Meningitis Caused by Streptococcus agalactiae in Nile Tilapia (Oreochromis niloticus): Infection and Inflammatory Response
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Bacteria and Experimental Infection
2.3. Immunofluorescence and Immunohistochemistry
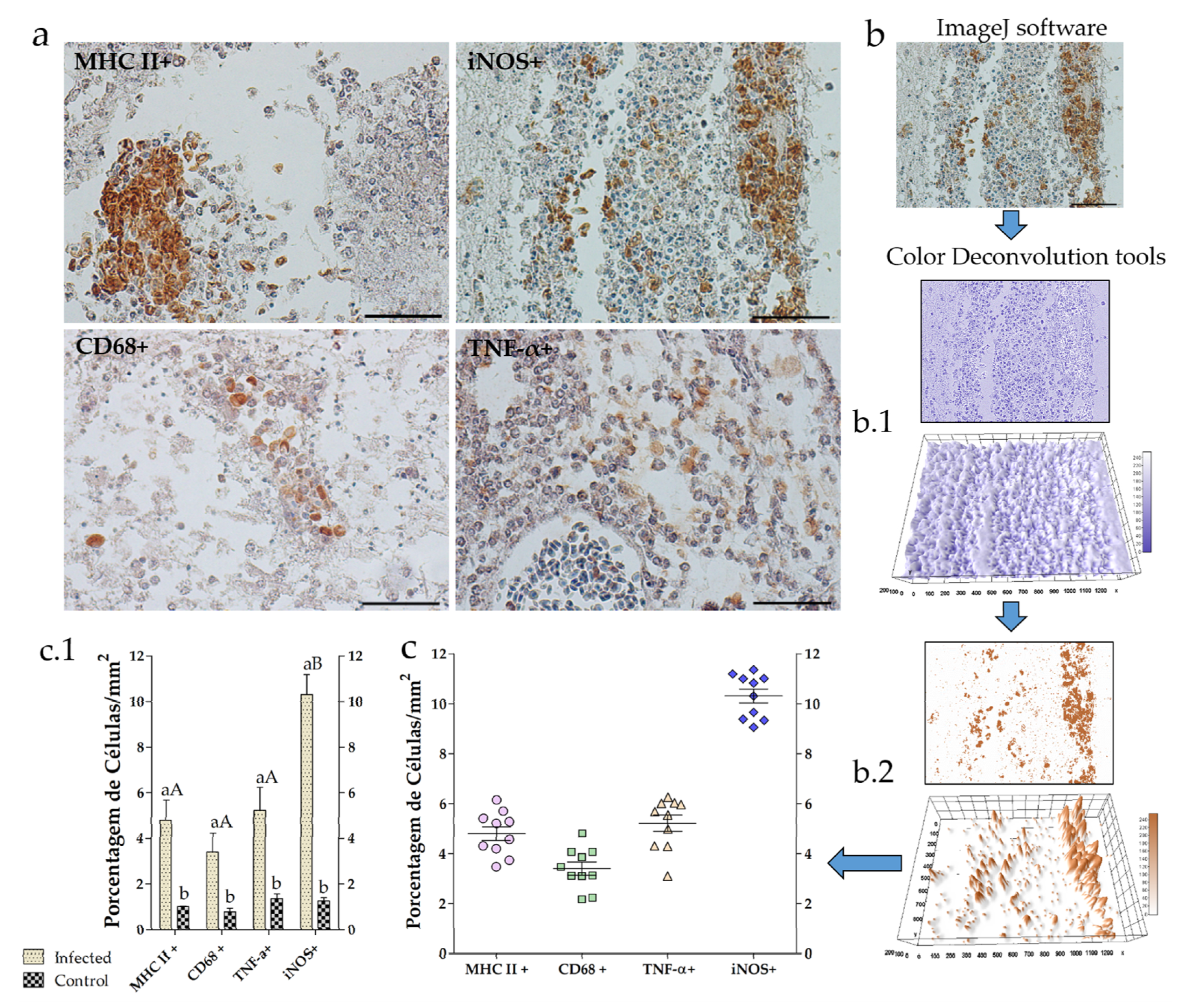
2.4. Image Analysis
2.5. Statistical Analysis
3. Results
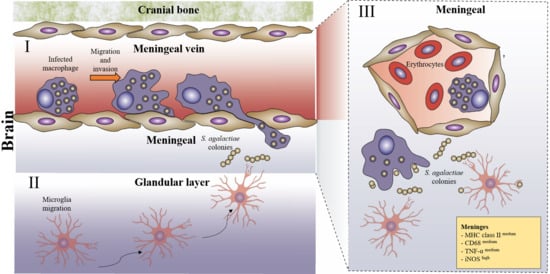
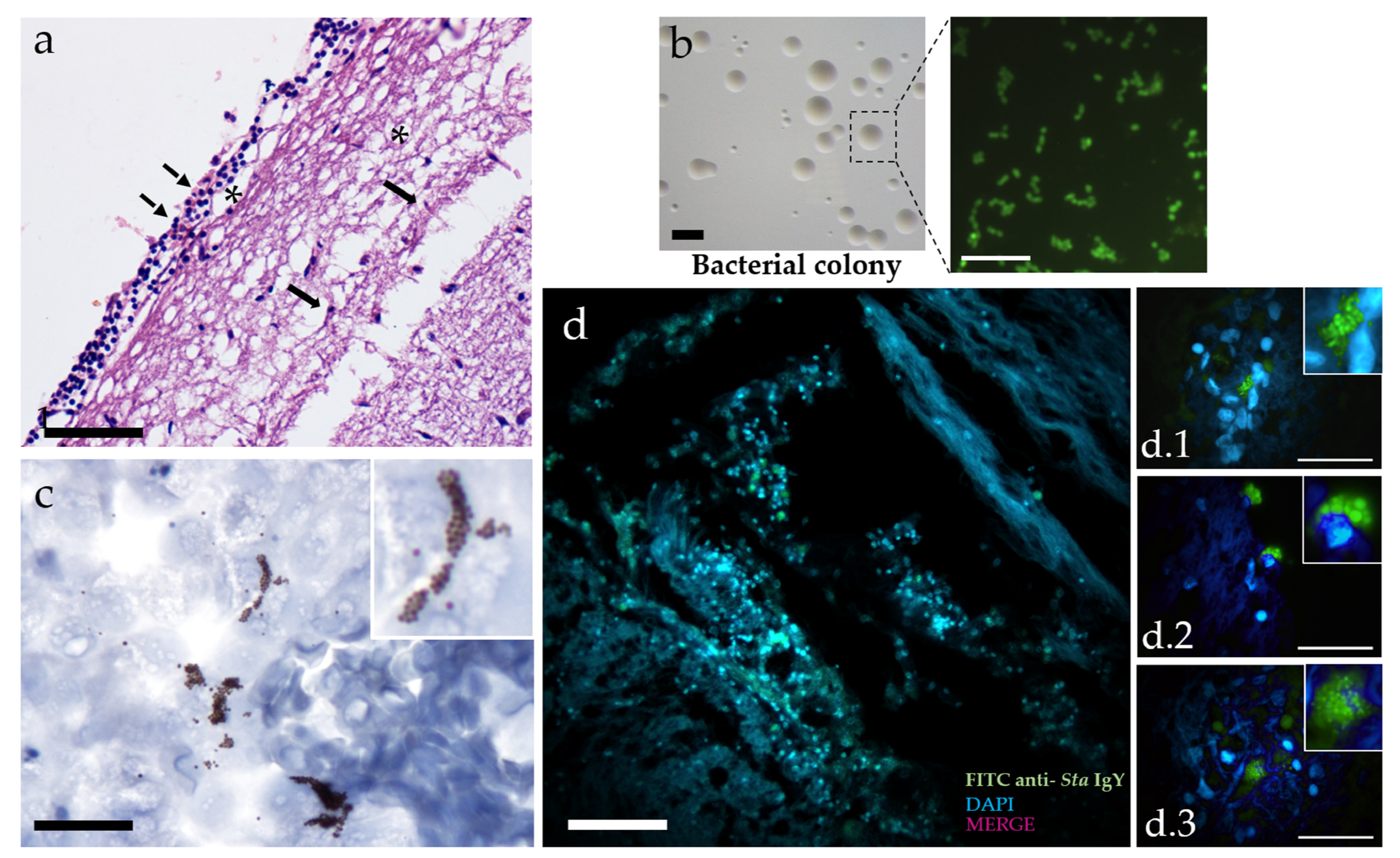
3.1. Infection
3.2. Neuroinflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability Statement
References
- Imperi, M.; Pataracchia, M.; Alfarone, G.; Baldassarri, L.; Orefici, G.; Creti, R. A multiplex PCR assay for the direct identification of the capsular type (Ia to IX) of Streptococcus agalactiae. J. Microbiol. Methods 2010, 80, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Delannoy, S.; Beutin, L.; Fach, P. Discrimination of enterohemorrhagic Escherichia coli (EHEC) from non-EHEC strains based on detection of various combinations of type III effector genes. J. Clin. Microbiol. 2013, 51, 3257–3262. [Google Scholar] [CrossRef] [PubMed]
- Rodkhum, C.; Kayansamruaj, P.; Pirarat, N.; Wongtawatchai, J. Duplex PCR for simultaneous and unambiguous detection of Streptococcus iniae and Streptococcus agalactiae associated with streptococcosis of cultured tilapia in Thailand. Thai. J. Vet. Med. 2012, 42, 153–158. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2014. [Google Scholar]
- El-Sayed, A.F.M. Tilapia Culture; Academic Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Li, L.; Wang, R.; Liang, W.; Gan, X.; Huang, T.; Huang, Y.; Li, J.; Shi, Y.; Chen, M.; Luo, H. Rare serotype occurrence and PFGE genotypic diversity of Streptococcus agalactiae isolated from tilapia in China. Vet. Microbiol. 2013, 167, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Mian, G.F.; Godoy, D.T.; Leal, C.A.; Yuhara, T.Y.; Costa, G.M.; Figueiredo, H.C. Aspects of the natural history and virulence of S. agalactiae infection in Nile tilapia. Vet. Microbiol. 