Nitric Oxide Synthase Is Involved in Follicular Development via the PI3K/AKT/FoxO3a Pathway in Neonatal and Immature Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Ethics Statement
2.2. Histological and Morphological Examination
2.3. Measurement of NOS Activity and NO Concentration
2.4. Western Blotting
2.5. Statistical Analysis
3. Results
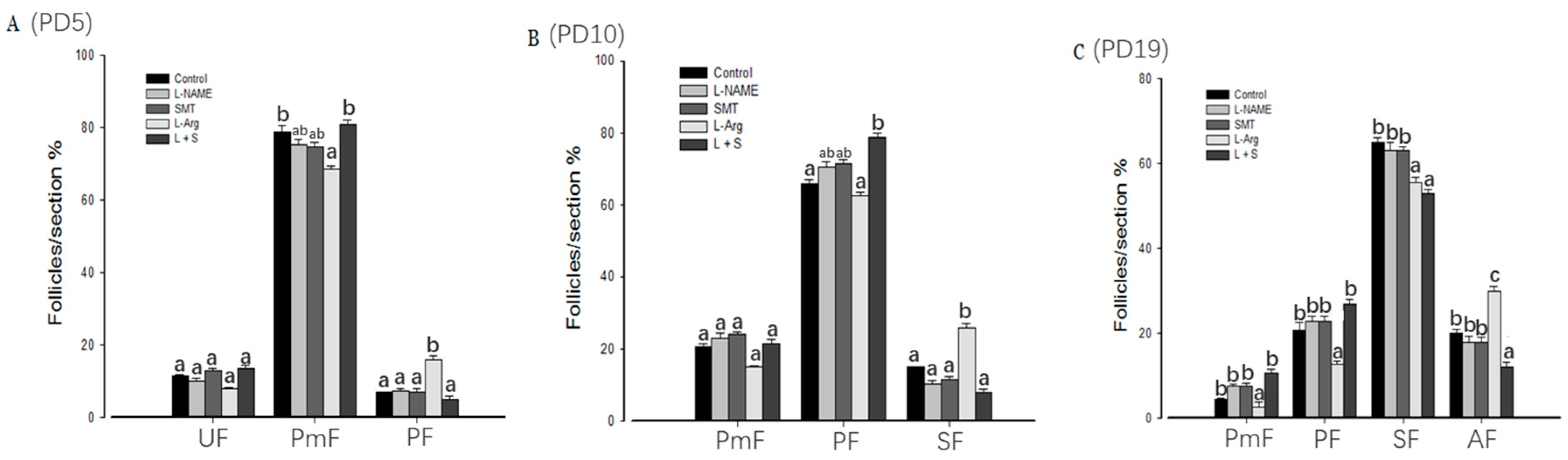
3.1. Follicular Development
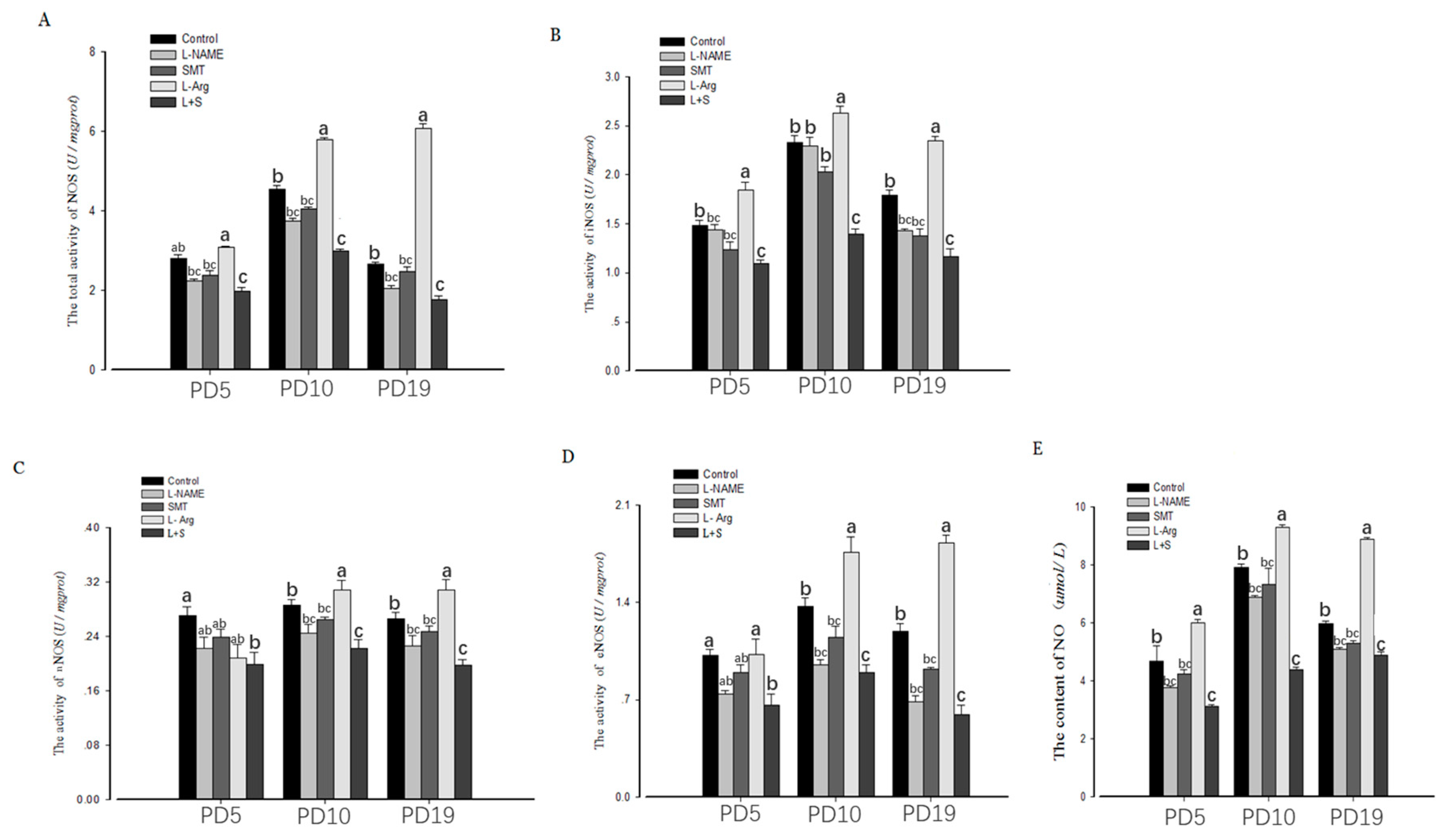
3.2. Ovarian NOS Activity and NO Content
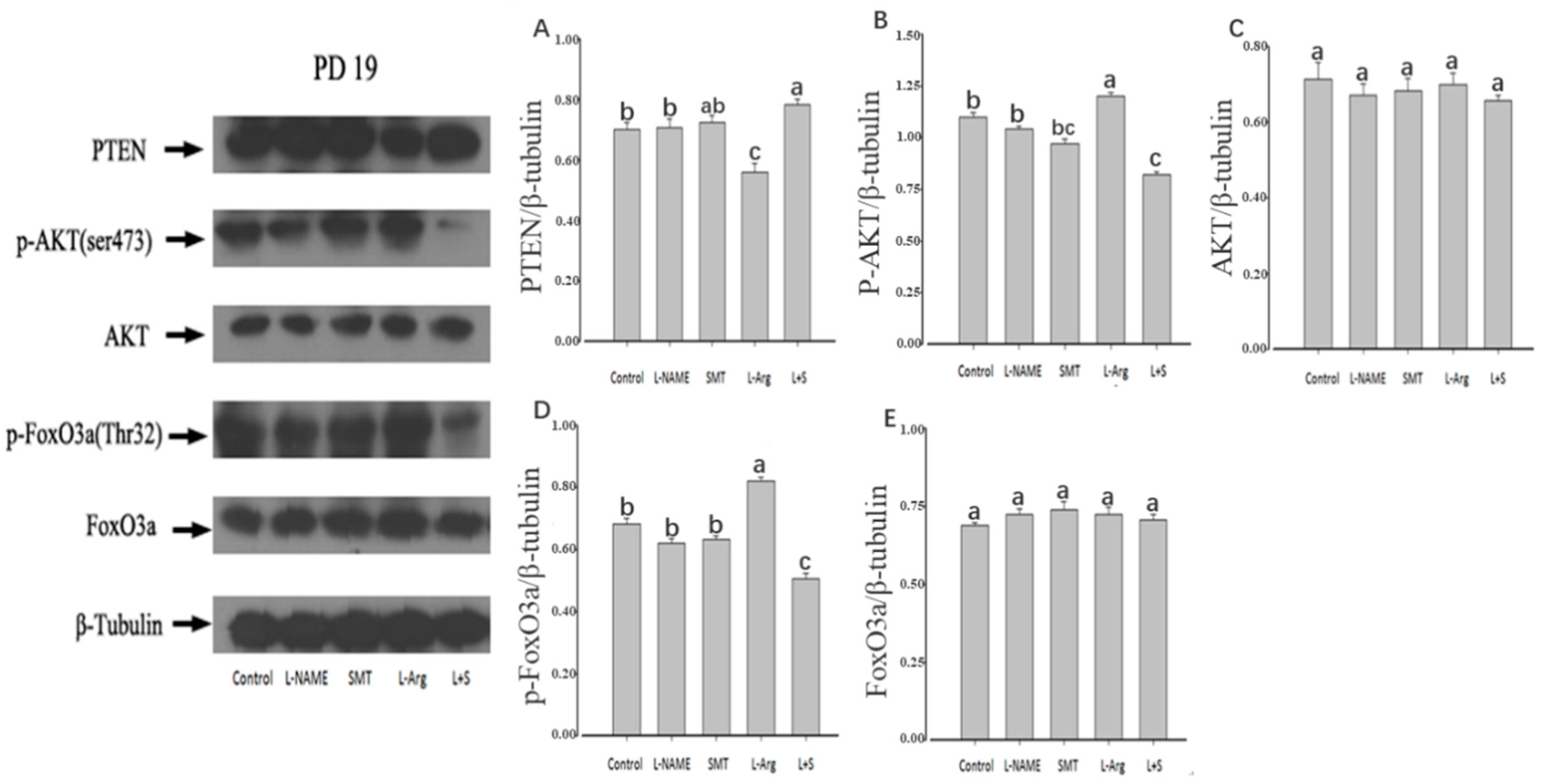
3.3. Ovarian Expression of PTEN, p-AKT, AKT, p-FoxO3a and FoxO3a
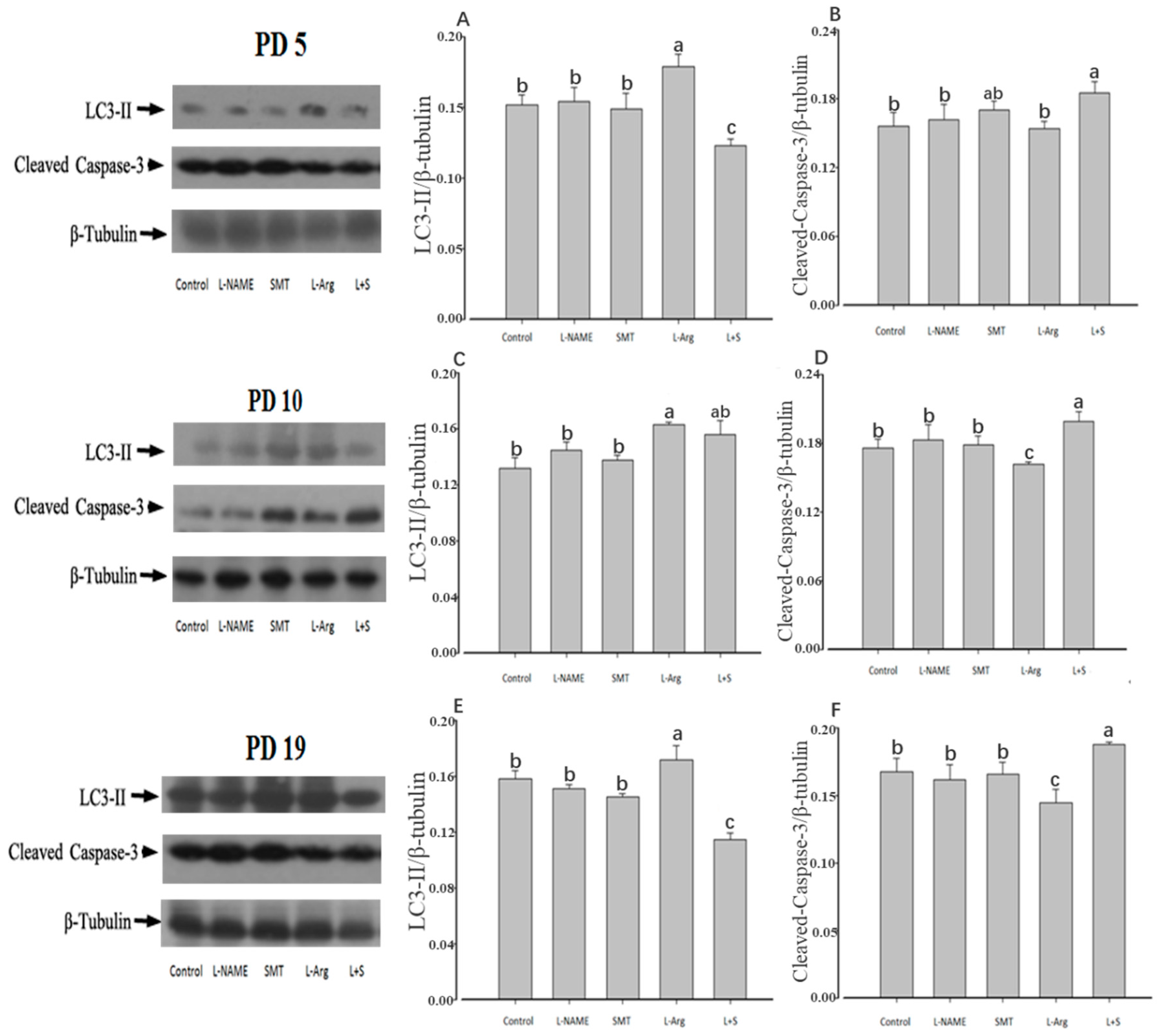
3.4. Ovarian Expression of LC3-II and Cleaved-Caspase-3
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maksoud, M.J.E.; Tellios, V.; Xiang, Y.Y.; Lu, W.Y. Nitric oxide signaling inhibits microglia proliferation by activation of protein kinase-G. Nitric. Oxide. 2019, 94, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Stuehr, D.J.; Vasquez-Vivar, J. Nitric oxide synthases-from genes to function. Nitric. Oxide. 2017, 63, 29. [Google Scholar] [CrossRef] [PubMed]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357 Pt 3, 593–615. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Olson, L.M.; Jones-Burton, C.M.; Jablonka-Shariff, A. Nitric oxide decreases estradiol synthesis of rat luteinized ovarian cells: Possible role for nitric oxide in functional luteal regression. Endocrinology 1996, 137, 3531–3539. [Google Scholar] [CrossRef]
- Xu, K.; Tian, Y.; Weng, X.; Hu, X.; Heng, D.; Xia, G.; Zhang, C. Effect of thyroid dysfunction on NOS expression in the female rat. Cell Tissue Res. 2019. [Google Scholar] [CrossRef]
- Becerril, S.; Rodríguez, A.; Catalán, V.; Ramírez, B.; Unamuno, X.; Portincasa, P.; Gómez-Ambrosi, J.; Frühbeck, G. Functional Relationship between Leptin and Nitric Oxide in Metabolism. Nutrients 2019, 11, 2129. [Google Scholar] [CrossRef]
- Zheng, K.; Sulieman, F.J.; Li, J.; Wei, Q.; Xu, M.; Shi, F. Nitric oxide and thyroid hormone receptor alpha 1 contribute to ovarian follicular development in immature hyper- and hypo-thyroid rats. Reprod. Biol. 2015, 15, 27–33. [Google Scholar] [CrossRef]
