Effects of Low ω6:ω3 Ratio in Sow Diet and Seaweed Supplement in Piglet Diet on Performance, Colostrum and Milk Fatty Acid Profiles, and Oxidative Status
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Experimental Diets
2.3. Recording and Sampling
2.4. Colostrum and Milk Fatty Acid Analysis
2.5. Leptin and Indicators of Oxidative Status in Plasma
2.6. Statistical Analysis
3. Results
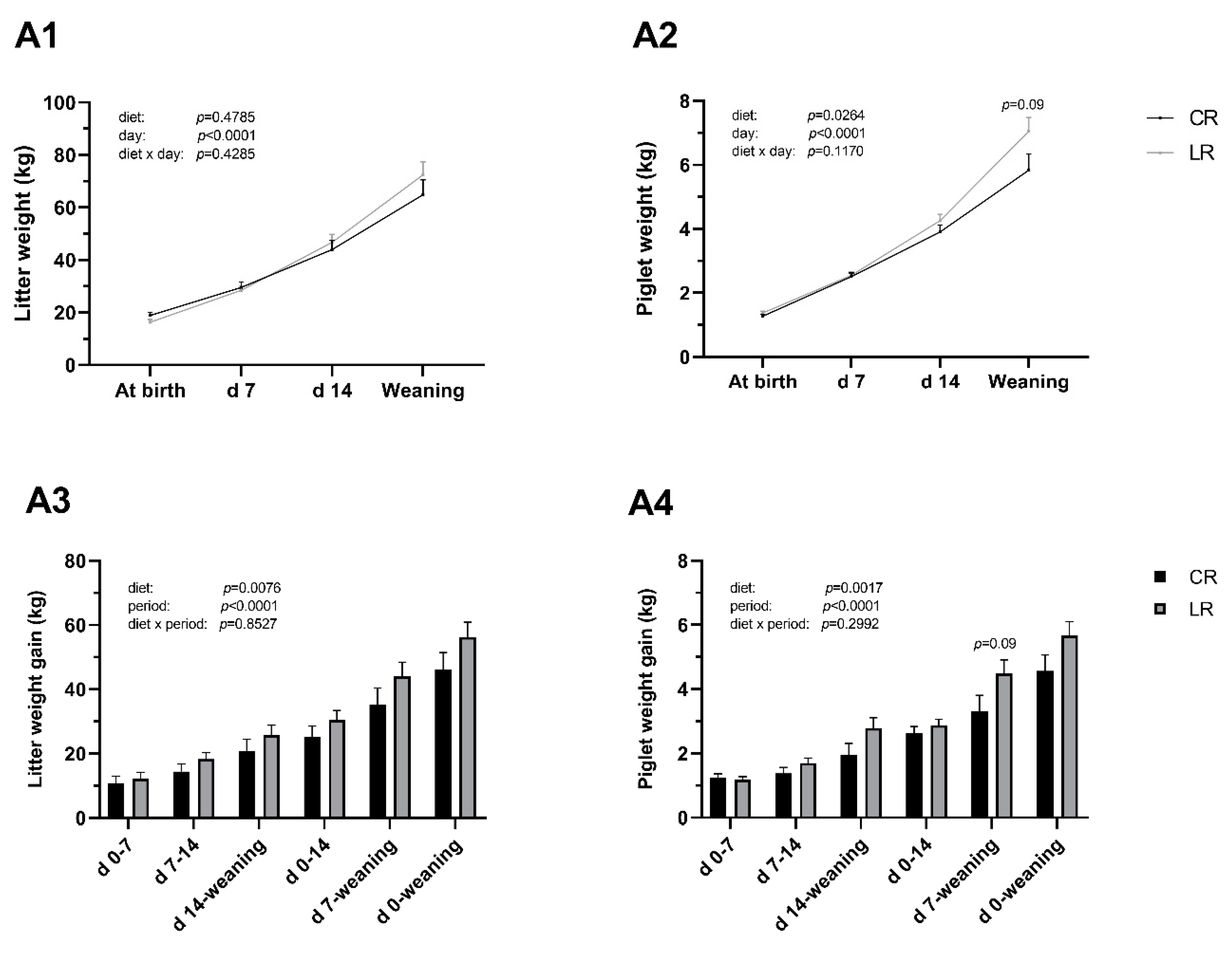
3.1. Sow Reproductive Performance
3.2. Growth Performance of Post-Weaning Piglets
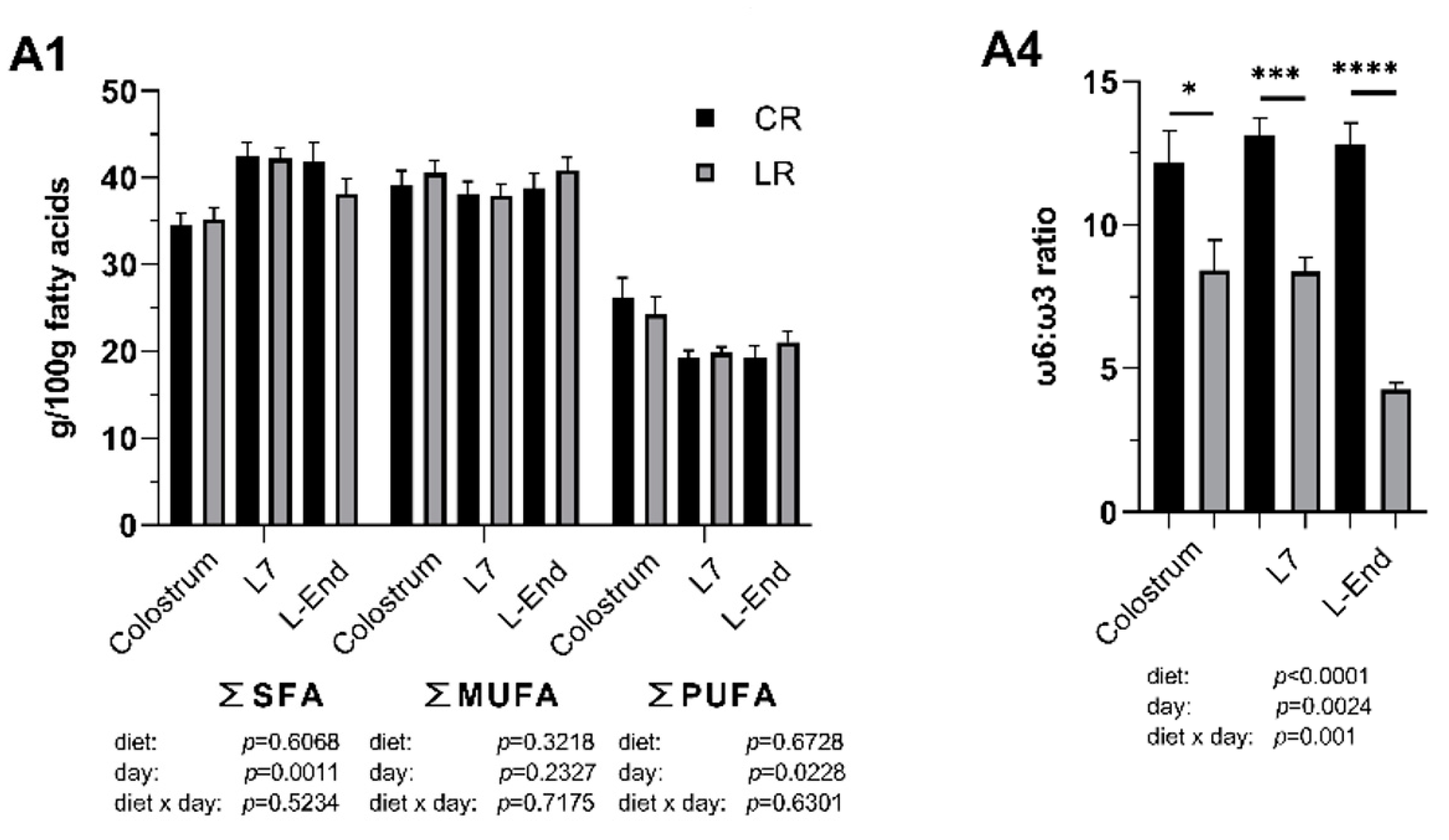
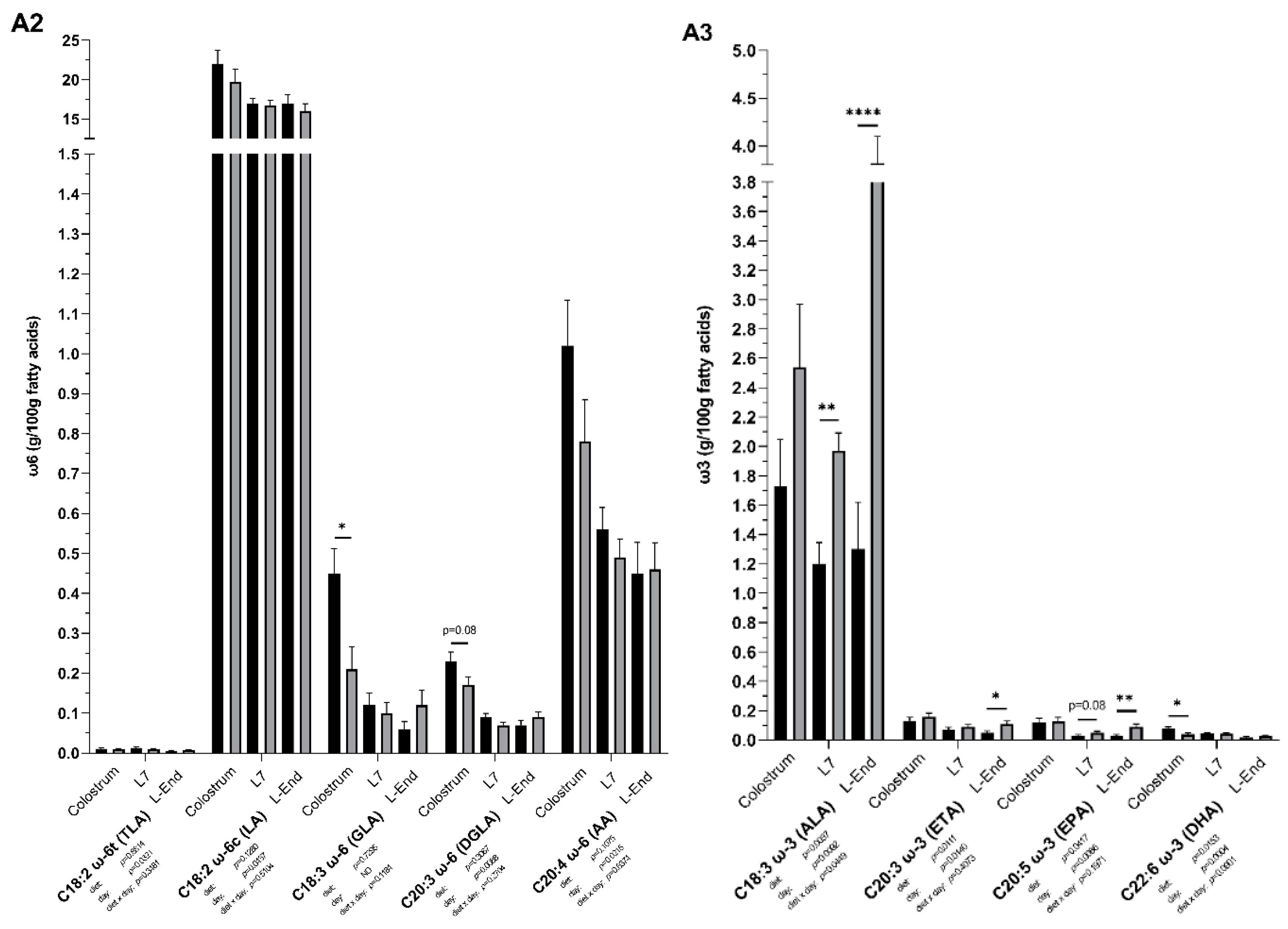
3.3. Fatty Acids Composition of Colostrum and Milk
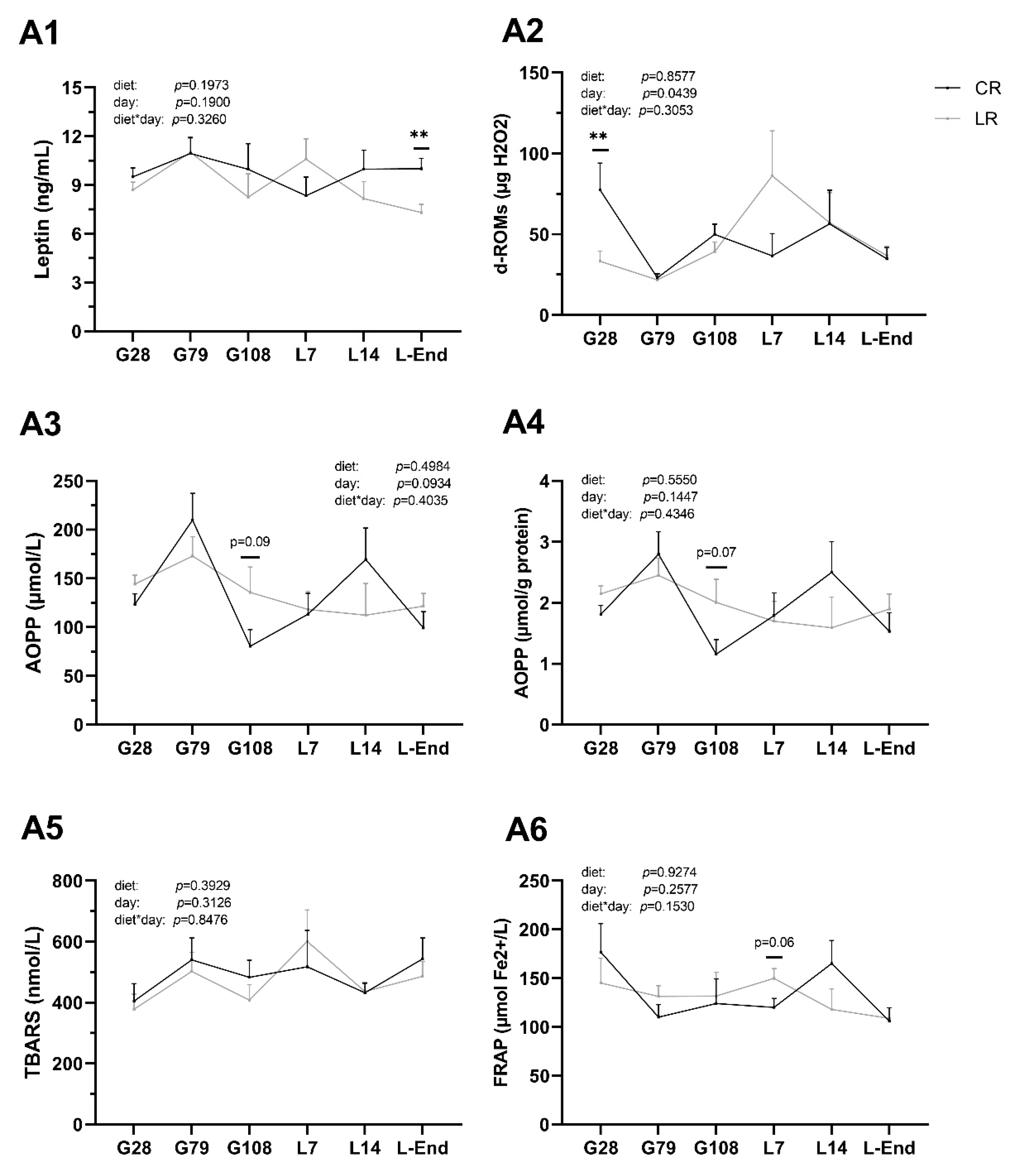
3.4. Leptin Concentrations and Oxidative Status in Sow Plasma
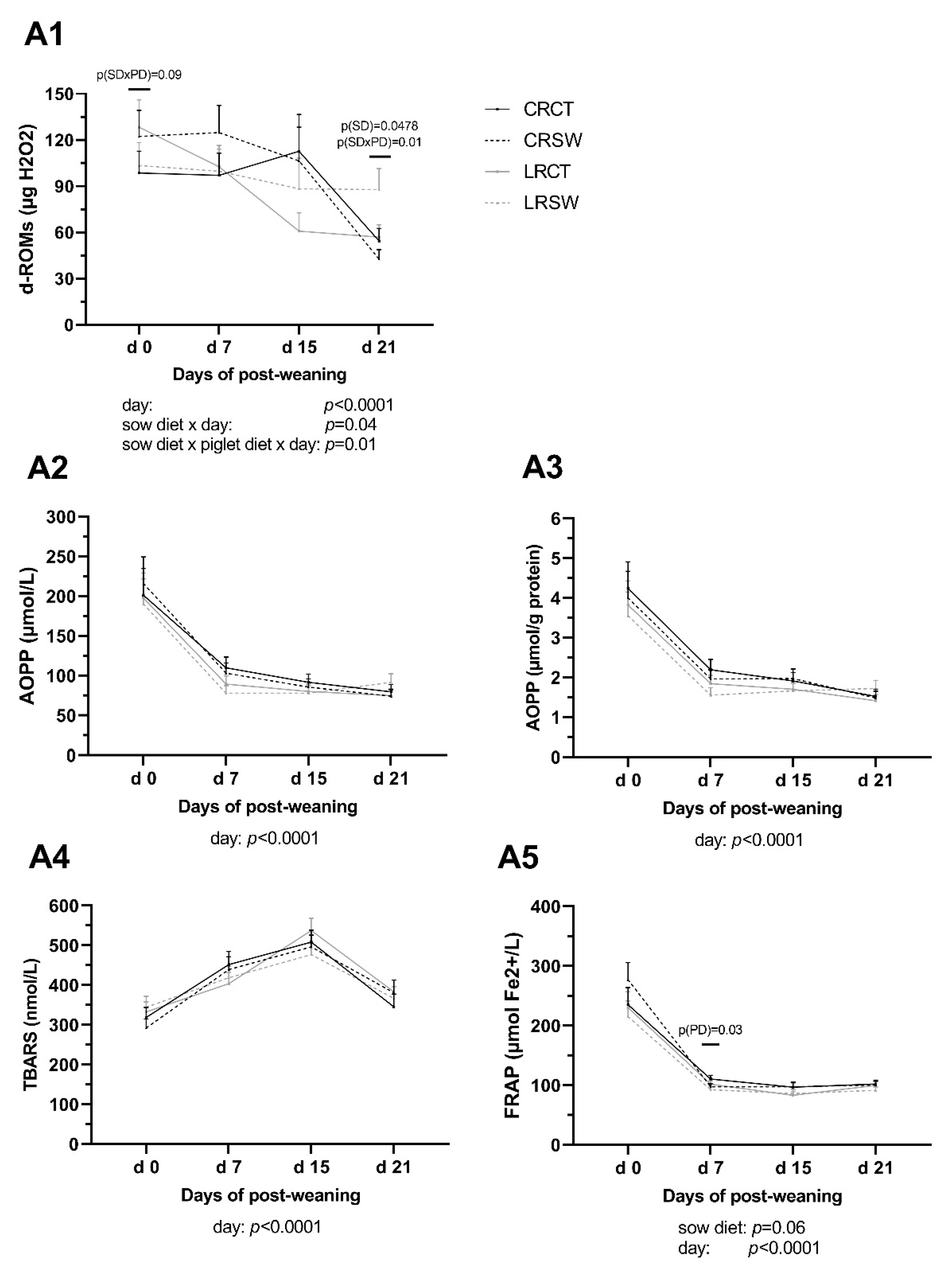
3.5. Oxidative Status in Plasma of Post-Weaning Piglets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yao, W.; Li, J.; Wang, J.; Zhou, W.; Wang, Q.; Zhu, R.; Wang, F.; Thacker, P. Effects of dietary ratio of n-6 to n-3 polyunsaturated fatty acids on immunoglobulins, cytokines, fatty acid composition, and performance of lactating sows and suckling piglets. J. Anim. Sci. Biotechnol. 2012, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- De Greeff, A.; Bikker, P.; Bruininx, E.; Zwolschen, H.; Fijten, H.; Zetteler, P. Increased fat and polyunsaturated fatty acid content in sow gestation diet has no effect on gene expression in progeny during the first 7 days of life. J. Anim. Physiol. Anim. Nutr. 2016, 100, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Lee, K.Y.; Kim, J.K.; Kim, I.H. Effects of different n-6 to n-3 polyunsaturated fatty acids ratio on reproductive performance, fecal microbiota and nutrient digestibility of gestation-lactating sows and suckling piglets. Anim. Sci. J. 2017, 88, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- McAfee, J.M.; Kattesh, H.G.; Lindemann, M.D.; Voy, B.H.; Kojima, C.J.; Burdick Sanchez, N.C.; Carroll, J.A.; Gillespie, B.E.; Saxton, A.M. Effect of omega-3 polyunsaturated fatty acid (n-3 PUFA) supplementation to lactating sows on growth and indicators of stress in the postweaned pig. J. Anim. Sci. 2019, 97, 4453–4463. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Hu, C.J.; Zhao, X.C.; Xiao, K.L.; Deng, M.; Zhang, L.; Qiu, X.G.; Deng, J.P.; Yin, Y.L.; Tan, C.Q. Dietary energy sources during late gestation and lactation of sows: Effects on performance, glucolipid metabolism, oxidative status of sows and their offspring. J. Anim. Sci. 2019, 97, 4608–4618. [Google Scholar] [CrossRef] [PubMed]