2009, 136, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Wang, M.; Li, Z.; Liu, Z.; Song, C.; Zhang, D.; Gao, F.; Ke, X.; Cao, J.; Lu, M. An investigation into the effects of Streptococcus agalactiae on the 5-HT system and the behavior of GIFT tilapia (Oreochromis niloticus). Aquaculture Rep. 2019, 15. [Google Scholar] [CrossRef]
- Maisey, H.C.; Doran, K.S.; Nizet, V. Recent advances in understanding the molecular basis of group B Streptococcus virulence. Expert Rev. Mol. Med. 2008, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuro-inflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef]
- Boche, D.; Perry, V.H.; Nicoll, J.A. Review: Activation patterns of microglia and their identification in the human brain. Neuropathol. Appl. Neurobiol. 2013, 39, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Norden, D.M.; Godbout, J.P. Microglia of the aged brain: Primed to be activated and resistant to regulation. Neuropathol. Appl. Neurobiol. 2013, 39, 19–34. [Google Scholar] [CrossRef]
- El-Razika, K.A.A.; Abdelrahmanb, K.A.; Ahmeda, Y.F.; Gomaac, A.M.; Eldebakya, H.A. Direct Identification of Major Pathogens of the Bubaline Subclinical Mastitis in Egypt using PCR. Am. J. Sci 2010, 6, 652–660. [Google Scholar]
- Eto, S.F.; Fernandes, D.C.; Moraes, A.C.; Prado, E.J.R.; Baldassi, A.C.; Manrique, W.G.; Silva, I.C.; Medeiros, A.S.R.; Belo, M.A.A.; Balbuena, T.S.; et al. Validation of IgY for the diagnosis of Streptococcus agalactiae-caused endocarditis and bacterial meningitis in Nile tilapia (Oreochromis niloticus). Fish. Shellfish. Immunol. 2018, 76, 153–160. [Google Scholar] [CrossRef] [PubMed]
- De Mateo, M.; Bovo, G.; Comuzzi, M.; Adams, A. Lectin histochemical studies on Sphaerospora sp. (Myxozoa) from Italian brown trout, Salmo trutta L. J. Fish. Dis. 1997, 20, 51–58. [Google Scholar] [CrossRef]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef] [PubMed]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods; Iowa State University Press: Ames, IA, USA, 1974. [Google Scholar]