- Grazul-Bilska, A.T.; Bass, C.S.; Kaminski, S.L.; Ebel, K.K.; Leke, E.; Thammasiri, J.; Kraisoon, A.; Navanukraw, C.; Holst, M.; Shelton, M.; et al. Effects of plane of nutrition and arginine on ovarian follicles in non-pregnant sheep: Cell proliferation, and expression of endothelial nitric oxide and its receptor. Acta. Histochem. 2019, 121, 189–197. [Google Scholar] [CrossRef]
- Basini, G.; Grasselli, F. Nitric oxide in follicle development and oocyte competence. Reproduction 2015, 150, R1–R9. [Google Scholar] [CrossRef]
- Grazul-Bilska, A.T.; Navanukraw, C.; Johnson, M.L.; Arnold, D.A.; Reynolds, L.P.; Redmer, D.A. Expression of endothelial nitric oxide synthase in the ovine ovary throughout the estrous cycle. Reproduction 2006, 132, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Delsouc, M.B.; Della Vedova, M.C.; Ramírez, D.; Delgado, S.M.; Casais, M. The production of nitric oxide in the coeliac ganglion modulates the effect of cholinergic neurotransmission on the rat ovary during the preovulatory period. Nitric. Oxide. 2018, 75, 85–94. [Google Scholar] [CrossRef]
- Singh, V.K.; Lal, B. Nitric oxide (NO) stimulates steroidogenesis and folliculogenesis in fish. Reproduction 2017, 153, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tan, J.; Xu, X.; Yang, H.; Wu, F.; Xu, B.; Liu, W.; Shi, P.; Xu, Z.; Deng, Y. Prepubertal overexposure to manganese induce precocious puberty through GABAA receptor/nitric oxide pathway in immature female rats. Ecotoxicol. Environ. Saf. 2019, 188, 109898. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.B.; Joseph, A.; Thomas, P.L.; Dsilva, B.; Pillai, S.M.; Laloraya, M. Impaired arginine metabolism coupled to a defective redox conduit contributes to low plasma nitric oxide in polycystic ovary syndrome. Cell. Physiol. Biochem. 2017, 43, 1880–1892. [Google Scholar] [CrossRef] [PubMed]
- Pallares, P.; Garcia-Fernandez, R.A.; Criado, L.M.; Letelier, C.A.; Esteban, D.; Fernandez-Toro, J.M.; Flores, J.M.; Gonzalez-Bulnes, A. Disruption of the endothelial nitric oxide synthase gene affects ovulation, fertilization and early embryo survival in a knockout mouse model. Reproduction 2008, 136, 573–579. [Google Scholar] [CrossRef]
