Comparative Analysis of Intestinal Helminth Infections in Colic and Non-Colic Control Equine Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participating Horses
2.2. Parasitological Examinations
2.3. Statistical Analyses
3. Results
3.1. Copromicroscopic Findings
3.2. Serological Findings
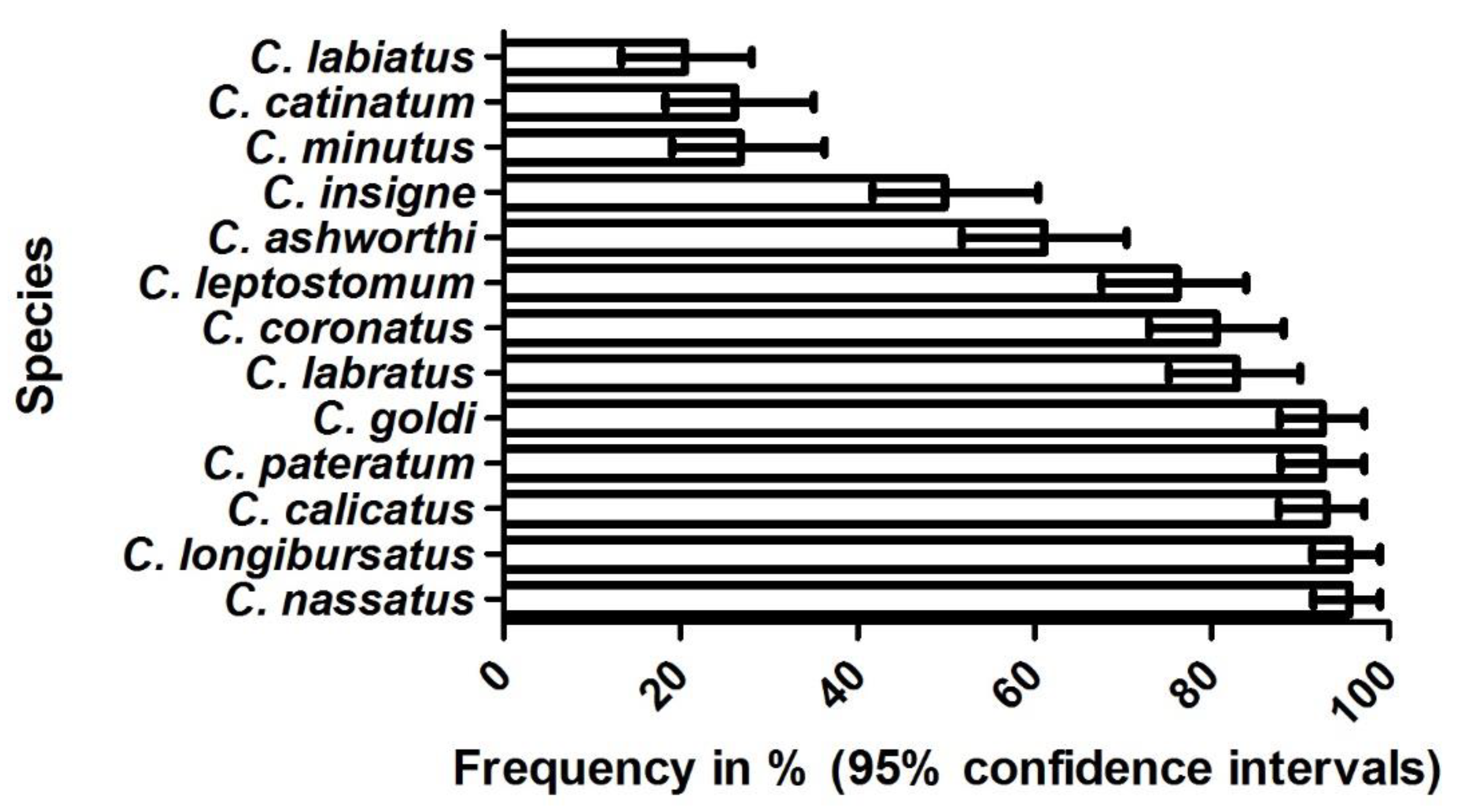
3.3. Molecular Findings
3.4. Clinical Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boswinkel, M.; Sloet van Oldruitenborgh-Oosterbaan, M.M. Correlation between colic and antibody levels against Anoplocephala perfoliata in horses in The Netherlands. Tijdschr. Diergeneeskd. 2007, 132, 508–512. [Google Scholar]
- Proudman, C.J.; French, N.P.; Trees, A.J. Tapeworm infection is a significant risk factor for spasmodic colic and ileal impaction colic in the horse. Equine Vet. J. 1998, 30, 194–199. [Google Scholar] [CrossRef]
- Nielsen, M.K.; Jacobsen, S.; Olsen, S.N.; Bousquet, E.; Pihl, T. Nonstrangulating intestinal infarction associated with Strongylus vulgaris in referred Danish equine cases. Equine Vet. J. 2016, 48, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Pihl, T.H.; Nielsen, M.; Olsen, S.N.; Leifsson, P.; Jacobsen, S. Nonstrangulating intestinal infarctions associated with Strongylus vulgaris: Clinical presentation and treatment outcomes of 30 horses (2008–2016). Equine Vet. J. 2018, 50, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.K. Evidence-based considerations for control of Parascaris spp. infections in horses. Equine Vet. Educ. 2016, 28, 224–231. [Google Scholar] [CrossRef]
- Cribb, N.C.; Cote, N.M.; Boure, L.P.; Peregrine, A.S. Acute small intestinal obstruction associated with Parascaris equorum infection in young horses: 25 cases (1985–2004). N. Z. Vet. J. 2006, 54, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Corning, S. Equine cyathostomins: A review of biology, clinical significance and therapy. Parasit. Vectors 2009, 2, S1. [Google Scholar] [CrossRef] [Green Version]
- Lyons, E.T.; Drudge, J.H.; Tolliver, S.C. Larval cyathostomiasis. Vet. Clin. N. Am. Equine Pract. 2000, 16, 501–513. [Google Scholar] [CrossRef]
- Uhlinger, C. Effects of 3 Anthelmintic Schedules on the Incidence of Colic in Horses. Equine Vet. J. 1990, 22, 251–254. [Google Scholar] [CrossRef]
- Kaspar, A.; Pfister, K.; Nielsen, M.K.; Silaghi, C.; Fink, H.; Scheuerle, M.C. Detection of Strongylus vulgaris in equine faecal samples by real-time PCR and larval culture - method comparison and occurrence assessment. BMC Vet. Res. 2017, 13, 19. [Google Scholar] [CrossRef] [Green Version]
- Hinney, B. Prävalenz von Helminthen und Risikofaktoren für ihre Befallsstärke bei Pferden in Brandenburg. Ph.D. Thesis, Freie Universität Berlin, Berlin, Germany, 2009. [Google Scholar]
