Simple Summary
Cities are the fastest developing ecosystems on the planet. The rapid expansion of urban areas is typically seen as a threat to global biodiversity, yet the role of cities in protecting species that may be rare in the wild remains poorly explored. Here, we report the use of environmental DNA (eDNA) to document the species present in one of the largest urban green spaces in Johannesburg, South Africa. We document a surprisingly large number of taxonomic groups, including some rare and threatened species. Our results support the notion that urban green spaces can provide refuge to a large number of species, and the species inventory provides critical information that can be used by city parks managers to conserve green spaces.
Abstract
Adaptation to environments that are changing as a result of human activities is critical to species’ survival. A large number of species are adapting to, and even thriving in, urban green spaces, but this diversity remains largely undocumented. In the current study, we explored the potential of environmental DNA (eDNA) to document species diversity in one of the largest green spaces in Johannesburg, South Africa. Using a novel metabarcoding approach that assembles short DNA fragments suitable for massively parallel sequencing platforms to the approximate standard ~710 bp COI barcoding fragment, we document the presence of 26 phyla, 52 classes, 134 orders, 289 families, 380 genera and 522 known species from the study site. Our results highlight the critical role that urban areas play in protecting the world’s declining biodiversity.
1. Introduction
Urban areas are the fastest growing ecosystems on the planet. As cities expand, the surrounding natural habitat is transformed, and wild species are either displaced or are forced to persist in shrinking pockets of fragmented habitats inside cities [,,]. As a consequence, parks, green spaces, architectural structures and wetlands within cities harbor a considerable level of concealed diversity [,,,] whose significance remains largely underappreciated. In cities, species face a combination of environmental stressors and selective anthropogenic pressures that differ from those found in natural environments []. However, for those species that can adapt to the challenges of living in urban areas, there are numerous benefits. Constant supply of food, shelter, fewer predators or competitors, and more stable micro-climatic conditions make cities an ecological hotspot for many species []. Species that have mastered living in urban areas can reach population densities that far exceed those of their conspecifics in the wild, making urban ecosystems particularly important for the survival of numerous threatened species across the globe [,,].
Johannesburg is the largest and most populated metropolitan area in South Africa []. Founded in the mid-1880s during the Witwatersrand gold rush, the city is situated in the Gauteng Province within the eastern Highveld plateau ecoregion [] of South Africa. Our study area, Delta Park, is one of the largest semi-natural green spaces in Johannesburg. Until the 1960s, Delta Park was used as an expansion of Johannesburg’s sewage scheme. Soon after the closure of the sewage site, an ecosystem restoration project started, and over time, several species colonized the area, with at least 200 bird species documented in Delta Park by 2011 [,]. However, the diversity of less conspicuous taxa that are associated with water, such as arthropods and mollusks, remains unexplored.
Traditional methods of monitoring biodiversity rely on visual observation and morphological identification of species that live in an area [,]. These approaches require extensive taxonomical and morphological expertise, and are unsuitable to monitor species that are rare, cryptic, secretive [], or that change phenotypically throughout their lives [,].
Recent developments in DNA sequencing technologies and high-performance computing have made it possible to study ecological diversity with unprecedented levels of accuracy. Particularly suitable for monitoring the diversity and abundance of species is the massively parallel sequencing of environmental DNA (eDNA) [], which is a non-destructive approach [] that utilizes trace amounts of DNA found in the environment []. This technique is highly sensitive and has considerable potential to identify scarce, cryptic, or elusive species that are otherwise overlooked [].
Most DNA-based methodologies are sensitive to the selection of appropriate DNA markers. Elbrecht and Leese (2015) [] showed that the selection of genetic markers directly influences the estimation of species diversity and abundance in an area. Since 2002, the cytochrome c oxidase subunit I gene (COI) has been the marker of choice for DNA-based biomonitoring []. The performance of this marker has been tested in a wide range of freshwater, marine, and terrestrial habitats [,,,,,,,] to address a wide array of ecological questions [,,,].
The approximate 710 bp length of the COI marker of Folmer et al. (1994) [], which is the most frequently amplified fragment, exceeds the current limits of most massively parallel sequencing platforms. While new sequencing platforms that produce longer sequences, such as PacBio and Oxford Nanopore technologies exist, the high costs of sequencing on these platforms makes the technology inaccessible to many laboratories in lower income countries. As an alternative, new sets of DNA primers that target shorter segments of DNA than the universal primers of Folmer and colleagues have been developed [,,,]. A trade off is that a combination of several barcodes is typically required to study the total diversity of an area (i.e., taxon/species-specific primers for different taxonomic groups), which involves extra effort and additional costs for optimizing amplification and sequencing.
The current study explores the potential of randomly shearing COI sequences into short fragments, followed by bioinformatic assembly of the complete length of the targeted product of Folmer’s primers. The aims of this study are two-fold. First, it constitutes the first DNA-based survey of species diversity in a South African urban green space, five decades after an ecological restoration project started. Second, we present a cost-effective method for monitoring biodiversity, which uses short shotgun fragments from commonly used sequencing platforms combined with high-throughput bioinformatic pipelines, to approximately the complete ~710 bp length of the COI barcode. The same methodology can be applied to reconstruct the full length of any other DNA fragments.
2. Materials and Methods
2.1. Study Area
Delta Park is located along the middle reaches of the Braamfontein Spruit (river), a tributary of the Jukskei River which falls within the larger Crocodile (West) and Marico Water Management Area. The upper reach of the river flows through urban areas in the west of Johannesburg while the middle reach of the Braamfontein Spruit is surrounded by formal residential areas. The two dams in the park (Top Dam and Middle Dam) are connected by a narrow channel whose water flows in a north-easterly direction through the park before it joins the Braamfontein Spruit (see Figure 1).
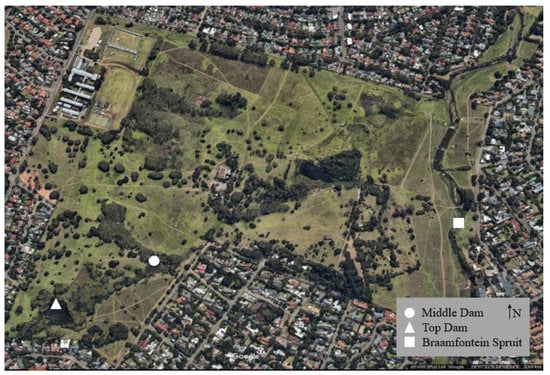
Figure 1.
Sampling localities within Delta Park, Johannesburg. The Braamfontein Spruit flows in a northerly direction. The three collection sites are indicated. Aerial photo taken from Google Earth.
