Extracellular Vesicles, the Road toward the Improvement of ART Outcomes
Abstract
:Simple Summary
Abstract
1. Assisted Reproductive Technologies and Their Handicaps
2. Extracellular Vesicles (EVs) and Their Role on ART Outcome Improvement
2.1. Relationship between Spermatozoa and EVs as a Tool to Enhance ART Results
2.2. Relationship between Oocyte Maturation and EVs Used as a Tool to Enhance ART Results
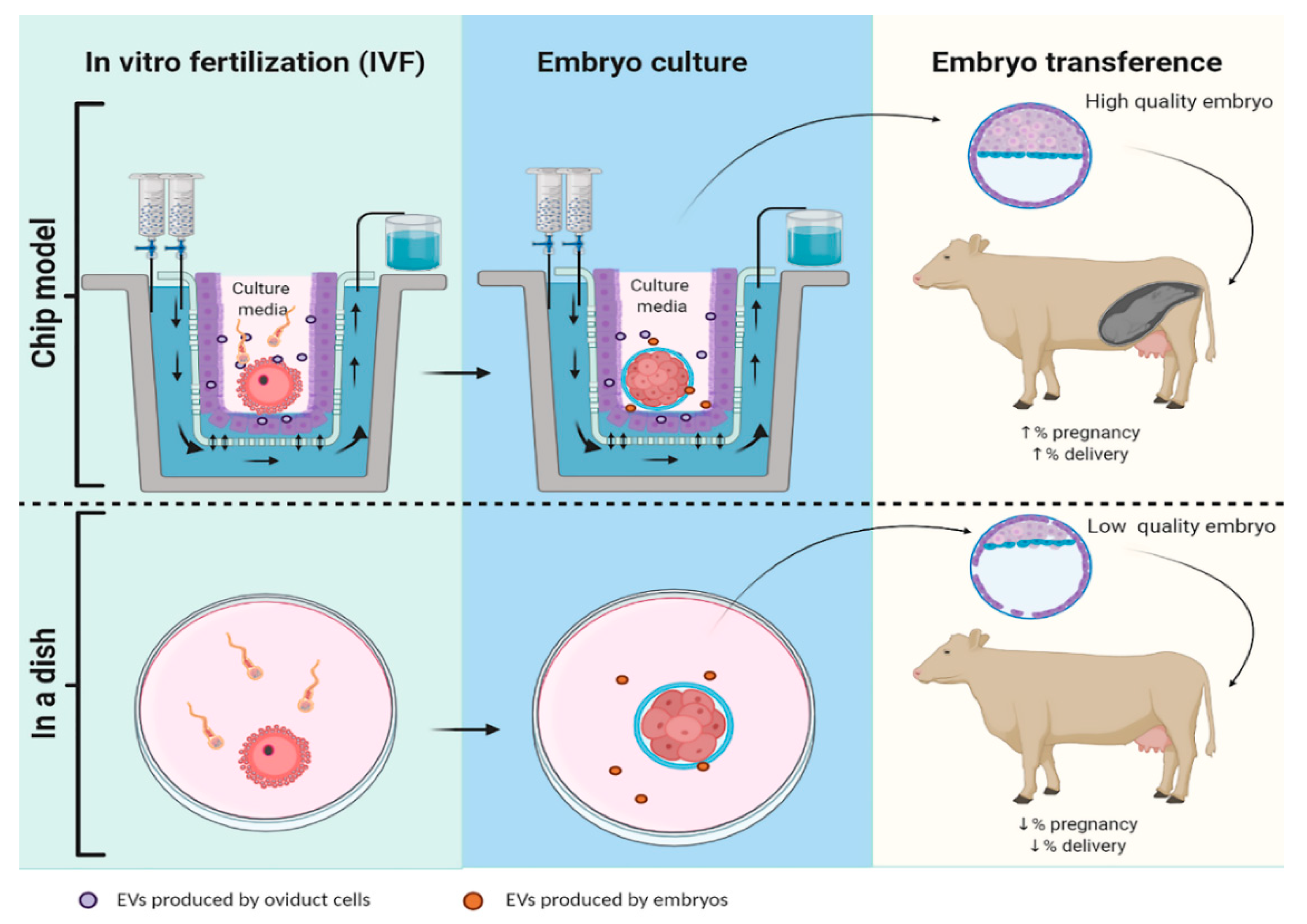
2.3. Relationship between EVs Used as a Tool to Enhance Embryos and Conceptus Development Obtained by ART
3. Challenges for the New Era of ART Development
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dahlen, C.; Larson, J.; Lamb, G.C. Impacts of reproductive technologies on beef production in the United States. Adv. Exp. Med. Biol. 2014, 752, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Bortolozzo, F.P.; Menegat, M.B.; Mellagi, A.P.; Bernardi, M.L.; Wentz, I. New Artificial Insemination Technologies for Swine. Reprod. Domest. Anim. 2015, 50, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.R. Observations on the penetration of the sperm in the mammalian egg. Aust. J. Sci. Res. B 1951, 4, 581–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, M.C. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 1951, 168, 697–698. [Google Scholar] [CrossRef]
- Austin, C.R. The capacitation of the mammalian sperm. Nature 1952, 170, 326. [Google Scholar] [CrossRef]
- Gervasi, M.G.; Visconti, P.E. Chang’s meaning of capacitation: A molecular perspective. Mol. Reprod. Dev. 2016, 83, 860–874. [Google Scholar] [CrossRef]
- Toyoda, Y.; Yokoyama, M.; Hosi, T. Studies on the fertilization of mouse eggs in vitro. J. STAGE 1971, 16, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.C. Fertilization of rabbit ova in vitro. Nature 1959, 184, 466–467. [Google Scholar] [CrossRef]
- Yanagimachi, R.; Chang, M.C. Fertilization of hamster eggs in vitro. Nature 1963, 200, 281–282. [Google Scholar] [CrossRef]
- Rizos, D.; Clemente, M.; Bermejo-Alvarez, P.; de La Fuente, J.; Lonergan, P.; Gutiérrez-Adán, A. Consequences of in vitro culture conditions on embryo development and quality. Reprod. Domest. Anim. 2008, 43, 44–50. [Google Scholar] [CrossRef]
- Rizos, D.; Ward, F.; Duffy, P.; Boland, M.P.; Lonergan, P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: Implications for blastocyst yield and blastocyst quality. Mol. Reprod. Dev. 2002, 61, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Leemans, B.; Gadella, B.M.; Stout, T.A.; De Schauwer, C.; Nelis, H.; Hoogewijs, M.; Van Soom, A. Why doesn’t conventional IVF work in the horse? The equine oviduct as a microenvironment for capacitation/fertilization. Reproduction 2016, 152, R233–r245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Moawad, A.R.; Wang, C.Y.; Li, H.F.; Ren, J.Y.; Dai, Y.F. Advances in in vitro production of sheep embryos. Int. J. Vet. Sci. Med. 2018, 6, S15–s26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell. Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Braun, R. Packaging paternal chromosomes with protamine. Nat. Genet. 2001, 28, 10–12. [Google Scholar] [CrossRef]
- Samanta, L.; Swain, N.; Ayaz, A.; Venugopal, V.; Agarwal, A. Post-Translational Modifications in sperm Proteome: The Chemistry of Proteome diversifications in the Pathophysiology of male factor infertility. Biochim. Biophys. Acta 2016, 1860, 1450–1465. [Google Scholar] [CrossRef]
- Caballero, J.; Frenette, G.; Sullivan, R. Post testicular sperm maturational changes in the bull: Important role of the epididymosomes and prostasomes. Vet. Med. Int. 2010, 2011, 757194. [Google Scholar] [CrossRef] [Green Version]