- Evans, J.J.; Pasnik, D.J.; Klesius, P.H.; AL-Ablani, S. First report of Streptococcus agalactiae and Lactococcus garvieae from a wild bottlenose dolphin (Tursiops truncatus). J. Wildl. Dis. 2006, 42, 561–569. [Google Scholar] [CrossRef]
- Abuseliana, A.; Hassan, D.; Saleha, A.A.; Siti-khairani, B.; Milud, A. Streptococcus agalactiae the etiological agent of mass mortality in farmed red tilapia (Oreochromis sp.). J. Anim. Vet. Adv. 2010, 20, 2640–2646. [Google Scholar] [CrossRef]
- Asencios, Y.O.; Sánchez, F.B.; Mendizábal, H.B.; Pusari, K.H.; Alfonso, H.O.; Sayán, A.M.; Figueiredo, M.A.P.; Manrique, W.G.; de Andrade Belo, M.A.; Chaupe, N.S. First report of Streptococcus agalactiae isolated from Oreochromis niloticus in Piura, Peru: Molecular identification and histopathological lesions. Aquacult. Rep. 2016, 4, 74–79. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Meningococcal Meningitis: Fact Sheet. 2017. Available online: http://www.who.int/mediacentre/factsheets/fs141/en/ (accessed on 9 November 2017).
- Barichello, T.; Generoso, J.S.; Simões, L.R.; Elias, S.G.; Quevedo, J. Role of oxidative stress in the pathophysiology of pneumococcal meningitis. Oxid. Med. Cell. Longev. 2013, 371465. [Google Scholar] [CrossRef]
- Melin, P. Neonatal group B streptococcal disease: From pathogenesis to preventive strategies. Clin. Microbiol. Infect. Dis. 2011, 17, 1294–1303. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Pargade, V.; Lamberet, G.; Gaudu, P.; Thomas, F.; Texereau, J.; Gruss, A.; Trieu-Cuot, P.; Poyart, C. The Group B Streptococcus NADH oxidase Nox-2 is involved in fatty acid biosynthesis during aerobic growth and contributes to virulence. Mol. Microbiol. 2006, 62, 772–785. [Google Scholar] [CrossRef]
- Ribes, S.; Abdullah, M.R.; Saleh, M.; Hanisch, U.; Nau, R.; Hammerschmidt, S. Thioredoxins and Methionine Sulfoxide Reductases in the Pathophysiology of Pneumococcal Meningitis. J. Infect. Dis. 2016, 214, 953–961. [Google Scholar] [CrossRef][Green Version]
