A Genomic Study of Myxomatous Mitral Valve Disease in Cavalier King Charles Spaniels
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- Cases (n = 16): Dogs with MMVD (class B1 or more severe) diagnosed before the age of 5 or with severe disease (class C or D) before the age of 8;
- Controls (n = 17): Dogs without MMVD (class A) or with extremely mild signs of MMVD (class B1 with a trivial mitral regurgitation characterized by a maximal ratio of the regurgitant jet area signal to left atrium area ≤ 20%) [25] over 5 years of age or those suffering from a mild form of disease (class B1) over 8 years of age.
2.1. Statistical Analysis
2.2. Genealogic Analysis
2.3. DNA Extraction and Genomic Analysis
3. Results
3.1. Genomic Analysis
3.2. Pathway Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Detweiler, D.K.; Patterson, D.F. The prevalence and types of cardiovascular disease in dogs. Ann. N. Y. Acad. Sci. 1965, 127, 481–516. [Google Scholar] [CrossRef] [PubMed]
- Borgarelli, M.; Crosara, S.; Lamb, K.; Savarino, P.; La Rosa, G.; Tarducci, A.; Häggström, J. Survival Characteristics and Prognostic Variables of Dogs with Preclinical Chronic Degenerative Mitral Valve Disease Attributable to Myxomatous Degeneration. J. Vet. Intern. Med. 2012, 26, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.B.; Olsen, L.H.; Häggström, J.; Höglund, K.; Ljungvall, I.; Falk, T.; Wess, G.; Stephenson, H.; Dukes-McEwan, J.; Chetboul, V.; et al. Identification of 2 Loci Associated with Development of Myxomatous Mitral Valve Disease in Cavalier King Charles Spaniels. J. Hered. 2011, 102, S62–S67. [Google Scholar] [CrossRef]
- Bagardi, M.; Bionda, A.; Locatelli, C.; Cortellari, M.; Frattini, S.; Negro, A.; Crepaldi, P.; Brambilla, P.G. Echocardiographic Evaluation of the Mitral Valve in Cavalier King Charles Spaniels. Animals 2020, 10, 1454. [Google Scholar] [CrossRef] [PubMed]
- Egenvall, A.; Bonnett, B.N.; Häggström, J. Heart disease as a cause of death in insured Swedish dogs younger than 10 years of age. J. Vet. Intern. Med. 2006, 20, 894–903. [Google Scholar] [CrossRef]
- Pedersen, H.D.; Häggström, J.; Falk, T.; Mow, T.; Olsen, L.H.; Iversen, L.; Jensen, A.L. Auscultation in mild mitral regurgitation in dogs: Observer variation, effects of physical maneuvers, and agreement with color Doppler echocardiography and phonocardiography. J. Vet. Intern. Med. 1999, 13, 56–64. [Google Scholar] [CrossRef]
- Keene, B.W.; Atkins, C.E.; Bonagura, J.D.; Fox, P.R.; Häggström, J.; Fuentes, V.L.; Oyama, M.A.; Rush, J.E.; Stepien, R.L.; Uechi, M. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J. Vet. Intern. Med. 2019, 1–14. [Google Scholar] [CrossRef]
- Olsen, L.H.; Fredholm, M.; Pedersen, H.D. Epidemiology an Inheritance of Mitral Valve Prolapse in Dachshunds. J. Vet. Intern. Med. 1999, 13, 448–456. [Google Scholar] [CrossRef]
- Lewis, T.W.; Swift, S.; Woolliams, J.A.; Blott, S.C. Heritability of premature mitral valve disease in Cavalier King Charles spaniels. Vet. J. 2011, 188, 73–76. [Google Scholar] [CrossRef]
- Swenson, L.; Häggström, J.; Kvart, C.; Juneja, R.K. Relationship between parental cardiac status in Cavalier King Charles spaniels and prevalence and severity of chronic valvular disease in offspring. J. Am. Vet. Med. Assoc. 1996, 208, 2009–2012. [Google Scholar]
- Lindblad-Toh, K.; Wade, C.M.; Mikkelsen, T.S.; Karlsson, E.K.; Jaffe, D.B.; Kamal, M.; Clamp, M.; Chang, J.L.; Kulbokas, E.J.; Zody, M.C.; et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 2005, 438, 803–819. [Google Scholar] [CrossRef] [PubMed]