- Jablonka-Shariff, A.; Ravi, S.; Beltsos, A.N.; Murphy, L.L.; Olson, L.M. Abnormal estrous cyclicity after disruption of endothelial and inducible nitric oxide synthase in mice. Biol. Reprod. 1999, 61, 171–177. [Google Scholar] [CrossRef]
- Moonmanee, T.; Navanukraw, C.; Uriyapongson, S.; Kraisoon, A.; Aiumlamai, S.; Guntaprom, S.; Rittirod, T.; Borowicz, P.P.; Redmer, D.A. Relationships among vasculature, mitotic activity, and endothelial nitric oxide synthase (eNOS) in bovine antral follicles of the first follicular wave. Domest. Anim. Endocrinol. 2013, 45, 11–21. [Google Scholar] [CrossRef]
- Matsumi, H.; Yano, T.; Osuga, Y.; Kugu, K.; Tang, X.; Xu, J.P.; Yano, N.; Kurashima, Y.; Ogura, T.; Tsutsumi, O.; et al. Regulation of nitric oxide synthase to promote cytostasis in ovarian follicular development. Biol. Reprod. 2000, 63, 141–146. [Google Scholar] [CrossRef]
- Tsutsui, M.; Shimokawa, H.; Morishita, T.; Nakashima, Y.; Yanagihara, N. Development of genetically engineered mice lacking all three nitric oxide synthases. Pharmacol. Sci. 2006, 102, 147–154. [Google Scholar] [CrossRef]
- Cantley, L.C. The phosphoinositide 3-kinase pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef] [PubMed]
- Cully, M.; You, H.; Levine, A.J.; Mak, T.W. Beyond PTEN mutations: The PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat. Rev. Cancer 2006, 6, 184–192. [Google Scholar] [CrossRef]
- Tran, H.; Brunet, A.; Griffith, E.C.; Greenberg, M.E. The many forks in FOXO’s road. Sci. STKE 2003, 172, RE5. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Mo, S.J.; Feng, Q.Q.; Zhan, M.L.; Ouyang, L.S.; Chen, J.C.; Ma, Y.X.; Wu, J.J.; Lei, W.L. EPO-dependent activation of PI3K/Akt/Foxo3a signalling mediates neuroprotection in in vitro and in vivo models of Parkinson’s disease. J. Mol. Neurosci. 2014, 53, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Castrillon, D.H.; Miao, L.; Kollipara, R.; Horner, J.W.; DePinho, R.A. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 2003, 301, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Rajareddy, S.; Liu, L.; Jagarlamudi, K.; Boman, K.; Selstam, G.; Reddy, P. Control of mammalian oocyte growth and early follicular development by the oocyte PI3 kinase pathway: New roles for an old timer. Dev. Biol. 2006, 299, 1–11. [Google Scholar] [CrossRef] [PubMed]
