Risk Assessment of Heavy Metals in Selected Marine Fish Species of Gadani Shipbreaking Area and Pakistan
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
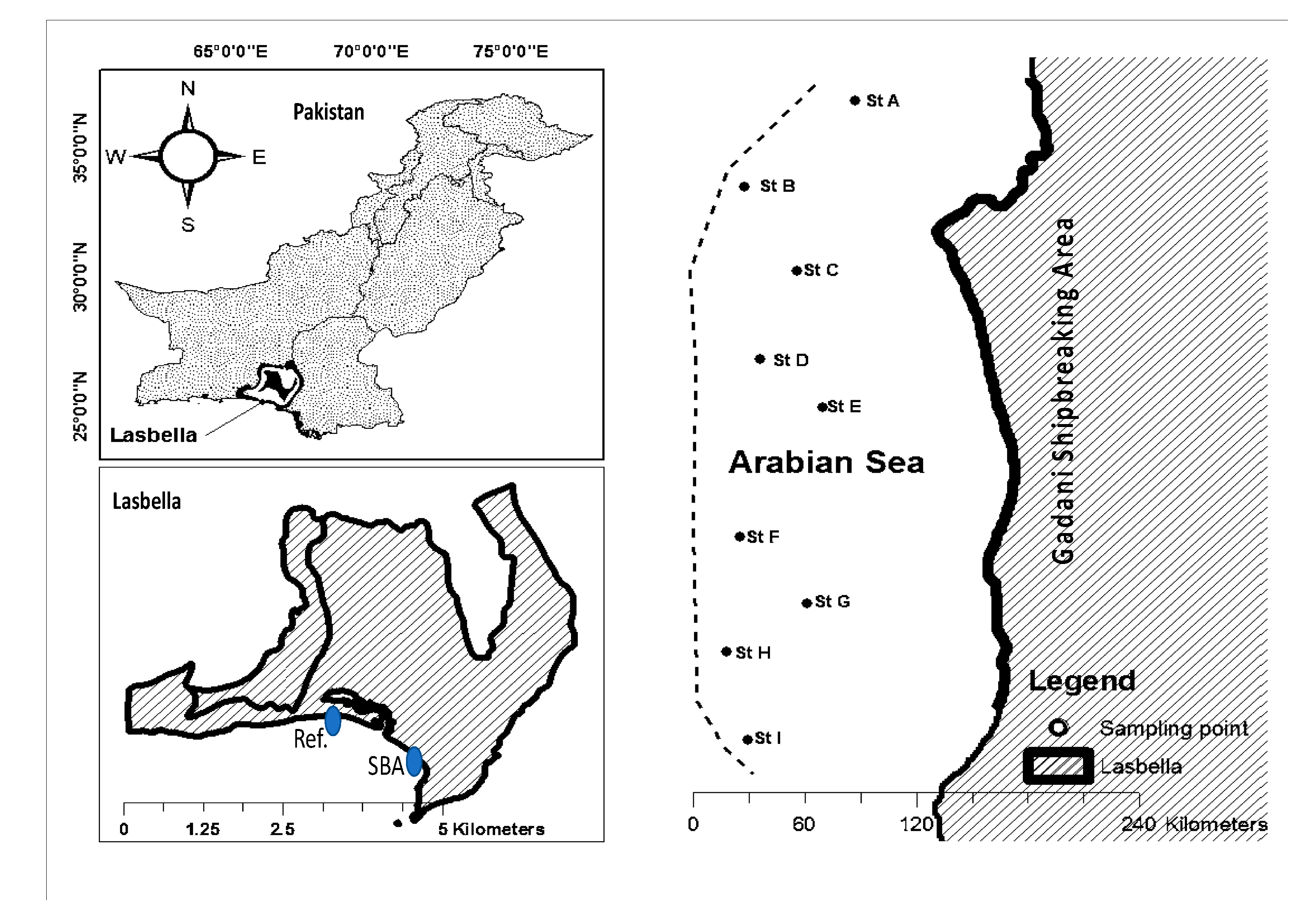
2.1. Study Area
2.2. Collection of Samples
- As fish are edible, and the killing of fish was indispensable, hence killing of fish samples was allowed to determine the heavy metals’ concentration in various body parts.
- The killing of fish samples followed procedures to avoid distress and caused rapid loss of consciousness without pain until death.
- After the experiment, the remains of body parts were properly disposed of in a landfill.