- Sutter, N.B.; Eberle, M.A.; Parker, H.G.; Pullar, B.J.; Kirkness, E.F.; Kruglyak, L.; Ostrander, E.A. Extensive and breed-specific linkage disequilibrium in Canis familiaris. Genome Res. 2004, 14, 2388–2396. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.G.; Meurs, K.M.; Ostrander, E.A. Finding cardiovascular disease genes in the dog. J. Vet. Cardiol. 2006, 8, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.G. The History and Relationships of Dog Breeds. In The Genetics of the Dog; Ostrander, E.A., Ruvinsky, A., Eds.; CAB International: Croydon, UK, 2012; pp. 38–56. [Google Scholar]
- Karlsson, E.K.; Baranowska, I.; Wade, C.M.; Salmon Hillbertz, N.H.; Zody, M.C.; Anderson, N.; Biagi, T.M.; Patterson, N.; Pielberg, G.R.; Kulbokas, E.J.; et al. Efficient Mapping of Mendelian Traits in Dogs Through Genome-Wide Association. Nat. Genet. 2007, 39, 1321–1328. [Google Scholar] [CrossRef]
- French, A.T.; Ogden, R.; Eland, C.; Hemani, G.; Pong-Wong, R.; Corcoran, B.M.; Summers, K.M. Genome-wide analysis of mitral valve disease in Cavalier King Charles Spaniels. Vet. J. 2012, 193, 283–286. [Google Scholar] [CrossRef]
- Meurs, K.M.; Friedenberg, S.G.; Williams, B.; Keene, B.W.; Atkins, C.E.; Adin, D.; Aona, B.; DeFrancesco, T.; Tou, S.; Mackay, T. Evaluation of genes associated with human myxomatous mitral valve disease in dogs with familial myxomatous mitral valve degeneration. Vet. J. 2018, 232, 16–19. [Google Scholar] [CrossRef]
- Lee, C.-M.; Han, J.-I.; Kang, M.-H.; Kim, S.-G.; Park, H.-M. Polymorphism in the serotonin transporter protein gene in Maltese dogs with degenerative mitral valve disease. J. Vet. Sci. 2018, 19, 129–135. [Google Scholar] [CrossRef]
- Lee, C.-M.; Song, D.-W.; Ro, W.-B.; Kang, M.-H.; Park, H.-M. Genome-wide association study of degenerative mitral valve disease in Maltese dogs. J. Vet. Sci. 2019, 20, 63–71. [Google Scholar] [CrossRef]
- Torres-García, O.; Rey-Buitrago, M.; Acosta-Virgüez, E.; Bernal-Rosas, Y.; Infante-González, J.; Gómez-Duarte, L. Role of COL1A2 Gene Polymorphisms in Myxomatous Mitral Valve Disease in Poodle Dogs Genetic Study of Mitral Valve Disease. J. Agric. Vet. Sci. 2016, 9, 113–118. [Google Scholar] [CrossRef]
- Stern, J.A.; Hsue, W.; Song, K.H.; Ontiveros, E.S.; Fuentes, V.L.; Stepien, R.L. Severity of mitral valve degeneration is associated with chromosome 15 loci in whippet dogs. PLoS ONE 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Lundin, T.; Kvart, C. Evaluation of the Swedish breeding program for cavalier King Charles spaniels. Acta Vet. Scand. 2010, 52, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Swift, S.; Baldin, A.; Cripps, P. Degenerative Valvular Disease in the Cavalier King Charles Spaniel: Results of the UK Breed Scheme 1991–2010. J. Vet. Intern. Med. 2017, 31, 9–14. [Google Scholar] [CrossRef]
- Birkegård, A.C.; Reimann, M.J.; Martinussen, T.; Häggström, J.; Pedersen, H.D.; Olsen, L.H. Breeding Restrictions Decrease the Prevalence of Myxomatous Mitral Valve Disease in Cavalier King Charles Spaniels over an 8- to 10-Year Period. J. Vet. Intern. Med. 2016, 30, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Chetboul, V.; Tissier, R. Echocardiographic assessment of canine degenerative mitral valve disease. J. Vet. Cardiol. 2012, 14, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.P.; Gaber, C.E.; Jacobs, G.J.; Kaplan, P.M.; Lombard, C.W.; Moise, N.S.; Moses, B.L. Recommendations for Standards in Transthoracic Two-Dimensional Echocardiography in the Dog and Cat. J. Vet. Intern. Med. 1993, 7, 247–252. [Google Scholar] [CrossRef]
- Wellmann, R. Optimum contribution selection for animal breeding and conservation: The R package optiSel. BMC Bioinformatics 2019, 20, 20–25. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A toolset for whole-genome association and population-based linkage analysis. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Browning, S.R.; Browning, B.L. Rapid and accurate haplotype phasing and missing data inference for whole genome association studies by use of localized haplotype clustering. Am. J. Hum. Genet. 2007, 81, 1084–1097. [Google Scholar] [CrossRef]