2.2. Sample Collection
Water and sediment samples were collected from three sites within Delta Park: Middle Dam (−26.127914, 28.006980), Top Dam (−26.129288, 28.003892) and the Braamfontein Spruit (−26.126860, 28.016090) (Figure 1). At each site, water was collected in two 500 mL bottles that had been sterilized through autoclaving (KT—2346A, ALP Co., Ltd. Tokyo, Japan) for 40 min at 115 °C under 1 atmosphere pressure. At the collection site, the lids were opened approximately 15 cm below the water surface, the bottles were filled with water, and the lids were closed while still underwater. For sediment collection, soil was collected into three 50 mL plastic tubes from various sites in the dams and river, approximately 1 m from the bank. All samples were immediately transferred to the Centre for Ecological Genomics and Wildlife Conservation at the University of Johannesburg. Water samples were placed in a refrigerator at 4 °C and sediment samples were preserved in a freezer at −20 °C. Environmental samples were processed within 24 h of collection.
Environmental samples were processed in a room that has not been used for any DNA work prior to this study. All surfaces were wiped down with 100% bleach, left to dry, and subsequently wiped with 70% EtOH. Approximately 500 mL of the collected water was filtered using a MicroFunnelTM Filter Unit with 0.2 µm Supor® Membrane (Pall Laboratory, Johannesburg, South Africa). Five filters were used for the Middle Dam site, two filters were used for Top Dam, and three filters were used for the Braamfontein Spruit. We changed filters when they became clogged, and DNA was extracted from all the filters. For the sediment, each falcon tube was shaken vigorously and approximately 5 mg of the homogenized slurry was subsampled for DNA extraction.
Metagenomic DNA was extracted by grinding sediment and water filters in 200 µL grinding buffer (0.5 M sorbitol, 0.2 M Tris-HCL, 7 mM TITRIPLEX® III EDTA, 20 mM Na-Bisulfit and 4% polyvinylpyrrolidone 40) using a Covaris sonicator (Whitehead Scientific, Cape Town, South Africa). Ground specimens were digested using 200 µL of lysis buffer (0.4 M Tris-HCL, 7 mM TITRIPLEX® III EDTA, 2 M NaCl and 2% of cetrimonium bromide), 10% SDS and 20 µL Proteinase K in a 1.5 µL Eppendorf tube. The samples were left to incubate for one hour at 60 °C. Lysates were centrifuged (4600 RPM) for 10 min at 4 °C. The supernatant was transferred into a new 1.5 µL Eppendorf tube, and 250 µL buffer mix III (3 M KAc) was added to each tube. The tubes were left to incubate on ice for 10 min followed by a 15 min centrifugation step at 4 °C. The supernatant was transferred onto a glass fibre filter plate and was centrifuged for an additional 5 min at 4 °C. Approximately 280 µL of isopropanol was added to the filtrate, which was then vortexed vigorously and left to incubate on the plate for 20 min at −20 °C. Each plate was centrifuged for an additional 25 min at 4 °C. Finally, 200 µL of ice cold 70% EtOH was added to each tube, which was centrifuged for 5 min at 4 °C. The resulting DNA pellets were dried at room temperature and then dissolved in 50 µL TE buffer.
A portion of the mitochondrial COI gene was amplified using 3 µL of template DNA, 6 µM of universal primers LCO1490 (forward primer, 5′–GGT CAA CAA ATC ATA AAG ATA TTG G–3′) and HCO2198 (reverse primer, 5′–TAA ACT TCA GGG TGA CCA AAA AAT CA–3′) [], 1× PCR buffer, 0.4 mM dNTPs, 2.5 mM MgCl2, and 1 unit of Taq DNA Polymerase (S7 Phusion; Biozym, Oldendorf, Germany). Thermal cycles for each reaction started with an initial denaturation step at 95 °C for 15 min, followed by 25 cycles of denaturing steps at 95 °C for 20 s, an annealing stage of 49 °C for 45 s, and an elongation step at 72 °C for 1 min. This was followed by a final elongation step at 72 °C for 10 min. To minimize the amplification of non-specific PCR product, we selected 25 PCR cycles, as preliminary analyses indicated that this produced sufficient product for Illumina library preparations.
Equimolar concentrations of amplicons from sediment and water samples were pooled into two separate tubes and randomly sheared into approximately 250–300 bp fragments using a Covaris sonicator (Whitehead Scientific, Cape Town, South Africa). To maximize the random shearing of the COI amplicons, multiple rounds of sonication with different intensities were performed, and the size of the resulting fragments after each round of sonication was checked using a Bioanalyzer 2100 (Agilent, Johannesburg, South Africa). Only fragments within the expected range were selected for genomic DNA library preparation. The targeted sequence was treated as a miniature genome that was sheared to a large number of smaller overlapping fragments that is necessary to assemble longer fragments.
Genomic libraries were generated using a NEBNext® UltraTM DNA Library Prep Kit (New England BioLabs, Ipswich, MA., USA) and sequenced on an Illumina HiSeq 4000 platform (San Diego, CA, USA) using 2 × 150 bp paired-end chemistry according to the manufacturer’s instructions.
2.3. Sequence Assembly and Analysis
Low-quality sequences and adapter contaminants were identified in FastQC [] and removed using Trimmomatic v0.39 []. MEGAHIT v1.1.1 [] was used to assemble metagenomic sequences into longer contigs using the program’s default settings. This metagenome assembler was chosen because it performs robustly in large and complex datasets that are typical of environmental samples []. Assembly statistics were estimated in QUAST v4.0 []. To check the quality of assemblies, the short read aligner Bowtie2 [] was used to map quality-filtered sequences against corresponding assembled contigs, and the mapping statistics for each alignment were computed in SAMtools v1.10 [].
The assembled sequences were dereplicated into unique sequence features using VSEARCH v2.4.2 [], chimeric reads were removed, and the remaining reads were subsumed into distinct clusters known as operational taxonomic units (or OTUs), by executing a VSEARCH smallmem command with a minimum sequence similarity of 98%. Consensus sequences from each cluster were extracted and sorted based on their size. Taking into account variation in the primers’ annealing sites between different taxa, and minor length variation that is typically observed in metagenome assemblies from complex communities, all sequences with a length exceeding 712 bp (the theoretical maximum product size length for the LCO1490 and HCO2198 primers is approximately 710 bp []) were considered assembly artefacts or non-target sequences and filtered from downstream analyses.
All consensus sequences were searched against an in-house database of COI sequences extracted from the NCBI non-redundant nucleotide database (https://www.ncbi.nlm.nih.gov/refseq/), using the MEGABLAST package (which searches for ‘highly similar’ matches) [] and somewhat similar BLASTn (which searches for ‘somewhat similar’ matches), with a minimum sequence similarity of 70% and an e-value of 10−5. The four best matches for each query were retained and reported. A Last Common Ancestor (LCA) consensus taxonomic rank was assigned to each sequence in BASTA v1.3.2.3 (https://github.com/timkahlke/BASTA) []. For each match, the NCBI taxonomy ID and scientific name were extracted, and a circular phylogenetic tree was constructed using the PhyloT online server (phylot.biobyte.de) [] and visualized in FigTree v1.4.4 [].