- Tanghe, S.; Missotten, J.; Raes, K.; De Smet, S. The effect of different concentrations of linseed oil or fish oil in the maternal diet on the fatty acid composition and oxidative status of sows and piglets. J. Anim. Physiol. Anim. Nutr. 2015, 99, 938–949. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An increase in the Omega-6/Omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 149. [Google Scholar] [CrossRef]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 2–5. [Google Scholar] [CrossRef]
- Ahasan, A.S.M.L.; Invernizzi, G.; Farina, G.; Pilotto, A.; Barbé, F.; Bontempo, V.; Rossi, R.; Bellagamba, F.; Lecchi, C.; Savoini, G.; et al. The effects of superoxide dismutase-rich melon pulp concentrate on inflammation, antioxidant status and growth performance of challenged post-weaning piglets. Animals 2019, 13, 136–143. [Google Scholar] [CrossRef]
- Xiong, X.; Tan, B.; Song, M.; Ji, P.; Kim, K.; Yin, Y.; Liu, Y. Nutritional intervention for the intestinal development and health of weaned pigs. Front. Vet. Sci. 2019, 6, 1–14. [Google Scholar] [CrossRef]
- Agazzi, A.; Perricone, V.; Zorini, F.O.; Sandrini, S.; Mariani, E.; Jiang, X.; Ferrari, A.; Crestani, M.; Nguyen, T.X.; Bontempo, V.; et al. Dietary Mannan Oligosaccharides Modulate Gut Inflammatory Response and Improve Duodenal Villi Height in Post-Weaning Piglets Improving Feed Efficiency. Animals 2020, 4, 1283. [Google Scholar] [CrossRef]
- Perricone, V.; Comi, M.; Bontempo, V.; Lecchi, C.; Ceciliani, F.; Crestani, M.; Ferrari, A.; Savoini, G.; Perricone, V.; Comi, M.; et al. Effects of nucleotides administration on growth performance and immune response of post- weaning piglets. Ital. J. Anim. Sci. 2020, 19, 295–301. [Google Scholar] [CrossRef]
- Jacobsen, C.; Sørensen, A.-D.M.; Holdt, S.L.; Akoh, C.C.; Hermund, D.B. Source, Extraction, Characterization, and Applications of Novel Antioxidants from Seaweed. Annu. Rev. Food Sci. Technol. 2019, 10, 541–568. [Google Scholar] [CrossRef] [PubMed]
- Ford, L.; Stratakos, A.C.; Theodoridou, K.; Dick, J.T.A.; Sheldrake, G.N.; Linton, M.; Corcionivoschi, N.; Walsh, P.J. Polyphenols from Brown Seaweeds as a Potential Antimicrobial Agent in Animal Feeds. ACS Omega 2020, 5, 9093–9103. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Turner, J.L.; Dritz, S.S.; Higgins, J.J.; Minton, J.E. Effects of Ascophyllum nodosum extract on growth performance and immune function of young pigs challenged with Salmonella typhimurium. J. Anim. Sci. 2002, 80, 1947–1953. [Google Scholar] [CrossRef]
