Phytopythium vexans Associated with Apple and Pear Decline in the Saïss Plain of Morocco
Abstract
:1. Introduction
2. Materials and Methods
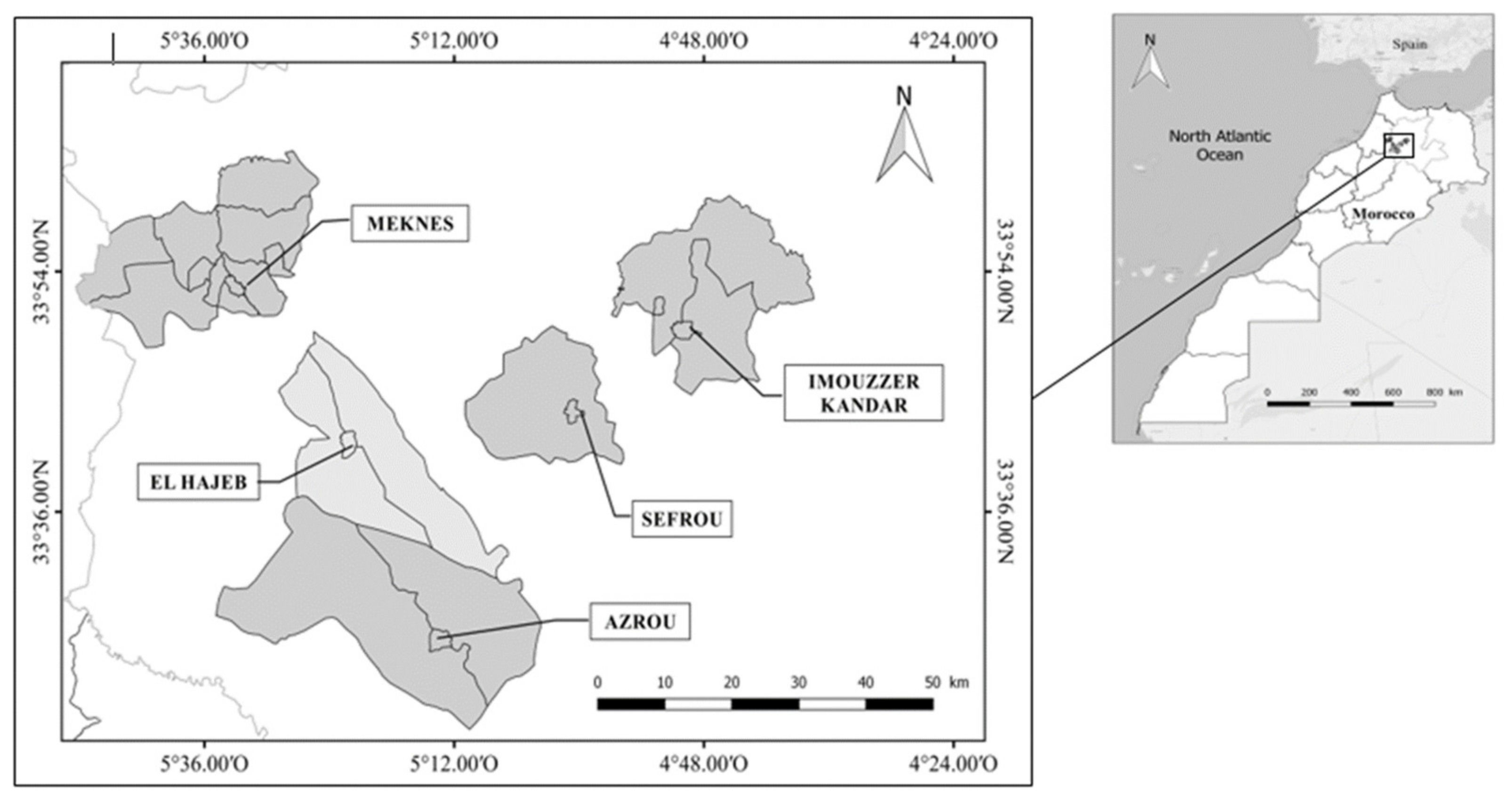
2.1. Sampling Sites
2.2. The Survey
2.3. Isolation of the Pathogenic Fungi: Fruit and Soil Baiting
2.4. Morphological Identification
2.5. Molecular Identification
2.6. Pathogenicity Test
2.7. Statistical Analysis
3. Results
3.1. Field Symptoms
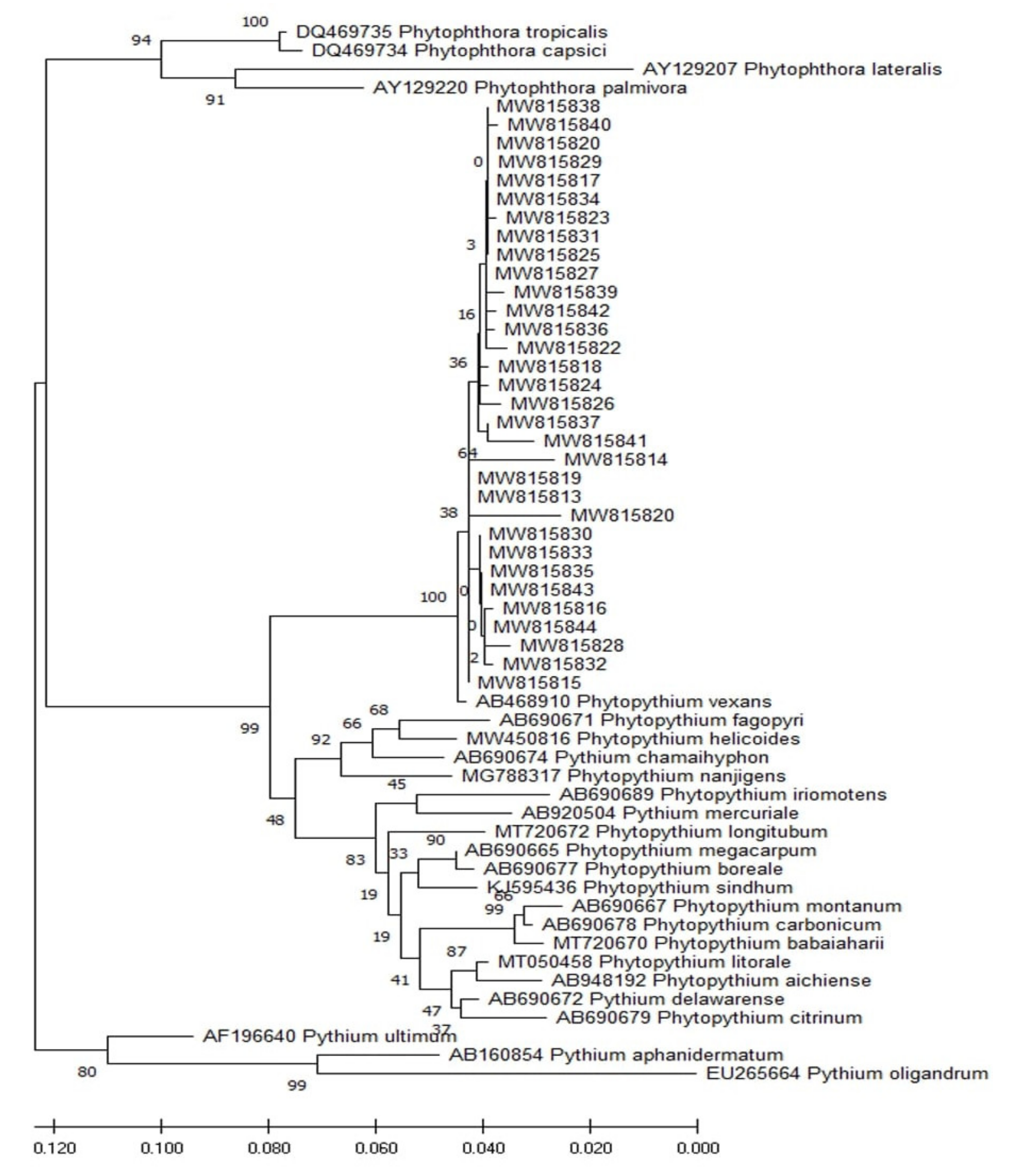
3.2. Isolation, Morphological, and Molecular Identification of Fungal Isolates
3.3. Distribution of the Pathogenic Oomycetes in the Surveyed Apple and Pears Orchards
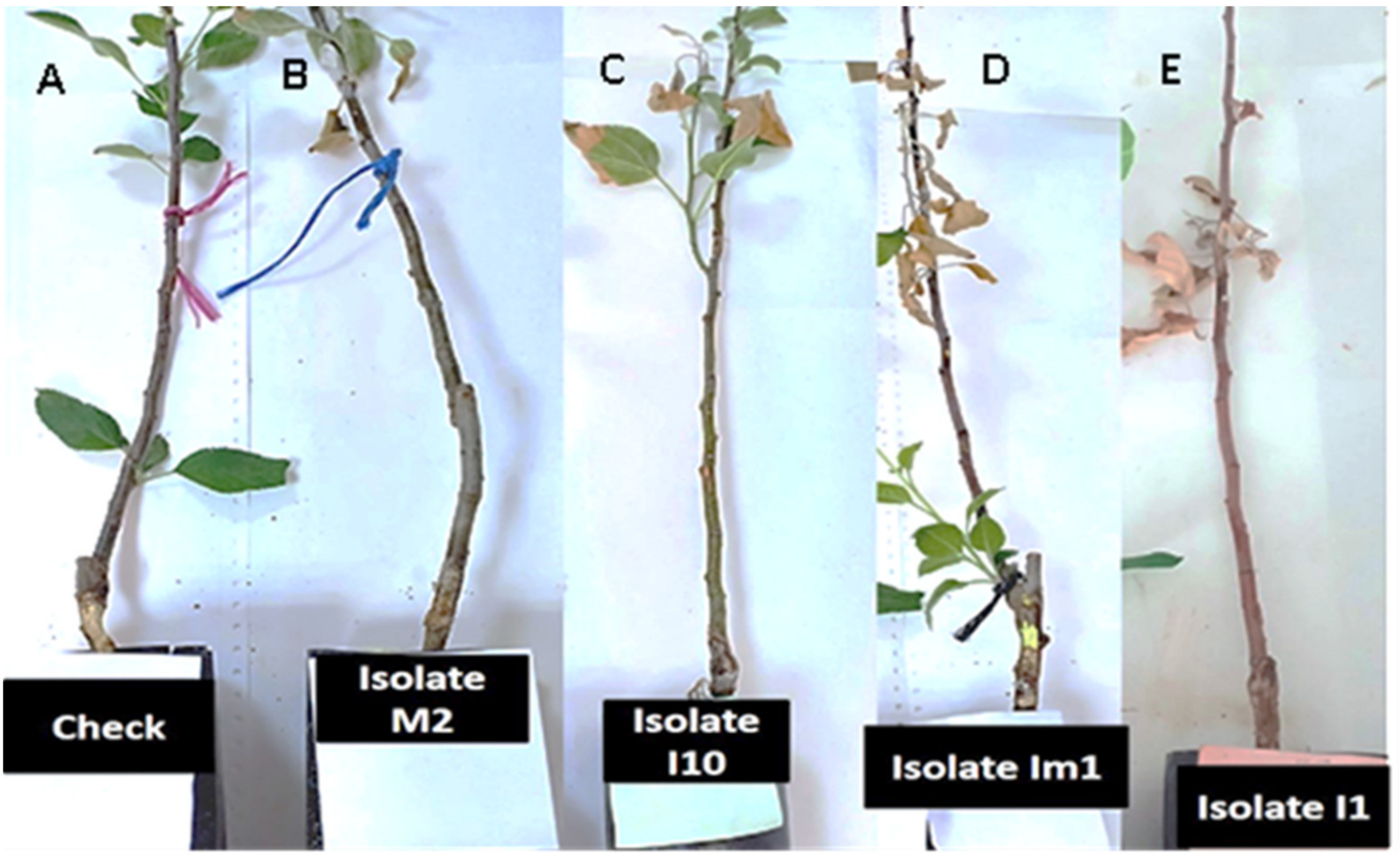
3.4. Pathogenicity Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazzola, M.; Manici, L.M. Apple replant disease: Role of microbial ecology in cause and control. Annu. Rev. Phytopathol. 2012, 50, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Derevnina, L.; Petre, B.; Kellner, R.; Dagdas, Y.F.; Sarowar, M.N.; Giannakopoulou, A.; De la Concepcion, J.C.; Chaparro-Garcia, A.; Pennington, H.G.; Van West, P.; et al. Emerging oomycete threats to plants and animals. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, H.H. The taxonomy and biology of phytophthora and pythium. J. Bacteriol. Mycol. Open Access 2018, 6, 00174. [Google Scholar] [CrossRef] [Green Version]
- Wheller, T.; Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; APS Press: Saint Paul, MN, USA, 1996. [Google Scholar]
- Tewoldemedhin, Y.T.; Mazzola, M.; Botha, W.J.; Spies, C.F.J.; McLeod, A. Characterization of fungi (Fusarium and Rhizoctonia) and oomycetes (Phytophthora and Pythium) associated with apple orchards in South Africa. Eur. J. Plant Pathol. 2011, 130, 215–229. [Google Scholar] [CrossRef]
- Porter, L.D.; Johnson, D.A. Survival of Phytophthora infestans in Surface Water. Phytopathology 2004, 94, 380–387. [Google Scholar] [CrossRef] [Green Version]
- Jung, T.; Orlikowski, L.; Henricot, B.; Abad-Campos, P.; Aday, A.G.; Casal, O.A.; Bakonyi, J.; Cacciola, S.O.; Cech, T.; Chavarriaga, D.; et al. Widespread phytophthora infestations in European nurseries put forest, semi-natural and horticultural ecosystems at high risk of phytophthora diseases. For. Pathol. 2015, 46, 134–163. [Google Scholar] [CrossRef] [Green Version]
- Goheen, D.J.; Filip, G.M. Root pathogen complexes in Pacific Northwest forests. Plant Dis. 1980, 64, 793–794. [Google Scholar] [CrossRef]
- Riolo, M.; Aloi, F.; La Spada, F.; Sciandrello, S.; Moricca, S.; Santilli, E.; Pane, A.; Cacciola, S.O. Diversity of Phytophthora communities across different types of mediterranean vegetation in a nature reserve area. Forests 2020, 11, 853. [Google Scholar] [CrossRef]
- Santa, O.C.; Maria, L.G. Emerging and re-emerging fungus and oomycete soil-borne plant diseases in Italy. Phytopathol. Mediterr. 2019, 58, 451–472. [Google Scholar]
- Anonymous Rosacées Fruitières. Available online: https://www.fellah-trade.com/fr/filiere-vegetale/chiffres-cles-rosacees-fruitieres?filiere=filiere_vegetale (accessed on 1 September 2020).
