Antagonistic Impact of Acrylamide and Ethanol on Biochemical and Morphological Parameters Consistent with Bone Health in Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Biochemical Analysis
2.3. Macroscopical Analysis
2.4. Micro-CT Analysis
2.5. Histomorphological Analysis
2.6. Statistics
3. Results
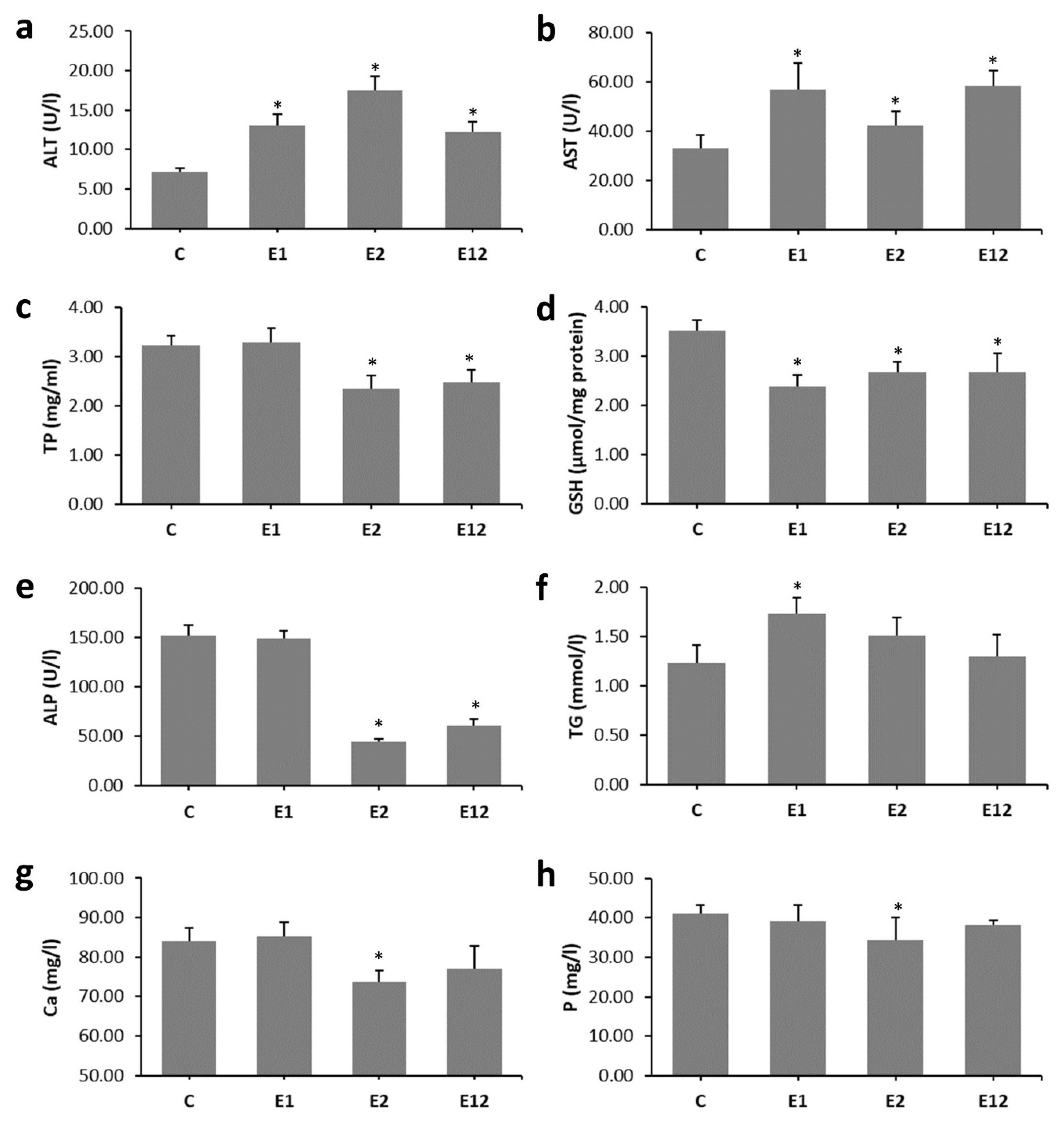
3.1. Biochemical Analysis
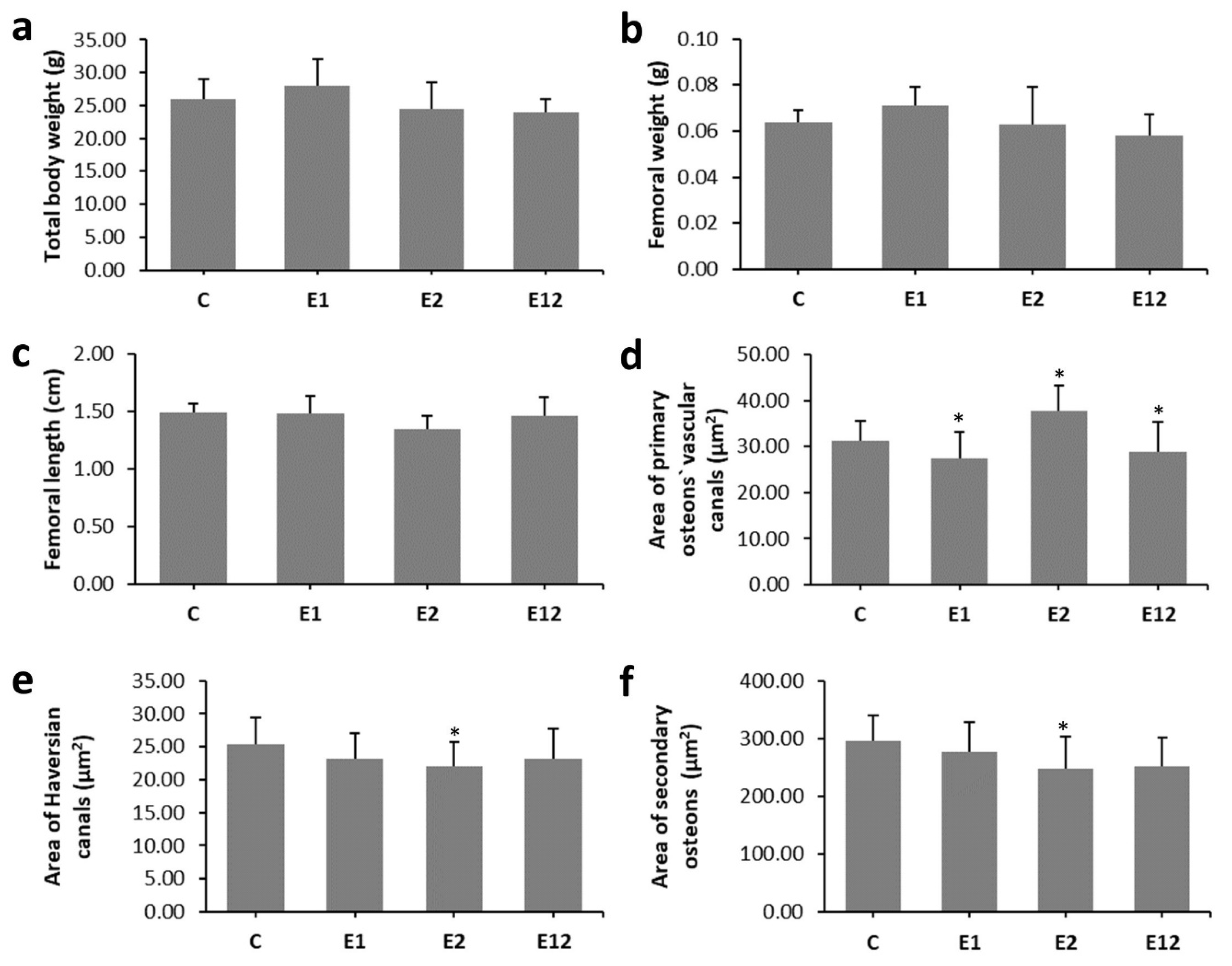
3.2. Macroscopical Analysis
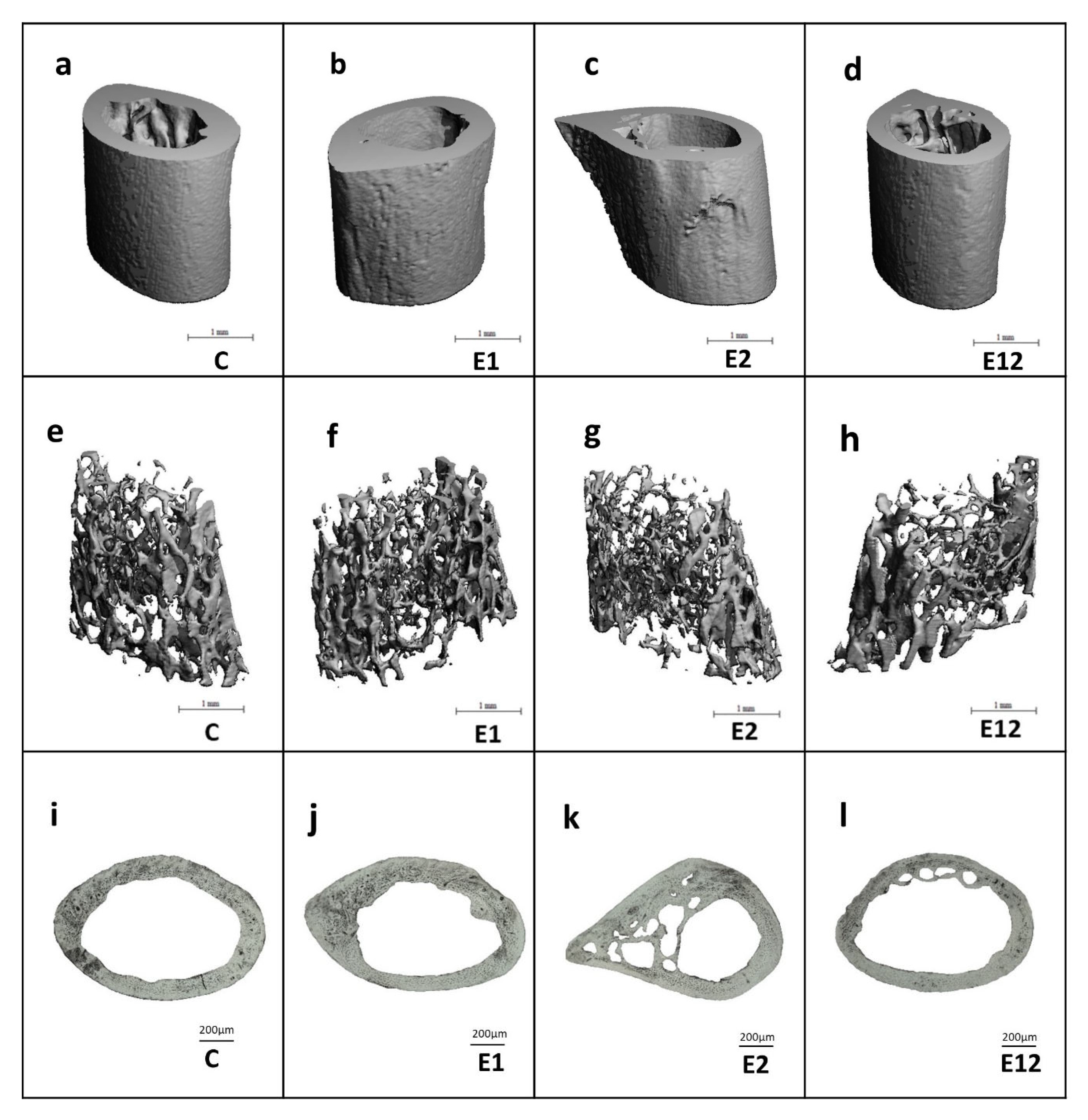
3.3. Micro-CT Analysis
3.4. Histomorphological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Semla, M.; Goc, Z.; Martiniaková, M.; Omelka, R.; Formicki, G. Acrylamide: A common food toxin related to physiological functions and health. Physiol. Res. 2017, 66, 205–217. [Google Scholar] [CrossRef]
- Broulík, P.D.; Vondrová, J.; Růzicka, P.; Sedlácek, R.; Zíma, T. The effect of chronic alcohol administration on bone mineral content and bone strength in male rats. Physiol. Res. 2010, 59, 599–604. [Google Scholar]
- Sarocka, A.; Babosova, R.; Kovacova, V.; Omelka, R.; Semla, M.; Kapusta, E.; Goc, Z.; Formicki, G.; Martiniakova, M. Acrylamide-induced changes in femoral bone microstructure of mice. Physiol. Res. 2017, 66, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Sarocka, A.; Babosova, R.; Grosskopf, B.; Kapusta, E.; Goc, Z.; Formicki, G.; Omelka, R. Changes in the microstructure of compact and trabecular bone tissues of mice subchronically exposed to alcohol. J. Biol Res. Thessal. 2018, 25, 8. [Google Scholar] [CrossRef] [Green Version]