To assess the efficiency of the sequence assembly and taxonomic rank assignments, all sequences in our dataset were divided into two approximately equally sized fragments using FASTX-Toolkit v 0.0.8 []. Small overlaps were allowed between two fragments, especially for shorter fragments (<400 bp). Each fragment was separately blast-searched using the same parameter settings for e-value and percentage identity as for the full-length dataset. The BASTA pipeline relies on unique NCBI accession numbers to assign a consensus taxonomic rank to the query sequences. To account for variations in NCBI accession numbers, an issue which typically arises when the first half of the sequence matches a specific accession number of a species and the second half matches the same species but from an entry with a different accession number, we verified whether the NCBI accession numbers for the best matches of the first half can be exactly matched among the four best matches reported for the second half. Furthermore, a database consisting of 100 chimeric sequences was manually created by adding random fragments of DNA from multiple arthropods and vertebrate species, and all these sequences were subjected to the same taxonomic rank assignments.
Alpha diversity indices, Shannon [], Simpson [] and Evenness [], were estimated using the R package diverse [].
3. Results
The Illumina sequencing run yielded 5,028,734 and 5,219,475 paired-end raw sequences from water and sediment samples, respectively. MEGAHIT assembled quality-filtered sequences from water samples into 2208 and 5443 contigs, respectively. The cumulative length distribution histogram of raw assemblies shows that the length of less than 5 percent of the sequences exceeded 712 bp (Figure 2).
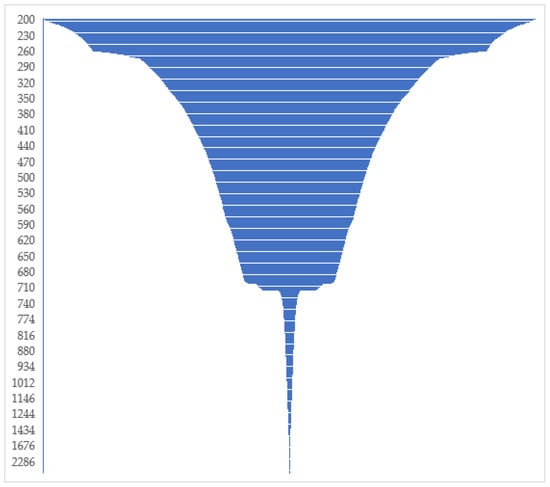
Figure 2.
A funnel graph showing the cumulative length distribution of the unfiltered assembled sequences. The Y axis represents the cumulative length of the scaffolds in base pair. The bottleneck in the figure reflects ~710 bp, the theoretical length of the target sequences. Less than 5% of sequences had a length exceeding 713 bp, and these were removed from the dataset.
After merging raw assemblies from water and sediment, VSEARCH dereplicated the pool into 6319 unique features. Subsequent clustering of unique sequence features with more than 98% identity produced 5582 clusters. The BASTA pipeline taxonomically ranked the resulting dataset, with a mean blast percentage identity of 85% (range 72–100), into 26 phyla, 52 classes, 134 orders, 289 families, 380 genera, and 522 known species. Among these, only 12 species, namely Achlya bisexualis, Aspergillus tubingensis, Biomphalaria glabrata, Bulinus natalensis, Cheyletus malaccensis, Chrysomya rufifacies, Drosophila hydei, Fannia canicularis, Homo sapiens, Opistophthalmus boehmi, Rattus norvegicus, and Tuberolachnus salignus, are known to occur in Delta Park based on earlier, non-genetic studies (Table 1, Figure 3; see Appendix A Table A1 for a complete taxonomic list). Our results show that the taxonomic rank assignment is sensitive to the selection of the blast algorithm, as 35.9% of species identified by highly sensitive MEGABLAST were absent when we used BLASTn. Similarly, 9.7% species that were identified by BLASTN were absent in MEGABLAST (Appendix A). More than 70% of the quality-filtered reads were properly mapped against the corresponding assemblies, which lies within the accepted range for an assembly.

Table 1.
Number of sequences identified at different taxonomic ranks for the water and sediment samples combined.
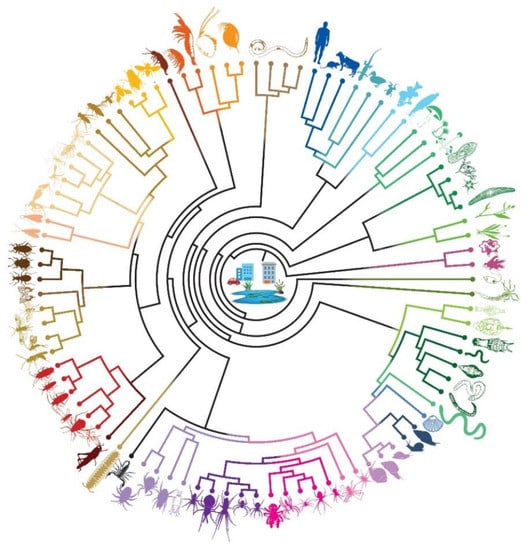
Figure 3.
A circular tree showing the different organisms found in the water and sediment samples in Delta Park, South Africa. This tree is based on the results from the BLASTN search results.
The exact NCBI accession number of the best matches for the first half of the assembled sequences were matched among four best matches of the second half in more than 98.4% of the pair-wise comparisons. In almost all cases, the exact match for NCBI accession number of the first half was found among the top eight blast hits for the second half. The BASTA pipeline assigns a taxonomic rank of “unknown” or “unknown-eukaryotic” to all manually generated chimeric sequences. All these confirm that the negative effects of chimeric sequences in our dataset are likely to be minimal.
Shannon’s diversity index was estimated slightly higher for sediment compared to water samples (sediment H = 2.13 and water H = 2.025), while both the Evenness index and the Simpson’s index were higher for the water communities (Table 2).

Table 2.
Diversity indices showing Simpson Diversity, Shannon Diversity and Evenness.
4. Discussion
Cities constitute the newest extensions to wild habitats. The role that urban ecosystems play in preserving the world’s declining biodiversity represents an underappreciated area of research [], yet it remains critical in ensuring the conservation of many species and the provisioning of ecosystem services. Living alongside wild populations exposes societies to health challenges; these challenges need to be addressed proactively using a combination of conventional and new tools. Here, we present and discuss the first application of environmental DNA to survey biological diversity in a semi-natural park in Johannesburg.
Not surprisingly given their abundance, arthropods dominated the aquatic biological diversity of Delta Park. Arthropods were followed by species belonging to the phyla Oomycota, Mollusca, Cnidaria, Chordata, Rotifera and Annelida. High diversity of arthropods has already been reported from urban areas in South Africa [] and elsewhere []. Among arthropods, the presence of the assassin spiders, Archaeidae, is of particular interest. With only one extant genus, Afrarchaea, reported from South Africa [], their presence in Delta Park highlights the importance of urban ecosystems for the survival of species that are comparatively rare in wild habitats [,,,,,,,,,,,,,].
Delta Park is home to several firefly species. Populations of fireflies are declining across the globe as a result of high intensity artificial light at night (ALAN) [,,] that exceeds the intensity of bioluminescent flashes of these nocturnal species during the mating season. The lower level of artificial light contamination in Delta Park compared to the densely populated surrounding residential areas makes it an ideal breeding habitat for this ecologically important species. The presence of members of Culicid mosquitos and Tabanid horse flies, among which there are several species that function as biological vectors for the causative agents of some diseases such as malaria, yellow and dengue fever, are also important.
