Mammary Cistern Size during the Dry Period in Healthy Dairy Cows: A Preliminary Study for an Ultrasonographic Evaluation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. Inclusion Criteria
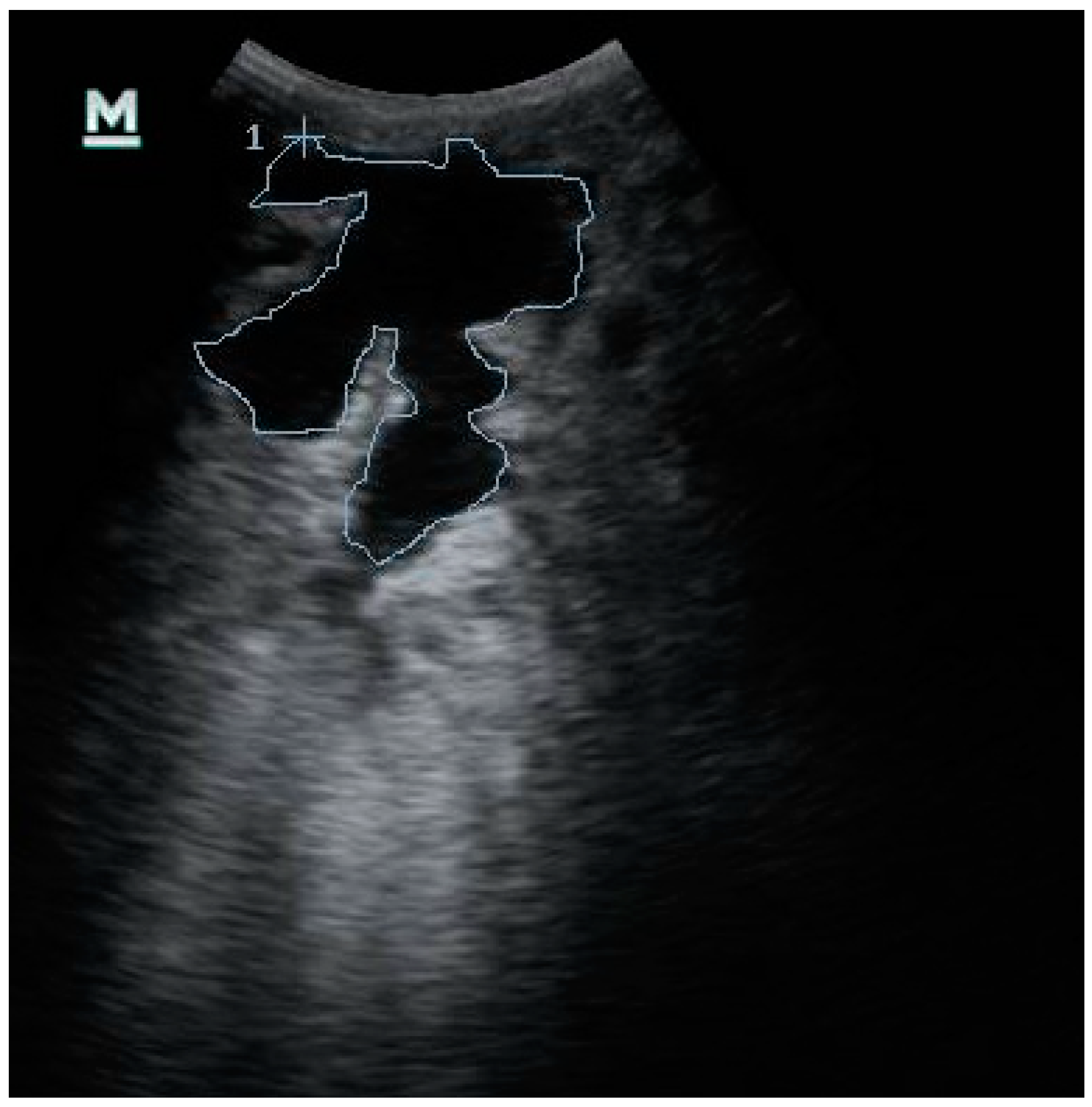
2.3. Ultrasonographic Examination of the Udder
2.4. Ultrasonographic Images Evaluation
2.5. Statistical Analysis
3. Results
3.1. Animals
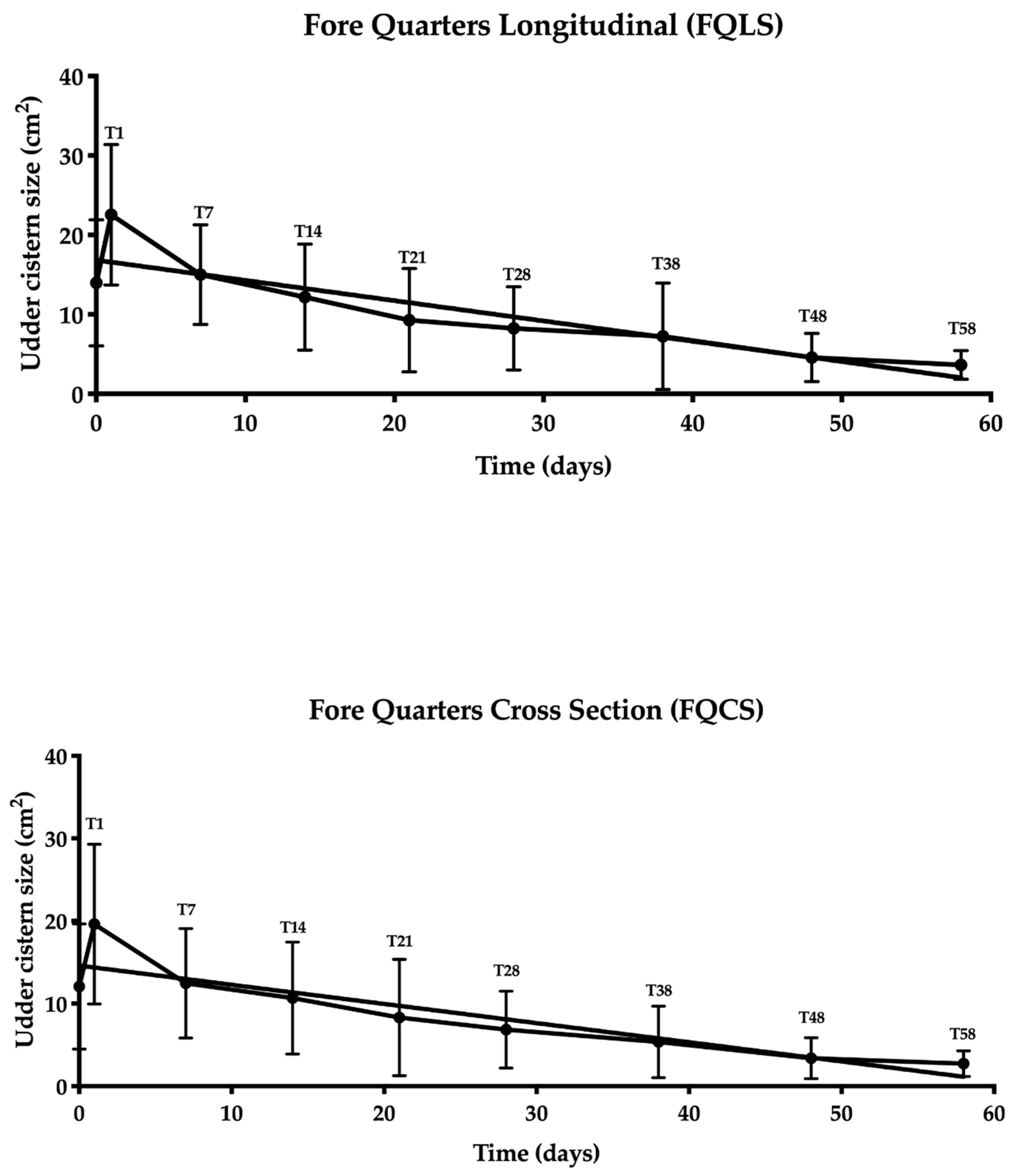
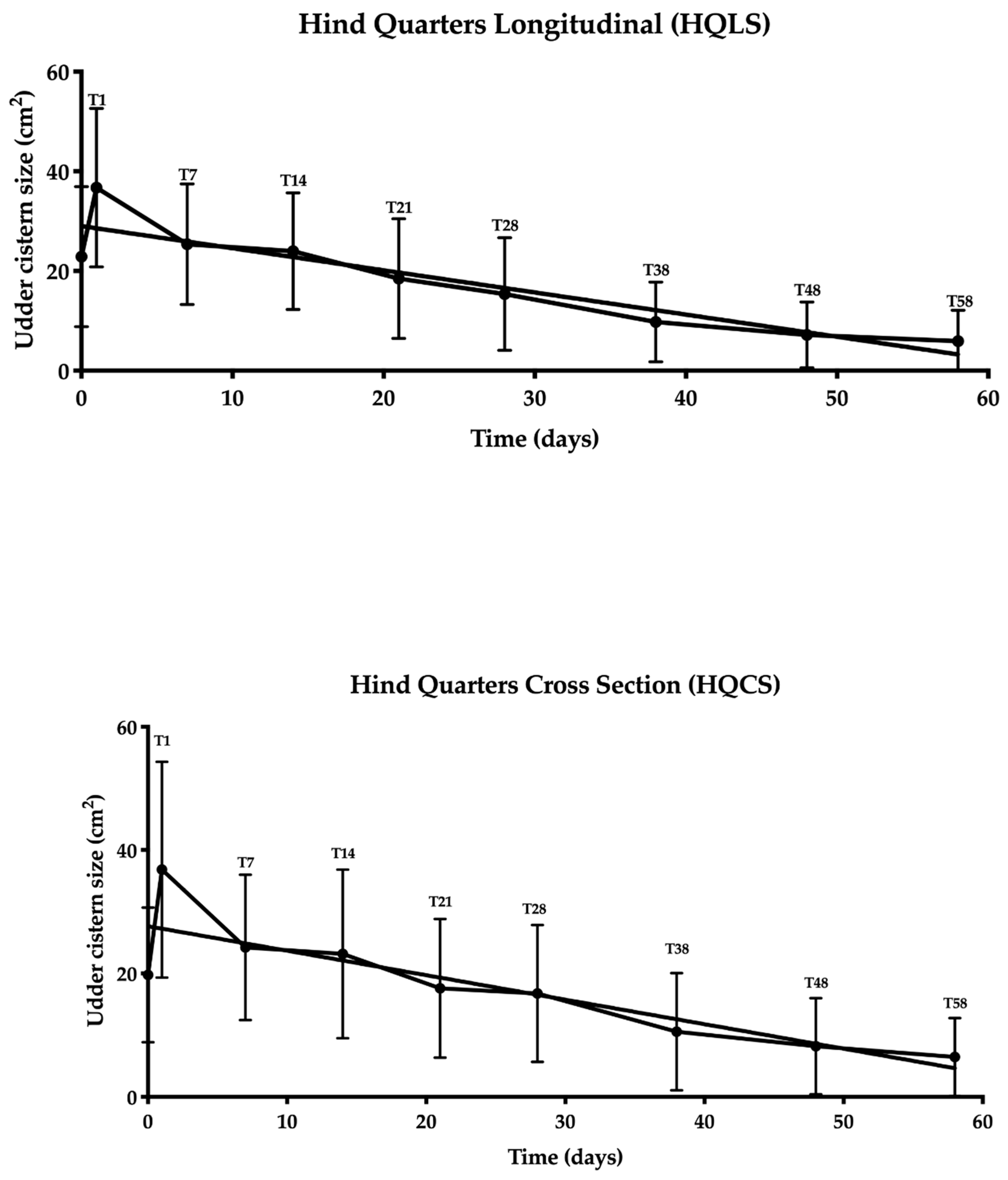
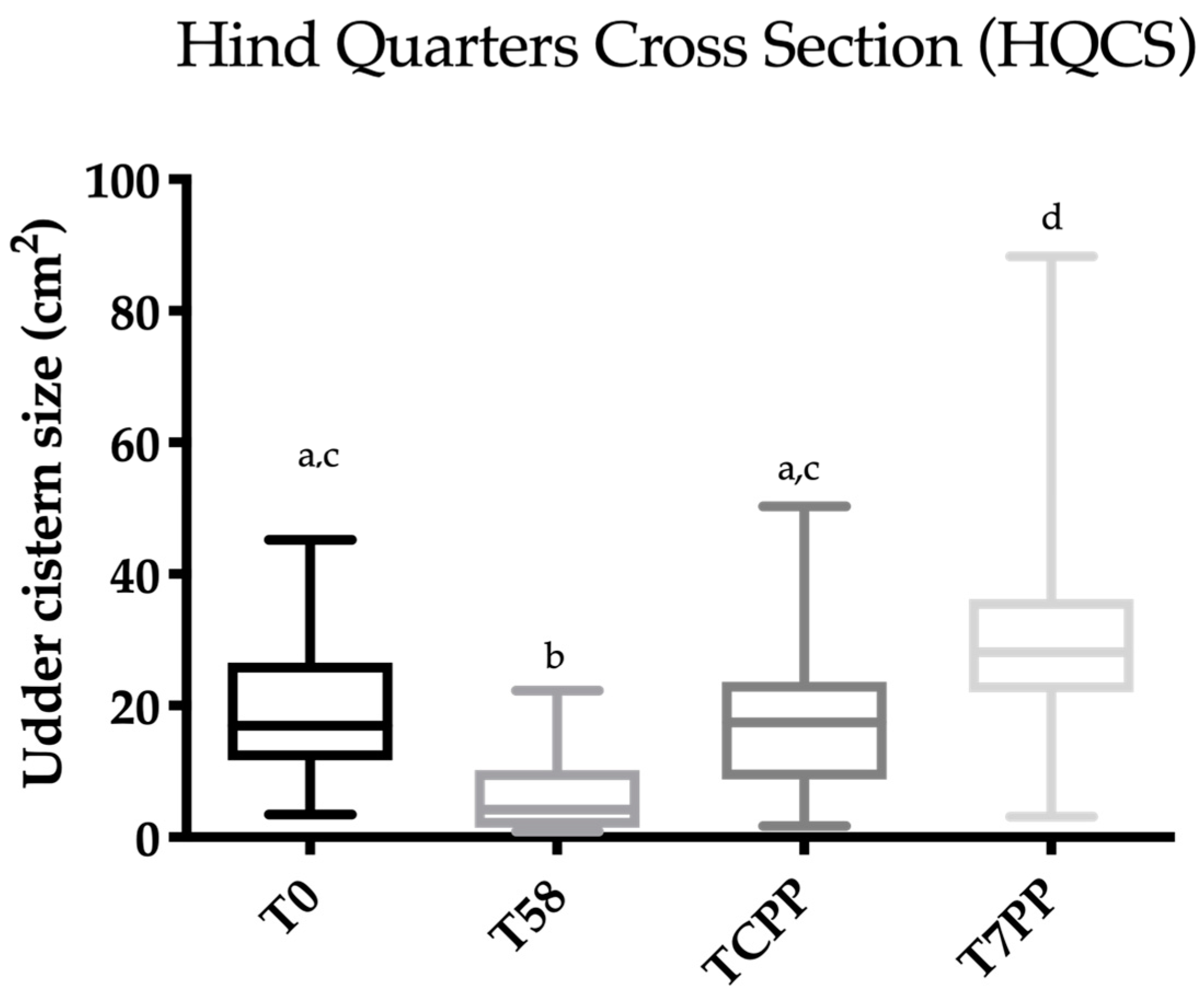
3.2. Udder Cistern Size
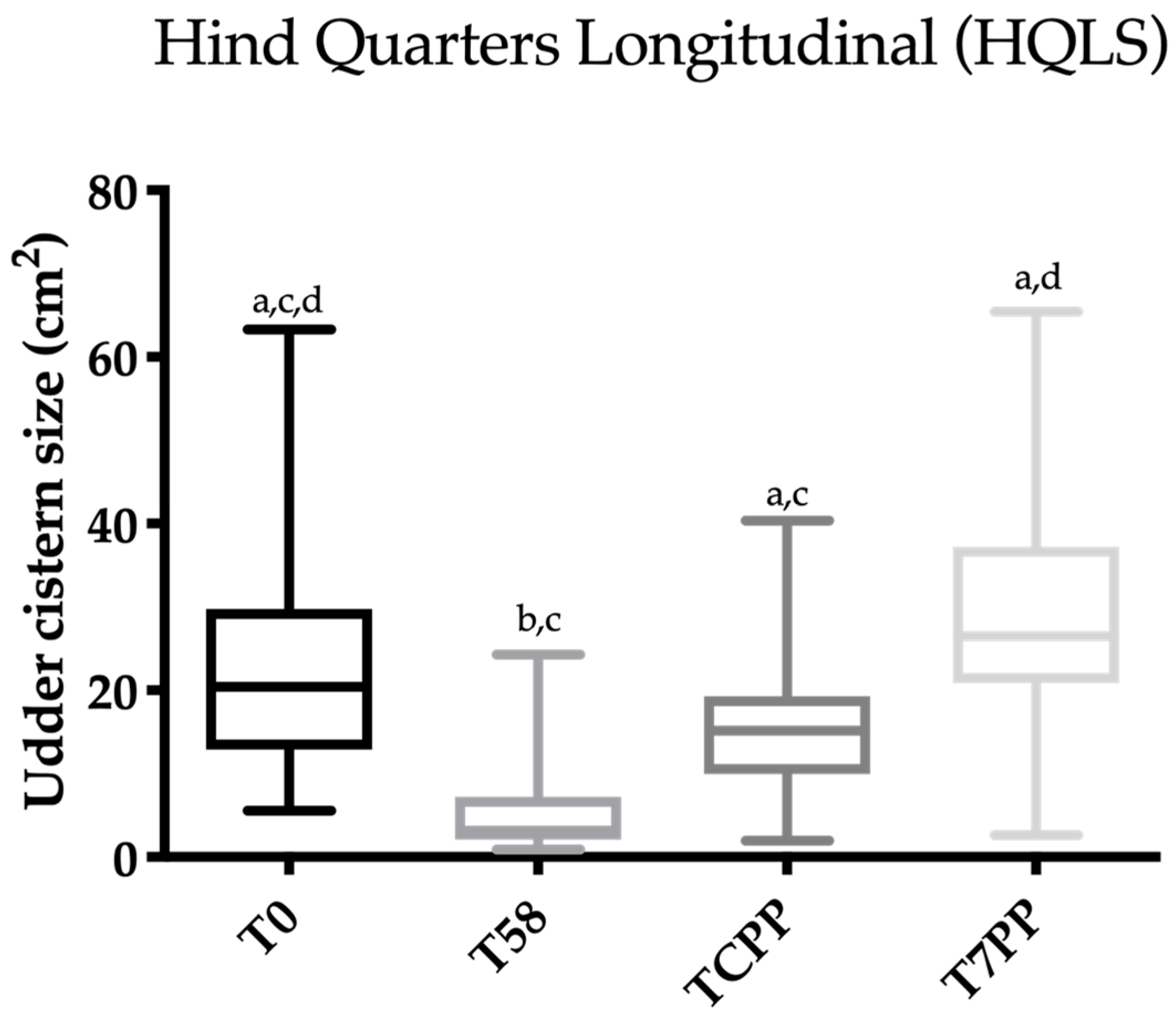
3.3. Statistical Analysis
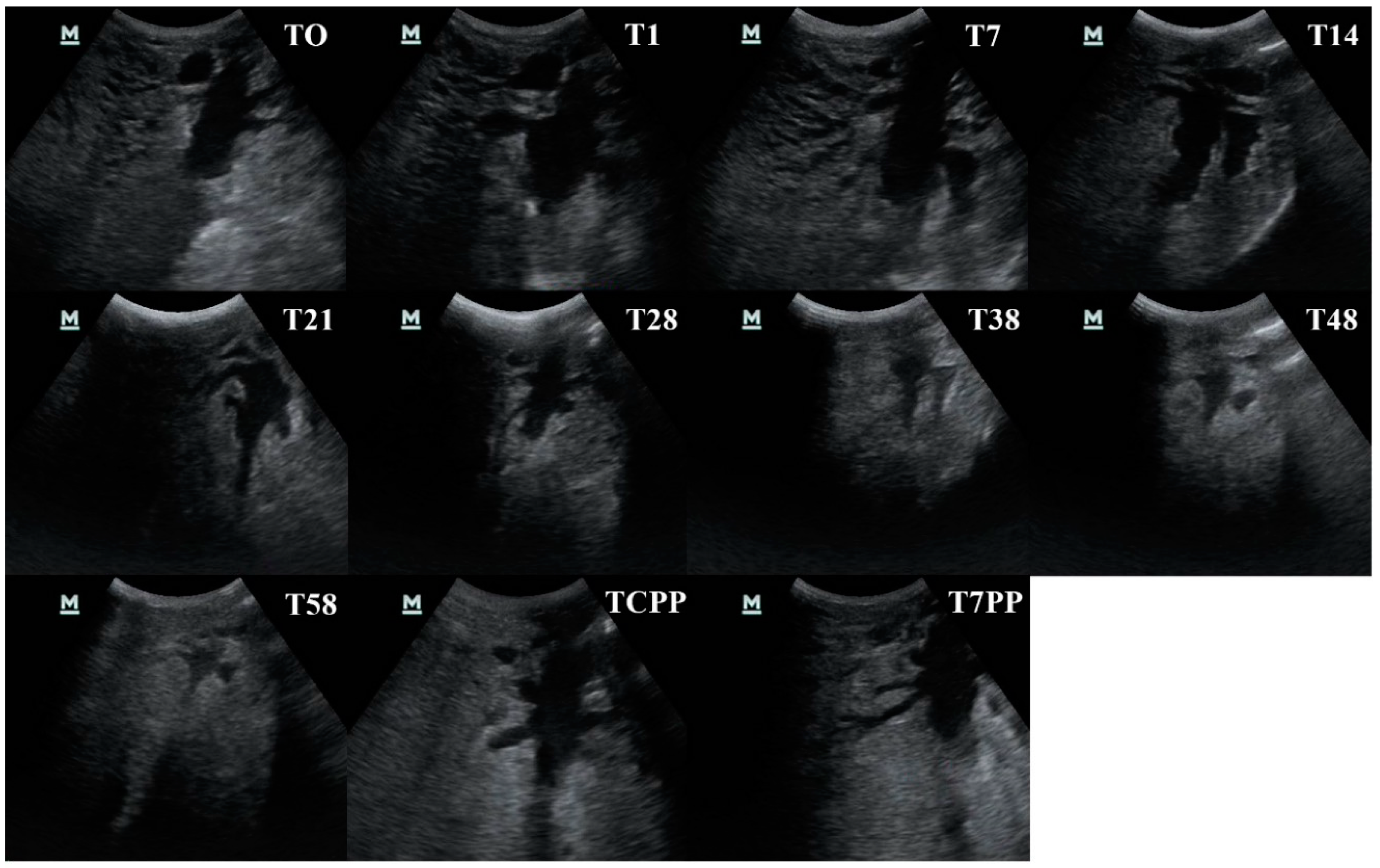
3.4. Evaluation of the Ultrasonography Images
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Capuco, A.V.; Akers, R.M.; Smith, J.J. Mammary growth in Holstein cows during the dry period: Quantification of nucleic acids and histology. J. Dairy Sci. 1997, 80, 477–487. [Google Scholar] [CrossRef]
- Annen, E.L.; Collier, R.J.; McGuire, M.A.; Vicini, J.L. Effects of dry period length on milk yield and mammary epithelial cells. J. Dairy Sci. 2004, 87, 66–76. [Google Scholar] [CrossRef]
- Annen, E.L.; Collier, R.J.; McGuire, M.A.; Vicini, J.L.; Ballam, J.M.; Lormore, M.J. Effect of modified dry period lengths and bovine somatotropin on yield and composition of milk from dairy cows. J. Dairy Sci. 2004, 87, 3746–3761. [Google Scholar] [CrossRef]