- Renato Romero, J.; Krause Neto, W.; Sabbag da Silva, A.; Luiz Dos Santos, E.; Aurélio Added, M.; Pianca, E.; Florencio Gama, E.; Rodrigues de Souza, R. Chronic cachaça consumption affects the structure of tibial bone by decreasing bone density and density of mature collagen fibers in middle-aged Wistar rats. Aging Male 2018, 1–6. [Google Scholar] [CrossRef]
- Sarocka, A.; Kovacova, V.; Omelka, R.; Grosskopf, B.; Kapusta, E.; Goc, Z.; Formicki, G.; Martiniakova, M. Single and simultaneous effects of acrylamide and ethanol on bone microstructure of mice after one remodeling cycle. BMC Pharm. Toxicol. 2019, 20, 38. [Google Scholar] [CrossRef]
- Belhadj Benziane, A.; Dilmi Bouras, A.; Mezaini, A.; Belhadri, A.; Benali, M. Effect of oral exposure to acrylamide on biochemical and hematologic parameters in Wistar rats. Drug Chem. Toxicol. 2019, 42, 157–166. [Google Scholar] [CrossRef]
- Goc, Z.; Kapusta, E.; Formicki, G.; Martiniaková, M.; Omelka, R. Effect of taurine on ethanol-induced oxidative stress in mouse liver and kidney. Chin. J. Physiol. 2019, 62, 148–156. [Google Scholar] [CrossRef]
- Bartlett, P.J.; Antony, A.N.; Agarwal, A.; Hilly, M.; Prince, V.L.; Combettes, L.; Hoek, J.B.; Gaspers, L.D. Chronic alcohol feeding potentiates hormone-induced calcium signalling in hepatocytes. J. Physiol. 2017, 595, 3143–3164. [Google Scholar] [CrossRef]