2.3. Physiochemical Analysis and Samples’ Treatment
2.4. Analytical Procedures and Quality Control
2.5. Human Exposure Assessment
2.5.1. Bioaccumulation Factor (BAF)
2.5.2. Consumption Data
2.5.3. Estimated Daily Intake (EDI)
2.5.4. Target Hazard Quotient (THQ)
2.5.5. Hazard Index (HI)
2.5.6. Target Cancer Risk (TR)
2.6. Statistical Analysis
3. Results
3.1. Physico-Chemical Analysis and Heavy Metal Concentration in Seawater
3.2. Heavy Metals’ Content in Fish
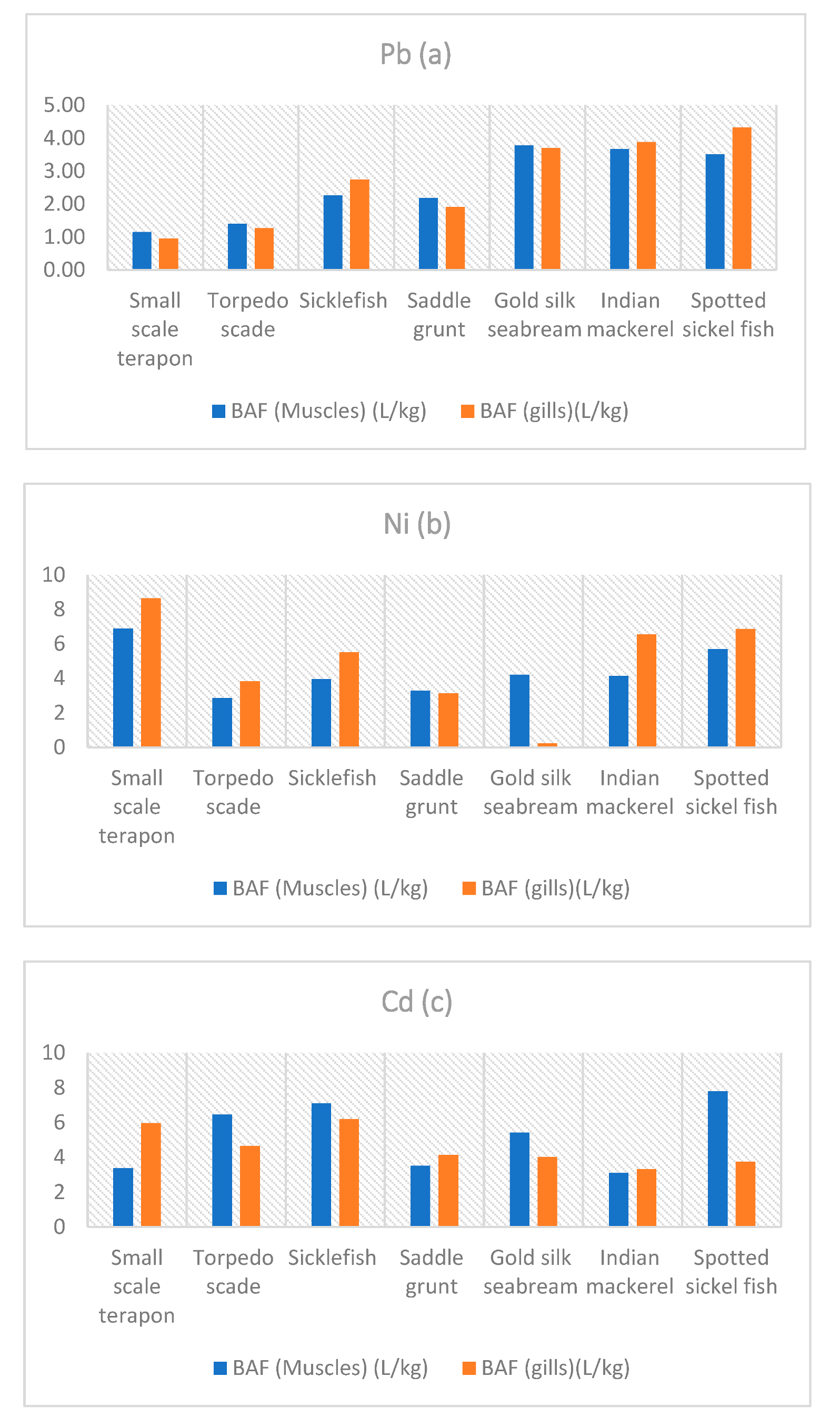
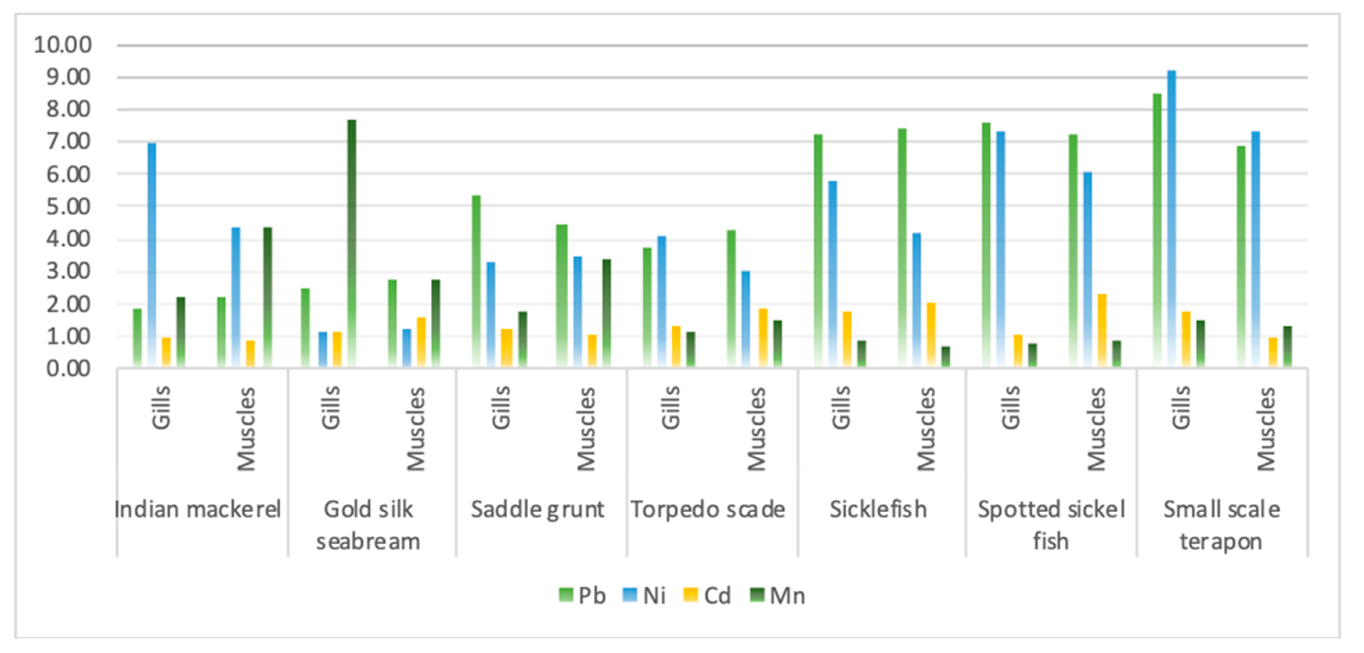
3.3. Bioaccumulation Factor
3.4. Risk Assessment
3.4.1. Estimated Daily Intake (EDI)
3.4.2. Target Hazard Quotient (THQ) and Hazard Index (HI)
3.4.3. Target Cancer Risk (TR)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- You, S.H.; Wang, S.L.; Pan, W.H.; Chan, W.C.; Fan, A.M.; Lin, P. Risk assessment of methylmercury based on internal exposure and fish and seafood consumption estimates in Taiwanese children. Int. J. Hyg. Environ. Health 2018, 221, 697–703. [Google Scholar] [CrossRef]
- Pal, J.; Shukla, B.N.; Maurya, A.K.; Verma, H.O.; Pandey, G.; Amitha, A. A review on role of fish in human nutrition with special emphasis to essential fatty acid. Int. J. Fish. Aq. St. 2018, 6, 427–430. [Google Scholar]
- Goretti, E.; Pallottini, M.; Ricciarini, M.I.; Selvaggi, R.; Cappelletti, D. Heavy metals bioaccumulation in selected tissues of red swamp crayfish: An easy tool for monitoring environmental contamination levels. Sci. Total Environ. 2016, 559, 339–346. [Google Scholar] [CrossRef]
- Mendoza-Carranza, M.; Sepúlveda-Lozada, A.; Dias-Ferreira, C.; Geissen, V. Distribution and bioconcentration of heavy metals in a tropical aquatic food web: A case study of a tropical estuarine lagoon in SE Mexico. Environ. Pollut. 2016, 210, 155–165. [Google Scholar] [CrossRef]
- Liu, J.-L.; Xu, X.-R.; Ding, Z.-H.; Peng, J.-X.; Jin, M.-H.; Wang, Y.-S.; Hong, Y.-G.; Yue, W.-Z. Heavy metals in wild marine fish from South China Sea: Levels, tissue-and species-specific accumulation and potential risk to humans. Ecotoxicology 2015, 24, 1583–1592. [Google Scholar] [CrossRef]
- Copat, C.; Arena, G.; Fiore, M.; Ledda, C.; Fallico, R.; Sciacca, S.; Ferrante, M. Heavy metals concentrations in fish and shellfish from eastern Mediterranean Sea: Consumption advisories. Food Chem. Toxicol. 2013, 53, 33–37. [Google Scholar] [CrossRef]