- Dingwell, R.T.; Kelton, D.F.; Leslie, K.E.; Edge, V.L. Deciding to dry off. Does level of production matter? In Proceedings of the National Mastitis Council 40th Annual Meeting, Reno, NV, USA, 11 February 2001; pp. 1–9. [Google Scholar]
- Dingwell, R.T.; Kelton, D.F.; Leslie, K.E. Management of the dry cow in control of peripartum disease and mastitis. Vet. Clin. N. Am. Food A 2003, 19, 235–265. [Google Scholar] [CrossRef]
- Hogan, J.; Smith, K.L. Coliform mastitis. Vet. Res. 2003, 34, 507–519. [Google Scholar] [CrossRef]
- Sordillo, L.M. Factors affecting mammary gland immunity and mastitis susceptibility. Livest. Sci. 2005, 98, 89–99. [Google Scholar] [CrossRef]
- Wendt, K.; Bostedt, H.; Mielke, H.; Fuchs, H.W. Euter-und Esäugekrankheiten; Gustav Fischer Verlag Jena: Stuttgart, Germany, 1994; pp. 229–231. [Google Scholar]
- Kossaibati, M.A. The costs of clinical mastitis in UK dairy herds. Cattle Pract. 2000, 8, 323–327. [Google Scholar]
- Klein, D.; Flöck, M.; Khol, J.L.; Franz, S.; Stüger, H.P.; Baumgartner, W. Ultrasonographic measurement of the bovine teat: Breed differences, and the significance of the measurements for udder health. J. Dairy Res. 2005, 72, 296–302. [Google Scholar] [CrossRef]
- Nishimura, M.; Yoshida, T.; El-Khodery, S.; Miyoshi, M.; Furuoka, H.; Yasuda, J.; Miyahara, K. Ultrasound imaging of mammary glands in dairy heifers at different stages of growth. J. Vet. Med. Sci. 2011, 73, 19–24. [Google Scholar] [CrossRef]
