14 ± 0.31 | 0.06–0.14 | 0.06–0.7 |
| α2-glob (g/dL) | 0.77 ± 0.11 | - | - | - | 0.97 ± 0.02 | 0.54–0.78 | 0.31–1.31 |
| β1-glob (g/dL) | 0.55 ± 0.14 | - | - | - | 0.75 ± 0.08 | 0.57–0.85 | 0.4–1.58 |
| β2-glob (g/dL) | 0.39 ± 0.10 | - | - | - | 0.56 ± 0.12 | 0.18–0.68 | 0.29–0.89 |
| γ-glob (g/dL) | 0.87 ± 0.14 | - | - | - | 1.18 ± 0.14 | 0.37–0.82 | 0.55–1.9 |
| A/G ratio | 1.12 ± 0.2 | 0.8 ± 0.1 | 0.6–1.46 | 0.6–1.46 | |||
| SAA(mg/L) | 1.9 ± 2.9 | 10.2 ± 11.1 | 0.0–20.0 | 0.0–20.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miglio, A.; Cappelli, K.; Capomaccio, S.; Mecocci, S.; Silvestrelli, M.; Antognoni, M.T. Metabolic and Biomolecular Changes Induced by Incremental Long-Term Training in Young Thoroughbred Racehorses during First Workout Season. Animals 2020, 10, 317. https://doi.org/10.3390/ani10020317
Miglio A, Cappelli K, Capomaccio S, Mecocci S, Silvestrelli M, Antognoni MT. Metabolic and Biomolecular Changes Induced by Incremental Long-Term Training in Young Thoroughbred Racehorses during First Workout Season. Animals. 2020; 10(2):317. https://doi.org/10.3390/ani10020317
Chicago/Turabian StyleMiglio, Arianna, Katia Cappelli, Stefano Capomaccio, Samanta Mecocci, Maurizio Silvestrelli, and Maria Teresa Antognoni. 2020. "Metabolic and Biomolecular Changes Induced by Incremental Long-Term Training in Young Thoroughbred Racehorses during First Workout Season" Animals 10, no. 2: 317. https://doi.org/10.3390/ani10020317
APA StyleMiglio, A., Cappelli, K., Capomaccio, S., Mecocci, S., Silvestrelli, M., & Antognoni, M. T. (2020). Metabolic and Biomolecular Changes Induced by Incremental Long-Term Training in Young Thoroughbred Racehorses during First Workout Season. Animals, 10(2), 317. https://doi.org/10.3390/ani10020317