- Al-Dossary, A.A.; Bathala, P.; Caplan, J.L.; Martin-DeLeon, P.A. Oviductosome-Sperm Membrane Interaction in Cargo Delivery: Detection of fusion and underlying molecular players using three-dimensional super-resolution structure illumination microscopy (SR-SIM). J. Biol. Chem. 2015, 290, 17710–17723. [Google Scholar] [CrossRef] [Green Version]
- Arienti, G.; Carlini, E.; Palmerini, C.A. Fusion of human sperm to prostasomes at acidic pH. J. Membr. Biol. 1997, 155, 89–94. [Google Scholar] [CrossRef]
- Murdica, V.; Giacomini, E.; Makieva, S.; Zarovni, N.; Candiani, M.; Salonia, A.; Vago, R.; Viganò, P. In vitro cultured human endometrial cells release extracellular vesicles that can be uptaken by spermatozoa. Sci. Rep. 2020, 10, 8856. [Google Scholar] [CrossRef] [PubMed]
- Gervasi, M.G.; Visconti, P.E. Molecular changes and signaling events occurring in spermatozoa during epididymal maturation. Andrology 2017, 5, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Trigg, N.A.; Eamens, A.L.; Nixon, B. The contribution of epididymosomes to the sperm small RNA profile. Reproduction 2019, 157, R209–r223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arienti, G.; Carlini, E.; Nicolucci, A.; Cosmi, E.V.; Santi, F.; Palmerini, C.A. The motility of human spermatozoa as influenced by prostasomes at various pH levels. Biol. Cell. 1999, 91, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Pons-Rejraji, H.; Artonne, C.; Sion, B.; Brugnon, F.; Canis, M.; Janny, L.; Grizard, G. Prostasomes: Inhibitors of capacitation and modulators of cellular signalling in human sperm. Int. J. Androl. 2011, 34, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Franchi, A.; Cubilla, M.; Guidobaldi, H.A.; Bravo, A.A.; Giojalas, L.C. Uterosome-like vesicles prompt human sperm fertilizing capability. Mol. Hum. Reprod. 2016, 22, 833–841. [Google Scholar] [CrossRef]
- Weigel Muñoz, M.; Battistone, M.A.; Carvajal, G.; Maldera, J.A.; Curci, L.; Torres, P.; Lombardo, D.; Pignataro, O.P.; Da Ros, V.G.; Cuasnicú, P.S. Influence of the genetic background on the reproductive phenotype of mice lacking Cysteine-Rich Secretory Protein 1 (CRISP1). Biol. Reprod. 2018, 99, 373–383. [Google Scholar] [CrossRef]
- Park, K.H.; Kim, B.J.; Kang, J.; Nam, T.S.; Lim, J.M.; Kim, H.T.; Park, J.K.; Kim, Y.G.; Chae, S.W.; Kim, U.H. Ca2+ signaling tools acquired from prostasomes are required for progesterone-induced sperm motility. Sci. Signal. 2011, 4, ra31. [Google Scholar] [CrossRef]
- Fereshteh, Z.; Bathala, P.; Galileo, D.S.; Martin-DeLeon, P.A. Detection of extracellular vesicles in the mouse vaginal fluid: Their delivery of sperm proteins that stimulate capacitation and modulate fertility. J. Cell. Physiol. 2019, 234, 12745–12756. [Google Scholar] [CrossRef]
- Siciliano, L.; Marcianò, V.; Carpino, A. Prostasome-like vesicles stimulate acrosome reaction of pig spermatozoa. Reprod. Biol. Endocrinol. 2008, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Shen, J.; Wang, Y.; Pan, C.; Pang, W.; Diao, H.; Dong, W. Boar seminal plasma exosomes maintain sperm function by infiltrating into the sperm membrane. Oncotarget 2016, 7, 58832–58847. [Google Scholar] [CrossRef] [PubMed]
- Aalberts, M.; Sostaric, E.; Wubbolts, R.; Wauben, M.W.; Nolte-’t Hoen, E.N.; Gadella, B.M.; Stout, T.A.; Stoorvogel, W. Spermatozoa recruit prostasomes in response to capacitation induction. Biochim. Biophys. Acta 2013, 1834, 2326–2335. [Google Scholar] [CrossRef] [PubMed]