- Michiels, J.; Skrivanova, E.; Missotten, J.; Ovyn, A.; Mrazek, J.; De Smet, S.; Dierick, N. Intact brown seaweed (Ascophyllum nodosum) in diets of weaned piglets: Effects on performance, gut bacteria and morphology and plasma oxidative status. J. Anim. Physiol. Anim. Nutr. 2012, 96, 1101–1111. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Swine, 11th ed.; The National Academies Press: Washington, DC, USA, 2012; ISBN 978-0-309-22423-9. [Google Scholar]
- Canadian Pork Council and the National Farm Animal Care Council. National Farm Animal Care Council Code of Practice: For the Care and Handling of Pigs; 2014; pp. 1–75. ISBN 978-0-993-61892-5. Available online: https://www.albertapork.com/wp-content/uploads/2017/06/pig_code_of_practice.pdf (accessed on 30 October 2018).
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Moltó-Puigmartí, C.; Castellote, A.I.; López-Sabater, M.C. Conjugated linoleic acid determination in human milk by fast-gas chromatography. Anal. Chim. Acta 2007, 602, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Sauerwein, H.; Heintges, U.; Hennies, M.; Selhorst, T.; Daxenberger, A. Growth hormone induced alterations of leptin serum concentrations in dairy cows as measured by a novel enzyme immunoassay. Livest. Prod. Sci. 2004, 87, 189–195. [Google Scholar] [CrossRef]
- Alberti, A.; Bolognini, L.; Macciantelli, D.; Caratelli, M. The radical cation of N,N-diethl-para-phenylendiamine: A possible indicator of oxidative stress in biological samples. Res. Chem. Intermed. 2000, 26, 253–267. [Google Scholar] [CrossRef]
- Regenhard, P.; Nakov, D.; Sauerwein, H. Applicability of a spectrophotometric method for assessment of oxidative stress in poultry. Maced. Vet. Rev. 2014, 37, 43–47. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C.; Quinlan, G.J. Malondialdehyde formation from lipid peroxides in the thiobarbituric acid test: The role of lipid radicals, iron salts, and metal chelators. J. Appl. Biochem. 1983, 5, 293–299. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Q.; Li, Y.; Tang, Z.; Sun, W.; Zhang, X.; Sun, J.; Sun, Z. Comparative effects of dietary supplementations with sodium butyrate, medium-chain fatty acids, and n-3 polyunsaturated fatty acids in late pregnancy and lactation on the reproductive performance of sows and growth performance of suckling piglets. J. Anim. Sci. 2019, 97, 4256–4267. [Google Scholar] [CrossRef]
- Cools, A.; Maes, D.; Papadopoulos, G.; Vandermeiren, J.A.; Meyer, E.; Demeyere, K.; De Smet, S.; Janssens, G.P.J. Dose-response effect of fish oil substitution in parturition feed on erythrocyte membrane characteristics and sow performance. J. Anim. Physiol. Anim. Nutr. 2011, 95, 125–136. [Google Scholar] [CrossRef]