- John, G.B.; Gallardo, T.D.; Shirley, L.J.; Castrillon, D.H. Foxo3 is a PI3Kdependent molecular switch controlling the initiation of oocyte growth. Dev. Biol. 2008, 321, 197–204. [Google Scholar] [CrossRef]
- Hou, Y.; Sun, G.; Jiang, X.; Zhu, Z.; Yang, J. Nuclear forkhead box O3a accumulation contributing to the proliferative suppression in liver cancer cells by PI3K/Akt signaling pathway. J. Cancer Res. Ther. 2018, 14, 1124. [Google Scholar]
- Wu, H.C.; Zhang, J.W.; Sun, Z.G.; Xiang, S.; Qiao, Y.; Lian, F. Effects of Electroacupuncture on Expression of PI3K/Akt/Foxo3a in Granulosa Cells from Women with Shen (Kidney) Deficiency Syndrome Undergoing in vitro Fertilization-Embryo Transfer. Chin. J. Integr. Med. 2019, 25, 252–258. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Wang, Y.; Zhao, K.; Chi, Y.; Wang, B. Pyrroloquinoline quinine protects HK-2 cells against high glucose-induced oxidative stress and apoptosis through Sirt3 and PI3K/Akt/FoxO3a signaling pathway. Biochem. Biophys. Res. Commun. 2019, 508, 398–404. [Google Scholar] [CrossRef]
- Borniquel, S.; García-Quintáns, N.; Valle, I.; Olmos, Y.; Wild, B.; Martínez-Granero, F.; Soria, E.; Lamas, S.; Monsalve, M. Inactivation of Foxo3a and subsequent downregulation of PGC-1 alpha mediate nitric oxide-induced endothelial cell migration. Mol. Cell. Biol. 2010, 30, 4035–4044. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Nagaraju, G.; Liu, Z.; Liu, K. Functional roles of the phosphatidylinositol 3-kinases (PI3Ks) signaling in the mammalian ovary. Mol. Cell. Endocrinol. 2012, 356, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ni, Z.; Liu, Y.; Ji, S.; Jin, F.; Jiang, K.; Ma, J.; Ren, C.; Zhang, H.; Hu, Z.; et al. Hyperactivated mTORC1 downregulation of FOXO3a/PDGFRα/AKT cascade restrains tuberous sclerosis complex-associated tumor development. Oncotarget 2017, 8, 54858–54872. [Google Scholar]