- Park, B.-J.; Lee, Y.-J.; Lee, H.-R. Chronic liver inflammation: Clinical implications beyond alcoholic liver disease. World J. Gastroenterol. 2014, 20, 2168–2175. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-R.; Lazarenko, O.P.; Shankar, K.; Blackburn, M.L.; Lumpkin, C.K.; Badger, T.M.; Ronis, M.J.J. Inhibition of NADPH oxidases prevents chronic ethanol-induced bone loss in female rats. J. Pharm. Exp. 2011, 336, 734–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burek, J.D.; Albee, R.R.; Beyer, J.E.; Bell, T.J.; Carreon, R.M.; Morden, D.C.; Wade, C.E.; Hermann, E.A.; Gorzinski, S.J. Subchronic toxicity of acrylamide administered to rats in the drinking water followed by up to 144 days of recovery. J. Env. Pathol. Toxicol. 1980, 4, 157–182. [Google Scholar]
- Bull, R.J.; Robinson, M.; Stober, J.A. Carcinogenic activity of acrylamide in the skin and lung of Swiss-ICR mice. Cancer Lett. 1984, 24, 209–212. [Google Scholar] [CrossRef]
- Doerge, D.R.; Gamboa da Costa, G.; McDaniel, L.P.; Churchwell, M.I.; Twaddle, N.C.; Beland, F.A. DNA adducts derived from administration of acrylamide and glycidamide to mice and rats. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2005, 580, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Martiniaková, M.; Boboňová, I.; Omelka, R.; Grosskopf, B.; Stawarz, R.; Toman, R. Structural changes in femoral bone tissue of rats after subchronic peroral exposure to selenium. Acta Vet. Scand. 2013, 55, 8. [Google Scholar] [CrossRef] [Green Version]