- Lund, M.S.; Puonti, M.; Rydhmer, L.; Jensen, J. Relationship between litter size and perinatal and pre-weaning survival in pigs. Anim. Sci. 2002, 74, 217–222. [Google Scholar] [CrossRef]
- Dobrzański, J.; Sell-kubiak, E.; Knol, E.F. Estimation of litter size variability phenotypes in Large White sows. J. Anim. Breed. Genet. 2020, 137, 559–570. [Google Scholar] [CrossRef]
- Camargo, E.G.; Marques, D.B.D.; De Figueiredo, E.A.P.; Silva, F.F.; Lopes, P.S. Genetic study of litter size and litter uniformity in Landrace pigs. Rev. Bras. Zootec. 2020, 49, 1–12. [Google Scholar] [CrossRef]
- Van Ginneken, V.J.T.; Helsper, J.P.F.G.; De Visser, W.; Van Keulen, H.; Brandenburg, W.A. Polyunsaturated fatty acids in various macroalgal species from north Atlantic and tropical seas. Lipids Health Dis. 2011, 10, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Leikus, R.; Juskiene, V.; Juska, R.; Juodka, R.; Stankeviciene, D.; Nainiene, R.; Siukscius, A. Effect of linseed oil sediment in the diet of pigs on the growth performance and fatty acid profile of meat. Rev. Bras. Zootec. 2018, 47, 1–8. [Google Scholar] [CrossRef]
- Tanghe, S.; Millet, S.; Missotten, J.; Vlaeminck, B.; De Smet, S. Effects of birth weight and maternal dietary fat source on the fatty acid profile of piglet tissue. Animals 2014, 8, 1857–1866. [Google Scholar] [CrossRef]
- De Quelen, F.; Boudry, G.; Mourot, J. Linseed oil in the maternal diet increases long chain-PUFA status of the foetus and the newborn during the suckling period in pigs. Br. J. Nutr. 2010, 104, 533–543. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, H.; Wang, S.; Tu, Z.; Zhang, L.; Wang, X.; Hou, Y.; Wang, C.; Chen, J.; Liu, Y. Flaxseed Oil Attenuates Intestinal Damage and Inflammation by Regulating Necroptosis and TLR4/NOD Signaling Pathways Following Lipopolysaccharide Challenge in a Piglet Model. Mol. Nutr. Food Res. 2018, 62, 1–11. [Google Scholar] [CrossRef]
- Greupner, T.; Kutzner, L.; Nolte, F.; Strangmann, A.; Kohrs, H.; Hahn, A.; Schebb, N.H.; Schuchardt, J.P. Effects of a 12-week high-α-linolenic acid intervention on EPA and DHA concentrations in red blood cells and plasma oxylipin pattern in subjects with a low EPA and DHA status. Food Funct. 2018, 9, 1587–1600. [Google Scholar] [CrossRef]
- Burdge, G.C.; Calder, P.C. Conversion of α-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef]
- Plourde, M.; Cunnane, S.C. Extremely limited synthesis of long chain polyunsaturates in adults: Implications for their dietary essentiality and use as supplements. Appl. Physiol. Nutr. Metab. 2007, 32, 619–634. [Google Scholar] [CrossRef]