- Moinina, A.; Boulif, M.; Lahlali, R. Important pests, diseases and weather conditions affecting apple production: Current state and perspectives. Rev. Mar. Sci. Agron. Vét. 2019, 7, 71–87. [Google Scholar]
- Moinina, A.; Lahlali, R.; MacLean, D.; Boulif, M. Farmers’ knowledge, perception and practices in apple pest management and climate change in the fes-meknes region, Morocco. Horticulturae 2018, 4, 42. [Google Scholar] [CrossRef] [Green Version]
- De Cock, A.; Lodhi, A.; Rintoul, T.; Bala, K.; Robideau, G.; Abad, Z.G.; Coffey, M.; Shahzad, S.; Lévesque, C. Phytopythium: Molecular phylogeny and systematics. Pers. Mol. Phylogeny Evol. Fungi 2015, 34, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Benfradj, N.; Migliorini, D.; Luchi, N.; Santini, A.; Boughalleb-M’Hamdi, N. Occurrence of Pythium and Phytopythium species isolated from citrus trees infected with gummosis disease in tunisia. Arch. Phytopathol. Plant Prot. 2017, 50, 286–302. [Google Scholar] [CrossRef]
- Polat, Z.; Awan, Q.N.; Hussain, M.; Akgül, D.S. First report of Phytopythium vexans causing root and collar rot of kiwi fruit in Turkey. Plant Dis. 2017, 101, 1058. [Google Scholar] [CrossRef]
- Rodriguez-Padron, C.; Siverio, F.; Perez-Sierra, A.; Rodriguez, A. Isolation and pathogenicity of Phytophthora species and Phytopythium vexans recovered from avocado orchards in the Canary Islands, including Phytophthora niederhauserii as a new pathogen of avocado. Phytopathol. Mediterr. 2018, 57, 89–106. [Google Scholar]
- Rodríguez-Padrón, C.; Rodríguez, A.; Siverio, F. Survey in nurseries and irrigation water reservoirs as sources of oomycetes found in avocado orchards in the Canary Islands. Plant Dis. 2019, 103, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Spies, C.F.J.; Mazzola, M.; McLeod, A. Characterisation and detection of Pythium and Phytophthora species associated with grapevines in South Africa. Eur. J. Plant Pathol. 2011, 131, 103–119. [Google Scholar] [CrossRef]
- Nam, B.; Choi, Y.-J. Phytopythium and Pythium species (Oomycota) isolated from freshwater environments of Korea. Mycobiology 2019, 47, 261–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabiri, S.; Lahlali, R.; Bahra, C.; Amraoui, M.B.; Tahiri, A.; Amiri, S. First report of Phytopythium vexans associated with dieback disease of apple trees in Morocco. J. Plant Pathol. 2020, 102, 1319. [Google Scholar] [CrossRef]
- Weiland, J.E.; Beck, B.R.; Davis, A. Pathogenicity and virulence of Pythium species obtained from forest nursery soils on douglas-fir seedlings. Plant Dis. 2013, 97, 744–748. [Google Scholar] [CrossRef] [Green Version]
- Zentmyer, G.; Gilatbick, J.; Thorn, W. Methods of isolating Phytophthora cinnamomi from soil and from host tissue. Phytopathology 1960, 50, 87–95. [Google Scholar]
- Tsao, P.H.; Ocana, G. Selective isolation of species of Phytophthora from natural soils on an improved antibiotic medium. Nature 1969, 223, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Tsao, P.H.; Guy, S.O. Inhibition of Mortierella and Pythium in a Phytophthora-isolation medium containing hymexazol. Phytopathology 1977, 67, 796–801. [Google Scholar] [CrossRef]
- Abad, Z.G.; Shew, H.D.; Lucas, L.T. Characterization and pathogenicity of Pythium species isolated from turfgrass with symptoms of root and crown rot in North Carolina. Phytopathology 1994, 84, 913–921. [Google Scholar] [CrossRef]
- Gallegly, M.E.; Hong, C.X. Phytophthora: Identifying Species with Morphology and DNA Fingerprints; The American Phytopathological Society Press: Saint Paul, MN, USA, 2008. [Google Scholar]
- Martin, F.N.; Abad, Z.G.; Balci, Y.; Ivors, K. Identification and detection of Phytophthora: Reviewing our progress, identifying our needs. Plant Dis. 2012, 96, 1080–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waterhouse, G.M.; Newhook, F.J.; Stamps, D.J. Present criteria for classification of Phytophthora. In Phytophthora. Its Biology, Taxonomy, Ecology, and Pathology; Erwin, D.C., Bartnicki-Garcia, S., Tsao, P., Eds.; The American Phytopathological Society Press: Saint Paul, MN, USA, 1983; pp. 139–147. [Google Scholar]