- Hossain, M.S.; Fakhruddin, A.N.M.; Chowdhury, M.A.Z.; Gan, S.H. Impact of ship-Breaking activities on the coastal environment of Bangladesh and a management system for its sustainability. Environ. Sci. Policy 2016, 60, 84–94. [Google Scholar] [CrossRef]
- Devault, D.A.; Beilvert, B.; Winterton, P. Ship breaking or scuttling? A review of environmental, economic and forensic issues for decision support. Environ. Sci. Pollut. Res. 2017, 24, 25741–25774. [Google Scholar] [CrossRef]
- Hiremath, A.M.; Tilwankar, A.K.; Asolekar, S.R. Significant steps in ship recycling vis-a-vis wastes generated in a cluster of yards in Alang: A case study. J. Clean. Prod. 2015, 87, 520–532. [Google Scholar] [CrossRef]
- Pejman, A.; Bidhendi, G.N.; Ardestani, M.; Saeedi, M.; Baghvand, A. A new index for assessing heavy metals contamination in sediments: A case study. Ecol. Indic. 2015, 58, 365–373. [Google Scholar] [CrossRef]
- Li, C.; Sun, M.; Song, C.; Tao, P.; Yin, Y.; Shao, M. Assessment of heavy metal contamination in the sediments of the Shuangtaizi estuary using multivariate statistical techniques. Soil Sediment Contam. Int. J. 2017, 26, 45–58. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M.; Alikunhi, N.; Al-Jahdali, H.; Al-Jebreen, D.; Al-Suwailem, A.; Aziz, M.A.M.; Batang, Z.B. Human health risk from metals in fish from Saudi Arabia: Consumption patterns for some species exceed allowable limits. Hum. Ecol. Risk Assess. Int. J. 2015, 21, 799–827. [Google Scholar] [CrossRef]
- Shaheen, A.; Baig, H.S.; Kazmi, S.U. Microbial Flora Isolated from Polluted and Non-Polluted Coastal Waters of Karachi. Pak. J. Bot. 2016, 48, 1703–1708. [Google Scholar]
- Saher, N.U.; Siddiqui, A.S. Comparison of heavy metal contamination during the last decade along the coastal sediment of Pakistan: Multiple pollution indices approach. Mar. Pollut. Bull. 2016, 105, 403–410. [Google Scholar] [CrossRef]
- Memon, A.A.; Zarar, M. Comprehensive Analysis of Existing Infrastructure Conditions Correlating Ship-Breaking Activities and its Implications on Workers and Community a Case Study of Gaddani Town and Ship-Breaking Industry, Baluchistan, Pakistan. Am. Sci. Res. J. Eng. Technol. Sci. 2016, 17, 245–257. [Google Scholar]
- Sarraf, M.; Stuer-Lauridsen, F.; Bloch, R.; Watkinson, R. The Ship Breaking and Recycling Industry in Bangladesh and Pakistan; Report No 58275-SAS.; World Bank: Washington, DC, USA, 2010; Available online: http://crossasia-repository.ub.uni-heidelberg.de/3749/ (accessed on 2 October 2019).