- Poumès-Ballihaut, C.; Langelier, B.; Houlier, F.; Alessandri, J.M.; Durand, G.; Latge, C.; Guesnet, P. Comparative bioavailability of dietary α-linolenic and docosahexaenoic acids in the growing rat. Lipids 2001, 36, 793–800. [Google Scholar] [CrossRef]
- Barceló-Coblijn, G.; Murphy, E.J. Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: Benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog. Lipid Res. 2009, 48, 355–374. [Google Scholar] [CrossRef]
- Greupner, T.; Kutzner, L.; Pagenkopf, S.; Kohrs, H.; Hahn, A.; Schebb, N.H.; Schuchardt, J.P. Effects of a low and a high dietary LA/ALA ratio on long-chain PUFA concentrations in red blood cells. Food Funct. 2018, 9, 4742–4754. [Google Scholar] [CrossRef]
- Gruzdeva, O.; Borodkina, D.; Uchasova, E.; Dyleva, Y.; Barbarash, O. Leptin resistance: Underlying mechanisms and diagnosis. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 191–198. [Google Scholar] [CrossRef]
- Fukumitsu, S.; Aida, K.; Ueno, N.; Ozawa, S.; Takahashi, Y.; Kobori, M. Flaxseed lignan attenuates high-fat diet-induced fat accumulation and induces adiponectin expression in mice. Br. J. Nutr. 2008, 100, 669–676. [Google Scholar] [CrossRef]
- Baranowski, M.; Enns, J.; Blewett, H.; Yakandawala, U.; Zahradka, P.; Taylor, C.G. Dietary flaxseed oil reduces adipocyte size, adipose monocyte chemoattractant protein-1 levels and T-cell infiltration in obese, insulin-resistant rats. Cytokine 2012, 59, 382–391. [Google Scholar] [CrossRef]
- Tekeleselassie, A.W.; Goh, Y.M.; Rajion, M.A.; Motshakeri, M.; Ebrahimi, M. A high-fat diet enriched with low omega-6 to omega-3 fatty acid ratio reduced fat cellularity and plasma leptin concentration in sprague-dawley rats. Sci. World J. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Ukropec, J.; Reseland, J.E.; Gasperikova, D.; Demcakova, E.; Madsen, L.; Berge, R.K.; Rustan, A.C.; Klimes, I.; Drevon, C.A.; Sebökova, E. The Hypotriglyceridemic Effect of Dietary n-3 FA Is Associated with Increased β-Oxidation and Reduced Leptin Expression. Lipids 2003, 38, 1023–1029. [Google Scholar] [CrossRef]
- Camkurt, M.A.; Findikli, E.; Tolun, F.I.; Bakacak, M.; Bal, N.G.; Sakalli, H.; Güneş, M. Probable preventive effects of placenta from oxidative stress; Evaluation of total antioxidant status, total oxidant status and oxidative stress index in fetal cord blood during the delivery. Psychiatry Res. 2016, 240, 222–225. [Google Scholar] [CrossRef]
- Celi, P.; Merlo, M.; Da Dalt, L.; Stefani, A.; Barbato, O.; Gabai, G. Relationship between late embryonic mortality and the increase in plasma advanced oxidised protein products (AOPP) in dairy cows. Reprod. Fertil. Dev. 2011, 23, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Lindblom, S.C. Impacts of Feeding Peroxidized Oils on Growth and Oxidative Status in Swine and Poultry. Master’s Thesis, Iwoa State University, Ames, IA, USA, 2017. [Google Scholar]