- Beaumont, M.A.; Balding, D.J. Identifying adaptive genetic divergence among populations from genome scans. Mol. Ecol. 2004, 13, 969–980. [Google Scholar] [CrossRef]
- Holsinger, K.E.; Weir, B.S. Genetics in geographically structured populations: Defining, estimating and interpreting FST. Nat. Rev. Genet. 2009, 10, 639–650. [Google Scholar] [CrossRef]
- Porto-Neto, L.R.; Sonstegard, T.S.; Liu, G.E.; Bickhart, D.M.; Da Silva, M.V.; Machado, M.A.; Utsunomiya, Y.T.; Garcia, J.F.; Gondro, C.; Van Tassell, C.P. Genomic divergence of zebu and taurine cattle identified through high-density SNP genotyping. BMC Genomics 2013, 14, 876. [Google Scholar] [CrossRef]
- Ablondi, M.; Viklund, Å.; Lindgren, G.; Eriksson, S.; Mikko, S. Signatures of selection in the genome of Swedish warmblood horses selected for sport performance. BMC Genomics 2019, 20, 717. [Google Scholar] [CrossRef]
- Sabeti, P.C.; Reich, D.E.; Higgins, J.M.; Levine, H.Z.P.; Richter, D.J.; Schaffner, S.F.; Gabriel, S.B.; Platko, J.V.; Patterson, N.J.; McDonald, G.J.; et al. Detecting recent positive selection in the human genome from haplotype structure. Nature 2002, 419, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Vitti, J.J.; Grossman, S.R.; Sabeti, P.C. Detecting Natural Selection in Genomic Data. Annu. Rev. Genet. 2013, 47, 97–120. [Google Scholar] [CrossRef]
- Szpiech, Z.A.; Hernandez, R.D. selscan: An Efficient Multithreaded Program to Perform EHH-Based Scans for Positive Selection. Mol. Biol. Evol. 2014, 31, 2824–2827. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Williams, F.J.; Dreger, D.L.; Plassais, J.; Davis, B.W.; Parker, H.G.; Ostrander, E.A. Genetic selection of athletic success in sport-hunting dogs. Proc. Natl. Acad. Sci. USA 2018, 115, E7212–E7221. [Google Scholar] [CrossRef]
- Liu, X.; Ong, R.T.-H.; Pillai, E.N.; Elzein, A.M.; Small, K.S.; Clark, T.G.; Kwiatkowski, D.P.; Teo, Y.-Y. Detecting and Characterizing Genomic Signatures of Positive Selection in Global Populations. Am. J. Hum. Genet. 2013, 92, 866–881. [Google Scholar] [CrossRef]
- Pitt, D.; Bruford, M.W.; Barbato, M.; Orozco-terWengel, P.; Martínez, R.; Sevane, N. Demography and rapid local adaptation shape Creole cattle genome diversity in the tropics. Evol. Appl. 2019, 12, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Grossman, S.R.; Shlyakhter, I.; Shylakhter, I.; Karlsson, E.K.; Byrne, E.H.; Morales, S.; Frieden, G.; Hostetter, E.; Angelino, E.; Garber, M.; et al. A composite of multiple signals distinguishes causal variants in regions of positive selection. Science 2010, 327, 883–886. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinforma. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef]
- Pemberton, T.J.; Absher, D.; Feldman, M.W.; Myers, R.M.; Rosenberg, N.A.; Li, J.Z. Genomic Patterns of Homozygosity in Worldwide Human Populations. Am. J. Hum. Genet. 2012, 91, 275–292. [Google Scholar] [CrossRef]
- Wiener, P.; Sánchez-Molano, E.; Clements, D.N.; Woolliams, J.A.; Haskell, M.J.; Blott, S.C. Genomic data illuminates demography, genetic structure and selection of a popular dog breed. BMC Genomics 2017, 18, 609. [Google Scholar] [CrossRef] [PubMed]
- Mortlock, S.-A.; Khatkar, M.S.; Williamson, P. Comparative Analysis of Genome Diversity in Bullmastiff Dogs. PLoS ONE 2016, 11, e0147941. [Google Scholar] [CrossRef]
- Sams, A.J.; Boyko, A.R. Fine-Scale Resolution of Runs of Homozygosity Reveal Patterns of Inbreeding and Substantial Overlap with Recessive Disease Genotypes in Domestic Dogs. G3 (Bethesda) 2019, 9, 117–123. [Google Scholar] [CrossRef]