- Tsopmbeng, G.; Fontem, D.; Yamde, K. Evaluation of culture media for growth and sporulation of Phytophthora colocasiae racib., causal agent of taro leaf blight. Int. J. Biol. Chem. Sci. 2012, 6, 1566–1573. [Google Scholar] [CrossRef] [Green Version]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Villa, N.; Kageyama, K.; Asano, T.; Suga, H. Phylogenetic relationships of Pythium and Phytophthora species based on ITS rDNA, cytochrome oxidase II and -tubulin gene sequences. Mycologia 2006, 98, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.J. Relationships of inoculum levels of several soilborne species of phytophthora and pythium to infection of several hosts. Phytopathology 1978, 68, 1754–1759. [Google Scholar] [CrossRef]
- Bala, K.; Robideau, G.; Désaulniers, N.; De Cock, A.; Lévesque, C. Taxonomy, DNA barcoding and phylogeny of three new species of Pythium from Canada. Persoonia 2010, 25, 22–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.-R.; Liu, B.-B.; Xing, Y.-P.; Cheng, B.-P.; Liu, M.-L.; Tong, Y.-H.; Xu, J.-Y. Identification and characterization of Phytopythium helicoides causing stem rot of Shatangju mandarin seedlings in China. Eur. J. Plant Pathol. 2016, 146, 715–727. [Google Scholar] [CrossRef]
- Hernández, P.A.; Chávez, E.C.; Ortiz, J.D.; Beache, M.B.; Vargas, L.T.; Fuentes, Y.O. First report of Phytopythium vexans causing the “avocado sadness” in Michoacan, Mexico. Phyton Int. J. Exp. Bot. 2019, 88, 11–13. [Google Scholar]
- Boari, A.J.; Cunha, E.M.; Quadros, A.F.F.; Barreto, R.W.; Fernandes, A.F. First report of Phytopythium sp. causing storage root rot and foliage blight of cassava in Brazil. Plant Dis. 2018, 102, 1042. [Google Scholar] [CrossRef]
- Thao, L.; Hien, L.; Liem, N.; Thanh, H.; Khanh, T.; Binh, V.; Trang, T.; Anh, P.; Tu, T. First report of Phytopythium vexans causing root rot disease on durian in Vietnam. New Dis. Rep. 2020, 41, 2. [Google Scholar] [CrossRef] [Green Version]
- Chastagner, G.A.; Hamm, P.B.; Riley, K.L. Symptoms and Phytophthora spp. associated with root rot and stem canker of Noble fir christmas trees in the Pacific Northwest. Plant Dis. 1995, 79, 290–293. [Google Scholar] [CrossRef]
- Rao, V.G. Influence of temperature upon growth and sporulation in two species of Phytophthora. Mycopathology 1970, 42, 39–48. [Google Scholar] [CrossRef]
- Ho, H.-H. The genus Pythium in Taiwan, China (1)—A synoptic review. Front. Biol. China 2009, 4, 15–28. [Google Scholar] [CrossRef]
- Yang, X.; Hong, C.X. Diversity and populations of Phytophthora, Phytopythium and Pythium species recovered from sediments in an agricultural run-off sedimentation reservoir. Plant Pathol. 2016, 65, 1118–1125. [Google Scholar] [CrossRef] [Green Version]
- Moein, S.; Mazzola, M.; Ntushelo, N.S.; McLeod, A. Apple nursery trees and irrigation water as potential external inoculum sources of apple replant disease in South Africa. Eur. J. Plant Pathol. 2019, 153, 1131–1147. [Google Scholar] [CrossRef]
- Moein, S. Quantification of Apple Replant Pathogens from Roots, and Their Occurrence in Irrigation Water and Nursery Trees; Stellenbosch University: Stellenbosch, South Africa, 2016. [Google Scholar]
- Gibbs, J.; Cech, T.; Jung, T.; Streito, J.C. Field studies on dissemination of the alder Phytophthora and disease development. In Forestry Commission Bulletin; Forestry Commission: Edinburgh, UK, 2003; Volume 126, pp. 55–64. [Google Scholar]
- Jung, T.; Blaschke, M. Phytophthora root and collar rot of alders in Bavaria: Distribution, modes of spread and possible management strategies. Plant Pathol. 2004, 53, 197–208. [Google Scholar] [CrossRef]