- Chernoff, B. A method for wet digestion of fish tissue for heavy metal analyses. Trans. Am. Fish. Soc. 1975, 104, 803–804. [Google Scholar] [CrossRef]
- Guerranti, C.; Grazioli, E.; Focardi, S.; Renzi, M.; Perra, G. Levels of chemicals in two fish species from four Italian fishing areas. Mar. Pollut. Bull. 2016, 111, 449–452. [Google Scholar] [CrossRef]
- Milošković, A.; Dojčinović, B.; Simić, V. Heavy metal and trace element bioaccumulation in target tissues of three edible predatory fish species from Bovan Reservoir (Serbia). Fresenius Environ. Bull. 2014, 23, 1884–1891. [Google Scholar]
- Rajeshkumar, S.; Liu, Y.; Zhang, X.; Ravikumar, B.; Bai, G.; Li, X. Studies on seasonal pollution of heavy metals in water, sediment, fish and oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere 2018, 191, 626–638. [Google Scholar] [CrossRef]
- Daziel, J.; Baker, C. Analytical methods for measuring metals by atomic absorption spectrometry. FAO Fish Tech. 1983, 212, 14–21. [Google Scholar]
- USEPA. Available online: https://www.epa.gov/sites/production/files/2015-08/documents/method_200-9_rev_2-2_1994.pdf (accessed on 5 July 2019).
- Tüzen, M. Determination of heavy metals in fish samples of the middle Black Sea (Turkey) by graphite furnace atomic absorption spectrometry. Food Chem. 2003, 80, 119–123. [Google Scholar] [CrossRef]
- Rathore, D.P.S. Trends in the Methods of Measurement in Analytical Chemistry. Explor. Res. Min. 2007, 17, 145–149. [Google Scholar]
- Crookes, M. Estimation Of Fish Bioconcentration Factor (BCF) From Depuration Data. Bristle: Environmental Agency Report. 2011. Available online: https://Assets.Publishing.Service.Gov.Uk/Government/Uploads/System/Uploads/Attachment_Data/File/291527/Scho0811buce-E-E.Pdf (accessed on 3 July 2019).
- Van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Statistics Pakistan Bureau of NBS Pakistan. Available online: http://www.pbs.gov.pk/content/pakistan-statistical-year-book-2012 (accessed on 5 July 2019).
- Ullah, A.K.M.A.; Maksud, M.A.; Khan, S.R.; Lutfa, L.N.; Quraishi, S.B. Dietary intake of heavy metals from eight highly consumed species of cultured fish and possible human health risk implications in Bangladesh. Toxicol. Rep. 2017, 4, 574–579. [Google Scholar] [CrossRef]
- EPA, U.S. Environmental Protection Agency 2011; Rep. No. EPA 430-R-11 5; EPA, U.S.: Washington, DC, USA, 2011. Available online: https://archive.epa.gov/epawaste/hazard/tsd/td/web/pdf/05hhrap7.pdf (accessed on 16 July 2019).
- USEPA. Region 3 Risk-Based Concentration Table: Technical Background Information. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls (accessed on 14 August 2019).
- Hasan, A.B.; Kabir, S.; Reza, A.S.; Zaman, M.N.; Ahsan, A.; Rashid, M. Enrichment factor and geo-accumulation index of trace metals in sediments of the ship breaking area of Sitakund Upazilla (Bhatiary–Kumira), Chittagong, Bangladesh. J. Geo. Expl. 2013, 125, 130–137. [Google Scholar] [CrossRef]
- Hossain, M.M.M.; Rahman, M.A. Ship breaking activities: Threats to coastal environment and fish biodiversity. In Eco-System Health and Management of Pollution in the Bay of Bengal Support to Sustainable Management of the BOBLME Project; Bangladesh Fisheries Research Institute (BFRI): Mymensingh, Bangladesh, 2010. [Google Scholar]
- Reddy, M.S.; Basha, S.; Joshi, H.V.; Ramachandraiah, G. Seasonal distribution and contamination levels of total PHCs, PAHs and heavy metals in coastal waters of the Alang–Sosiya ship scrapping yard. Chemosphere 2005, 61, 1587–1593. [Google Scholar] [CrossRef]
- FAO. Compilation of Legal Limits for Hazardous Substance in Fish and Fishery Products. Available online: http://www.fao.org/inland-fisheries/topics/detail/en/c/1150083/ (accessed on 5 September 2019).
- WHO. Heavy metals environmental aspects. In Environmental Health Criteria. Available online: http://wedocs.unep.org/handle/20.500.11822/29400 (accessed on 5 July 2019).