- Rowlison, T.; Ottinger, M.A.; Comizzoli, P. Key factors enhancing sperm fertilizing ability are transferred from the epididymis to the spermatozoa via epididymosomes in the domestic cat model. J. Assist. Reprod. Genet. 2018, 35, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.d.A.M.M.; Carothers, A.; Dahal, R.; Noonan, M.J.; Songsasen, N. Oviductal extracellular vesicles interact with the spermatozoon’s head and mid-piece and improves its motility and fertilizing ability in the domestic cat. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Qamar, A.Y.; Fang, X.; Kim, M.J.; Cho, J. Improved Post-Thaw Quality of Canine Semen after Treatment with Exosomes from Conditioned Medium of Adipose-Derived Mesenchymal Stem Cells. Animals 2019, 9, 865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franchi, A.; Moreno-Irusta, A.; Dominguez, E.M.; Adre, A.J.; Giojalas, L.C. Extracellular vesicles from oviductal isthmus and ampulla stimulate the induced acrosome reaction and signaling events associated with capacitation in bovine spermatozoa. J. Cell. Biochem. 2020, 121, 2877–2888. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Bailey, J.L.; Moore, G.D.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 1995, 121, 1129–1137. [Google Scholar]
- Yanagimachi, R. The movement of golden hamster spermatozoa before and after capacitation. J. Reprod. Fertil. 1970, 23, 193–196. [Google Scholar] [CrossRef]
- Arienti, G.; Carlini, E.; De Cosmo, A.M.; Di Profio, P.; Palmerini, C.A. Prostasome-like particles in stallion semen. Biol. Reprod. 1998, 59, 309–313. [Google Scholar] [CrossRef] [Green Version]
- Arienti, G.; Carlini, E.; Polci, A.; Cosmi, E.V.; Palmerini, C.A. Fatty acid pattern of human prostasome lipid. Arch. Biochem. Biophys. 1998, 358, 391–395. [Google Scholar] [CrossRef]
- Carlini, E.; Palmerini, C.A.; Cosmi, E.V.; Arienti, G. Fusion of sperm with prostasomes: Effects on membrane fluidity. Arch. Biochem. Biophys. 1997, 343, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Kravets, F.G.; Lee, J.; Singh, B.; Trocchia, A.; Pentyala, S.N.; Khan, S.A. Prostasomes: Current concepts. Prostate 2000, 43, 169–174. [Google Scholar] [CrossRef]
- Murdica, V.; Giacomini, E.; Alteri, A.; Bartolacci, A.; Cermisoni, G.C.; Zarovni, N.; Papaleo, E.; Montorsi, F.; Salonia, A.; Viganò, P.; et al. Seminal plasma of men with severe asthenozoospermia contain exosomes that affect spermatozoa motility and capacitation. Fertil. Steril. 2019, 111, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Maldera, J.A.; Weigel Muñoz, M.; Chirinos, M.; Busso, D.; Raffo, F.G.E.; Battistone, M.A.; Blaquier, J.A.; Larrea, F.; Cuasnicu, P.S. Human fertilization: Epididymal hCRISP1 mediates sperm-zona pellucida binding through its interaction with ZP3. Mol. Hum. Reprod. 2014, 20, 341–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, R.; Al-Dossary, A.A.; Stabley, D.L.; Barone, C.; Galileo, D.S.; Strehler, E.E.; Martin-DeLeon, P.A. Plasma membrane Ca2+-ATPase 4 in murine epididymis: Secretion of splice variants in the luminal fluid and a role in sperm maturation. Biol. Reprod. 2013, 89, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, R.E.; Galileo, D.S.; Martin-DeLeon, P.A. Plasma membrane Ca2+-ATPase 4: Interaction with constitutive nitric oxide synthases in human sperm and prostasomes which carry Ca2+/CaM-dependent serine kinase. Mol. Hum. Reprod. 2015, 21, 832–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvau, A.; Battistone, M.A.; Gervasi, M.G.; Navarrete, F.A.; Xu, X.; Sánchez-Cárdenas, C.; De la Vega-Beltran, J.L.; Da Ros, V.G.; Greer, P.A.; Darszon, A.; et al. The tyrosine kinase FER is responsible for the capacitation-associated increase in tyrosine phosphorylation in murine sperm. Development 2016, 143, 2325–2333. [Google Scholar] [CrossRef] [Green Version]