- Traversa, D.; Milillo, P.; Barnes, H.; von Samson-Himmelstjerna, G.; Schurmann, S.; Demeler, J.; Otranto, D.; Lia, R.P.; Perrucci, S.; Frangipane di Regalbono, A.; et al. Distribution and species-specific occurrence of cyathostomins (Nematoda, Strongylida) in naturally infected horses from Italy, United Kingdom and Germany. Vet. Parasitol. 2010, 168, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.K.; Vidyashankar, A.N.; Olsen, S.N.; Monrad, J.; Thamsborg, S.M. Strongylus vulgaris associated with usage of selective therapy on Danish horse farms-is it reemerging? Vet. Parasitol. 2012, 189, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Tydén, E.; Enemark, H.L.; Franko, M.A.; Höglund, J.; Osterman-Lind, E. Prevalence of Strongylus vulgaris in horses after ten years of prescription usage of anthelmintics in Sweden. Vet. Parasitol. 2019, 2, 100013. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M. Equine tapeworm infections: Disease, diagnosis and control. Equine Vet. E 2016, 28, 388–395. [Google Scholar] [CrossRef]
- Hinney, B.; Wirtherle, N.C.; Kyule, M.; Miethe, N.; Zessin, K.H.; Clausen, P.H. Prevalence of helminths in horses in the state of Brandenburg, Germany. Parasitol. Res. 2011, 108, 1083–1091. [Google Scholar] [CrossRef]
- Behrens, T. Bandwürmer (Anoplocephaliden) beim Pferd. Ph.D. Thesis, Tierärztliche Hochschule Hannover, Hanover, Germany, 2001. [Google Scholar]
- Engell-Sørensen, K.; Pall, A.; Damgaard, C.; Holmstrup, M. Seasonal variation in the prevalence of equine tapeworms using coprological diagnosis during a seven-year period in Denmark. Vet. Parasitol. Reg. Stud. Rep. 2018, 12, 22–25. [Google Scholar]
- Rehbein, S.; Visser, M.; Winter, R. Prevalence, intensity and seasonality of gastrointestinal parasites in abattoir horses in Germany. Parasitol. Res. 2013, 112, 407–413. [Google Scholar] [CrossRef]
- Lightbody, K.L.; Davis, P.J.; Austin, C.J. Validation of a novel saliva-based ELISA test for diagnosing tapeworm burden in horses. Vet. Clin. Path. 2016, 45, 335–346. [Google Scholar] [CrossRef]
- Lightbody, K.L.; Matthews, J.B.; Kemp-Symonds, J.G.; Lambert, P.A.; Austin, C.J. Use of a saliva-based diagnostic test to identify tapeworm infection in horses in the UK. Equine Vet. J. 2018, 50, 213–219. [Google Scholar] [CrossRef]
- Jürgenschellert, L.; Krücken, J.; Austin, C.J.; Lightbody, K.L.; Bousquet, E.; von Samson-Himmelstjerna, G. Investigations on the occurrence of tapeworm infections in German horse populations with comparison of different antibody detection methods based on saliva and serum and serum samples. Parasit. Vectors 2020, 13, 462. [Google Scholar] [CrossRef]