- Themann, K.; Werres, S.; Luttmann, R.; Diener, H.-A. Observations of Phytophthora spp. in water recirculation systems in commercial hardy ornamental nursery stock. Eur. J. Plant Pathol. 2002, 108, 337–343. [Google Scholar] [CrossRef]
- Hickman, C.J.; Ho, H.H. Behaviour of zoospores in plant-pathogenic Phycomycetes. Annu. Rev. Phytopathol. 1966, 4, 195–214. [Google Scholar] [CrossRef]
- Martin, F.N.; Loper, J.E. Soilborne plant diseases caused by Pythium spp.: Ecology, epidemiology, and prospects for biological control. Crit. Rev. Plant Sci. 1999, 18, 111–181. [Google Scholar] [CrossRef]
- Broders, K.D.; Lipps, P.E.; Ellis, M.L.; Dorrance, A.E. Pythium delawarii—A new species isolated from soybean in Ohio. Mycologia 2009, 101, 232–238. [Google Scholar] [CrossRef]
- Van der Plaats-Niterink, A.J. Monograph of the genus Pythium. Stud. Mycol. 1981, 21, 1–242. [Google Scholar]
- Carlile, M.J. Motility, taxis, and tropism in Phytophthora. In Phytophthora, It’s Biology Taxonomy, Ecology and Pathology; The American Phytopathological Society: Saint Paul, MN, USA, 1983; pp. 95–107. [Google Scholar]
- Thomson, S.; Allen, R. Occurrence of Phytophthora species and other potential plant pathogens in recycled irrigation water. Plant Dis. Rep. 1974, 58, 945–949. [Google Scholar]
- Weste, G.; Marks, G.C. The biology of Phytophthora cinnamomi in Australasian forests. Annu. Rev. Phytopathol. 1987, 25, 207–229. [Google Scholar] [CrossRef]
- Workneh, F.; Yang, X.B.; Tylka, G.L. Soybean brown stem rot, Phytophthora sojae, and Heterodera glycines affected by soil texture and tillage relations. Phytopathology 1999, 89, 844–850. [Google Scholar] [CrossRef] [Green Version]
- Thomson, T.; Athow, K.; Laviolette, F. The effect of temperature on the pathogenicity of Pythium aphanidermatum, Pythium debaryanum, and Pythium ultimum on soybean. Phytopathology 1971, 61, 933–935. [Google Scholar] [CrossRef]
- Ingram, D.M.; Cook, R.J. Pathogenicity of four Pythium species to wheat, barley, peas and lentils. Plant Pathol. 1990, 39, 110–117. [Google Scholar] [CrossRef]
- Nyoni, M.; Lötze, E.; Mazzola, M.; Wessels, J.P.B.; McLeod, A. Evaluating different approaches in the application of phosphonates for the control of apple root diseases. Australas. Plant Pathol. 2019, 48, 461–472. [Google Scholar] [CrossRef]
- Lu, X.H.; Michael, D.R.; Livingston, S.; Nunez, J.; Hao, J.J. Fungicide sensitivity of Pythium spp. associated with cavity spot of carrot in California and Michigan. Plant Dis. 2012, 96, 384–388. [Google Scholar] [CrossRef] [Green Version]
- Weiland, J.E.; Santamaria, L.; Grünwald, N.J. Sensitivity of Pythium irregulare, P. sylvaticum and P. ultimum from forest nurseries to mefenoxam and fosetyl-Al, and control of Pythium damping-off. Plant Dis. 2014, 98, 937–942. [Google Scholar] [PubMed]
- Matthiesen, R.L.; Ahmad, A.A.; Robertson, A.E. Temperature affects aggressiveness and fungicide sensitivity of four Pythium spp. that cause soybean and corn damping off in Iowa. Plant Dis. 2016, 100, 583–591. [Google Scholar] [CrossRef] [Green Version]
- Radmer, L.; Anderson, G.; Malvick, D.; Kurle, J.E.; Rendahl, A.; Mallik, A. Pythium, Phytophthora, and Phytopythium spp. associated with soybean in Minnesota, their relative aggressiveness on soybean and corn, and their sensitivity to seed treatment fungicides. Plant Dis. 2017, 101, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M.D.; Hausbeck, M.K. Using soil-applied fungicides to manage Phytophthora crown and root rot on summer squash. Plant Dis. 2013, 97, 107–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Fungal Isolate | Origin | Type of Plantation | Rootstock | Type of Soil | Identified Species | Genbank COX II an. 1 |
|---|---|---|---|---|---|---|
| E4 | El-Hajeb | Pear tree | M106 | Clay | Phytopythium vexans | MW815816 |
| E5 | El-Hajeb | Apple tree | Pajam | Clay | Phytopythium vexans | - |