- Bathala, P.; Fereshteh, Z.; Li, K.; Al-Dossary, A.A.; Galileo, D.S.; Martin-DeLeon, P.A. Oviductal extracellular vesicles (oviductosomes, OVS) are conserved in humans: Murine OVS play a pivotal role in sperm capacitation and fertility. Mol. Hum. Reprod. 2018, 24, 143–157. [Google Scholar] [CrossRef]
- Reilly, J.N.; McLaughlin, E.A.; Stanger, S.J.; Anderson, A.L.; Hutcheon, K.; Church, K.; Mihalas, B.P.; Tyagi, S.; Holt, J.E.; Eamens, A.L.; et al. Characterisation of mouse epididymosomes reveals a complex profile of microRNAs and a potential mechanism for modification of the sperm epigenome. Sci. Rep. 2016, 6, 31794. [Google Scholar] [CrossRef]
- Fereshteh, Z.; Schmidt, S.A.; Al-Dossary, A.A.; Accerbi, M.; Arighi, C.; Cowart, J.; Song, J.L.; Green, P.J.; Choi, K.; Yoo, S.; et al. Murine Oviductosomes (OVS) microRNA profiling during the estrous cycle: Delivery of OVS-borne microRNAs to sperm where miR-34c-5p localizes at the centrosome. Sci. Rep. 2018, 8, 16094. [Google Scholar] [CrossRef]
- Aguila, L.; Felmer, R.; Arias, M.E.; Navarrete, F.; Martin-Hidalgo, D.; Lee, H.C.; Visconti, P.; Fissore, R. Defective sperm head decondensation undermines the success of ICSI in the bovine. Reproduction 2017, 154, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Lange-Consiglio, A.; Perrini, C.; Albini, G.; Modina, S.; Lodde, V.; Orsini, E.; Esposti, P.; Cremonesi, F. Oviductal microvesicles and their effect on in vitro maturation of canine oocytes. Reproduction 2017, 154, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Luvoni, G.C.; Chigioni, S.; Allievi, E.; Macis, D. Factors involved in vivo and in vitro maturation of canine oocytes. Theriogenology 2005, 63, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Oh, H.J.; Kim, M.J.; Lee, B.C. Exosomes derived from oviduct cells mediate the EGFR/MAPK signaling pathway in cumulus cells. J. Cell. Physiol. 2020, 235, 1386–1404. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Oh, H.J.; Kim, M.J.; Lee, B.C. Canine oviductal exosomes improve oocyte development via EGFR/MAPK signaling pathway. Reproduction 2020, 160, 613–625. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, J.C.; Veeramachaneni, D.N.; Winger, Q.A.; Carnevale, E.M.; Bouma, G.J. Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: A possible new form of cell communication within the ovarian follicle. Biol. Reprod. 2012, 86, 71. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, M.; Vento, M.; Guglielmino, M.R.; Battaglia, R.; Wahlgren, J.; Ragusa, M.; Barbagallo, D.; Borzì, P.; Rizzari, S.; Maugeri, M.; et al. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: Bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil. Steril. 2014, 102, 1751–1761. [Google Scholar] [CrossRef]
- Hung, W.T.; Navakanitworakul, R.; Khan, T.; Zhang, P.; Davis, J.S.; McGinnis, L.K.; Christenson, L.K. Stage-specific follicular extracellular vesicle uptake and regulation of bovine granulosa cell proliferation. Biol. Reprod. 2017, 97, 644–655. [Google Scholar] [CrossRef]
- da Silveira, J.C.; Andrade, G.M.; Del Collado, M.; Sampaio, R.V.; Sangalli, J.R.; Silva, L.A.; Pinaffi, F.V.L.; Jardim, I.B.; Cesar, M.C.; Nogueira, M.F.G.; et al. Supplementation with small-extracellular vesicles from ovarian follicular fluid during in vitro production modulates bovine embryo development. PLoS ONE 2017, 12, e0179451. [Google Scholar] [CrossRef]
- da Silveira, J.C.; Winger, Q.A.; Bouma, G.J.; Carnevale, E.M. Effects of age on follicular fluid exosomal microRNAs and granulosa cell transforming growth factor-β signalling during follicle development in the mare. Reprod. Fertil. Dev. 2015, 27, 897–905. [Google Scholar] [CrossRef]