- Reinemeyer, C.R.; Nielsen, M.K. Parasitism and colic. Vet. Clin. N. Am. Equine Pract. 2009, 25, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Cringoli, G.; Rinaldi, L.; Maurelli, M.P.; Utzinger, J. FLOTAC: New multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat. Protoc. 2010, 5, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Demeler, J.; Ramünke, S.; Wolken, S.; Ianiello, D.; Rinaldi, L.; Gahutu, J.B.; Cringoli, G.; von Samson-Himmelstjerna, G.; Krücken, J. Discrimination of gastrointestinal nematode eggs from crude fecal egg preparations by inhibitor-resistant conventional and real-time PCR. PLoS ONE 2013, 8, e61285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, M.K.; Peterson, D.S.; Monrad, J.; Thamsborg, S.M.; Olsen, S.N.; Kaplan, R.M. Detection and semi-quantification of Strongylus vulgaris DNA in equine faeces by real-time quantitative PCR. Int. J. Parasitol. 2008, 38, 443–453. [Google Scholar] [CrossRef]
- Traversa, D.; Iorio, R.; Klei, T.R.; Kharchenko, V.A.; Gawor, J.; Otranto, D.; Sparagano, O.A. New method for simultaneous species-specific identification of equine strongyles (nematoda, strongylida) by reverse line blot hybridization. J. Clin. Microbiol. 2007, 45, 2937–2942. [Google Scholar] [CrossRef] [Green Version]
- Andersen, U.V.; Howe, D.K.; Dangoudoubiyam, S.; Toft, N.; Reinemeyer, C.R.; Lyons, E.T.; Olsen, S.N.; Monrad, J.; Nejsum, P.; Nielsen, M.K. SvSXP: A Strongylus vulgaris antigen with potential for prepatent diagnosis. Parasit. Vectors 2013, 6, 84. [Google Scholar] [CrossRef] [Green Version]
- Pittaway, C.E.; Lawson, A.L.; Coles, G.C.; Wilson, A.D. Systemic and mucosal IgE antibody responses of horses to infection with Anoplocephala perfoliata. Vet. Parasitol. 2014, 199, 32–41. [Google Scholar] [CrossRef]
- Grosche, A. Kolik bei Pferden-Retrospektive Studie aus dem Patientengut der Medizinischen Tierklinik Leipzig 1994–1998. Ph.D. Thesis, Universität Leipzig, Leipzig, Germany, 2000. [Google Scholar]
- Wirtherle, N.C. Untersuchungen zur Verbreitung von Anthelminthikaresistenzen bei Pferden in Niedersachsen. Ph.D. Thesis, Tierärztliche Hochschule Hannover, Hanover, Germany, 2003. [Google Scholar]
- Wirtherle, N.; Schnieder, I.; von Samson-Himmelstjerna, G. Prevalence of benzimidazole resistance on horse farms in Germany. Vet. Rec. 2004, 154, 39–41. [Google Scholar] [CrossRef]
- Fritzen, B.M. Untersuchungen zum Vorkommen von Anthelminthika-Resistenz in nordrhein-westfälischen Pferdebeständen. Ph.D. Thesis, Tierärztliche Hochschule Hannover, Hanover, Germany, 2005. [Google Scholar]
- Fritzen, B.; Rohn, K.; Schnieder, T.; von Samson-Himmelstjerna, G. Endoparasite control management on horse farms–lessons from worm prevalence and questionnaire data. Equine Vet. J. 2010, 42, 79–83. [Google Scholar] [CrossRef]
- Menzel, M. Selektive Entwurmung der Pferde in einer Pferdepraxis. Ph.D. Thesis, Ludwig-Maximilians-Universität München, Munich, Germany, 2013. [Google Scholar]