- Ding, W.; Zhang, W.; Hui, F.M.; Zhang, Y.H.; Zhang, F.F.; Li, X.M.; Shi, F.X. Cell-specific expression and immunolocalization of nitric oxide synthase isoforms and soluble guanylyl cyclase α1 and β1 subunits in the ovary of fetal, neonatal and immature pigs. Anim. Reprod. Sci. 2012, 131, 172–180. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, Q.W.; Wang, Z.C.; Ding, W.; Wang, W.; Shi, F.X. Cell-specific expression and immunolocalization of nitric oxide synthase isoforms and the related nitric oxide/cyclic GMP signaling pathway in the ovaries of neonatal and immature rats. J. Zhejiang Univ. Sci. B 2011, 12, 55–64. [Google Scholar] [CrossRef]
- Castardo-de-Paula, J.C.; de Campos, B.H.; Amorim, E.D.T.; da Silva, R.V.; de Farias, C.C.; Higachi, L.; Pinge-Filho, P.; Barbosa, D.S.; Martins-Pinge, M.C. Cardiovascular risk and the effect of nitric oxide synthase inhibition in female rats: The role of estrogen. Exp. Gerontol. 2017, 97, 38–48. [Google Scholar] [CrossRef]
- Hasarmeh, M.; Itzik, A.; Weidenfeld, J.; Ovadia, H. Modulation of Hyperosmotic and Immune-Induced Disruption of the Blood-Brain Barrier by the Nitric Oxide System. Neuroimmunomodulation 2016, 23, 1–7. [Google Scholar] [CrossRef]
- Hallemeesch, M.M.; Cobben, D.C.; Soeters, P.B.; Deutz, N.E. Differential effects of selective and non-selective NOS inhibition on renal arginine and protein metabolism during endotoxemia in rats. Clin. Nutr. 2002, 21, 111–117. [Google Scholar] [CrossRef]
- Vrankova, S.; Zemancikova, A.; Torok, J.; Pechanova, O. Effect of low dose L-NAME pretreatment on nitric oxide/reactive oxygen species balance and vasoactivity in L-NAME/salt-induced hypertensive rats. J. Physiol. Pharmacol. 2019, 70. [Google Scholar] [CrossRef]
- Nath, P.; Mukherjee, U.; Biswas, S.; Pal, S.; Das, S.; Ghosh, S.; Samanta, A.; Maitra, S. Expression of nitric oxide synthase (NOS) in Anabas testudineus ovary and participation of nitric oxide-cyclic GMP cascade in maintenance of meiotic arrest. Mol. Cell. Endocrinol. 2019, 496, 110544. [Google Scholar] [CrossRef]
- Bulbul, T.; Akosman, M.S.; Yilmaz, O.; Ulutas, E.; Bulbul, A. Supplementary dietary nitric oxide donor (sodium nitroprusside) or inhibitor (NG-nitro-l-arginine methyl ester) depressed growth performance and ovarian primordial and primary follicles in Japanese quail (Coturnix coturnix japonica) in a dose-dependent manner. Br. Poult. Sci. 2015, 56, 113–120. [Google Scholar] [PubMed]