- USEPA. Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories, Risk Assessment and Fish Consumption Limit. Available online: https://www.epa.gov/sites/production/files/2015-06/documents/volume2.pdf (accessed on 5 September 2019).
- Bayen, S. Occurrence, bioavailability and toxic effects of trace metals and organic contaminants in mangrove ecosystems: A review. Environ. Int. 2012, 48, 84–101. [Google Scholar] [CrossRef]
- Alam, S.; Faruque, A. Legal regulation of the shipbreaking industry in Bangladesh: The international regulatory framework and domestic implementation challenges. Mar. Policy 2014, 47, 46–56. [Google Scholar] [CrossRef]
- Demaria, F. Shipbreaking at Alang-Sosiya (India): An ecological distribution conflict. Ecol. Econ. 2010, 70, 250–260. [Google Scholar] [CrossRef]
- Martín-Torre, M.C.; Payán, M.C.; Verbinnen, B.; Coz, A.; Ruiz, G.; Vandecasteele, C.; Viguri, J.R. Metal Release from Contaminated Estuarine Sediment under pH Changes in the Marine Environment. Arch. Environ. Contam. Toxicol. 2015, 68, 577–587. [Google Scholar] [CrossRef]
- Gambrell, R.P.; Wiesepape, J.B.; Patrick, W.H.; Duff, M.C. The effects of pH, redox, and salinity on metal release from a contaminated sediment. Water. Air. Soil Pollut. 1991, 57, 359–367. [Google Scholar] [CrossRef]
- García-Seoane, E.; Coelho, J.P.; Mieiro, C.; Dolbeth, M.; Ereira, T.; Rebelo, J.E.; Pereira, E. Effect of historical contamination in the fish community structure of a recovering temperate coastal lagoon. Mar. Pollut. Bull. 2016, 111, 221–230. [Google Scholar] [CrossRef]
- Copat, C.; Bella, F.; Castaing, M.; Fallico, R.; Sciacca, S.; Ferrante, M. Heavy metals concentrations in fish from Sicily (Mediterranean Sea) and evaluation of possible health risks to consumers. Bull. Environ. Contam. Toxicol. 2012, 88, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Islam, M. Ship Breaking Activities and Its Impact on the Coastal Zone of Chittagong. Available online: http://ypsa.org/publications/Impact.pdf (accessed on 13 December 2019).
- Hanna, R.G.M. Levels of heavy metals in some Red Sea fish before hot brine pools mining. Mar. Pollut. Bull. 1989, 20, 631–635. [Google Scholar] [CrossRef]
- Jordao, C.P.; Pereira, J.L.; Jham, G.N.; Bellato, R. Distribution of heavy metals in environmental samples near smelters and mining areas in Brazil. Environ. Technol. 1999, 20, 489–498. [Google Scholar] [CrossRef]
- Ismail, N.S.; Abu-Hilal, A.H. Heavy metals in three commonly available coral reef fish species from the Jordan Gulf of Aqaba, Red Sea. Jordan J. Biol. Sci. 2008, 2, 61–66. [Google Scholar]
- Ali, A.A.; Elazein, E.M.; Alian, M.A. Investigation of heavy metals pollution in water, sediment and fish at Red Sea-Jeddah Coast-KSA at two different locations. J. Appl. Environ. Biol. Sci. 2011, 1, 630–637. [Google Scholar]
- Rejomon, G.; Nair, M.; Joseph, T. Trace metal dynamics in fishes from the southwest coast of India. Environ. Monit. Assess. 2010, 167, 243–255. [Google Scholar] [CrossRef]
- Rahman, M.S.; Molla, A.H.; Saha, N.; Rahman, A. Study on heavy metals levels and its risk assessment in some edible fishes from Bangshi River, Savar, Dhaka, Bangladesh. Food Chem. 2012, 134, 1847–1854. [Google Scholar] [CrossRef]
- Alipour, H.; Pourkhabbaz, A.; Hassanpour, M. Estimation of potential health risks for some metallic elements by consumption of fish. Water Qual. Expo Health 2015, 7, 179–185. [Google Scholar] [CrossRef]