- Barba-Vidal, E.; Martín-Orué, S.M.; Castillejos, L. Review: Are we using probiotics correctly in post-weaning piglets? Animals 2018, 12, 2489–2498. [Google Scholar] [CrossRef]
- Sauerwein, H.; Schmitz, S.; Hiss, S. Effects of a dietary application of a yeast cell wall extract on innate and acquired immunity, on oxidative status and growth performance in weanling piglets and on the ileal epithelium in fattened pigs. J. Anim. Physiol. Anim. Nutr. 2007, 91, 369–380. [Google Scholar] [CrossRef]
- Zhu, L.H.; Zhao, K.L.; Chen, X.L.; Xu, J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 2012, 90, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Silva-Guillen, Y.V.; Arellano, C.; Boyd, R.D.; Martinez, G.; Van Heugten, E. Growth performance, oxidative stress and immune status of newly weaned pigs fed peroxidized lipids with or without supplemental vitamin e or polyphenols. J. Anim. Sci. Biotechnol. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 2016, 212, 1–17. [Google Scholar] [CrossRef]
- Lee, A.V.; You, L.; Oh, S.Y.; Li, Z.; Fisher-Heffernan, R.E.; Regnault, T.R.H.; de Lange, C.F.M.; Huber, L.; Karrow, N.A. Microalgae supplementation to late gestation sows and its effects on the health status of weaned piglets fed diets containing high- or low-quality protein sources. Vet. Immunol. Immunopathol. 2019, 218. [Google Scholar] [CrossRef]
- Bahar, B.; O’Doherty, J.V.; Smyth, T.J.; Sweeney, T. A comparison of the effects of an Ascophyllum nodosum ethanol extract and its molecular weight fractions on the inflammatory immune gene expression in-vitro and ex-vivo. Innov. Food Sci. Emerg. Technol. 2016, 37, 276–285. [Google Scholar] [CrossRef]
- Corona, G.; Ji, Y.; Anegboonlap, P.; Hotchkiss, S.; Gill, C.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Gastrointestinal modifications and bioavailability of brown seaweed phlorotannins and effects on inflammatory markers. Br. J. Nutr. 2016, 115, 1240–1253. [Google Scholar] [CrossRef]
- Dell’Anno, M.; Sotira, S.; Rebucci, R.; Reggi, S.; Castiglioni, B.; Rossi, L. In vitro evaluation of antimicrobial and antioxidant activities of algal extracts. Ital. J. Anim. Sci. 2020, 19, 103–113. [Google Scholar] [CrossRef]
- Leskovec, J.; Rezar, V.; Svete, A.N. Antioxidative effects of olive polyphenols compared to vitamin e in piglets fed a diet rich in n-3 pufa. Animals 2019, 9, 161. [Google Scholar] [CrossRef]