- Bosada, F.M.; Devasthali, V.; Jones, K.A.; Stankunas, K. Wnt/β-catenin signaling enables developmental transitions during valvulogenesis. Development 2016, 143, 1041–1054. [Google Scholar] [CrossRef]
- Gabriel, L.A.R.; Wang, L.W.; Bader, H.; Ho, J.C.; Majors, A.K.; Hollyfield, J.G.; Traboulsi, E.I.; Apte, S.S. ADAMTSL4, a Secreted Glycoprotein Widely Distributed in the Eye, Binds Fibrillin-1 Microfibrils and Accelerates Microfibril Biogenesis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 461. [Google Scholar] [CrossRef] [PubMed]
- Dietz, H.C.; Cutting, C.R.; Pyeritz, R.E.; Maslen, C.L.; Sakai, L.Y.; Corson, G.M.; Puffenberger, E.G.; Hamosh, A.; Nanthakumar, E.J.; Curristin, S.M.; et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 1991, 352, 337–339. [Google Scholar] [CrossRef]
- Motterle, A.; Pu, X.; Wood, H.; Xiao, Q.; Gor, S.; Ng, F.L.; Chan, K.; Cross, F.; Shohreh, B.; Poston, R.N.; et al. Functional analyses of coronary artery disease associated variation on chromosome 9p21 in vascular smooth muscle cells. Hum. Mol. Genet. 2012, 21, 4021–4029. [Google Scholar] [CrossRef]
- Bosanquet, D.C.; Ye, L.; Harding, K.G.; Jiang, W.G. Expressed in high metastatic cells (Ehm2) is a positive regulator of keratinocyte adhesion and motility: The implication for wound healing. J. Dermatol. Sci. 2013, 71, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Otsu, K.; Fujii, J.; Periasamy, M.; Difilippantonio, M.; Uppender, M.; Ward, D.C.; MacLennan, D.H. Chromosome mapping of five human cardiac and skeletal muscle sarcoplasmic reticulum protein genes. Genomics 1993, 17, 507–509. [Google Scholar] [CrossRef] [PubMed]
- di Barletta, M.R.; Viatchenko-Karpinski, S.; Nori, A.; Memmi, M.; Terentyev, D.; Turcato, F.; Valle, G.; Rizzi, N.; Napolitano, C.; Gyorke, S.; et al. Clinical phenotype and functional characterization of CASQ2 mutations associated with catecholaminergic polymorphic ventricular tachycardia. Circulation 2006, 114, 1012–1019. [Google Scholar] [CrossRef]
- Serfass, P.; Chetboul, V.; Carlos Sampedrano, C.; Nicolle, A.P.; Benalloul, T.; Laforge, H.; Gau, C.; Hébert, C.; Pouchelon, J.-L.; Tissier, R. Retrospective study of 942 small-sized dogs: Prevalence of left apical systolic heart murmur and left-sided heart failure, critical effects of breed and sex. J. Vet. Cardiol. 2006, 8, 11–18. [Google Scholar] [CrossRef]
- Thrusfield, M.V.; Aitken, C.G.G.; Darker, P.G.G. Observations on breed and sex in relation to canine heart valve incompetence. J. Small Anim. Pract. 1985, 26, 709–717. [Google Scholar] [CrossRef]
- Schutte, J.E.; Gaffney, F.A.; Blend, L.; Blomqvist, C.G. Distinctive anthropometric characteristics of women with mitral valve prolapse. Am. J. Med. 1981, 71, 533–538. [Google Scholar] [CrossRef]
- Freed, L.A.; Levy, D.; Levine, R.A.; Larson, M.G.; Evans, J.C.; Fuller, D.L.; Lehman, B.; Benjamin, E.J. Prevalence and clinical outcome of mitral-valve prolapse. N. Engl. J. Med. 1999, 341, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Potts, J.D.; Runyan, R.B. Epithelial-mesenchymal cell transformation in the embryonic heart can be mediated, in part, by transforming growth factor beta. Dev. Biol. 1989, 134, 392–401. [Google Scholar] [CrossRef]
- Azhar, M.; Runyan, R.B.; Gard, C.; Sanford, L.P.; Miller, M.L.; Andringa, A.; Pawlowski, S.; Rajan, S.; Doetschman, T. Ligand-specific function of transforming growth factor beta in epithelial-mesenchymal transition in heart development. Dev. Dyn. 2009, 238, 431–442. [Google Scholar] [CrossRef]
- Camenisch, T.D.; Schroeder, J.A.; Bradley, J.; Klewer, S.E.; McDonald, J.A. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2–ErbB3 receptors. Nat. Med. 2002, 8, 850–855. [Google Scholar] [CrossRef]