- Martiniaková, M.; Chovancová, H.; Omelka, R.; Grosskopf, B.; Toman, R. Effects of a single intraperitoneal administration of cadmium on femoral bone structure in male rats. Acta Vet. Scand 2011, 53, 49. [Google Scholar] [CrossRef] [Green Version]
- Enlow, D.H.; Brown, S.O. A comparative histological study of fossil and recent bone tissues. Part I. Tex. J. Sci. 1956, 8, 405–412. [Google Scholar]
- De Ricqles, A. Comparative microstructure of bone. In Bone Matrix and Bone Specific Products; CRC Press Inc.: Boca Raton, FL, USA, 1991; pp. 1–78. [Google Scholar]
- Breitling, L.P. Liver enzymes and bone mineral density in the general population. J. Clin. Endocrinol. Metab. 2015, 100, 3832–3840. [Google Scholar] [CrossRef]
- Allam, A.A.; El-Ghareeb, A.W.; Abdul-Hamid, M.; Bakery, A.E.; Gad, M.; Sabri, M. Effect of prenatal and perinatal acrylamide on the biochemical and morphological changes in liver of developing albino rat. Arch. Toxicol. 2010, 84, 129–141. [Google Scholar] [CrossRef]
- Gao, B.; Xu, M.-J.; Bertola, A.; Wang, H.; Zhou, Z.; Liangpunsakul, S. Animal models of alcoholic liver disease: Pathogenesis and clinical relevance. Gene Expr. 2017, 17, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Nakchbandi, I.A. Osteoporosis and fractures in liver disease: Relevance, pathogenesis and therapeutic implications. World J. Gastroenterol. 