| M1 | Meknès | Apple tree | M111 | Calcimagnesic | Phytopythium vexans | MW815828 |
| M2 | Meknès | Apple tree | Pajam | Calcimagnesic | Phytopythium vexans | MW815829 |
| S1 | Sefrou | Pear tree | Wild | Calcimagnesic-clay | Phytopythium vexans | MW815836 |
| S2 | Sefrou | Pear tree | Wild | Calcimagnesic-clay | Phytopythium vexans | MW815837 |
| S3 | Sefrou | Apple tree | M7 | Sandy-clay | Phytopythium vexans | MW815838 |
| S4 | Sefrou | Apple tree | M9 | Sandy-clay | Phytopythium vexans | MW815839 |
| S5 | Sefrou | Apple tree | M7 | Sandy-clay | Phytopythium vexans | MW815840 |
| S8 | Sefrou | Apple tree | M9 | Sandy-clay | Phytopythium vexans | MW815841 |
| S9 | Sefrou | Apple tree | M9 | Sandy-clay | Phytopythium vexans | MW815842 |
| S10 | Sefrou | Apple tree | M7 | Sandy-clay | Phytopythium vexans | MW815843 |
| A1 | Azrou | Apple tree | M9 | Calcareous-clay | Phytopythium vexans | MW815813 |
| A6 | Azrou | Apple tree | Wild | Clay-silty | Phytopythium vexans | MW815814 |
| A7 | Azrou | Apple tree | Pajam | Clay-silty | Phytopythium vexans | MW815815 |
| I1 | Imouzzer | Apple tree | Wild | Sandy-clay | Phytopythium vexans | - |
| Im1 | Imouzzer | Apple tree | Pajam | Sandy-clay | Phytopythium vexans | MW815817 |
| I2 | Imouzzer | Pear tree | Wild | Sandy-clay | Phytopythium vexans | - |
| I3 | Imouzzer | Apple tree | Wild | Sandy-clay | Phytopythium vexans | MW815819 |
| Im3 | Imouzzer | Apple tree | Wild | Sandy-clay | Phytopythium vexans | MW815818 |
| I5 | Imouzzer | Apple tree | M106 | Sandy-clay | Phytopythium vexans | MW815830 |
| I6 | Imouzzer | Apple tree | M111 | Sandy-clay | Phytopythium vexans | MW815831 |
| I7 | Imouzzer | Apple tree | Wild | Sandy-clay | Phytopythium vexans | MW815832 |
| I8 | Imouzzer | Apple tree | Wild | Sandy-clay | Phytopythium vexans | MW815833 |
| I10 | Imouzzer | Apple tree | Wild | Sandy-clay | Phytopythium vexans | MW815834 |
| I12 | Imouzzer | Apple tree | Wild | Sandy-clay | Phytopythium vexans | MW815825 |
| I13 | Imouzzer | Apple tree | Wild | Sandy-clay | Phytopythium vexans | MW815835 |
| I14 | Imouzzer | Apple tree | Wild | Sandy-clay | Phytopythium vexans | MW815844 |
| I15 | Imouzzer | Apple tree | Wild | Sandy-clay | Phytopythium vexans | MW815820 |
| I16 | Imouzzer | Apple tree | Wild | Sandy-clay | Phytopythium vexans | MW815821 |
| I17 | Imouzzer | Apple tree | Wild | Sandy-clay | Phytopythium vexans | MW815822 |
| I18 | Imouzzer | Apple tree | Wild | Sandy-clay | Phytopythium vexans | MW815823 |
| I19 | Imouzzer | Apple tree | Wild | Sandy-clay | Phytopythium vexans | MW815824 |
| I20 | Imouzzer | Apple tree | Wild | Sandy-clay | Phytopythium vexans | MW815826 |
| I21 | Imouzzer | Apple tree | Wild | Sandy-clay | Phytopythium vexans | MW815827 |
| Location | District | No. Orchards a | Positive Isolations | |
|---|---|---|---|---|
| Phytopythium vexans | Disease Prevalence (%) | |||
| Azrou | Tigrigra | 2 | 1 | 33 |
| Sidi Lmakhfi | 2 | - | ||
| Ain Louh | 5 | 2 | ||
| El-Hajeb | Tamchachate | 3 | 2 | 40 |
| Chlihat | 2 | - | ||
| Imouzzer | Ain Chifa | 9 | 8 | 83 |
| Aït Sbaà | 14 | 11 | ||
| Farha | 1 | 1 | ||
| Meknès | Majjat | 2 | 2 | 100 |
| Sefrou | Aghbalou Akourar | 2 | 2 | 80 |
| Laanoucer | 8 | 6 | ||
| Total | 50 | 35 | 70 | |
| Fungal Isolates | Lesion Length (cm) | Scale Severity Symptoms | ||
|---|---|---|---|---|
| Collar | Stem | Leaves Wilting 2 | Roots Necrosis 3 | |
| Pp. vexans E4 | 4.20 1 ± 0.1 ghij | 2.93 ± 0.15 c | 1.00 ± 0.00 b | 4.66 ± 0.57 cd |
| Pp. vexans E5 | 3.60 ± 0.15 cd | 3.06 ± 0.11 cd | 1.00 ± 0.00 b | 3.66 ± 0.57 b |
| Pp. vexans M1 | 5.06± 0.05 opq | 3.57 ± 0.57 efgh | 1.66 ± 0.57 bcd | 4.00 ± 1.00 bc |
| Pp. vexans M2 | 3.60 ± 0.10 cd | 3.03 ± 0.05 cd | 2.00 ± 0.00 cde | 4.33 ± 0.57 bcd |
| Pp. vexans S1 | 4.53 ± 0.05 jklm | 3.33 ± 0.11 cdef | 1.33 ± 0.57 bc | 4.33 ± 0.57 bcd |