- Sonja, F.; Martina, F.; Margarete, H.P. Ultrasonography of bovine udder and teat. Vet. Clin. N. Am. Food Anim. Pract. 2009, 25, 669–685. [Google Scholar] [CrossRef]
- Ruberte, J.; Carretero, A.; Fernàndez, M.; Navarro, M.; Caja, G.; Kirchner, F.; Such, X. Ultrasound mammography in the lactating ewe and its correspondence to anatomical section. Small Rumin. Res. 1994, 13, 199–204. [Google Scholar] [CrossRef]
- Pulina, G.; Nudda, A.; Rassu, S.P.G.; Vallebella, R. Measurement of the mammary gland cistern of dairy ewes. Inf. Agrar. 1996, 52, 77–78. [Google Scholar]
- Bruckmaier, R.M.; Paul, G.; Mayer, H.; Schams, D. Machine milking of Ostfriesian and Lacaune dairy sheep: Udder anatomy, milk ejection and milking characteristics. J. Dairy Res. 1997, 64, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Bruckmaier, R.M.; Rothenanger, E.; Blum, J.W. Measurement of mammary gland cistern size and determination of the cisternal milk fraction in dairy cows. Milchwissenschaft 1994, 49, 543–546. [Google Scholar]
- Bruckmaier, R.M.; Ritter, C.; Schams, D.; Blum, J.W. Machine milking of dairy goats during lactation: Udder anatomy, milking characteristics, and blood concentrations of oxytocin and prolactin. J. Dairy Res. 1994, 61, 457–466. [Google Scholar] [CrossRef]
- Franz, S.; Hofmann-Parisot, M.; Baumgartner, W.; Windischbauer, G.; Suchy, A.; Bauder, B. Ultrasonography of the teat canal in cows and sheep. Vet. Rec. 2001, 149, 109–112. [Google Scholar] [CrossRef]