- Peri, F.; Nüsslein-Volhard, C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell 2008, 133, 916–927. [Google Scholar] [CrossRef]
- Patterson, H.; Saralahti, A.; Parikka, M.; Dramsi, S.; Trieu-Cuot, P.; Poyart, C.; Rounioja, S.; Rämet, M. Adult zebrafish model of bacterial meningitis in Streptococcus agalactiae infection. Dev. Comp. Immunol. 2012, 38, 447–455. [Google Scholar] [CrossRef]
- Fernandes, D.C.; Eto, S.F.; Funnicelli, M.I.G.; Fernandes, C.C.; Charlie-Silva, I.; Belo, M.A.A.; Pizauro, J.M. Immunoglobulin Y in the diagnosis of Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus). Aquaculture 2019, 500, 576–585. [Google Scholar] [CrossRef]
- Charlie-Silva, I.; Klein, A.; Gomes, J.M.M.; Prado, E.J.R.; Moraes, A.C.; Eto, S.F.; Fernandes, D.C.; Fagliari, J.; Corrêa Junior, J.D.; Lima, C.; et al. Acute-phase proteins during inflammatory reaction by bacterial infection: Fish-model. Sci. Rep. 2019, 9, 4776. [Google Scholar] [CrossRef] [PubMed]
- Nurani, F.S.; Sukenda, S.; Nuryati, S. Maternal immunity of tilapia broodstock vaccinated with polyvalent vaccine and resistance of their offspring against Streptococcus agalactiae. Aquacult. Res. 2020, 51, 1513–1522. [Google Scholar] [CrossRef]
- Kim, K.S. Mechanisms of microbial traversal of the blood-brain barrier. Nat. Rev. Microbiol. 2008, 6, 625–634. [Google Scholar] [CrossRef]
- Santiago-Tirado, F.H.; Onken, M.D.; Cooper, J.A.; Klein, R.S.; Doering, T.L. Trojan horse transit contributes to blood-brain barrier crossing of a eukaryotic pathogen. mBio 2017, 8. [Google Scholar] [CrossRef]
- Zlotkin, A.; Chilmonczyk, S.; Eyngor, M.; Hurvitz, A.; Ghittino, C.; Eldar, A. Trojan horse effect: Phagocyte-mediated Streptococcus iniae infection of fish. Infect. Immun. 2003, 71, 2318–2325. [Google Scholar] [CrossRef]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology; Saunders/Elsevier: Philadelphia, PA, USA, 2010. [Google Scholar]
- Caffo, M.; Caruso, G.; Germano, A.; Galatioto, S.; Meli, F.; Tomasello, F. CD68 and CR3/43 immunohistochemical expression in secretory meningiomas. Neurosurgery 2005, 57, 551–557. [Google Scholar] [CrossRef]