- de Ávila, A.; Bridi, A.; Andrade, G.M.; Del Collado, M.; Sangalli, J.R.; Nociti, R.P.; da Silva Junior, W.A.; Bastien, A.; Robert, C.; Meirelles, F.V.; et al. Estrous cycle impacts microRNA content in extracellular vesicles that modulate bovine cumulus cell transcripts during in vitro maturation†. Biol. Reprod. 2020, 102, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Laezer, I.; Palma-Vera, S.E.; Liu, F.; Frank, M.; Trakooljul, N.; Vernunft, A.; Schoen, J.; Chen, S. Dynamic profile of EVs in porcine oviductal fluid during the periovulatory period. Reproduction 2020, 159, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Navakanitworakul, R.; Hung, W.T.; Gunewardena, S.; Davis, J.S.; Chotigeat, W.; Christenson, L.K. Characterization and Small RNA Content of Extracellular Vesicles in Follicular Fluid of Developing Bovine Antral Follicles. Sci. Rep. 2016, 6, 25486. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.d.A.M.M.; Fujihara, M.; Nagashima, J.B.; Noonan, M.J.; Inoue-Murayama, M.; Songsasen, N. Follicular extracellular vesicles enhance meiotic resumption of domestic cat vitrified oocytes. Sci. Rep. 2020, 10, 8619. [Google Scholar] [CrossRef]
- O’Doherty, E.M.; Wade, M.G.; Hill, J.L.; Boland, M.P. Effects of culturing bovine oocytes either singly or in groups on development to blastocysts. Theriogenology 1997, 48, 161–169. [Google Scholar] [CrossRef]
- Cortezzi, S.S.; Garcia, J.S.; Ferreira, C.R.; Braga, D.P.; Figueira, R.C.; Iaconelli, A., Jr.; Souza, G.H.; Borges, E., Jr.; Eberlin, M.N. Secretome of the preimplantation human embryo by bottom-up label-free proteomics. Anal. Bioanal. Chem. 2011, 401, 1331–1339. [Google Scholar] [CrossRef]
- Katz-Jaffe, M.G.; Schoolcraft, W.B.; Gardner, D.K. Analysis of protein expression (secretome) by human and mouse preimplantation embryos. Fertil. Steril. 2006, 86, 678–685. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Kim, S.J.; Choi, Y.B.; Lee, B.C. Improvement of cloned embryos development by co-culturing with parthenotes: A possible role of exosomes/microvesicles for embryos paracrine communication. Cell. Reprogram. 2014, 16, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Alcântara-Neto, A.S.; Fernandez-Rufete, M.; Corbin, E.; Tsikis, G.; Uzbekov, R.; Garanina, A.S.; Coy, P.; Almiñana, C.; Mermillod, P. Oviduct fluid extracellular vesicles regulate polyspermy during porcine in vitro fertilisation. Reprod. Fertil. Dev. 2020, 32, 409–418. [Google Scholar] [CrossRef]
- Alminana, C.; Corbin, E.; Tsikis, G.; Alcantara-Neto, A.S.; Labas, V.; Reynaud, K.; Galio, L.; Uzbekov, R.; Garanina, A.S.; Druart, X.; et al. Oviduct extracellular vesicles protein content and their role during oviduct-embryo cross-talk. Reproduction 2017, 154, 153–168. [Google Scholar] [CrossRef]
- Alminana, C.; Tsikis, G.; Labas, V.; Uzbekov, R.; da Silveira, J.C.; Bauersachs, S.; Mermillod, P. Deciphering the oviductal extracellular vesicles content across the estrous cycle: Implications for the gametes-oviduct interactions and the environment of the potential embryo. BMC Genom. 2018, 19, 622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wennemuth, G.; Babcock, D.F.; Hille, B. Calcium clearance mechanisms of mouse sperm. J. Gen. Physiol. 2003, 122, 115–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gervasi, M.G.; Rapanelli, M.; Ribeiro, M.L.; Farina, M.; Billi, S.; Franchi, A.M.; Perez Martinez, S. The endocannabinoid system in bull sperm and bovine oviductal epithelium: Role of anandamide in sperm-oviduct interaction. Reproduction 2009, 137, 403–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, I. Culture of bovine oviduct epithelial cells (BOEC). Anat. Rec. 1995, 243, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Lopera-Vásquez, R.; Hamdi, M.; Fernandez-Fuertes, B.; Maillo, V.; Beltrán-Breña, P.; Calle, A.; Redruello, A.; López-Martín, S.; Gutierrez-Adán, A.; Yañez-Mó, M.; et al. Extracellular Vesicles from BOEC in In Vitro Embryo Development and Quality. PLoS ONE 2016, 11, e0148083. [Google Scholar] [CrossRef] [PubMed]