Environmental DNA highlighted the presence of several vertebrate species in Delta Park. These include rats (Rattus sp.), cattle (Bos taurus; not physically present in the park, but likely the result of DNA carried by water), and a number of unidentified species belonging to Gekkonidae, Scincidae and Eulipotyphla families (all present in the park). Among aquatic vertebrates, species of Perciformes, Blenniidae, Cypriniformes, Clarias sp. and Galaxiidae fish were identified. While some of these species such Clarias sp. are common inhabitants of freshwater ecosystems worldwide and in southern Africa, the presence of some other species most likely represents non-native species released into dams by aquarium owners.
Delta Park is home to two species of freshwater aquatic snails, Bulinus natalensis and Biomphalaria glabrata. Both are intermediate hosts for parasitic flatworms (Schistosoma sp.). Infection with schistosome flatworms can progress to the development of schistosomiasis, the world’s third most devastating parasitic disease []. The identification of a pathogenic protist, Acanthamoeba sp., the causative agent for ocular keratitis and granulomatous encephalitis of the central nervous system, is also a major health concern [].
The primers used in the current study were initially developed for animals. However, the identification of the several species of fungi and algae in our dataset points to the potential of the COI marker for the identification of additional taxa, although only at higher taxonomic ranks [], because in line with previous studies [], there was little taxonomic resolution at lower taxonomic levels such as genus and species level. We demonstrated that environmental DNA obtained from both water and sediment samples can be used to estimate species diversity in a wetland even if species are present at low numbers and were not detected during visual surveys. Our study had higher biodiversity compared to another river in South Africa (H′ = 1.028) [], and was similar in comparison to a study on an urban temporary pond (H′ = 2.72) [], and that of fish in large rivers (H′ = 2.21) []. This is because the environment can retain a molecular imprint of species inhabiting the area [,]. Determining when the species were present in the system is difficult, and it cannot be reliably expressed whether the species identified are still present in the system. Our study is a snapshot of the eDNA that was present in the system at the time of sampling, and over time, temporal communities can change in response to seasons and water quality, which needs to be a focus of future studies.
While we cannot rule out that some of the sequences that have failed conclusive assignment to lower taxonomic ranks are artefacts of the bioinformatic assembly, others undoubtedly reflect the lack of publicly available records for the species in question, or for their close relatives.
Methods that are based on the last common ancestor to assign taxonomic rank perform poorly when local databases are not complete, which is a common problem in underrepresented geographic areas such as Africa [], as well as when the geographical records of the specimens in a database are heterogeneous. In both cases, conservative rank assignments to a higher taxonomic rank or to a taxonomic rank that does not occur within the study area will negatively affect the accuracy of such surveys. It is expected that underrepresented taxa and regions will be affected disproportionately.
As scientific efforts to characterize global biodiversity using environmental DNA intensify, our study emphasizes that the need for a comprehensive taxonomically-curated reference database is equally important. A reference database to address such shortcomings requires close cooperation between experts from different fields, such as systematics, morphology, biochemistry and molecular biology, at both regional and global scales. Successful collaborations can have far-reaching implications for better characterizing global biodiversity [].
5. Conclusions
We demonstrated that environmental DNA obtained from water and sediment samples can be used to detect the presence of species in aquatic habitats, even if a species is present at densities undetected during visual biodiversity surveys. This reaffirms that aquatic habitats retain a molecular imprint of species inhabiting the area []. However, determining when the species were present in the system cannot be reliably assessed.
The specific methodology applied in the current study, which is based on the sequence assembly, results in different sections of the markers being covered with different number of raw sequences. This heterogeneous coverage across the length of the amplicon limits the power of such methodologies to estimate the abundance of each species. However, presence or absence of species in an area can be reliably investigated.
Author Contributions
Conceptualization, B.J.v.V. and P.R.T.; methodology and formal analysis, H.J.W. and A.E.-K.; data curation, H.J.W. and A.E.-K.; writing—original draft preparation, H.J.W. and A.E.-K.; writing—review and editing, P.R.T., J.C.v.D. and B.J.v.V.; supervision, A.E.-K., P.R.T., J.C.v.D. and B.J.v.V.; funding, B.J.v.V. and P.R.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Johannesburg (FRC and URC grants) to B.J.v.V. and P.R.T. A.E.-K. was funded through a University of Johannesburg Global Excellence and Stature bursary.
Acknowledgments
We thank Geoff Lockwood and Shirley Tebbutt (Delta Park) for permission to collect samples. Our manuscript benefitted from the input from an editor and two anonymous reviewers.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Comparison of species identified using two different blast algorithms: highly sensitive MEGABLAST and BLASTn.