2014, 20, 9427–9438. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.J.; Babbs, C.; Warnes, T.W.; Neary, R.H. Hypophosphataemia in acute liver failure. Br. Med. J. Clin. Res. Ed. 1987, 295, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Camilli, J.A.; da Cunha, M.R.; Bertran, C.A.; Kawachi, E.Y. Subperiosteal hydroxyapatite implants in rats submitted to ethanol ingestion. Arch. Oral Biol. 2004, 49, 747–753. [Google Scholar] [CrossRef]
- González-Reimers, E.; Quintero-Platt, G.; Rodríguez-Rodríguez, E.; Martínez-Riera, A.; Alvisa-Negrín, J.; Santolaria-Fernández, F. Bone changes in alcoholic liver disease. World J. Hepatol. 2015, 7, 1258–1264. [Google Scholar] [CrossRef]
- Ghorbel, I.; Elwej, A.; Chaabene, M.; Boudawara, O.; marrakchi, R.; Jamoussi, K.; Boudawara, T.S.; Zeghal, N. Effects of acrylamide graded doses on metallothioneins I and II induction and DNA fragmentation: Bochemical and histomorphological changes in the liver of adult rats. Toxicol. Ind. Health 2017. [Google Scholar] [CrossRef]
- Mahmood, S.A.F.; Amin, K.; Rahman, H.S.; Othman, H.H. The pathophysiological effects of acrylamide in albino wister rats. Int. J. Med. Res. Health Sci. 2016, 5, 42–48. [Google Scholar]
- TR-575: Technical Report Pathology Tables and Curves. Available online: https://ntp.niehs.nih.gov/data/tables/tr/500s/tr575/index.html (accessed on 8 September 2020).