- Honeder, A. Selektive anthelmintische Therapie von Pferden im Raum Salzburg und Oberbayern. Ph.D. Thesis, Ludwig-Maximilians-Universität München, Munich, Germany, 2015. [Google Scholar]
- Hedberg-Alm, Y.; Penell, J.; Riihimaki, M.; Osterman-Lind, E.; Nielsen, M.K.; Tyden, E. Parasite Occurrence and Parasite Management in Swedish Horses Presenting with Gastrointestinal Disease-A Case-Control Study. Animals 2020, 10, 638. [Google Scholar] [CrossRef] [Green Version]
- Stancampiano, L.; Usai, F.; Marigo, A.; Rinnovati, R. Are small strongyles (Cyathostominae) involved in horse colic occurrence? Vet. Parasitol. 2017, 247, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Proudman, C.J. A 2 Year, Prospective Survey of Equine Colic in General-Practice. Equine Vet. J. 1992, 24, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.K.; Reinemeyer, C.R.; Sellon, D.C. Nematodes. In Equine Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2014; pp. 475–489.e4. [Google Scholar]
- Back, H.; Nyman, A.; Osterman-Lind, E. The association between Anoplocephala perfoliata and colic in Swedish horses—A case control study. Vet. Parasitol. 2013, 197, 580–585. [Google Scholar] [CrossRef]
- Rinaldi, L.; Coles, G.; Maurelli, M.; Musella, V.; Cringoli, G. Calibration and diagnostic accuracy of simple flotation, McMaster and FLOTAC for parasite egg counts in sheep. Vet. Parasitol. 2011, 177, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Trotz-Williams, L.; Physick-Sheard, P.; McFarlane, H.; Pearl, D.L.; Martin, S.W.; Peregrine, A.S. Occurrence of Anoplocephala perfoliata infection in horses in Ontario, Canada and associations with colic and management practices. Vet. Parasitol. 2008, 153, 73–84. [Google Scholar] [CrossRef]
- Barrett, E.J.; Blair, C.W.; Farlam, J.; Proudman, C.J. Postdosing colic and diarrhoea in horses with serological evidence of tapeworm infection. Vet. Rec. 2005, 156, 252–253. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Symptoms | Grading |
|---|---|---|
| Bowel peristalsis | Normal motility. | 0 |
| Reduced motility. | 1 | |
| No motility. | 2 | |
| Hypermotility. | 3 | |
| Kicking to the stomach | Horse stands still, no kicking to the stomach. | 0 |
| Occasionally kick against the stomach (1–2 times in 5 min). | 1 | |
| Kicks regularly against the stomach (3–4 times in 5 min). | 2 | |
| Kicks excessively against the stomach (>5 times in 5 min). | 3 | |
| Pawing | Horse stands still, no pawing. | 0 |
| Occasional pawing (1–2 times in 5 min). | 1 | |
| Regular pawing (3–4 times in 5 min). | 2 | |
| Excessive pawing (>5 times in 5 min). | 3 | |
| Head movements | No sign of discomfort, head is mainly held straight in front of the body. | 0 |
| Intermittent, lateral, or vertical head movements, occasionally looking at the flank (1–2 times in 5 min) and/or lifting the lips (1–2 times in 5 min). | 1 | |