- Baki, M.A.; Hossain, M.M.; Akter, J.; Quraishi, S.B.; Shojib, M.F.H.; Ullah, A.K.M.A.; Khan, M.F. Concentration of heavy metals in seafood (fishes, shrimp, lobster and crabs) and human health assessment in Saint Martin Island, Bangladesh. Ecotoxicol. Environ. Saf. 2018, 159, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Kalay, M.; Ay, Ö.; Canli, M. Heavy metal concentrations in fish tissues from the Northeast Mediterranean Sea. Bull. Environ. Contam. Toxicol. 1999, 63, 673–681. [Google Scholar] [CrossRef]
- Sharif, A.K.M.; Mustafa, A.I.; Amin, M.N.; Akter, J.; Quraishi, S.B.; Shojib, M.F.H.; Ullah, A.K.M.A.; Khan, M.F. Trace element concentrations in tropical marine fish from the Bay of Bengal. Sci. Total Environ. 1993, 138, 223–234. [Google Scholar] [CrossRef]
- Arulkumar, A.; Paramasivam, S.; Rajaram, R. Toxic heavy metals in commercially important food fishes collected from Palk Bay, Southeastern India. Mar. Pollut. Bull. 2017, 119, 454–459. [Google Scholar] [CrossRef]
- Ateş, A.; Türkmen, M.; Tepe, Y. Assessment of heavy metals in fourteen marine fish species of four turkish seas. Indian J. Mar Sci. 2015, 44, 49–55. [Google Scholar]
- Biswas, S.; Prabhu, R.K.; Hussain, K.J.; Selvanayagam, M.; Satpathy, K.K. Heavy metals concentration in edible fishes from coastal region of Kalpakkam, southeastern part of India. Environ. Monit. Assess. 2012, 184, 5097–5104. [Google Scholar] [CrossRef] [PubMed]
- Jonathan, M.P.; Aurioles-Gamboa, D.; Villegas, L.E.C.; Bohórquez-Herrera, J.; Hernández-Camacho, C.J.; Sujitha, S.B. Metal concentrations in demersal fish species from Santa Maria Bay, Baja California Sur, Mexico (Pacific coast). Mar. Pollut. Bull. 2015, 99, 356–361. [Google Scholar] [CrossRef]
- El-Moselhy, K.M.; Othman, A.I.; El-Azem, H.A.; El-Metwally, M.E.A. Bioaccumulation of heavy metals in some tissues of fish in the Red Sea, Egypt. Egypt. J. Basic Appl. Sci. 2014, 1, 97–105. [Google Scholar] [CrossRef]
- Sharif, A.K.M.; Mustafa, A.I.; Mirza, A.H.; Safiullah, S. Trace metals in tropical marine fish from the Bay of Bengal. Sci. Total Environ. 1991, 107, 135–142. [Google Scholar] [CrossRef]
- Abarshi, M.M.; Dantala, E.O.; Mada, S.B. Bioaccumulation of heavy metals in some tissues of croaker fish from oil spilled rivers of Niger Delta region, Nigeria. Asian Pac. J. Trop. Biomed. 2017, 7, 563–568. [Google Scholar] [CrossRef]
- EC. Maximum Levels of Cadmium in Foodstuffs. Available online: https://www.ecolex.org/details/legislation/commission-regulation-ec-no-18812006-setting-maximum-levels-for-certain-contaminants-in-foodstuffs-lex-faoc068134/? (accessed on 12 December 2019).
- Aderinola, O.J.; Clarke, E.O.; Olarinmoye, O.M.; Kusemiju, V.; Anatekhai, M.A. Heavy metals in surface water, sediments, fish and Perwinklesof Lagos Lagoon. Am.-Eurasian, J. Agric. Environ. Sci. 2009, 5, 609–617. [Google Scholar]
- McGeer, J.C.; Brix, K.V.; Skeaff, J.M.; DeForest, D.K.; Brigham, S.I.; Adams, W.J.; Green, A. Inverse relationship between bioconcentration factor and exposure concentration for metals: Implications for hazard assessment of metals in the aquatic environment. Environ. Toxicol. Chem. 2003, 22, 1017–1037. [Google Scholar] [CrossRef]

| Scientific Name | Common Name | Habitant | No. Of Samples | Average Weight (g) | Length (cm) (Total Lenght) |
|---|---|---|---|---|---|
| Terapon puta Cuvier | Small-scale terapon | Pelagic | 12 | 45.359 ± 5.4 | 16 |
| Megalaspis cordyla) (IUCN red list) | Torpedo scade | Pelagic | 8 | 861.826 ± 4.3 | 25 |
| Drepane | Sicklefish | Benthic | 12 | 635.029 ± 6.2 | 25 |
| Pomadasys maculatus (IUCN red list) | Saddle grunt | Pelagic | 10 | 90.718 ± 5.3 | 16 |
| Acanthopagrus australis | Gold silk seabream | Demersal/coastal waters | 8 | 1859.73 ± 4.6 | 23 |
| Rastrelliger kanagurta | Indian mackerel | Shallow coastal waters | 12 | 226.796 ± 6.6 | 30 |
| Drepane punctata | Spotted sickle fish | shallow coastal waters | 12 | 635.029 ± 6.3 | 25 |