| Pp. vexans S2 | 4.56 ± 0.11 klm | 3.80 ± 0.10 ghij | 2.33 ± 0.57 def | 4.66 ± 0.57 cd |
| Pp. vexans S3 | 3.93 ± 0.15 defg | 4.10 ± 0.10 ijk | 2.00 ± 0.00 cde | 5.00 ± 0.00 c |
| Pp. vexans S4 | 4.8 ± 0.10 mno | 3.6 0± 0.26 efgh | 5.00 ± 0.00 l | 4.66 ± 0.57 cd |
| Pp. vexans S5 | 5.93 ± 0.15 t | 4.20 ± 0.10 jk | 4.66 ± 0.57 kl | 4.66 ± 0.57 cd |
| Pp. vexans S8 | 5.16 ± 0.15 pqr | 3.70 ± 0.10 fghi | 3.00 ± 0.00 fgh | 4.66 ± 0.57 cd |
| Pp. vexans S9 | 4.40 ± 0.10 ijkl | 3.40 ± 0.10 defg | 2.66 ± 0.57 efg | 5.00 ± 0.00 c |
| Pp. vexans S10 | 4.96 ± 0.11 nop | 3.43 ± 0.57 defg | 4.67 ± 0.57 kl | 4.66 ± 0.57 cd |
| Pp. vexans A1 | 3.50 ± 0.52 bc | 3.36 ± 0.49 cdefg | 1.33 ± 0.57 bc | 4.66 ± 0.57 cd |
| Pp. vexans A6 | 3.60 ± 0.10 cd | 3.10 ± 0.10 cd | 1.00 ± 0.00 b | 4.33 ± 0.00 bcd |
| Pp. vexans A7 | 4.46 ± 0.32 ijklm | 3.62 ± 0.15 efgh | 1.00 ± 0.00 b | 4.66 ± 0.57 cd |
| Pp. vexans I1 | 5.56 ± 0.60 s | 6.13 ± 0.15 m | 4.33 ± 0.57 jkl | 4.33 ± 0.57 bcd |
| Pp. vexans Im1 | 4.30 ± 0.20 hijk | 4.00 ± 0.10 hij | 4.66 ± 0.57 kl | 4.66 ± 0.57 cd |
| Pp. vexans I2 | 5.10 ± 0.20 opqr | 4.53 ± 0.30 k | 2 ± 1.00 cde | 4.66 ± 0.57 cd |
| Pp. vexans I3 | 4.67 ± 0.15 lmn | 7.23 ± 0.55 n | 2.33 ± 0.57 def | 5.00 ± 0.00 c |
| Pp. vexans Im3 | 5.36 ± 0.32 qrs | 4.06 ± 0.11 ij | 4.00 ± 0.00 ijk | 5.00 ± 0.00 c |
| Pp. vexans I5 | 4.16 ± 0.11 ghi | 3.80 ± 0.1 ghij | 1.66 ± 0.57 bcd | 4.66 ± 0.57 cd |
| Pp. vexans I6 | 3.76 ± 0.25 cdef | 3.06 ± 0.11 cd | 1.00 ± 0.00 b | 5.00 ± 0.00 c |
| Pp. vexans I7 | 5.03 ± 0.15 opq | 2.13 ± 0.15 b | 4.00 ± 0.00 ijk | 4.33 ± 0.57 bcd |
| Pp. vexans I8 | 4.00 ± 0.10 efgh | 3.23 ± 0.25 cde | 5.00 ± 0.00 l | 4.33 ± 0.57 bcd |
| Pp. vexans I10 | 4.80 ± 0.20 mno | 3.68 ± 0.05 fghi | 3.00 ± 0.00 fgh | 4.66 ± 0.57 cd |
| Pp. vexans I12 | 4.66 ± 0.41 lmn | 6.20 ± 0.75 m | 3.66 ± 0.57 hij | 5.00 ± 0.00 c |
| Pp. vexans I13 | 3.93 ± 0.15 defg | 3.70 ± 0.10 fghi | 5.00 ± 0.00 l | 5.00 ± 0.00 c |
| Pp. vexans I14 | 4.03 ± 0.05 fgh | 3.26 ± 0.28 cdef | 1.00 ± 0.00 b | 4.66 ± 0.57 cd |
| Pp. vexans I15 | 5.40 ± 0.15 rs | 3.20 ± 0.20 cde | 4.66 ± 0.57 kl | 5.00 ± 0.00 c |
| Pp. vexans I16 | 3.93 ± 0.15 defg | 3.30 ± 0.20 cdef | 5.00 ± 0.00 l | 4.33 ± 0.57 bcd |
| Pp. vexans I17 | 3.23 ± 0.15 b | 5.46 ± 0.11 l | 3.33 ± 0.57 ghi | 4.33 ± 0.57 bcd |
| Pp. vexans I18 | 4.73 ± 0.15 mno | 3.70 ± 0.10 fghi | 4.66 ± 0.57 kl | 4.66 ± 0.57 cd |
| Pp. vexans I19 | 4.30 ± 0.20 hijk | 3.70 ± 0.20 fghi | 5.00 ± 0.00 l | 4.66 ± 0.57 cd |
| Pp. vexans I20 | 3.66 ± 0.15 cde | 3.40 ± 0.25 defg | 3.00 ± 0.00 fgh | 4.00 ± 0.00 bc |
| Pp. vexans I21 | 4.16 ± 0.15 ghi | 5.23 ± 0.68 l | 4.00 ± 1.00 ijk | 4.33 ± 0.57 bcd |
| Un-inoculated control | 0.00 a | 0.00 a | 0.00 a | 0.00 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabiri, S.; Bahra, C.; MacLean, D.; Radouane, N.; Barka, E.A.; Bendriss Amraoui, M.; Lahlali, R. Phytopythium vexans Associated with Apple and Pear Decline in the Saïss Plain of Morocco. Microorganisms 2021, 9, 1916. https://doi.org/10.3390/microorganisms9091916
Jabiri S, Bahra C, MacLean D, Radouane N, Barka EA, Bendriss Amraoui M, Lahlali R. Phytopythium vexans Associated with Apple and Pear Decline in the Saïss Plain of Morocco. Microorganisms. 2021; 9(9):1916. https://doi.org/10.3390/microorganisms9091916
Chicago/Turabian StyleJabiri, Salma, Chaimaa Bahra, Dustin MacLean, Nabil Radouane, Essaid Ait Barka, Mohamed Bendriss Amraoui, and Rachid Lahlali. 2021. "Phytopythium vexans Associated with Apple and Pear Decline in the Saïss Plain of Morocco" Microorganisms 9, no. 9: 1916. https://doi.org/10.3390/microorganisms9091916
APA StyleJabiri, S., Bahra, C., MacLean, D., Radouane, N., Barka, E. A., Bendriss Amraoui, M., & Lahlali, R. (2021). Phytopythium vexans Associated with Apple and Pear Decline in the Saïss Plain of Morocco. Microorganisms, 9(9), 1916. https://doi.org/10.3390/microorganisms9091916