Table A1.
Comparison of species identified using two different blast algorithms: highly sensitive MEGABLAST and BLASTn.
| Species | BLASTN | MEGABLAST |
|---|---|---|
| Acanthamoeba | Yes | Yes |
| Achatinellidae | Yes | Yes |
| Achlya | Yes | Yes |
| Achlya bisexualis | Yes | Yes |
| Acrididae | Yes | No |
| Actinopteri | Yes | Yes |
| Agaricomycetes | Yes | Yes |
| Agelenidae | Yes | Yes |
| Agelenopsis | Yes | Yes |
| Aglaoctenus | Yes | No |
| Agromyzidae | Yes | Yes |
| Alona | No | Yes |
| Amaurobiidae | Yes | Yes |
| Amaurobioides | Yes | Yes |
| Amphibia | Yes | Yes |
| Amphipoda | Yes | Yes |
| Anacaena | No | Yes |
| Anatidae | Yes | No |
| Anelosimus | Yes | Yes |
| Anisakidae | Yes | Yes |
| Annelida | Yes | Yes |
| Anthocoridae | Yes | No |
| Antrodiaetidae | Yes | Yes |
| Anura | Yes | Yes |
| Aphelenchoides | Yes | Yes |
| Aphelenchoididae | Yes | Yes |
| Aphididae | Yes | Yes |
| Apodemus | No | Yes |
| Arachnida | Yes | Yes |
| Araneae | Yes | Yes |
| Araneidae | Yes | Yes |
| Archaeidae | No | Yes |
| Arthropoda | Yes | Yes |
| Ascomycota | Yes | Yes |
| Aspergillus tubingensis | Yes | No |
| Atemnidae | No | Yes |
| Atomaria | No | Yes |
| Aulodrilus | No | Yes |
| Aves | Yes | No |
| Bacillariaceae | Yes | Yes |
| Bacillariophyta | Yes | Yes |
| Basidiomycota | Yes | Yes |
| Bdelloidea | Yes | Yes |
| Bellamya | No | Yes |
| Biomphalaria glabrata | Yes | Yes |
| Bivalvia | Yes | Yes |
| Blenniidae | No | Yes |
| Boletaceae | No | Yes |
| Bos taurus | Yes | Yes |
| Bosmina | Yes | Yes |
| Bourletiella | No | Yes |
| Bovidae | Yes | Yes |
| Bracon | Yes | Yes |
| Braconidae | Yes | Yes |
| Branchiodrilus | Yes | Yes |
| Branchiopoda | Yes | Yes |
| Bulinus natalensis | Yes | Yes |
| Buthidae | Yes | Yes |
| Calliphoridae | Yes | No |
| Camisiidae | No | Yes |
| Caponiidae | No | Yes |
| Carabidae | Yes | Yes |
| Caraboctonidae | Yes | Yes |
| Cecidomyiidae | Yes | Yes |
| Cerambycidae | No | Yes |
| Ceratopogonidae | Yes | Yes |
| Chaetocerotales | No | Yes |
| Chaetonotida | Yes | Yes |
| Chaoboridae | No | Yes |
| Cheiracanthium | Yes | Yes |
| Cheyletus | Yes | Yes |
| Cheyletus malaccensis | Yes | No |
| Chironomidae | Yes | Yes |
| Chlaenius | No | Yes |
| Chlorophyta | Yes | Yes |
| Chloropidae | Yes | Yes |
| Chordata | Yes | Yes |
| Chromadorea | Yes | Yes |
| Chrysomelidae | Yes | Yes |
| Chrysomya rufifacies | No | Yes |
| Chrysoperla | Yes | Yes |
| Chrysophyceae | Yes | Yes |
| Chydoridae | No | Yes |
| Cicadellidae | No | Yes |
| Cicadidae | No | Yes |
| Ciliophora | Yes | Yes |
| Clarias | Yes | Yes |
| Clitellata | Yes | Yes |
| Clubionidae | No | Yes |
| Cochliopodium | Yes | Yes |
| Coleoptera | Yes | Yes |
| Colletidae | No | Yes |
| Columella | No | Yes |
| Conioscinella | No | Yes |
| Contracaecum | No | Yes |
| Corinnidae | No | Yes |
| Corydalidae | Yes | No |
| Cotesia | Yes | Yes |
| Cottus | No | Yes |
| Crabronidae | Yes | Yes |
| Crambidae | Yes | Yes |
| Cricotopus | Yes | No |
| Cryptophyceae | Yes | Yes |
| Culex | No | Yes |
| Culicidae | Yes | Yes |
| Cybaeus | Yes | No |
| Cyclopidae | Yes | Yes |
| Cyclopoida | Yes | Yes |
| Cypriniformes | No | Yes |
| Daphnia | No | Yes |
| Daphniidae | Yes | Yes |
| Decapoda | Yes | Yes |
| Dictyuchus | No | Yes |
| Dinotrema | No | Yes |
| Diplopoda | Yes | Yes |
| Diptera | Yes | Yes |
| Discosea | Yes | Yes |
| Dolichopodidae | Yes | No |
| Dorylaimida | Yes | Yes |
| Drosophila | Yes | Yes |
| Drosophila hydei | Yes | Yes |
| Drosophilidae | Yes | Yes |
| Elateridae | No | Yes |
| Ellobiidae | No | Yes |
| Ellobium | No | Yes |
| Empoasca | No | Yes |
| Endomychidae | Yes | No |
| Enoplea | Yes | No |
| Enoplognatha | No | Yes |
| Entomobrya | No | Yes |
| Entomobryomorpha | Yes | Yes |
| Erigone | No | Yes |
| Eulipotyphla | No | Yes |
| Eunotiales | No | Yes |
| Euonychophora | No | Yes |
| Fannia canicularis | Yes | No |
| Folsomia | Yes | Yes |
| Formicidae | No | Yes |
| Galaxiidae | No | Yes |
| Gastropoda | Yes | Yes |
| Gastrotricha | Yes | Yes |
| Geckolepis | No | Yes |
| Gekkonidae | Yes | Yes |
| Geometridae | No | Yes |
| Geomitridae | Yes | Yes |
| Glomerida | No | Yes |
| Glyptapanteles | No | Yes |
| Gnaphosidae | Yes | Yes |
| Gyponana | No | Yes |
| Haplotaxida | Yes | Yes |
| Helobdella | Yes | Yes |
| Hemiptera | Yes | Yes |
| Heptageniidae | No | Yes |
| Heterolobosea | Yes | Yes |
| Heteronemertea | Yes | Yes |
| Himatismenida | Yes | Yes |
| Hirudinida | Yes | Yes |
| Homo sapiens | Yes | Yes |
| Hydropsychidae | No | Yes |
| Hydrozetes | No | Yes |
| Hydrozoa | Yes | Yes |
| Hymenoptera | Yes | Yes |
| Insecta | Yes | Yes |
| Isotomidae | Yes | Yes |
| Ixodidae | Yes | Yes |
| Laelapidae | No | Yes |
| Lampyridae | No | Yes |
| Lasiocampidae | No | Yes |
| Lauriidae | No | Yes |
| Lepidoptera | Yes | Yes |
| Leptonetidae | Yes | Yes |
| Linyphiidae | Yes | Yes |
| Longitarsus | No | Yes |
| Luciola | No | Yes |
| Lumbricidae | Yes | No |
| Lutzomyia | No | Yes |
| Lycosidae | Yes | Yes |
| Lygaeidae | Yes | No |
| Lymnaea | No | Yes |
| Lymnaeidae | Yes | Yes |
| Lysianassidae | Yes | Yes |
| Lysiphlebus | Yes | No |
| Macrocentrus | No | Yes |
| Magnoliopsida | Yes | No |
| Maiestas | No | Yes |
| Malacostraca | Yes | Yes |
| Mammalia | Yes | Yes |
| Mecoptera | No | Yes |
| Mesostigmata | Yes | Yes |
| Micropholcommatidae | Yes | No |
| Miridae | Yes | No |
| Moinidae | Yes | Yes |
| Mollusca | Yes | Yes |
| Monogononta | Yes | Yes |
| Mucorales | Yes | Yes |
| Mucoromycota | Yes | Yes |
| Muridae | Yes | Yes |
| Mycetophilidae | Yes | Yes |
| Mycomya | Yes | Yes |
| Naididae | Yes | Yes |