| Parameters | Shipbreaking Zone (Gadani) | Reference Zone (Miani Hor) | Other Regional Shipbreaking Areas | ||||
|---|---|---|---|---|---|---|---|
| Min–Max | Average ± SD | Min–Max | Average ± SD 1 | Average a | Range b | Average c | |
| Pb (mg/L) | 1.81–2.11 | 1.96 ± 0.07 | 0.92–1.01 | 0.97 ± 0.03 | 0.07 | 0.9–1.05 | 1.77 |
| Cr (mg/L) | 0.017–0.34 | 0.107 ± 0.08 | 0.02–0.11 | 0.066 ± 0.04 | 0.04 | 0.36–0.45 | 0.678 |
| Ni (mg/L) | 0.49–1.235 | 1.06 ± 0.15 | 0.71–0.95 | 0.84 ± 0.09 | 0.08 | 0.41–0.72 | 0.696 |
| Cd (mg/L) | 0.18–0.38 | 1.06 ± 0.15 | 0.20–0.26 | 0.23 ± 0.02 | 0.0034 | 0.04–0.06 | 0.446 |
| Mn (mg/L) | 0.027–0.25 | 0.05 ± 0.04 | 0.01–0.06 | 0.03 ± 0.02 | 0.48 | ND 2 | 4.36 |
| Temperature (°C) | 20.1–22.90 | 21.80 ± 1.31 | 19.98–22.89 | 22.13 ± 1.44 | |||
| Conductivity (μS/cm) | 36,500–38,500 | 37,280 ± 0.45 | 35,800–36,500 | 36,210 ± 0.27 | |||
| pH | 7.00–8.00 | 7.76 ± 0.06 | 7.01–8.00 | 7.89 ± 0.07 | |||
| Salinity (ppt) | 36.3–39.7 | 37.4 | 38–39 | 38.5 ± 0.3 | |||
| Pb (μg/g) | Ni (μg/g) | Cd (μg/g) | Mn (μg/g) | |||||
|---|---|---|---|---|---|---|---|---|
| Gills | Muscles | Gills | Muscles | Gills | Muscles | Gills | Muscles | |
| Indian mackerel | ||||||||
| Mean | 1.87 | 2.24 | 6.95 | 4.38 | 0.96 | 0.9 | 2.18 | 4.34 |
| SD | 0.77 | 1.18 | 5.02 | 1.62 | 0.64 | 0.27 | 4.40 | 4.52 |
| Minimum | 0.89 | 1.15 | 1.41 | 1.54 | 0.37 | 0.37 | 0.01 | 1.3 |
| Maximum | 2.85 | 4.04 | 13.7 | 6.2 | 2.16 | 1.12 | 11.16 | 10.59 |
| Gold silk Seabream | ||||||||
| Mean | 2.49 | 2.74 | 0.241 | 4.47 | 1.165 | 1.57 | 7.69 | 2.73 |
| SD | 1.29 | 1.07 | 3.07 | 2.60 | 0.38 | 1.46 | 12.86 | 8.90 |
| Minimum | 0.61 | 0.15 | 1.17 | 1.24 | 0.57 | 0.84 | 0.46 | 0.22 |
| Maximum | 4.65 | 4.99 | 11.86 | 12.37 | 2.02 | 1.7 | 44.6 | 44.4 |
| Saddle grunt | ||||||||
| Mean | 5.37 | 4.43 | 3.32 | 3.47 | 1.2 | 1.019 | 1.8 | 3.37 |
| SD | 1.42 | 1.31 | 2.05 | 2.63 | 0.80 | 0.45 | 1.16 | 4.75 |
| Minimum | 3.1 | 3.09 | 0.37 | 0.12 | 0.19 | 0.26 | 0.82 | 0.55 |
| Maximum | 7.34 | 7.13 | 5.84 | 8.14 | 3.34 | 1.56 | 3.94 | 17.64 |
| Torpedo scade | ||||||||
| Mean | 3.72 | 4.27 | 4.06 | 3.03 | 1.35 | 1.87 | 1.12 | 1.49 |
| SD | 2.27 | 1.86 | 3.53 | 2.43 | 0.44 | 1.18 | 0.62 | 1.07 |
| Minimum | 0.26 | 0.375 | 1.65 | 0.16 | 0.84 | 0.07 | 0.64 | 0.66 |
| Maximum | 7.05 | 6.89 | 13.85 | 7.32 | 2.24 | 4.19 | 2.84 | 3.64 |
| Sicklefish | ||||||||
| Mean | 7.59 | 7.40 | 5.83 | 4.18 | 1.8 | 2.06 | 0.91 | 0.73 |
| SD | 1.16 | 1.55 | 2.34 | 2.21 | 0.63 | 0.50 | 0.63 | 0.34 |
| Minimum | 5.87 | 4.15 | 9.4 | 6.81 | 0.84 | 1.17 | 0.09 | 0.28 |
| Maximum | 8.96 | 8.78 | 1.84 | 0.2 | 2.82 | 2.73 | 1.66 | 1.38 |
| Spotted sickle fish | ||||||||
| Mean | 8.45 | 6.87 | 7.28 | 6.041 | 1.09 | 2.26 | 0.81 | 0.83 |
| SD | 0.66 | 3.04 | 3.57 | 4.24 | 0.38 | 1.02 | 0.37 | 0.32 |
| Minimum | 7.56 | 2.71 | 3.75 | 1.5 | 0.71 | 1.11 | 0.12 | 0.35 |
| Maximum | 9.42 | 9.44 | 12.42 | 11.74 | 1.69 | 4.0 | 1.19 | 1.24 |
| Small-scale terapon | ||||||||
| Mean | 7.59 | 7.18 | 9.15 | 7.29 | 1.73 | 0.98 | 1.47 | 1.31 |
| SD | 1.16 | 2.89 | 1.98 | 3.07 | 1.05 | 0.49 | 1.25 | 0.85 |
| Minimum | 5.87 | 1.6 | 6.3 | 1.8 | 0.54 | 0.34 | 0.12 | 0.01 |
| Maximum | 8.96 | 9.17 | 11.41 | 10.76 | 3.12 | 1.64 | 3.35 | 2.41 |
| Permissible limitμg/g (WW) | 0.52 1 | 0.53 2 | 0.052 1 | 0.51 3 | ||||
| Fish Name | Pb | Ni | Cd | Mn |
|---|---|---|---|---|
| Small-scale terapon | 0.0006 | 0.002 | 0.00027 | 0.00036 |
| Torpedo scade | 0.0007 | 0.001 | 0.00051 | 0.00040 |
| Sicklefish | 0.0013 | 0.001 | 0.00056 | 0.00020 |
| Saddle grunt | 0.0011 | 0.001 | 0.00028 | 0.00091 |
| Gold silk seabream | 0.0020 | 0.001 | 0.00043 | 0.00074 |