- Lopera-Vasquez, R.; Hamdi, M.; Maillo, V.; Gutierrez-Adan, A.; Bermejo-Alvarez, P.; Ramírez, M.; Yáñez-Mó, M.; Rizos, D. Effect of bovine oviductal extracellular vesicles on embryo development and quality in vitro. Reproduction 2017, 153, 461–470. [Google Scholar] [CrossRef] [Green Version]
- Kouba, A.J.; Abeydeera, L.R.; Alvarez, I.M.; Day, B.N.; Buhi, W.C. Effects of the porcine oviduct-specific glycoprotein on fertilization, polyspermy, and embryonic development in vitro. Biol. Reprod. 2000, 63, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Algarra, B.; Maillo, V.; Aviles, M.; Gutierrez-Adan, A.; Rizos, D.; Jimenez-Movilla, M. Effects of recombinant OVGP1 protein on in vitro bovine embryo development. J. Reprod. Dev. 2018, 64, 433–443. [Google Scholar] [CrossRef] [Green Version]
- Ferraz, M.d.A.M.M.; Rho, H.S.; Hemerich, D.; Henning, H.H.W.; van Tol, H.T.A.; Hölker, M.; Besenfelder, U.; Mokry, M.; Vos, P.; Stout, T.A.E.; et al. An oviduct-on-a-chip provides an enhanced in vitro environment for zygote genome reprogramming. Nat. Commun. 2018, 9, 4934. [Google Scholar] [CrossRef] [Green Version]
- Qu, P.; Luo, S.; Du, Y.; Zhang, Y.; Song, X.; Yuan, X.; Lin, Z.; Li, Y.; Liu, E. Extracellular vesicles and melatonin benefit embryonic develop by regulating reactive oxygen species and 5-methylcytosine. J. Pineal Res. 2020, 68, e12635. [Google Scholar] [CrossRef]
- Qu, P.; Zhao, Y.; Wang, R.; Zhang, Y.; Li, L.; Fan, J.; Liu, E. Extracellular vesicles derived from donor oviduct fluid improved birth rates after embryo transfer in mice. Reprod. Fertil. Dev. 2019, 31, 324–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blázquez, R.; Sánchez-Margallo, F.M.; Álvarez, V.; Matilla, E.; Hernández, N.; Marinaro, F.; Gómez-Serrano, M.; Jorge, I.; Casado, J.G.; Macías-García, B. Murine embryos exposed to human endometrial MSCs-derived extracellular vesicles exhibit higher VEGF/PDGF AA release, increased blastomere count and hatching rates. PLoS ONE 2018, 13, e0196080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinaro, F.; Macias-Garcia, B.; Sanchez-Margallo, F.M.; Blazquez, R.; Alvarez, V.; Matilla, E.; Hernandez, N.; Gomez-Serrano, M.; Jorge, I.; Vazquez, J.; et al. Extracellular vesicles derived from endometrial human mesenchymal stem cells enhance embryo yield and quality in an aged murine model. Biol. Reprod. 2019, 100, 1180–1192. [Google Scholar] [CrossRef] [PubMed]
- Marinaro, F.; Pericuesta, E.; Sanchez-Margallo, F.M.; Casado, J.G.; Alvarez, V.; Matilla, E.; Hernandez, N.; Blazquez, R.; Gonzalez-Fernandez, L.; Gutierrez-Adan, A.; et al. Extracellular vesicles derived from endometrial human mesenchymal stem cells improve IVF outcome in an aged murine model. Reprod. Domest. Anim. 2018, 53, 46–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Yin, H.; Jiang, H.; Du, X.; Wang, C.; Liu, Y.; Li, Y.; Yang, Z. Extracellular Vesicles Derived from Mesenchymal Stem Cells Recover Fertility of Premature Ovarian Insufficiency Mice and the Effects on their Offspring. Cell Transplant. 2020, 29. [Google Scholar] [CrossRef]
- Wang, H.; Dey, S.K. Roadmap to embryo implantation: Clues from mouse models. Nat. Rev. Genet. 2006, 7, 185–199. [Google Scholar] [CrossRef]