| Item | Gestation | Lactation |
|---|---|---|
| Ingredients (g/kg as fed basis) | ||
| Corn | 284.60 | 249.10 |
| Barley | 224.20 | 216.70 |
| Wheat bran | 208.00 | 115.80 |
| Distillers grains | 125.00 | 40.00 |
| Biscuit | 50.20 | 52.10 |
| Rice | 35.00 | 35.00 |
| Commercial concentrate * | 25.00 | 250.00 |
| Soybean oil | 12.90 | 14.00 |
| Fish meal | - | 14.50 |
| Mineral-vitamin premix ** | 20.00 | 11.70 |
| HCl-Lysine | 11.20 | 15.00 |
| Composition (% DM) | ||
| Crude protein | 15.85 | 19.92 |
| Crude fat | 4.55 | 4.93 |
| Crude fiber | 5.69 | 5.66 |
| Ash | 5.68 | 4.46 |
| Ca | 1.70 | 1.21 |
| P | 0.56 | 0.57 |
| Ca/P | 3.04 | 2.12 |
| Lysine | 1.04 | 1.34 |
| Methionine | 0.18 | 0.22 |
| Met +Cis | 0.37 | 0.50 |
| Item | Gestation CR | Gestation LR | Lactation CR | Lactation LR |
|---|---|---|---|---|
| 10:0 | 0.26 | 0.22 | - | - |
| 12:0 | 0.35 | 0.30 | 1.80 | 1.56 |
| 14:0 | 0.25 | 0.21 | - | - |
| 16:0 | 20.23 | 18.08 | 17.58 | 16.06 |
| 16:1 ω7 | 0.50 | 0.42 | - | - |
| 18:0 | 3.06 | 3.30 | 4.36 | 4.40 |
| 18:1 ω9 cis | 19.88 | 19.80 | 24.51 | 23.84 |
| 18:1 ω7 | 1.05 | 1.03 | - | 0.12 |
| 18:2 ω6 cis 9,12 | 49.79 | 44.73 | 46.92 | 42.90 |
| 18:3 ω3 | 3.72 | 11.12 | 4.83 | 11.12 |
| 20:0 | 0.28 | 0.24 | - | - |
| 20:1 ω9 | 0.41 | 0.35 | - | - |
| 22:0 | 0.23 | 0.20 | - | - |
| ω6 | 49.79 | 44.73 | 46.92 | 42.90 |
| ω3 | 3.72 | 11.12 | 4.83 | 11.12 |
| ω6:ω3 | 13.40 | 4.02 | 9.71 | 3.88 |
| Item | Post-Weaning Basal Diet |
|---|---|
| Ingredients (g/kg as fed basis) | |
| Barley | 220.0 |
| Wheat | 161.7 |
| Soy protein concentrate (Soicomil R) | 98.0 |
| Wheat, flaked | 80.0 |
| Corn | 80.0 |
| Corn, flaked | 60.0 |
| Soybean, meal | 59.0 |
| Biscuits | 50.0 |
| Whey | 50.0 |
| Dextrose, mono | 40.0 |
| Barley, flaked | 40.0 |
| Soybean oil | 20.0 |
| Dicalcium phosphate | 10.0 |
| Cocoa oil | 10.0 |
| L-Lysine | 6.0 |
| DL-Methionine | 3.6 |
| L-Threonine | 3.5 |
| Sodium chloride | 2.7 |
| Vitamin + trace elements | 2.5 |
| L-Valine (96.5%) | 2.2 |
| L-Tryptophan | 0.8 |
| Composition (%, DM) | |
| Dry matter (DM) | 89.60 |
| Crude protein | 20.10 |
| Crude fat | 5.68 |
| Fiber | 3.29 |
| Neutral detergent fiber (NDF) | 12.91 |
| Acid detergent fiber (ADF) | 4.67 |
| Acid detergent lignin (ADL) | 0.97 |
| Lysine (Lys), total | 1.57 |
| Cystine | 0.32 |
| Methionine (Met), total | 0.66 |
| Threonine, total | 1.09 |
| Tryptophan, total | 0.32 |
| Valine | 1.08 |
| Phenylalanine | 0.85 |
| Tyrosine | 0.55 |
| Isoleucine | 0.74 |
| Leucine | 0.32 |
| Net energy (NE, Mcal/kg) | 2.90 |
| Sow Diets (SD) Piglet Diets (PD) * | CR | LR | SEM 1 | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| CT | SW | CT | SW | SD | PD | SD × PD | ||