| Naviculales | Yes | Yes |
| Nematoda | Yes | Yes |
| Nemertea | Yes | Yes |
| Nemesiidae | Yes | Yes |
| Neogastropoda | No | Yes |
| Neuroptera | Yes | Yes |
| Nymphalidae | Yes | Yes |
| Oedemera | No | Yes |
| Oomycota | Yes | Yes |
| Opiliones | No | Yes |
| Opistophthalmus boehmi | No | Yes |
| Oppiella | No | Yes |
| Oppiidae | No | Yes |
| Oroperipatus | No | Yes |
| Orthetrum | No | Yes |
| Orthocladius | No | Yes |
| Ostracoda | Yes | Yes |
| Ototyphlonemertes | No | Yes |
| Oxyopes | No | Yes |
| Parabathynellidae | Yes | Yes |
| Paracalliopiidae | No | Yes |
| Paramecium | No | Yes |
| Paraphaenocladius | No | Yes |
| Paratanytarsus | Yes | Yes |
| Perciformes | Yes | Yes |
| Peronosporales | Yes | Yes |
| Philodromus | Yes | Yes |
| Philopotamidae | Yes | Yes |
| Philotrypesis | No | Yes |
| Phyllodocida | Yes | Yes |
| Phytomyza | No | Yes |
| Pinnularia | Yes | Yes |
| Planorbidae | Yes | Yes |
| Plantago | Yes | Yes |
| Platyhelminthes | No | Yes |
| Ploima | Yes | Yes |
| Podocopida | Yes | Yes |
| Poduromorpha | No | Yes |
| Polychaeta | Yes | Yes |
| Polydesmida | Yes | Yes |
| Polygonaceae | Yes | No |
| Polyxenidae | No | Yes |
| Proctophyllodidae | Yes | No |
| Pseudomallada | No | Yes |
| Pseudopoda | No | Yes |
| Psyllidae | Yes | No |
| Pteromalidae | No | Yes |
| Ptiliidae | No | Yes |
| Pythiales | Yes | Yes |
| Rattus | No | Yes |
| Rattus norvegicus | Yes | Yes |
| Resseliella | Yes | No |
| Rhabdias | Yes | Yes |
| Rhabditida | Yes | Yes |
| Rhizoglyphus | No | Yes |
| Rhodophyta | Yes | Yes |
| Rotifera | Yes | Yes |
| Salix | No | Yes |
| Salticidae | Yes | Yes |
| Saprolegniaceae | Yes | Yes |
| Sarcoptiformes | Yes | Yes |
| Scarabaeidae | No | Yes |
| Scatopsidae | No | Yes |
| Schizomida | No | Yes |
| Sciaridae | No | Yes |
| Scincidae | No | Yes |
| Scolopendromorpha | No | Yes |
| Scorpiones | Yes | Yes |
| Selenopidae | Yes | Yes |
| Selenops | Yes | Yes |
| Sergiolus | Yes | No |
| Silphidae | Yes | Yes |
| Simulium | Yes | Yes |
| Streblidae | No | Yes |
| Strongylida | Yes | Yes |
| Synurophyceae | Yes | Yes |
| Tabanidae | No | Yes |
| Tachinidae | Yes | Yes |
| Tardigrada | No | Yes |
| Tectocepheus | No | Yes |
| Tenebrionidae | Yes | No |
| Tenthredinidae | Yes | Yes |
| Tetragnatha | Yes | Yes |
| Tetragnathidae | Yes | Yes |
| Theridiidae | Yes | Yes |
| Thomisidae | No | Yes |
| Tipulidae | No | Yes |
| Tomoceridae | No | Yes |
| Torrenticolidae | No | Yes |
| Trichoceridae | No | Yes |
| Trombidiformes | Yes | Yes |
| Tuberolachnus | Yes | Yes |
| Tuberolachnus salignus | Yes | No |
| Tubulinea | Yes | Yes |
| Uropygi | No | Yes |
| Veneroida | Yes | Yes |
| Xyelidae | Yes | No |
| Xystodesmidae | No | Yes |
References
- Gong, C.; Chen, J.; Yu, S. Biotic homogenization and differentiation of the flora in artificial and near-natural habitats across urban green spaces. Landsc. Urban. Plan. 2013, 120, 158–169. [Google Scholar] [CrossRef]
- Mckinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Czech, B.; Krausman, P.R.; Devers, P.K. Economic associations among causes of species endangerment in the United States. Bioscience 2000, 50, 593–601. [Google Scholar] [CrossRef]
- Dale, S. Urban bird community composition influenced by size of urban green spaces, presence of native forest, and urbanization. Urban. Ecosyst. 2018, 1–14. [Google Scholar] [CrossRef]
- Angold, P.G.; Sadler, J.P.; Hill, M.O.; Pullin, A.; Rushton, S.; Austin, K.; Small, E.; Wood, B.; Wadsworth, R.; Sanderson, R.; et al. Biodiversity in urban habitat patches. Sci. Total Environ. 2006, 360, 196–204. [Google Scholar] [CrossRef]
- Goddard, M.A.; Dougill, A.J.; Benton, T.G. Scaling up from gardens: Biodiversity conservation in urban environments. Trends Ecol. Evol. 2009, 25, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Alvey, A.A. Promoting and preserving biodiversity in the urban forest. Urban. For. Urban. Green. 2006, 5, 195–201. [Google Scholar] [CrossRef]
- Ditchkoff, S.S.; Saalfeld, S.T.; Gibson, C.J. Animal behavior in urban ecosystems: Modifications due to human-induced stress. Urban. Ecosyst. 2006, 9, 5–12. [Google Scholar] [CrossRef]
- Hindmarch, S.; Elliott, J.E. A specialist in the city: The diet of barn owls along a rural to urban gradient. Urban. Ecosyst. 2015, 18, 477–488. [Google Scholar] [CrossRef]
- Baxter-Gilbert, J.H.; Whiting, M.J. Street fighters: Bite force, injury rates, and density of urban Australian water dragons (Intellagama lesueurii). Austral. Ecol. 2018, 44, 1–10. [Google Scholar] [CrossRef]
- Luniak, M. Synurbization—Adaptation of animal wildlife to urban development. In Proceedings of the 4th International Urban Wildlife Symposium, Tucson, AZ, USA, 1–5 May 1999; pp. 50–55. [Google Scholar]
- Ives, C.D.; Lentini, P.E.; Threlfall, C.G.; Ikin, K.; Shanahan, D.F.; Garrard, G.E.; Bekessy, S.A.; Fuller, R.A.; Mumaw, L.; Rayner, L.; et al. Cities are hotspots for threatened species. Glob. Ecol. Biogeogr. 2016, 25, 117–126. [Google Scholar] [CrossRef]
- Satterthwaite, D. The impact of urban development on risk in sub-Saharan Africa’s cities with a focus on small and intermediate urban centres. Int. J. Disaster Risk Reduct. 2017, 26, 16–23. [Google Scholar] [CrossRef]
- Mucina, L.; Rutherford, M.C. The Vegetation of South Africa, Lesotho and Swaziland; South African National Biodiversity Institute: Pretoria, South Africa, 2006. [Google Scholar]
- JCP. Birds flock to Delta Park. Available online: https://www.jhbcityparks.com/index.php/news-mainmenu-56/725-birds-flock-to-delta-park (accessed on 19 June 2019).
- Lockwood, G. Florence Bloom Bird Sanctuary. Available online: http://deltaenviro.org.za/florence-bloom-bird-sanctuary/ (accessed on 3 December 2019).