- Lemstra, A.W.; Groen In’t Woud, J.C.M.; Hoozemans, J.J.M.; Van Haastert, E.S.; Rozemuller, A.J.M.; Eikelenboom, P.; Van Gool, W.A. Microglia activation in sepsis: A case-control study. J. Neuroinflamm. 2007, 4, 1–8. [Google Scholar] [CrossRef][Green Version]
- Jiang-Shieh, Y.F.; Yeh, K.Y.; Wei, I.H.; Chang, C.Y.; Chien, H.F.; Tsai, R.Y.; Chang, M.L.; Lee, A.W.; Pai, M.H.; Wu, C.H. Responses of microglia in vitro to the gram-positive bacterial component, lipoteichoic acid. J. Neurosci. Res. 2005, 82, 515–524. [Google Scholar] [CrossRef]
- Kuno, R.; Wang, J.; Kawanokuchi, J.; Takeuchi, H.; Mizuno, T.; Suzumura, A. Autocrine activation of microglia by tumor necrosis factor-alpha. J. Neuroimmunol. 2005, 162, 89–96. [Google Scholar] [CrossRef]
- Olmos, G.; Llado, J. Tumor necrosis factor alpha: A link between neuroinflammation and excitotoxicity. Mediat. Inflamm. 2014, 861231. [Google Scholar] [CrossRef]
- Golan, H.; Levav, T.; Mendelsohn, A.; Huleihel, M. Involvement of tumor necrosis factor alpha in hippocampal development and function. Cereb. Cortex 2004, 14, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Skovronsky, D.M.; Fath, S.; Lee, V.M.-Y.; Milla, M.E. Neuronal localization of the TNFα converting enzyme (TACE) in brain tissue and its correlation to amyloid plaques. J. Neurobiol. 2001, 49, 40–46. [Google Scholar] [CrossRef]
- Allan, R.N.; Morgan, S.; Brito-Mutunayagam, S.; Skipp, P.; Feelisch, M.; Hayes, S.M.; Hellier, W.; Clarke, S.C.; Stoodley, P.; Burgess, A.; et al. Low concentrations of nitric oxide modulate Streptococcus pneumoniae biofilm metabolism and antibiotic tolerance. Antimicrob. Agents Chemother. 2016, 60, 2456–2466; [Google Scholar] [CrossRef]
- Leib, S.L.; Kim, Y.S.; Black, S.M.; Tureen, J.H.; Täuber, M.G. Inducible Nitric Oxide Synthase and the Effect of Aminoguanidine in Experimental Neonatal Meningitis. J. Infect. Dis. 1998, 177, 692–700. [Google Scholar] [CrossRef] [PubMed]