- Neijenhuis, F.; Klungel, G.; Hogeveen, H. Recovery of cow teats after milking as determined by ultrasonographic scanning. J. Dairy Sci. 2001, 84, 2599–2606. [Google Scholar] [CrossRef]
- Seker, I.; Risvanli, A.; Yuksel, M.; Saatb, N.; Ozmena, O. Relationship between California Mastitis Test score and ultrasonographic teat measurements in dairy cows. Aust. Vet. J. 2009, 87, 480–483. [Google Scholar] [CrossRef]
- Ayadi, M.; Caja, G.; Such, X.; Knight, C. Use of ultrasonography to estimate cistern size and milk storage at different milking intervals in the udder of dairy cows. J. Dairy Res. 2003, 70, 1–7. [Google Scholar] [CrossRef]
- Pulina, G.; Nudda, A. Technical and physiological aspects of the mechanical milking of sheep. Inf. Agrar. 1996, 52, 69–74. [Google Scholar]
- Nudda, A.; Pulina, G.; Valebella, R.; Bencini, R.; Enne, G. Ultrasound technique for measuring mammary cistern size of dairy ewes. J. Dairy Res. 2000, 64, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Neijenhuis, F.; Barkema, H.W.; Hogeveen, H.; Noordhuizen, J.P.T.M. Classification and longitudinal examination of callused teat ends in dairy cow. J. Dairy Sci. 2000, 83, 2795–2804. [Google Scholar] [CrossRef]
- Harmon, R.J.; Eberhart, R.J.; Jasper, D.E.; Langlois, B.E.; Wilson, R.A. Microbiological Procedures for the Diagnosis of Bovine Udder Infection, 3rd ed.; National Mastitis Council: Arlington, VA, USA, 1990; p. 34. [Google Scholar]