| No. of piglets ** | 10 | 10 | 10 | 10 | ||||
| BW 2 (kg) | ||||||||
| d 0 | 6.19 | 6.19 | 6.66 | 6.36 | 0.55 | ns 4 | ns | ns |
| d 7 | 6.86 | 7.21 | 7.58 | 6.89 | 0.59 | ns | ns | ns |
| d 15 | 9.56 | 10.72 | 10.62 | 9.32 | 0.98 | ns | ns | 0.018 |
| d 21 | 12.41 b | 14.14 a | 13.64 | 12.13 | 1.17 | ns | ns | 0.010 |
| ADG 2 (g/d) | ||||||||
| d 0 to 7 | 51.86 | 101.30 | 157.50 | 100.20 | 62.74 | ns | ns | ns |
| d 0 to 15 | 202.76 | 279.59 | 275.82 | 211.51 | 55.73 | ns | ns | 0.017 |
| d 0 to 21 | 276.15 b | 358.55 a | 345.63 | 289.71 | 52.20 | ns | ns | 0.013 |
| ADFI 2 (g/d) | ||||||||
| d 0 to 7 | 154.46 | 194.17 | 222.59 | 196.79 | 44.58 | ns | ns | ns |
| d 0 to 15 | 345.07 | 394.71 | 373.65 | 313.38 | 56.11 | ns | ns | ns |
| d 0 to 21 | 458.59 | 527.04 a | 474.17 a | 387.27 b | 55.81 | ns | ns | 0.009 |
| G:F 3 | ||||||||
| d 0 to 7 | 0.59 | 0.48 | 0.70 | 0.57 | 0.27 | ns | ns | ns |
| d 0 to 15 | 0.55 b | 0.75 a | 0.71 | 0.67 | 0.16 | ns | ns | 0.033 |
| d 0 to 21 | 0.62 | 0.73 | 0.70 | 0.70 | 0.08 | ns | ns | 0.050 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.X.; Agazzi, A.; Comi, M.; Bontempo, V.; Guido, I.; Panseri, S.; Sauerwein, H.; Eckersall, P.D.; Burchmore, R.; Savoini, G. Effects of Low ω6:ω3 Ratio in Sow Diet and Seaweed Supplement in Piglet Diet on Performance, Colostrum and Milk Fatty Acid Profiles, and Oxidative Status. Animals 2020, 10, 2049. https://doi.org/10.3390/ani10112049
Nguyen TX, Agazzi A, Comi M, Bontempo V, Guido I, Panseri S, Sauerwein H, Eckersall PD, Burchmore R, Savoini G. Effects of Low ω6:ω3 Ratio in Sow Diet and Seaweed Supplement in Piglet Diet on Performance, Colostrum and Milk Fatty Acid Profiles, and Oxidative Status. Animals. 2020; 10(11):2049. https://doi.org/10.3390/ani10112049
Chicago/Turabian StyleNguyen, Thi Xuan, Alessandro Agazzi, Marcello Comi, Valentino Bontempo, Invernizzi Guido, Sara Panseri, Helga Sauerwein, Peter David Eckersall, Richard Burchmore, and Giovanni Savoini. 2020. "Effects of Low ω6:ω3 Ratio in Sow Diet and Seaweed Supplement in Piglet Diet on Performance, Colostrum and Milk Fatty Acid Profiles, and Oxidative Status" Animals 10, no. 11: 2049. https://doi.org/10.3390/ani10112049
APA StyleNguyen, T. X., Agazzi, A., Comi, M., Bontempo, V., Guido, I., Panseri, S., Sauerwein, H., Eckersall, P. D., Burchmore, R., & Savoini, G. (2020). Effects of Low ω6:ω3 Ratio in Sow Diet and Seaweed Supplement in Piglet Diet on Performance, Colostrum and Milk Fatty Acid Profiles, and Oxidative Status. Animals, 10(11), 2049. https://doi.org/10.3390/ani10112049