- Witlin, A.G.; Gangula, P.R.; Thompson, M.L.; Yallampalli, C. Growth and fertility rates in the offspring of pregnant rats treated with L-omega nitro-L-arginine methyl ester (L-NAME), a nitric oxide inhibitor. Am. J. Obstet. Gynecol. 2002, 186, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Bednov, A.; Espinoza, J.; Betancourt, A.; Vedernikov, Y.; Belfort, M.; Yallampalli, C. L-arginine prevents hypoxia-induced vasoconstriction in dual-perfused human placental cotyledons. Placenta 2015, 36, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Differ, C.; Klatte-Schulz, F.; Bormann, N.; Minkwitz, S.; Knaus, P.; Wildemann, B. Is NO the Answer? The Nitric Oxide Pathway Can Support Bone Morphogenetic Protein 2 Mediated Signaling. Cells 2019, 8, 1273. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, H.; Peng, A.; Guo, S.; Wang, M.; Loor, J.J.; Wang, H. N-carbamylglutamate and l-arginine promote intestinal function in suckling lambs with intrauterine growth restriction by regulating antioxidant capacity via a nitric oxide-dependent pathway. Food Funct. 2019, 10, 6374–6384. [Google Scholar] [CrossRef]
- Vitt, U.A.; McGee, E.A.; Hayashi, M.; Hsueh, A.J. In vivo treatment with GDF-9 stimulates primordial and primary follicle progression and theca cell marker CYP17 in ovaries of immature rats. Endocrinology 2000, 141, 3814–3820. [Google Scholar] [CrossRef]
- Wei, Q.; Korejo, N.A.; Jiang, J.; Xu, M.; Zheng, K.; Mao, D.; Shi, F. Mitigation of stress from gastric mucosal injuries by mulberry extract may occur via nitric oxide synthase signaling in mice. Tissue Cell 2018, 54, 59–64. [Google Scholar] [CrossRef]
- Sheshpari, S.; Shahnazi, M.; Mobarak, H.; Ahmadian, S.; Bedate, A.M.; Nariman-Saleh-Fam, Z.; Nouri, M.; Rahbarghazi, R.; Mahdipour, M. Ovarian function and reproductive outcome after ovarian tissue transplantation: A systematic review. J. Transl. Med. 2019, 17, 396. [Google Scholar] [CrossRef]
- Berlinguer, F.; Porcu, C.; Molle, G.; Cabiddu, A.; Dattena, M.; Gallus, M.; Pasciu, V.; Succu, S.; Sotgiu, F.D.; Paliogiannis, P.; et al. Circulating concentrations of key regulators of nitric oxide production in undernourished sheep carrying single and multiple fetuses. Animals 2020, 10, 65. [Google Scholar] [CrossRef]