- Chen, B.; Bronson, R.T.; Klaman, L.D.; Hampton, T.G.; Wang, J.; Green, P.J.; Magnuson, T.; Douglas, P.S.; Morgan, J.P.; Neel, B.G. Mice mutant for Egfr and Shp2 have defective cardiac semilunar valvulogenesis. Nat. Genet. 2000, 24, 296–299. [Google Scholar] [CrossRef]
- Lee, K.-F.; Simon, H.; Chen, H.; Bates, B.; Hung, M.-C.; Hauser, C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature 1995, 378, 394–398. [Google Scholar] [CrossRef]
- Walker, G.A.; Masters, K.S.; Shah, D.N.; Anseth, K.S.; Leinwand, L.A. Valvular myofibroblast activation by transforming growth factor-beta: Implications for pathological extracellular matrix remodeling in heart valve disease. Circ. Res. 2004, 95, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Pchejetski, D.; Foussal, C.; Alfarano, C.; Lairez, O.; Calise, D.; Guilbeau-Frugier, C.; Schaak, S.; Seguelas, M.-H.; Wanecq, E.; Valet, P.; et al. Apelin prevents cardiac fibroblast activation and collagen production through inhibition of sphingosine kinase 1. Eur. Heart J. 2012, 33, 2360–2369. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, K.; Miyagawa-Tomita, S.; Matsumoto, H.; Koyama, H.; Nakanishi, T.; Hirose, H. Effects of transforming growth factor-β3 and matrix metalloproteinase-3 on the pathogenesis of chronic mitral valvular disease in dogs. Am. J. Vet. Res. 2011, 72, 194–202. [Google Scholar] [CrossRef]
- Barrick, C.J.; Roberts, R.B.; Rojas, M.; Rajamannan, N.M.; Suitt, C.B.; O’Brien, K.D.; Smyth, S.S.; Threadgill, D.W. Reduced EGFR causes abnormal valvular differentiation leading to calcific aortic stenosis and left ventricular hypertrophy in C57BL/6J but not 129S1/SvImJ mice. Am. J. Physiol. Circ. Physiol. 2009, 297, H65–H75. [Google Scholar] [CrossRef]
- Hulin, A.; Moore, V.; James, J.M.; Yutzey, K.E. Loss of Axin2 results in impaired heart valve maturation and subsequentmyxomatous valve disease. Cardiovasc. Res. 2017, 113, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Loeys, B.L.; Schwarze, U.; Holm, T.; Callewaert, B.L.; Thomas, G.H.; Pannu, H.; De Backer, J.F.; Oswald, G.L.; Symoens, S.; Manouvrier, S.; et al. Aneurysm Syndromes Caused by Mutations in the TGF-β Receptor. N. Engl. J. Med. 2006, 355, 788–798. [Google Scholar] [CrossRef]
- van de Laar, I.M.B.H.; Oldenburg, R.A.; Pals, G.; Roos-Hesselink, J.W.; de Graaf, B.M.; Verhagen, J.M.A.; Hoedemaekers, Y.M.; Willemsen, R.; Severijnen, L.-A.; Venselaar, H.; et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat. Genet. 2011, 43, 121–126. [Google Scholar] [CrossRef]
- Matt, P.; Schoenhoff, F.; Habashi, J.; Holm, T.; Van Erp, C.; Loch, D.; Carlson, O.D.; Griswold, B.F.; Fu, Q.; De Backer, J.F.; et al. Circulating transforming growth factor-beta in Marfan syndrome. Circulation 2009, 120, 526–532. [Google Scholar] [CrossRef]
- Aupperle, H.; März, I.; Thielebein, J.; Schoon, H.A. Expression of Transforming Growth Factor-β1, -β2 and -β3 in Normal and Diseased Canine Mitral Valves. J. Comp. Pathol. 2008, 139, 97–107. [Google Scholar] [CrossRef]
- Guarda, E.; Katwa, L.C.; Myers, P.R.; Tyagi, S.C.; Weber, K.T. Effects of endothelins on collagen turnover in cardiac fibroblasts. Cardiovasc. Res. 2008, 27, 2130–2134. [Google Scholar] [CrossRef]
- Myers, P.R.; Tanner, M.A. Vascular endothelial cell regulation of extracellular matrix collagen: Role of nitric oxide. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.G.; Zhao, J.; Yang, J.; Thomsen, P.D.; Gregersen, H.; Hasenkam, J.M.; Smerup, M.; Pedersen, H.D.; Olsen, L.H. Increased expression of endothelin B receptor in static stretch exposed porcine mitral valve leaflets. Res. Vet. Sci. 2007, 82, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.G.; Offenberg, H.; Moesgaard, S.G.; Thomsen, P.D.; Pedersen, H.D.; Olsen, L.H. Transcription levels of endothelin-1 and endothelin receptors are associated with age and leaflet location in porcine mitral valves. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 2007, 54, 113–118. [Google Scholar] [CrossRef]