- Blowey, R.; Edmondson, P. Somatic cell count. In Mastitis Control in Dairy Herds, 2nd ed.; Blowey, R., Edmondson, P., Eds.; CABI: Wallingford (Oxfordshire), UK, 2010; pp. 153–170. [Google Scholar]
- Bradley, A.; Barkema, H.; Biggs, A.; Green, M.; Lam, T. Control of Mastitis and Enhancement of Milk Quality. In Dairy Herd Health, 1st ed.; Green, M., Ed.; CABI: Wallingford (Oxfordshire), UK, 2012; pp. 117–168. [Google Scholar]
- Albino, R.L.; Guimarães, S.E.F.; Daniels, K.M.; Fontes, M.M.S.; Machado, A.F.; Dos Santos, G.B.; Marcondes, M.I. Mammary gland ultrasonography to evaluate mammary parenchymal composition in prepubertal heifers. J. Dairy Sci. 2017, 100, 1588–1591. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Knight, C.H. Relationship between milk storage characteristics and the short-term response of dairy cows to thrice-daily milking. Anim. Sci. 1994, 58, 181–187. [Google Scholar] [CrossRef]
- Caja, G.; Ayadi, M.; Knight, C.H. Changes in cisternal compartment based on stage of lactation and time since milk ejection in the udder of dairy cows. J. Dairy Sci. 2004, 87, 2409–2415. [Google Scholar] [CrossRef]
- Flöck, M.; Winter, P. Diagnostic ultrasonography in cattle with diseases of the mammary gland. Vet. J. 2006, 171, 314–321. [Google Scholar] [CrossRef]
- Oliver, S.P.; Sordillo, L.M. Approaches to the manipulation of mammary involution. J. Dairy Sci. 1989, 72, 1647–1664. [Google Scholar] [CrossRef]