- Klohonatz, K.M.; Cameron, A.D.; Hergenreder, J.R.; da Silveira, J.C.; Belk, A.D.; Veeramachaneni, D.N.; Bouma, G.J.; Bruemmer, J.E. Circulating miRNAs as Potential Alternative Cell Signaling Associated with Maternal Recognition of Pregnancy in the Mare. Biol. Reprod. 2016, 95, 124. [Google Scholar] [CrossRef]
- Vilella, F.; Moreno-Moya, J.M.; Balaguer, N.; Grasso, A.; Herrero, M.; Martínez, S.; Marcilla, A.; Simón, C. Hsa-miR-30d, secreted by the human endometrium, is taken up by the pre-implantation embryo and might modify its transcriptome. Development 2015, 142, 3210–3221. [Google Scholar] [CrossRef] [Green Version]
- Burns, G.W.; Brooks, K.E.; Spencer, T.E. Extracellular Vesicles Originate from the Conceptus and Uterus During Early Pregnancy in Sheep. Biol. Reprod. 2016, 94, 56. [Google Scholar] [CrossRef] [Green Version]
- Pallinger, E.; Bognar, Z.; Bodis, J.; Csabai, T.; Farkas, N.; Godony, K.; Varnagy, A.; Buzas, E.; Szekeres-Bartho, J. A simple and rapid flow cytometry-based assay to identify a competent embryo prior to embryo transfer. Sci. Rep. 2017, 7, 39927. [Google Scholar] [CrossRef]
- Mellisho, E.A.; Briones, M.A.; Velásquez, A.E.; Cabezas, J.; Castro, F.O.; Rodríguez-Álvarez, L. Extracellular vesicles secreted during blastulation show viability of bovine embryos. Reproduction 2019, 158, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, K.; Nõmm, M.; Lättekivi, F.; Ressaissi, Y.; Godakumara, K.; Lavrits, A.; Midekessa, G.; Viil, J.; Bæk, R.; Jørgensen, M.M.; et al. Individually cultured bovine embryos produce extracellular vesicles that have the potential to be used as non-invasive embryo quality markers. Theriogenology 2020, 149, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, E.; Makieva, S.; Murdica, V.; Vago, R.; Viganó, P. Extracellular vesicles as a potential diagnostic tool in assisted reproduction. Curr. Opin. Obstet. Gynecol. 2020, 32, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Pomatto, M.A.C.; Bussolati, B.; D’Antico, S.; Ghiotto, S.; Tetta, C.; Brizzi, M.F.; Camussi, G. Improved Loading of Plasma-Derived Extracellular Vesicles to Encapsulate Antitumor miRNAs. Mol. Ther. Methods Clin. Dev. 2019, 13, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Riou, C.; Brionne, A.; Cordeiro, L.; Harichaux, G.; Gargaros, A.; Labas, V.; Gautron, J.; Gérard, N. Avian uterine fluid proteome: Exosomes and biological processes potentially involved in sperm survival. Mol. Reprod. Dev. 2020, 87, 454–470. [Google Scholar] [CrossRef]

| Subsection | Name |
|---|---|
| Epididymis | Epididymosome |
| Prostate | Prostasome |
| Vagina | Vaginosome |
| Uterus | Uterosome |
| Oviduct | Oviductosome |
| Size (nm) | Name |
| 100–1000 | Microvesicles or ectosomes |
| 30–100 | Exosomes |
| Specie | EVs Origen | ART used | Output | Reference |
|---|---|---|---|---|
| Human | Prostate | In vitro incubation in acidic media | ↑ % of motile spermatozoa | [24] |
| Human | Prostate | In vitro capacitation | Inhibit sperm capacitation Inhibit spontaneous acrosome reaction | [25] |
| Human | EECs | In vitro capacitation | Enhance sperm capacitation status | [26] |
| Human | Prostate | In vitro capacitation | Enhance acrosome reaction response to calcium ionophore | [27] |
| Human/ mouse | Prostate | In vitro incubation | ↑ Hypermotility ↑ IVF fertility | [28] |
| Mouse | Vagina from superovulated females | In vitro capacitation | Enhance sperm responsiveness to progesterone Incorporation of several sperm proteins with roles on calcium homeostasis (SPAM1, PMCA1/4, PMCA4) and capacitation process (protein tyrosine phosphorylation) | [29] |
| Pig | Prostate | In vitro incubation | Enhance sperm acrosome reaction | [30] |