- Borgarelli, M.; Crosara, S. Malattia cronica mitralica. In Manuale di cardiologia del cane e del gatto; Santilli, R.A., Bussadori, C., Borgarelli, M., Eds.; Elsevier Srl: Milan, Italy, 2012; pp. 153–164. [Google Scholar]
- Riegger, G.A.J.; Liebau, G.; Holzschuh, M.; Witkowski, D.; Steilner, H.; Kochsiek, K. Role of the renin-angiotensin system in the development of congestive heart failure in the dog as assessed by chronic converting-enzyme blockade. Am. J. Cardiol. 1984, 53, 614–618. [Google Scholar] [CrossRef]
- Brilla, C.G.; Rupp, H.; Funck, R.; Maisch, B. The renin-angiotensin-aldosterone system and myocardial collagen matrix remodelling in congestive heart failure. Eur. Heart J. 1995, 16 (Suppl. O), 107–109. [Google Scholar] [CrossRef]
- Avierinos, J.-F.; Brown, R.D.; Foley, D.A.; Nkomo, V.; Petty, G.W.; Scott, C.; Enriquez-Sarano, M. Cerebral ischemic events after diagnosis of mitral valve prolapse: A community-based study of incidence and predictive factors. Stroke 2003, 34, 1339–1344. [Google Scholar] [CrossRef][Green Version]
- Caltrider, N.D.; Irvine, A.R.; Kline, H.J.; Rosenblatt, A. Retinal Emboli in Patients with Mitral Valve Prolapse. Am. J. Ophthalmol. 1980, 90, 534–539. [Google Scholar] [CrossRef]
- Walsh, P.N.; Kansu, T.A.; Corbett, J.J.; Savion, P.J.; Goldburgh, W.P.; Schatz, N.J. Platelets, thromboembolism and mitral valve prolapse. Circulation 1981, 63, 552–559. [Google Scholar] [CrossRef]
- Riddle, J.M.; Stein, P.D.; Magilligan, D.J.; McElroy, H.H. Evaluation of platelet reactivity in patients with valvular heart disease. J. Am. Coll. Cardiol. 1983, 1, 1381–1384. [Google Scholar] [CrossRef]
- Tse, H.F.; Lau, C.P.; Cheng, G. Relation between mitral regurgitation and platelet activation. J. Am. Coll. Cardiol. 1997, 30, 1813–1818. [Google Scholar] [CrossRef]
- Corcoran, B.M.; Black, A.; Anderson, H.; Dukes-McEwan, J.; French, A.T.; Smith, P.; Devine, C. Identification of surface morphologic changes in the mitral valve leaflets and chordae tendineae of dogs with myxomatous degeneration. Am. J. Vet. Res. 2004, 65, 198–206. [Google Scholar] [CrossRef]
- Tanaka, R.; Murota, A.; Nagashima, Y.; Yamane, Y. Changes in Platelet Life Span in Dogs with Mitral Valve Regurgitation. J. Vet. Intern. Med. 2002, 16, 446–451. [Google Scholar] [CrossRef]
- Tarnow, I.; Kristensen, A.T.; Texel, H.; Olsen, L.H.; Pedersen, H.D. Decreased Platelet Function in Cavalier King Charles Spaniels with Mitral Valve Regurgitation. J. Vet. Intern. Med. 2003, 17, 680–686. [Google Scholar] [CrossRef]
- Tanaka, R.; Yamane, Y. Platelet aggregation in dogs with mitral valve regurgitation. Am. J. Vet. Res. 2000, 61, 1248–1251. [Google Scholar] [CrossRef]
- Lu, C.-C.; Liu, M.-M.; Culshaw, G.J.; Clinton, M.; Argyle, D.J.; Corcoran, B.M. Gene network and canonical pathway analysis in canine myxomatous mitral valve disease: A microarray study. Vet. J. 2015, 204, 23–31. [Google Scholar] [CrossRef]
- Oyama, M.A.; Chittur, S.V. Genomic expression patterns of mitral valve tissues from dogs with degenerative mitral valve disease. Am. J. Vet. Res. 2006, 67, 1307–1318. [Google Scholar] [CrossRef]
- Cremer, S.E.; Moesgaard, S.G.; Rasmussen, C.E.; Zois, N.E.; Falk, T.; Reimann, M.J.; Cirera, S.; Aupperle, H.; Oyama, M.A.; Olsen, L.H. Alpha-smooth muscle actin and serotonin receptors 2A and 2B in dogs with myxomatous mitral valve disease. Res. Vet. Sci. 2015, 100, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Arndt, J.W.; Reynolds, C.A.; Singletary, G.E.; Connolly, J.M.; Levy, R.J.; Oyama, M.A. Serum Serotonin Concentrations in Dogs with Degenerative Mitral Valve Disease. J. Vet. Intern. Med. 2009, 23, 1208–1213. [Google Scholar] [CrossRef]