- Shi, F.; Stewart, R.L.; Pere, E.; Chen, J.Y.; LaPolt, P.S. Cell-specific expression and regulation of soluble guanylyl cyclase alpha and beta subunit in the rat ovary. Bio Reprod. 2004, 70, 1552–1561. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Zhou, Y.; Graves, D.T. FOXO transcription factors: Their clinical significance and regulation. Biomed. Res. Int. 2014, 2014, 925350. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, Y.; Lee, W.L.; Inoue, K.; Ida, H.; Ito, Y. RUNX3 cooperates with FoxO3a to induce apoptosis in gastric cancer cells. J. Biol. Chem. 2006, 281, 5267–5276. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ma, L.; Liu, N.; Tang, X.; Guo, S.; Zhang, B.; Jiang, Z. Autophagy and apoptosis of porcine ovarian granulosa cells during follicular development. Animals 2019, 9, 1111. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Zhang, R.; Guan, H.; Zhang, W. Follicle loss and PTEN/PI3K/mTOR signaling pathway activated in LepR-mutated mice. Gynecol. Endocrinol. 2019, 35, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.S.; Kim, K.M.; Kwon, Y.G.; Bai, S.K.; Nam, W.D.; Yoo, Y.M.; Kim, P.K.; Chung, H.T.; Billiar, T.R.; Kim, Y.M. Nitric oxide prevents 6-hydroxydopamine-induced apoptosis in PC12 cells through cGMP-dependent PI3 kinase/Akt activation. FASEB J. 2003, 17, 1036–1347. [Google Scholar] [CrossRef] [PubMed]
- Devos, M.; Grosbois, J.; Demeestere, I. Interaction between PI3K/AKT and Hippo pathways during in vitro follicular activation and response to fragmentation and chemotherapy exposure using a mouse immature ovary model. Biol. Reprod. 2019, ioz215. [Google Scholar] [CrossRef]
- Carver, D.J.; Gaston, B.; Deronde, K.; Palmer, L.A. Akt-Mediated Activation of HIF-1 in Pulmonary Vascular Endothelial Cells by S-Nitrosoglutathione. Am. J. Respir. Cell Mol. Biol. 2007, 37, 255–263. [Google Scholar] [CrossRef]
- Choi, J.Y.; Jo, M.W.; Lee, E.Y.; Yoon, B.K.; Choi, D.S. The role of autophagy in follicular development and atresia in rat granulosa cells. Fertil. Steril. 2010, 93, 2532–2537. [Google Scholar] [CrossRef]
- Rosselli, M.; Keller, P.J.; Dubey, R.K. Role of nitric oxide in the biology, physiology and pathophysiology of reproduction. Hum. Reprod. Update 1998, 4, 3–24. [Google Scholar] [CrossRef]