- Wilde, C.J.; Addey, C.V.; Li, P.; Fernig, D.G. Programmed cell death in bovine mammary tissue during lactation and involution. Exp. Physiol. 1997, 82, 943–953. [Google Scholar] [CrossRef]
- Heikkila, A.M.; Liski, E.; Pyorala, S.; Taponen, S. Pathogen-specific production losses in bovine mastitis. J. Dairy Sci. 2018, 101, 9493–9504. [Google Scholar] [CrossRef]
- Goncalves, J.L.; Kamphuis, C.; Martins, C.M.M.R.; Barreiro, J.R.; Tomazi, T.; Gameiro, A.H.; Hogeveen, H.; dos Santos, M.V. Bovine subclinical mastitis reduces milk yield and economic return. Livest. Sci. 2018, 210, 25–32. [Google Scholar] [CrossRef]


| UC Longitudinal Section (cm2) | T0 | T1 | T7 | T14 | T21 | T28 | T38 | T48 | T58 | TCPP | T7PP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FQLS—left | Min | 3.48 | 10.11 | 7.35 | 5.93 | 2.33 | 1.87 | 1.47 | 1.28 | 1.34 | 1.67 | 3.03 |
| Median | 14.36 | 24.76 | 15.26 | 13.59 | 7.94 | 9.95 | 5.74 | 3.72 | 3.79 | 13.34 | 21.19 | |
| Max | 28.82 | 35.02 | 24.88 | 18.39 | 20.57 | 15.58 | 16.93 | 9.05 | 5.58 | 34.73 | 51.00 | |
| FQLS—right | Min | 3.43 | 4.12 | 2.96 | 2.11 | 1.06 | 0.72 | 0.83 | 1.02 | 1.08 | 4.15 | 13.20 |
| Median | 8.94 | 24.75 | 15.20 | 10.56 | 6.30 | 6.35 | 5.30 | 4.06 | 4.28 | 14.09 | 19.05 | |
| Max | 24.65 | 36.48 | 25.22 | 27.26 | 23.88 | 17.70 | 28.95 | 11.16 | 6.28 | 25.54 | 32.21 | |
| HQLS—left | Min | 7.82 | 19.23 | 10.33 | 14.92 | 6.56 | 4.20 | 0.83 | 0.88 | 0.89 | 4.64 | 11.24 |
| Median | 26.63 | 37.71 | 22.86 | 20.45 | 14.64 | 10.88 | 7.23 | 3.99 | 4.93 | 16.02 | 26.77 | |