- Aupperle, H.; Disatian, S. Pathology, protein expression and signaling in myxomatous mitral valve degeneration: Comparison of dogs and humans. J. Vet. Cardiol. 2012, 14, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-C.; Liu, M.-M.; Culshaw, G.J.; French, A.T.; Corcoran, B.M. Comparison of cellular changes in Cavalier King Charles spaniel and mixed breed dogs with myxomatous mitral valve disease. J. Vet. Cardiol. 2016, 18, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Takuwa, N.; Takuwa, Y.; Yanagisawa, M.; Yamashita, K.; Masaki, T. A novel vasoactive peptide endothelin stimulates mitogenesis through inositol lipid turnover in Swiss 3T3 fibroblasts. J. Biol. Chem. 1989, 264, 7856–7861. [Google Scholar] [PubMed]
- Mow, T.; Pedersen, H.D. Increased Endothelin-Receptor Density in Myxomatous Canine Mitral Valve Leaflets. J. Cardiovasc. Pharmacol. 1999, 34, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Hedhammar, Å.A.; Indrebø, A. Rules, regulations, strategies and activities within the Fédération Cynologique Internationale (FCI) to promote canine genetic health. Vet. J. 2011, 189, 141–146. [Google Scholar] [CrossRef]
- Häggström, J. Chronic Valvular Disease in Cavalier King Charles Spaniels—Epidemiology, Inheritance and Pathophysiology. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala Sweden, 1996. [Google Scholar]
- Kyndt, F.; Gueffet, J.-P.; Probst, V.; Jaafar, P.; Legendre, A.; Le Bouffant, F.; Toquet, C.; Roy, E.; McGregor, L.; Lynch, S.A.; et al. Mutations in the gene encoding filamin A as a cause for familial cardiac valvular dystrophy. Circulation 2007, 115, 40–49. [Google Scholar] [CrossRef]
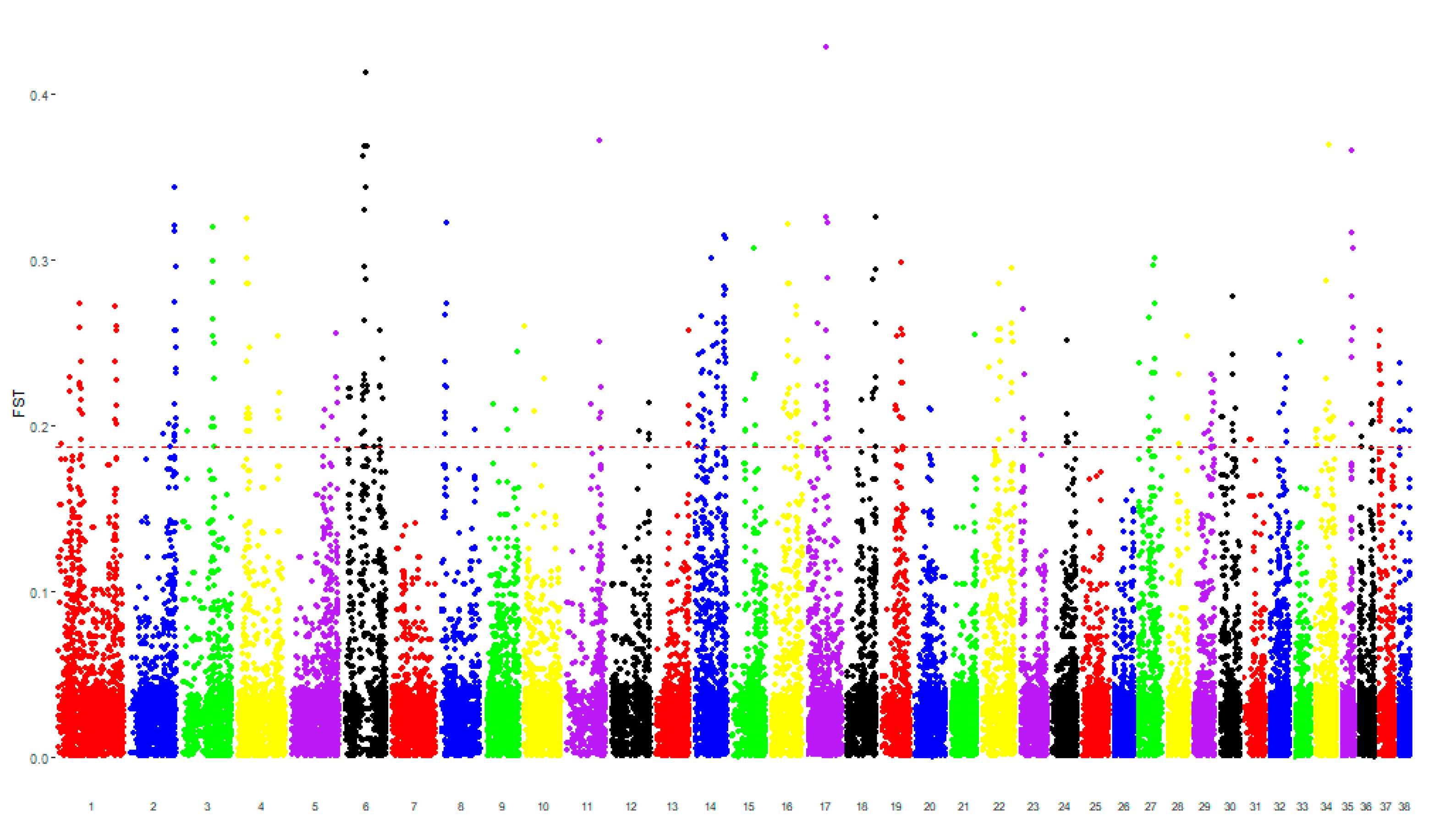
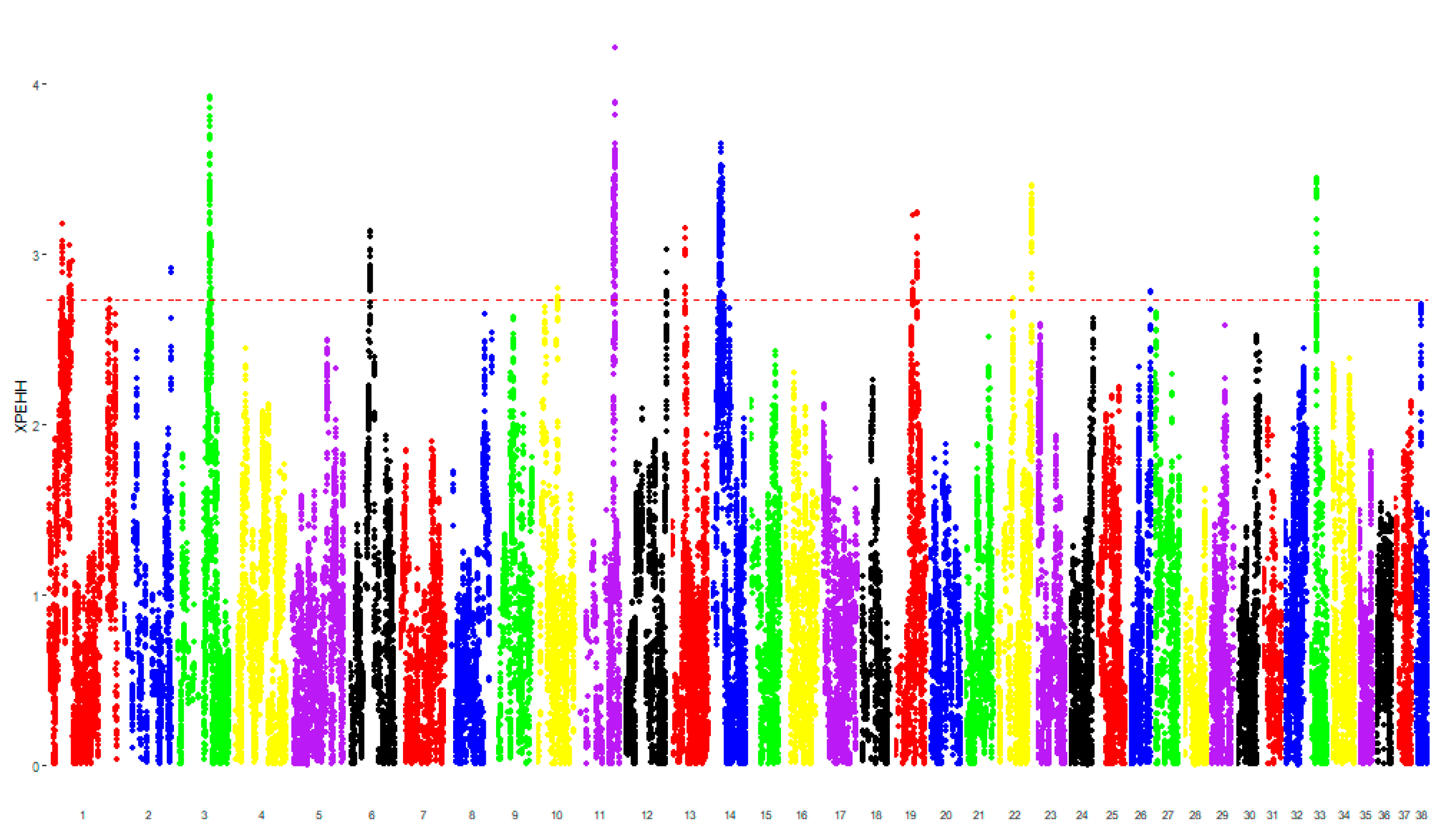

| Authors | Results | Dog Breed | Sample Size | Criteria for the Inclusion | Diagnostic Techniques | Genomic Analysis | |
|---|---|---|---|---|---|---|---|
| Age | Diagnosis | ||||||
| Madsen et al., 2011 | CFA 13q2.2.3 CFA 14q1.3 | CKCS | 139 cases | <4.5 years <8 years | Murmur ≥1/6 and ARJ/LAA ≥20% Heart failure symptoms | Auscultation Echocardiography | GWAS |
| 102 controls | >8 years | Murmur ≤2/6 and ARJ/LAA ≤50% | |||||
| French et al., 2012 | No mutations at a single genetic locus were found | CKCS | 18 early-onset | <5 years | Detectable murmur | Auscultation | Homozygosity mapping GWAS |
| 18 late-onset | >7 years | Detectable murmur | |||||
| Stern et al., 2015 | FSTL5, EEF1a1a, NAF1, NPY1R, NPY5R, TMA16, March1, ARHGAP26 | Whippet | 138 dogs | 5 years cut-off | Scoring based on age, presence and degree of mitral valve prolapse, regurgitation, and left heart enlargement | Auscultation Echocardiography Cumulative echocardiographic score system | GWAS |
| Torres-García et al., 2016 | Allele T of the rs22372411 variant of COL1A2 | Poodle | 50 cases | No restrictions >8 years | Diagnosis of MMVD MMVD absent or mild | Auscultation Echocardiography | Candidate gene polymorphisms |
| 80 controls | >8 years | MMVD absent or mild | |||||
| Meurs et al., 2018 | A missense mutation of COL5A1, predicted to be benign, was present in CKCS | CKCS and dachshund | 10 CKCSs and 10 dachshunds as cases | No restrictions | Diagnosis of MMVD | Auscultation Echocardiography | Candidate gene approach, whole genome sequencing |
| 98 medium to large dog breeds as controls | No restrictions | Phenotype not evaluated; low prevalence of MMVD | |||||
| Lee et al., 2018 | SERT (SLC6A4): c.1193delT (p.Val397Gly) | Maltese | 20 cases | No restrictions | Diagnosis of MMVD | Echocardiography | Candidate gene polymorphisms |