| Pig | Prostate | Preservation at low temperature | Prolonged sperm motility ↑ Sperm antioxidative capacity ↓ Lipid peroxidation Protect plasma membrane Protect against premature capacitation | [31] |
| Stallion | Prostate | In vitro capacitation | Inhibit sperm capacitation events as protein tyrosine phosphorylation | [32] |
| Feline | Epididymis | In vitro incubation | ↑ % of motile spermatozoa for a short period of time (up to 1 h) ↑ Forward motility (1.5 to 3 h of co-incubation) | [33] |
| Feline | Oviduct (different follicular phases) | IVF | ↑ % Motile spermatozoa Protect again premature acrosome reaction Enhanced IVF outcome | [34] |
| Dog | ASCs | Cryopreservation | ↑ Sperm motility and viability ↑ Mucus penetration ability or ↓ Acrosome and chromatin damaged | [35] |
| Bovine | Oviduct (different sections) | Cryopreservation | ↑Protein tyrosine phosphorylation ↑ Responsiveness to progesterone Maintain sperm survival | [36] |
| Specie | EVs Origen | ART Used | Output | Reference |
|---|---|---|---|---|
| Bovine | BOEC | IVC Vitrification | No differences on embryo development Enhance vitrification outcome: ↑ Embryo quality ↑ Cryo-survival rate ↑ Number of cells | [75] |
| Bovine | BOEC | IVP IVC | Enhanced the embryo quality ↑ Number of cells ↑ Hatching rate = Fertilization rate | [70] |
| Bovine | Oviduct (ampulla and isthmus) | IVC Vitrification | No differences on embryo development ↑ Cryo-survival rate | [76] |
| Mouse | endMSCs | Embryo culture obtained in vivo | ↑ Number of total cells by blastocyst ↑ Hatching rate | [82] |
| Mouse (ageing) | endMSCs | IVF IVC | Enhance embryo competence and quality ×2 blastocyst rate ↑ mRNA expression of Sod1, Gadph, Vegfa and Sox2 | [83,84] |
| Mouse | Oviduct from pregnant females | IVF ET | ↑ Embryo quality (↑ Bcl-2; Oct-4↓Bax) ↑ ICM ↑ Blastocyst and birth rates | [81] |
| Mouse (POI) | HUCMSCs | IVF | Rescue ovary function, hormones levels (FSH and E2), natural fertility. ↑ oocytes retrieved, fertilized zygotes, cleaved embryos and blastocysts | [85] |
| Porcine | Oviduct | IVF | ↓ Polyspermia | [69] |
| Feline | Ovarian fluid | Vitrification IVM | = Vitrification survival rate ↑ Oocyte IVM from 8.6% in control to 28.3% in supplemented with EVs | [64] |
| Canine | Oviduct | IVM | ↑ Oocyte IVM 21.82% vs. control 8.66% | [52] |
| Canine | Oviduct | IVM | ↑ Cumulus cell viability, and proliferation rate ↓ ROS and apoptotic rate | [54] |
| Canine | Oviduct | IVM | ↑ Maturation rate of oocytes | [55] |
| Rabbit | Oviduct | IVF IVC | ↓ ROS and DNA methylation levels ↑ Blastocyst rate | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gervasi, M.G.; Soler, A.J.; González-Fernández, L.; Alves, M.G.; Oliveira, P.F.; Martín-Hidalgo, D. Extracellular Vesicles, the Road toward the Improvement of ART Outcomes. Animals 2020, 10, 2171. https://doi.org/10.3390/ani10112171
Gervasi MG, Soler AJ, González-Fernández L, Alves MG, Oliveira PF, Martín-Hidalgo D. Extracellular Vesicles, the Road toward the Improvement of ART Outcomes. Animals. 2020; 10(11):2171. https://doi.org/10.3390/ani10112171
Chicago/Turabian StyleGervasi, Maria G., Ana J. Soler, Lauro González-Fernández, Marco G. Alves, Pedro F. Oliveira, and David Martín-Hidalgo. 2020. "Extracellular Vesicles, the Road toward the Improvement of ART Outcomes" Animals 10, no. 11: 2171. https://doi.org/10.3390/ani10112171
APA StyleGervasi, M. G., Soler, A. J., González-Fernández, L., Alves, M. G., Oliveira, P. F., & Martín-Hidalgo, D. (2020). Extracellular Vesicles, the Road toward the Improvement of ART Outcomes. Animals, 10(11), 2171. https://doi.org/10.3390/ani10112171