| Max | 63.28 | 63.81 | 54.50 | 53.41 | 49.06 | 40.05 | 27.53 | 20.81 | 24.32 | 40.34 | 65.46 | |
| HQLS—right | Min | 5.58 | 13.57 | 8.34 | 6.49 | 4.35 | 3.12 | 1.21 | 1.37 | 1.42 | 1.94 | 2.61 |
| Median | 17.98 | 30.53 | 22.57 | 18.95 | 13.68 | 10.29 | 7.78 | 2.92 | 2.80 | 14.31 | 25.23 | |
| Max | 39.15 | 61.56 | 39.96 | 37.16 | 35.35 | 28.89 | 21.71 | 16.16 | 9.32 | 28.57 | 47.89 | |
| UC Cross Section (cm2) | T0 | T1 | T7 | T14 | T21 | T28 | T38 | T48 | T58 | TCPP | T7PP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FQLS—left | Min | 2.12 | 6.43 | 7.66 | 2.43 | 1.26 | 1.81 | 0.68 | 0.65 | 0.55 | 1.06 | 3.22 |
| Median | 12.15 | 17.42 | 12.73 | 11.31 | 8.09 | 6.93 | 4.10 | 2.73 | 2.66 | 8.26 | 17.41 | |
| Max | 26.38 | 35.13 | 21.22 | 19.54 | 17.88 | 14.69 | 11.55 | 8.82 | 6.85 | 26.28 | 37.61 | |
| FQLS—right | Min | 2.31 | 2.80 | 1.25 | 1.28 | 0.68 | 0.41 | 1.62 | 1.21 | 1.22 | 3.31 | 5.33 |
| Median | 7.82 | 18.32 | 10.08 | 9.21 | 7.95 | 6.69 | 4.11 | 2.94 | 2.42 | 9.27 | 17.75 | |
| Max | 29.08 | 29.00 | 25.21 | 22.02 | 26.52 | 15.11 | 18.84 | 8.91 | 4.18 | 31.87 | 31.88 | |
| HQLS—left | Min | 9.33 | 17.34 | 9.88 | 9.72 | 4.61 | 3.01 | 1.00 | 0.90 | 0.88 | 1.74 | 3.09 |
| Median | 18.79 | 33.43 | 21.32 | 15.43 | 14.57 | 10.95 | 6.23 | 6.86 | 6.64 | 18.60 | 28.67 | |
| Max | 45.23 | 88.78 | 49.54 | 51.17 | 35.08 | 34.60 | 29.12 | 24.24 | 22.30 | 50.29 | 88.25 | |
| HQLS—right | Min | 3.45 | 17.33 | 9.79 | 4.97 | 5.06 | 5.22 | 1.00 | 1.20 | 1.08 | 5.61 | 8.02 |