- O’ Brien, K.L.; Wolfson, L.J.; Watt, J.P.; Henkle, E.; Deloria-knoll, M.; Mccall, N.; Lee, E.; Mulholland, K.; Levine, O.S.; Cherian, T. Burden of disease caused by Streptococcus pneumoni in childrens younger than 5 years: Global estimates. Lancet 2009, 12, 893–902. [Google Scholar] [CrossRef]
- Doran, K.S.; Fulde, M.; Gratz, N.; Kim, B.J.; Nemani Prasadarao, R.N.; Schubert-Unkmeir, A.; Tuomanen, E.I.; Valentin-Weigand, P. Host-pathogen interactions in bacterial meningitis. Acta Neuropathol. 2016, 131, 185–209. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eto, S.F.; Fernandes, D.C.; Moraes, A.C.d.; Alecrim, J.V.d.C.; Souza, P.G.d.; Carvalho, F.C.A.d.; Charlie-Silva, I.; Belo, M.A.d.A.; Pizauro, J.M. Meningitis Caused by Streptococcus agalactiae in Nile Tilapia (Oreochromis niloticus): Infection and Inflammatory Response. Animals 2020, 10, 2166. https://doi.org/10.3390/ani10112166
Eto SF, Fernandes DC, Moraes ACd, Alecrim JVdC, Souza PGd, Carvalho FCAd, Charlie-Silva I, Belo MAdA, Pizauro JM. Meningitis Caused by Streptococcus agalactiae in Nile Tilapia (Oreochromis niloticus): Infection and Inflammatory Response. Animals. 2020; 10(11):2166. https://doi.org/10.3390/ani10112166
Chicago/Turabian StyleEto, Silas Fernandes, Dayanne Carla Fernandes, Alessandra Cristina de Moraes, João Victor da Costa Alecrim, Pedro Galdino de Souza, Fabíola Christian Almeida de Carvalho, Ives Charlie-Silva, Marco Antonio de Andrade Belo, and João Martins Pizauro. 2020. "Meningitis Caused by Streptococcus agalactiae in Nile Tilapia (Oreochromis niloticus): Infection and Inflammatory Response" Animals 10, no. 11: 2166. https://doi.org/10.3390/ani10112166
APA StyleEto, S. F., Fernandes, D. C., Moraes, A. C. d., Alecrim, J. V. d. C., Souza, P. G. d., Carvalho, F. C. A. d., Charlie-Silva, I., Belo, M. A. d. A., & Pizauro, J. M. (2020). Meningitis Caused by Streptococcus agalactiae in Nile Tilapia (Oreochromis niloticus): Infection and Inflammatory Response. Animals, 10(11), 2166. https://doi.org/10.3390/ani10112166