| Intermittent, violent, lateral, or vertical head movements, looking regularly at the flank (3–4 times in 5 min) and/or lifting the lips (3–4 times in 5 min). | 2 | |
| Continuous head movements, looking excessively at the flank (>5 times in 5 min) and/or lifting the lips (>5 times in 5 min). | 3 | |
| Lying down, rolling | Horse stands quietly in the box. | 0 |
| Occasionally laying down. | 1 | |
| Regularly lying down and getting up again, rolling. | 2 | |
| Horse repeatedly throws itself down uncontrollably and rolls on the ground. | 3 |
| Prevalence (%) [95% Confidence Interval] | |||
|---|---|---|---|
| Parasite(s) | Total | Colic patients | Non-colic controls |
| Helminths | 50.5 [45.9; 55.2] | 49.5 [41.8; 56.3] | 51.4 [45.1; 57.6] |
| Strongyles | 50.2 [45.7; 54.9] | 49.5 [41.6; 56.3] | 50.9 [44.7; 57.2] |
| A. perfoliata | 1 [0.2; 2] | 0.5 [0; 1.6] | 1.4 [0; 3.3] |
| Parascaris spp. | 0.5 [0; 1.2] | 0 [0; 0] | 0.9 [0; 2.3] |
| Percent of Colic Patients with Clinical Signs Listed Below | |||||
| Last deworming | Coughing | Diarrhea | Emaciation | Recurrent colic | Reduced performance |
| Irrespective (n = 82) | 12.2 | 8.5 | 22 | 68.3 | 4.9 |
| ≥8 weeks (n = 53) | 9.4 | 9.4 | 17 | 77.4 | 3.8 |
| Percent of Control Patients with Clinical Signs Listed Below | |||||
| Last deworming | Coughing | Diarrhea | Emaciation | Recurrent colic | Reduced performance |
| Irrespective (n = 306) | 3.3 | 2.3 | 5.9 | 18.3 | 1.3 |
| ≥8 weeks (n = 196) | 2.6 | 4.5 | 4.6 | 35.9 | 2.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gehlen, H.; Wulke, N.; Ertelt, A.; Nielsen, M.K.; Morelli, S.; Traversa, D.; Merle, R.; Wilson, D.; Samson-Himmelstjerna, G.v. Comparative Analysis of Intestinal Helminth Infections in Colic and Non-Colic Control Equine Patients. Animals 2020, 10, 1916. https://doi.org/10.3390/ani10101916
Gehlen H, Wulke N, Ertelt A, Nielsen MK, Morelli S, Traversa D, Merle R, Wilson D, Samson-Himmelstjerna Gv. Comparative Analysis of Intestinal Helminth Infections in Colic and Non-Colic Control Equine Patients. Animals. 2020; 10(10):1916. https://doi.org/10.3390/ani10101916
Chicago/Turabian StyleGehlen, Heidrun, Nadine Wulke, Antonia Ertelt, Martin K. Nielsen, Simone Morelli, Donato Traversa, Roswitha Merle, Douglas Wilson, and Georg von Samson-Himmelstjerna. 2020. "Comparative Analysis of Intestinal Helminth Infections in Colic and Non-Colic Control Equine Patients" Animals 10, no. 10: 1916. https://doi.org/10.3390/ani10101916
APA StyleGehlen, H., Wulke, N., Ertelt, A., Nielsen, M. K., Morelli, S., Traversa, D., Merle, R., Wilson, D., & Samson-Himmelstjerna, G. v. (2020). Comparative Analysis of Intestinal Helminth Infections in Colic and Non-Colic Control Equine Patients. Animals, 10(10), 1916. https://doi.org/10.3390/ani10101916