- Kim, Y.M.; Bombeck, C.A.; Billiar, T.R. Nitric Oxide as a bifunctional regulator of apoptosis. Circ. Res. 1999, 84, 253–256. [Google Scholar] [CrossRef]
- Sarkar, S.; Korolchuk, V.I.; Renna, M.; Imarisio, S.; Fleming, A.; Williams, A.; Garcia-Arencibia, M.; Rose, C.; Luo, S.; Underwood, B.R.; et al. Complex inhibitory effects of nitric oxide on autophagy. Mol. Cell 2011, 43, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, S.W.; Klassen, S.S. Nitric oxide differentially regulates the gene expression of caspase genes but not some autophagic genes. Nitric Oxide 2007, 16, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, S.W. Nitric oxide-induced cell death in the heart: The role of autophagy. Autophagy 2007, 3, 347–349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, M.; Zhang, H.L. Death and survival of neuronal and astrocytic cells in ischemic brain injury: A role of autophagy. Acta Pharmacol. Sin. 2011, 32, 1089–1099. [Google Scholar] [CrossRef]
- Boya, P.; González-Polo, R.A.; Casares, N.; Perfettini, J.L.; Dessen, P.; Larochette, N.; Métivier, D.; Meley, D.; Souquere, S.; Yoshimori, T.; et al. Inhibition of macroautophagy triggers apoptosis. Mol. Cell. Biol. 2005, 25, 1025–1040. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhang, W.; Zhu, S.; Shi, F. Nitric Oxide Synthase Is Involved in Follicular Development via the PI3K/AKT/FoxO3a Pathway in Neonatal and Immature Rats. Animals 2020, 10, 248. https://doi.org/10.3390/ani10020248
Li J, Zhang W, Zhu S, Shi F. Nitric Oxide Synthase Is Involved in Follicular Development via the PI3K/AKT/FoxO3a Pathway in Neonatal and Immature Rats. Animals. 2020; 10(2):248. https://doi.org/10.3390/ani10020248
Chicago/Turabian StyleLi, Junrong, Wei Zhang, Shanli Zhu, and Fangxiong Shi. 2020. "Nitric Oxide Synthase Is Involved in Follicular Development via the PI3K/AKT/FoxO3a Pathway in Neonatal and Immature Rats" Animals 10, no. 2: 248. https://doi.org/10.3390/ani10020248
APA StyleLi, J., Zhang, W., Zhu, S., & Shi, F. (2020). Nitric Oxide Synthase Is Involved in Follicular Development via the PI3K/AKT/FoxO3a Pathway in Neonatal and Immature Rats. Animals, 10(2), 248. https://doi.org/10.3390/ani10020248