| Median | 15.74 | 35.40 | 21.22 | 20.74 | 13.13 | 8.73 | 7.70 | 3.23 | 2.82 | 12.61 | 27.82 | |
| Max | 43.04 | 57.41 | 42.83 | 47.35 | 41.42 | 37.00 | 29.24 | 21.36 | 14.14 | 35.58 | 64.74 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonelli, F.; Orsetti, C.; Turini, L.; Meucci, V.; Pierattini, A.; Sgorbini, M.; Citi, S. Mammary Cistern Size during the Dry Period in Healthy Dairy Cows: A Preliminary Study for an Ultrasonographic Evaluation. Animals 2020, 10, 2082. https://doi.org/10.3390/ani10112082
Bonelli F, Orsetti C, Turini L, Meucci V, Pierattini A, Sgorbini M, Citi S. Mammary Cistern Size during the Dry Period in Healthy Dairy Cows: A Preliminary Study for an Ultrasonographic Evaluation. Animals. 2020; 10(11):2082. https://doi.org/10.3390/ani10112082
Chicago/Turabian StyleBonelli, Francesca, Chiara Orsetti, Luca Turini, Valentina Meucci, Alessio Pierattini, Micaela Sgorbini, and Simonetta Citi. 2020. "Mammary Cistern Size during the Dry Period in Healthy Dairy Cows: A Preliminary Study for an Ultrasonographic Evaluation" Animals 10, no. 11: 2082. https://doi.org/10.3390/ani10112082
APA StyleBonelli, F., Orsetti, C., Turini, L., Meucci, V., Pierattini, A., Sgorbini, M., & Citi, S. (2020). Mammary Cistern Size during the Dry Period in Healthy Dairy Cows: A Preliminary Study for an Ultrasonographic Evaluation. Animals, 10(11), 2082. https://doi.org/10.3390/ani10112082