| Indian mackerel | 0.0020 | 0.001 | 0.00024 | 0.00118 |
| Spotted sickle fish | 0.0021 | 0.002 | 0.00061 | 0.00023 |
| Tolerable daily intake (TDI) (mg/kg/day) in fish | 0.002 | 0.012 | 0.00080 | 0.14000 |
| Mean EDI (mg/kg/day) | 0.0014 | 0.001 | 0.0004 | 0.0006 |
| Maximum permissible limit (mg/kg wet weight) in Fish | 2 (a) | 0.5–1 (a) | 2 (b) | 1 (a) |
| THQ | HI | Target Cancer Risk (TR) | ||||
|---|---|---|---|---|---|---|
| Fish Name | Pb | Ni | Cd | Mn | (Hazard Index) | Ni |
| Small-scale terapon | 0.17 | 1.07 * | 2.86 * | 0.2736 | 4.374 | 3.7−5 |
| Torpedo scade | 0.21 | 0.44 | 5.46 * | 0.3112 | 6.437 | 1.5−5 |
| Sicklefish | 0.40 | 0.61 | 6.02 * | 0.1525 | 7.190 | 2.1−5 |
| Saddle grunt | 0.33 | 0.51 | 2.98 * | 0.7040 | 4.519 | 1.8−5 |
| Gold silk seabream | 0.60 | 0.65 | 4.59 * | 0.5703 | 6.415 | 2.3−5 |
| Indian mackerel | 0.61 | 0.64 | 2.63 * | 0.9066 | 4.784 | 2.2−5 |
| Spotted sickle fish | 0.63 | 0.88 | 6.61 * | 0.1734 | 8.294 | 3.1−5 |
| Non-carcinogenic Reference Dose (RfD) for fish (mg/kg-day) | 0.04 (ASTDR) | 0.02 (IRIS) | 0.001 (IRIS) | 0.014 (IRIS) | THQ > 1, may cause potential health risk HI > 1, adverse health effects are expected TR from 10−4 to 10−6 (acceptable range) BAF > 1 > 100, potential accumulation BAF > 100 > 1000, significant accumulation BAF > 1000, hazardous accumulation | |
| Locations | Pb | Ni | Cd | Mn | Reference |
|---|---|---|---|---|---|
| Red Sea (Mining Site) (ww) | 0.2 | 5.33 | - | 9.34 | [45] |
| Mining Area in Brazil (Muscles) (ww) | 2.1 | - | - | 13.0 | [46] |
| Gulf of Aqaba, Red Sea (ww) (Muscles) | 4.52 | 2.20 | 0.66 | 0.96 | [47] |
| Jedda coast (Dw) | 6.1 | _ | 1.06 | - | [48] |
| South West coast of India (ww) | 1.5 | 0.41 | 0.11 | 0.4 | [49] |
| Bangladesh (Bangshi River) (ww) | 4.64 | 2.59 | 0.3 | 23.7 | [50] |
| Iran (Mian kaley Lake) (ww) | 0.67 | 0.21 | 0.26 | - | [51] |
| Saint Martin Island (ww) | 0.11–8.92 | - | 1.52–14.09 | 0.59–0.74 | [52] |
| Mediterranean Sea (Dw) | 7.33–9.11 | 4.25–6.07 | 1.07–1.43 | - | [53] |
| Bay of Bengal (Dw) | 1.67–2.58 | 6.43–7.57 | 0.01–0.16 | 5.00–11.14 | [54] |
| Palk Bay India (Dw) | 0.1–0.12 | - | 0.02–0.28 | - | [55] |
| Turkish Sea (ww) | 0.15–1.15 | 0.01–3.43 | 0.01–0.43 | 0.07–3.62 | [56] |
| Gadani Shipbreaking Area | |||||
| Average Concentration (Gills and Muscles) | 4.51 | 5.27 | 1.33 | 2.29 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakar, A.; Hayat, M.T.; Abbasi, A.M.; Pervez, A.; Mahmood, Q.; Farooq, U.; Akbar, T.A.; Ali, S.; Rizwan, M.; El-Serehy, H.A.; et al. Risk Assessment of Heavy Metals in Selected Marine Fish Species of Gadani Shipbreaking Area and Pakistan. Animals 2020, 10, 1738. https://doi.org/10.3390/ani10101738
Kakar A, Hayat MT, Abbasi AM, Pervez A, Mahmood Q, Farooq U, Akbar TA, Ali S, Rizwan M, El-Serehy HA, et al. Risk Assessment of Heavy Metals in Selected Marine Fish Species of Gadani Shipbreaking Area and Pakistan. Animals. 2020; 10(10):1738. https://doi.org/10.3390/ani10101738
Chicago/Turabian StyleKakar, Allauddin, Malik Tahir Hayat, Arshad Mahmood Abbasi, Arshid Pervez, Qaisar Mahmood, Umar Farooq, Tahir Ali Akbar, Shafaqat Ali, Muhammad Rizwan, Hamed A. El-Serehy, and et al. 2020. "Risk Assessment of Heavy Metals in Selected Marine Fish Species of Gadani Shipbreaking Area and Pakistan" Animals 10, no. 10: 1738. https://doi.org/10.3390/ani10101738
APA StyleKakar, A., Hayat, M. T., Abbasi, A. M., Pervez, A., Mahmood, Q., Farooq, U., Akbar, T. A., Ali, S., Rizwan, M., El-Serehy, H. A., & Abdel-Daim, M. M. (2020). Risk Assessment of Heavy Metals in Selected Marine Fish Species of Gadani Shipbreaking Area and Pakistan. Animals, 10(10), 1738. https://doi.org/10.3390/ani10101738