- Sayon-Orea, C.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Alcohol consumption and body weight: A systematic review. Nutr. Rev. 2011, 69, 419–431. [Google Scholar] [CrossRef]
- Turner, R.T.; Kidder, L.S.; Kennedy, A.; Evans, G.L.; Sibonga, J.D. Moderate alcohol consumption suppresses bone turnover in adult female rats. J. Bone Miner. Res. 2001, 16, 589–594. [Google Scholar] [CrossRef]
- Mercer, K.E.; Wynne, R.A.; Lazarenko, O.P.; Lumpkin, C.K.; Hogue, W.R.; Suva, L.J.; Chen, J.-R.; Mason, A.Z.; Badger, T.M.; Ronis, M.J.J. Vitamin D Supplementation protects against bone loss associated with chronic alcohol administration in female mice. J. Pharm. Exp. 2012, 343, 401–412. [Google Scholar] [CrossRef] [Green Version]
- Trevisiol, C.H.; Turner, R.T.; Pfaff, J.E.; Hunter, J.C.; Menagh, P.J.; Hardin, K.; Ho, E.; Iwaniec, U.T. Impaired osteoinduction in a rat model for chronic alcohol abuse. Bone 2007, 41, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bao, Q.; Chen, S.; Liu, H.; Feng, J.; Qin, H.; Li, A.; Liu, D.; Shen, Y.; Zhao, Y.; et al. Different bone remodeling levels of trabecular and cortical bone in response to changes in Wnt/β-catenin signaling in mice. J. Orthop. Res. 2017, 35, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, E.F. Cellular mechanisms of bone remodeling. Rev. Endocr. Metab. Disord. 2010, 11, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaddini, G.W.; Turner, R.T.; Grant, K.A.; Iwaniec, U.T. Alcohol: A simple nutrient with complex actions on bone in the adult skeleton. Alcohol. Clin. Exp. Res. 2016, 40, 657–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piemontese, M.; Almeida, M.; Robling, A.G.; Kim, H.-N.; Xiong, J.; Thostenson, J.D.; Weinstein, R.S.; Manolagas, S.C.; O’Brien, C.A.; Jilka, R.L. Old age causes de novo intracortical bone remodeling and porosity in mice. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
- Greenlee, D.M.; Dunnell, R.C. Identification of fragmentary bone from the Pacific. J. Archaeol. Sci. 2010, 37, 957–970. [Google Scholar] [CrossRef]
- Pries, A.R.; Reglin, B.; Secomb, T.W. Remodeling of blood vessels: Responses of diameter and wall thickness to hemodynamic and metabolic stimuli. Hypertension 2005, 46, 725–731. [Google Scholar] [CrossRef] [Green Version]
- Kuchay, M.S.; Mishra, S.K.; Farooqui, K.J.; Bansal, B.; Wasir, J.S.; Mithal, A. Hypercalcemia of advanced chronic liver disease: A forgotten clinical entity! Clin. Cases Min. Bone Metab. 2016, 13, 15–18. [Google Scholar] [CrossRef]
- Miller, M.; Seidler, A.; Kwiterovich, P.O.; Pearson, T.A. Long-term predictors of subsequent cardiovascular events with coronary artery disease and “desirable” levels of plasma total cholesterol. Circulation 1992, 86, 1165–1170. [Google Scholar] [CrossRef] [Green Version]
- Palaparthy, R.; Saini, B.K.; Gulati, A. Modulation of diaspirin crosslinked hemoglobin induced systemic and regional hemodynamic response by ethanol in normal rats. Life Sci. 2001, 68, 1383–1394. [Google Scholar] [CrossRef]
- Felder, A.A.; Phillips, C.; Cornish, H.; Cooke, M.; Hutchinson, J.R.; Doube, M. Secondary osteons scale allometrically in mammalian humerus and femur. R. Soc. Open Sci. 2017, 4, 170431. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martiniakova, M.; Sarocka, A.; Kovacova, V.; Kapusta, E.; Goc, Z.; Gren, A.; Formicki, G.; Omelka, R. Antagonistic Impact of Acrylamide and Ethanol on Biochemical and Morphological Parameters Consistent with Bone Health in Mice. Animals 2020, 10, 1835. https://doi.org/10.3390/ani10101835
Martiniakova M, Sarocka A, Kovacova V, Kapusta E, Goc Z, Gren A, Formicki G, Omelka R. Antagonistic Impact of Acrylamide and Ethanol on Biochemical and Morphological Parameters Consistent with Bone Health in Mice. Animals. 2020; 10(10):1835. https://doi.org/10.3390/ani10101835
Chicago/Turabian StyleMartiniakova, Monika, Anna Sarocka, Veronika Kovacova, Edyta Kapusta, Zofia Goc, Agnieszka Gren, Grzegorz Formicki, and Radoslav Omelka. 2020. "Antagonistic Impact of Acrylamide and Ethanol on Biochemical and Morphological Parameters Consistent with Bone Health in Mice" Animals 10, no. 10: 1835. https://doi.org/10.3390/ani10101835
APA StyleMartiniakova, M., Sarocka, A., Kovacova, V., Kapusta, E., Goc, Z., Gren, A., Formicki, G., & Omelka, R. (2020). Antagonistic Impact of Acrylamide and Ethanol on Biochemical and Morphological Parameters Consistent with Bone Health in Mice. Animals, 10(10), 1835. https://doi.org/10.3390/ani10101835