- Brunialti, G.; Giordani, P.; Ferretti, M. Discriminating between the good and the bad: Quality assurance is central in biomonitoring studies. In Environmental Monitoring; Wiersma, B., Ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 443–464. [Google Scholar]
- Thomsen, P.F.; Willerslev, E. Environmental DNA—An emerging tool in conservation for monitoring past and present biodiversity. Biol. Conserv. 2015, 183, 4–18. [Google Scholar] [CrossRef]
- Taberlet, P.; Coissac, E.; Pompanon, F.; Brochmann, C.; Willerslev, E. Towards next-generation biodiversity assessment using DNA metabarcoding. Mol. Ecol. 2012, 21, 2045–2050. [Google Scholar] [CrossRef] [PubMed]
- Bonk, M.; Bobrek, R.; Dołêga, J.; Strużyński, W. Evaluation of visual encounter surveys of the noble crayfish, Astacus astacus, and the spiny-cheek crayfish, Orconectes limosus. Fish. Aquat. Life 2019, 27, 112–117. [Google Scholar] [CrossRef]
- Lefort, M.C.; Cruickshank, R.H.; Descovich, K.; Adams, N.J.; Barun, A.; Emami-Khoyi, A.; Ridden, J.; Smith, V.R.; Sprague, R.; Waterhouse, B.R.; et al. Blood, sweat and tears: A review of non-invasive DNA sampling. bioRxiv 2019. [Google Scholar] [CrossRef]
- Klymus, K.E.; Marshall, N.T.; Stepien, C.A. Environmental DNA (eDNA) metabarcoding assays to detect invasive invertebrate species in the Great Lakes. PLoS ONE 2017, 12, e0177643. [Google Scholar] [CrossRef]
- Bohmann, K.; Evans, A.; Gilbert, M.T.P.; Carvalho, G.R.; Creer, S.; Knapp, M.; Yu, D.W.; de Bruyn, M. Environmental DNA for wildlife biology and biodiversity monitoring. Trends Ecol. Evol. 2014, 29, 358–367. [Google Scholar] [CrossRef]
- Elbrecht, V.; Leese, F. Can DNA-based ecosystem assessments quantify species abundance? Testing primer bias and biomass—Sequence relationships with an innovative metabarcoding protocol. PLoS ONE 2015, 10, e0130124. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Mohammadi, S.; Lutermann, H.; Hoffmann, S.; Emami-Khoyi, A.; Webster, H.J.; Fagir, D.; Bennett, N.C.; Jansen van Vuuren, B. Morphological and molecular characterization of the plague vector Xenopsylla brasiliensis. J. Parasitol. 2020, accepted. [Google Scholar]
- Porco, D.; Bedos, A.; Greenslade, P.; Janion, C.; Skarżyński, D.; Stevens, M.I.; Jansen van Vuuren, B.; Deharveng, L. Challenging species delimitation in Collembola: Cryptic diversity among common springtails unveiled by DNA barcoding. Invertebr. Syst. 2012, 26, 470–477. [Google Scholar] [CrossRef]
- Pieterse, W.; Muller, D.L.; Jansen van Vuuren, B. A molecular identification approach for five species of mealybug (Hemiptera: Pseudococcidae) on citrus fruit exported from South Africa. Afr. Entomol. 2010, 18, 23–28. [Google Scholar] [CrossRef]
- Chown, S.L.; Sinclair, B.J.; Jansen van Vuuren, B. DNA barcoding and the documentation of alien species establishment on sub-Antarctic Marion Island. Polar Biol. 2008, 31, 651–655. [Google Scholar] [CrossRef]
- Voua Otomo, P.; Jansen Van Vuuren, B.; Reinecke, S.A. Usefulness of DNA barcoding in ecotoxicological investigations: Resolving taxonomic uncertainties using Eisenia Malm 1877 as an example. Bull. Environ. Contam. Toxicol. 2009, 82, 261–264. [Google Scholar] [CrossRef]
- Karim, A.; Iqbal, A.; Akhtar, R.; Rizwan, M.; Amar, A.; Qamar, U.; Jahan, S. Barcoding of fresh water fishes from Pakistan. Mitochondrial DNA Part A 2016, 27, 2685–2688. [Google Scholar] [CrossRef]
- Macheriotou, L.; Guilini, K.; Bezerra, T.N.; Tytat, B.; Nguyen, D.T.; Nguyen, T.X.P.; Noppe, F.; Armenteros, M.; Boufahja, F.; Rigaux, A.; et al. Metabarcoding free-living marine nematodes using curated 18S and CO1 reference sequence databases for species-level taxonomic assignments. Ecol. Evol. 2018, 9, 1211–1226. [Google Scholar] [CrossRef]
- Dopheide, A.; Tooman, L.K.; Grosser, S.; Agabiti, B.; Rhode, B.; Xie, D.; Stevens, M.I.; Nelson, N.; Buckley, T.R.; Drummond, A.J.; et al. Estimating the biodiversity of terrestrial invertebrates on a forested island using DNA barcodes and metabarcoding data. Ecol. Appl. 2019, 29, e01877. [Google Scholar] [CrossRef] [PubMed]
- Jerde, C.L.; Mahon, A.R.; Chadderton, W.L.; Lodge, D.M. “Sight-unseen” detection of rare aquatic species using environmental DNA. Conserv. Lett. 2011, 4, 150–157. [Google Scholar] [CrossRef]
- Dejean, T.; Valentini, A.; Miquel, C.; Taberlet, P.; Bellemain, E.; Miaud, C. Improved detection of an alien invasive species through environmental DNA barcoding: The example of the American bullfrog Lithobates catesbeianus. J. Appl. Ecol. 2012, 49, 953–959. [Google Scholar] [CrossRef]
- Turner, C.R.; Uy, K.L.; Everhart, R.C. Fish environmental DNA is more concentrated in aquatic sediments than surface water. Biol. Conserv. 2015, 183, 93–102. [Google Scholar] [CrossRef]
- Andruszkiewicz, E.A.; Starks, H.A.; Chavez, F.P.; Sassoubre, L.M.; Block, B.A.; Boehm, A.B. Biomonitoring of marine vertebrates in Monterey Bay using eDNA metabarcoding. PLoS ONE 2017, 12, e0176343. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Amaral-Zettler, L.A.; McCliment, E.A.; Ducklow, H.W.; Huse, S.M. A method for studying protistan diversity using massively parallel sequencing of V9 hypervariable regions of small-subunit ribosomal RNA genes. PLoS ONE 2009, 4, e6372. [Google Scholar] [CrossRef]
- Leray, M.; Yang, J.Y.; Meyer, C.P.; Mills, S.C.; Agudelo, N.; Ranwez, V.; Boehm, J.T.; Machida, R.J. A new versatile primer set targeting a short fragment of the mitochondrial COI region for metabarcoding metazoan diversity: Application for characterizing coral reef fish gut contents. Front. Zool. 2013, 10, 1–14. [Google Scholar] [CrossRef]
- Geller, J.; Meyer, C.; Parker, M.; Hawk, H. Redesign of PCR primers for mitochondrial cytochrome c oxidase subunit I for marine invertebrates and application in all-taxa biotic surveys. Mol. Ecol. Resour. 2013, 13, 851–861. [Google Scholar] [CrossRef]
- Hajibabaei, M.; McKenna, C. DNA Mini-barcodes. In DNA Barcodes. Methods in Molecular Biology (Methods and Protocols); Kress, W., Erickson, D., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 858. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for Higher Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/%0Afastqc (accessed on 13 April 2020).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. BMC Inform. 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.-M.; Luo, R.; Sadakane, K.; Lam, T. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2013, 9, 357–359. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. Subgroup, 1000 Genome Project Data Processing Subgroup The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, 1–22. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, W.; Zhang, Y.; Xu, Y. High speed BLASTN: An accelerated MegaBLAST search tool. Nucleic Acids Res. 2015, 43, 7762–7768. [Google Scholar] [CrossRef]
- Kahlke, T.; Ralph, P.J. BASTA—Taxonomic classification of sequences and sequence bins using last common ancestor estimations. Methods Ecol. Evol. 2018, 10, 100–103. [Google Scholar] [CrossRef]
- Letunic, I. phyloT: Phylogenetic Tree Generator. Available online: https://phylot.biobyte.de/ (accessed on 4 March 2020).