| 10 controls | No restrictions | Echocardiographically healthy | |||||
| Lee et al., 2019 | PDZD2, CTNNA3, LDLRAD4, ARVCF | Maltese | 32 cases | No restrictions | Diagnosis of MMVD | Echocardiography Radiography | GWAS |
| 16 controls | >10 years | Echocardiographically healthy | |||||
| Gene Name | CFA | Start | End | Complete Name |
|---|---|---|---|---|
| KIAA1024 | 3 | 57739740 | 57748234 | KIAA1024 |
| TBC1D14 | 3 | 59003766 | 59097331 | TBC1 domain family member 14 |
| FAH | 3 | 57300583 | 57326453 | Fumarylacetoacetate hydrolase |
| FRRS1L | 11 | 64281895 | 64447650 | Ferric chelate reductase 1 like |
| EPB41L4B | 11 | 64312862 | 64447436 | Erythrocyte membrane protein band 4.1 like 4B |
| CDK6 | 14 | 18188429 | 18420100 | Cyclin dependent kinase 6 |
| HEPACAM2 | 14 | 18695744 | 18735539 | HEPACAM family member 2 |
| RAB3GAP1 | 19 | 37861985 | 37957007 | RAB3 GTPase activating protein catalytic subunit 1 |
| ZRANB3 | 19 | 38002194 | 38302593 | Zinc finger RANBP2-type containing 3 |
| UBXN4 | 19 | 38519927 | 38568223 | UBX domain protein 4 |
| Pathway or Disease | p-Value | Adjusted p-Value | Associated Group of Genes | Library | Associated Genes |
|---|---|---|---|---|---|
| Wnt signaling pathway | 0.002 | 0.040 | Top 1% FST | KEGG 2019 human | CREBBP, TCFL1, SMAD3, AXIN1, WNT2, PLCB2 |
| 0.0003 | 0.031 | Top 1% FST + XP-EHH | Wikipathways 2019, mouse | CREBBP, TCFL1, PPP2R2C, AXIN1, PRKD1, WNT2 | |
| Hippo signaling pathway | 0.002 | 0.040 | Top 1% FST | KEGG 2019 Human | LATS1, TCF7L1, SMAD3, AXIN1, CTNNA3, WNT2 |
| 0.001 | 0.028 | Top 1% FST + XP-EHH | KEGG 2019 Human | LATS1, TCFL1, SMAD3, PPP2R2C, AXIN1, CTNNA3, WNNT2 | |
| Apelin | 0.001 | 0.026 | Top 1% FST | KEGG 2019 Human | ADCY9, SMAD3, ITPR2, ADCY2, PLCB2, SLC8A2 |
| 0.002 | 0.050 | Top 1% FST + XP-EHH | KEGG 2019 Human | ADCY9, SMAD3, ITPR2, ADCY2, PLCB2, SLC8A2 | |
| ErbB and EGFR | 0.0002 | 0.047 | Top 1% FST | BioPlanet 2019 | ADCY9, PDPK1, PDE1A, ITPR2, NRG1, ADCY2 |
| TGF-β | 0.009 | 1.000 | Top 1% XP-EHH | BioPlanet 2019 | ARNT2, CDK6, LPAR1, CTNNAL1, STEAP2 |
| Body Mass Index | 0.0002 | 0.313 | Consensus | GWAS catalog 2019 | ZRANB3, EPB41L4B, FRRS1L, UBXN4, RAB3GAP1 |
| Endothelins | 0.003 | 0.158 | Top 1% FST + XP-EHH | BioPlanet 2019 | ADCY9, ADCY2, PLCB2, BCAR1 |
| Aldosterone | 0.0001 | 0.008 | Top 1% FST | KEGG 2019 Human | ADCY9, ITPR2, ADCY2, PRKD1, PLCB2, CACNA1H |
| Renin | 0.0002 | 0.008 | Top 1% FST | KEGG 2019 Human | ADCYAP1R1, PDE1A, PDE3A, ITPR2, PLCB2 |
| Platelet activation | 0.003 | 0.046 | Top 1% FST | KEGG 2019 Human | ADCY9, ITPR2, ADCY2, TLN2, PLCB2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bionda, A.; Cortellari, M.; Bagardi, M.; Frattini, S.; Negro, A.; Locatelli, C.; Brambilla, P.G.; Crepaldi, P. A Genomic Study of Myxomatous Mitral Valve Disease in Cavalier King Charles Spaniels. Animals 2020, 10, 1895. https://doi.org/10.3390/ani10101895
Bionda A, Cortellari M, Bagardi M, Frattini S, Negro A, Locatelli C, Brambilla PG, Crepaldi P. A Genomic Study of Myxomatous Mitral Valve Disease in Cavalier King Charles Spaniels. Animals. 2020; 10(10):1895. https://doi.org/10.3390/ani10101895
Chicago/Turabian StyleBionda, Arianna, Matteo Cortellari, Mara Bagardi, Stefano Frattini, Alessio Negro, Chiara Locatelli, Paola Giuseppina Brambilla, and Paola Crepaldi. 2020. "A Genomic Study of Myxomatous Mitral Valve Disease in Cavalier King Charles Spaniels" Animals 10, no. 10: 1895. https://doi.org/10.3390/ani10101895
APA StyleBionda, A., Cortellari, M., Bagardi, M., Frattini, S., Negro, A., Locatelli, C., Brambilla, P. G., & Crepaldi, P. (2020). A Genomic Study of Myxomatous Mitral Valve Disease in Cavalier King Charles Spaniels. Animals, 10(10), 1895. https://doi.org/10.3390/ani10101895