- Bouckaert, R.; Heled, J.; Kühnert, D.; Vaughan, T.; Wu, C.-H.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS ONE 2014, 10, e1003537. [Google Scholar] [CrossRef]
- FASTX-Toolkit: FASTQ/a Short-Reads Pre-Processing Tools. Available online: http://hannonlab.cshl.edu/fastx_toolkit/ (accessed on 23 October 2020).
- Keylock, C.J. Simpson diversity and the Shannon-Wiener index as special cases of a generalized entropy. Oikos 2005, 109, 203–208. [Google Scholar] [CrossRef]
- Jost, L. The relation between evenness and diversity. Diversity 2010, 2, 207–232. [Google Scholar] [CrossRef]
- Guevara, M.R.; Hartmann, D.; Mendoza, M. Diverse: An r package to analyze diversity in complex systems. R J. 2016, 8, 60–78. [Google Scholar] [CrossRef]
- Dearborn, D.C.; Kark, S. Motivations for conserving urban biodiversity. Conserv. Biol. 2009, 24, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.E.; Samways, M.J. Sampling arthropod diversity for urban ecological landscaping in a species-rich southern hemisphere botanic garden. J. Insect Conserv. 1997, 1, 221–234. [Google Scholar] [CrossRef]
- Majumder, J.; Das, R.K.; Majumder, P.; Ghosh, D.; Agarwala, B.K. Aquatic insect fauna and diversity in urban fresh water lakes of Tripura, northeast India. Middle East. J. Sci. Res. 2013, 13, 25–32. [Google Scholar] [CrossRef]
- Lotz, L.N. A new species of Afrarchaea (Araneae: Archaeidae) from South Africa. Afr. Invertebr. 2015, 56, 409–414. [Google Scholar] [CrossRef]
- Wood, H.M.; Griswold, C.E.; Gillespie, R.G. Phylogenetic placement of pelian spider (Archaeidae, Araneae), with insight into evoluation of the “neck” and predatory behaviours of the superfamily Palpimanoidea. Cladistics 2012, 1–29. [Google Scholar] [CrossRef]
- Blair, R.B. Land use and avian species diversity along an urban gradient. Ecol. Appl. 1996, 6, 506–519. [Google Scholar] [CrossRef]
- Francis, R.A.; Chadwick, M.A. What makes a species synurbic? Appl. Geogr. 2012, 32, 514–521. [Google Scholar] [CrossRef]
- Teixeira, B.; Hirsch, A.; Goulart, V.D.L.R.; Passos, L.; Teixeira, C.P.; James, P.; Young, R. Good neighbours: Distribution of black-tufted marmoset (Callithrix penicillata) in an urban environment. Wildl. Res. 2015, 42, 579–589. [Google Scholar] [CrossRef]
- Owens, A.C.S.; Cochard, P.; Durrant, J.; Farnworth, B.; Perkin, E.K.; Seymoure, B. Light pollution is a driver of insect declines. Biol. Conserv. 2020, 241, 108259. [Google Scholar] [CrossRef]
- Owens, A.C.S.; Meyer-Rochow, V.B.; Yang, E.-C. Short- and mid-wavelength artificial light influences the flash signals of Aquatica ficta fireflies (Coleoptera: Lampyridae). PLoS ONE 2018, 13, e0191576. [Google Scholar] [CrossRef]
- Firebaugh, A.; Haynes, K.J. Experimental tests of light-pollution impacts on nocturnal insect courtship and dispersal. Oecologia 2016, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- WHO Schistosomiasis. Available online: http://www.who.int/mediacentre/factsheets/fs115/en/ (accessed on 4 May 2020).
- CDC Parasites—Acanthamoeba—Granulomtous Amebix Encephalitis (GAE); Keratitis. Available online: https://www.cdc.gov/parasites/acanthamoeba/index.html (accessed on 4 May 2020).
- Seifert, K.A.; Samson, R.A.; deWaard, J.R.; Houbraken, J.; Lévesque, C.A.; Moncalvo, J.M.; Louis-Seize, G.; Hebert, P.D.N. Prospects for fungus identification using CO1 DNA barcodes, with Penicillium as a test case. Proc. Natl. Acad. Sci. USA 2007, 104, 3901–3906. [Google Scholar] [CrossRef]
- Derycke, S.; Vanaverbeke, J.; Rigaux, A.; Backeljau, T.; Moens, T. Exploring the use of cytochrome oxidase c subunit 1 (COI) for DNA barcoding of free-living marine nematodes. PLoS ONE 2010, 5, e13716. [Google Scholar] [CrossRef]
- Midgley, J.M.; Hill, M.P.; Villet, M.H. The effect of water hyacinth, Eichhornia crassipes (Martius) Solms-Laubach (Pontederiaceae), on benthic biodiversity in two impoundments on the New Year’s River, South Africa. Afr. J. Aquat. Sci. 2006, 31, 25–30. [Google Scholar] [CrossRef]
- Martins, K.P.; Bandeira, M.G.d.S.; Palma-Silva, C.; Albertoni, E.F. Microcrustacean metacommunities in urban temporary ponds. Aquat. Sci. 2019, 81, 1–12. [Google Scholar] [CrossRef]
- Pont, D.; Rocle, M.; Valentini, A.; Civade, R.; Jean, P.; Maire, A.; Roset, N.; Schabuss, M.; Zornig, H.; Dejean, T. Environmental DNA reveals quantitative patterns of fish biodiversity in large rivers despite its downstream transportation. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Hofreiter, M.; Mead, J.I.; Martin, P.; Poinar, H.N. Molecular caving. Curr. Biol. 2003, 13, 10–13. [Google Scholar] [CrossRef]
- Stoeckle, B.C.; Beggel, S.; Cerwenka, A.F.; Motivans, E.; Kuehn, R.; Geist, J. A systematic approach to evaluate the influence of environmental conditions on eDNA detection success in aquatic ecosystems. PLoS ONE 2017, 12, e0189119. [Google Scholar] [CrossRef]
- Belle, C.C.; Stoeckle, B.C.; Geist, J. Taxonomic and geographical representation of freshwater environmental DNA research in aquatic conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 1996–2009. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. Bold: The barcode of life data system (www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).