Whole-Genome Sequencing-Based Characterization of a Listeria monocytogenes Strain from an Aborted Water Buffalo in Southern Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. L. monocytogenes Isolation, DNA Isolation, Library Preparation, and Sequencing
2.2. WGS-Based Characterization of L. monoytogenes
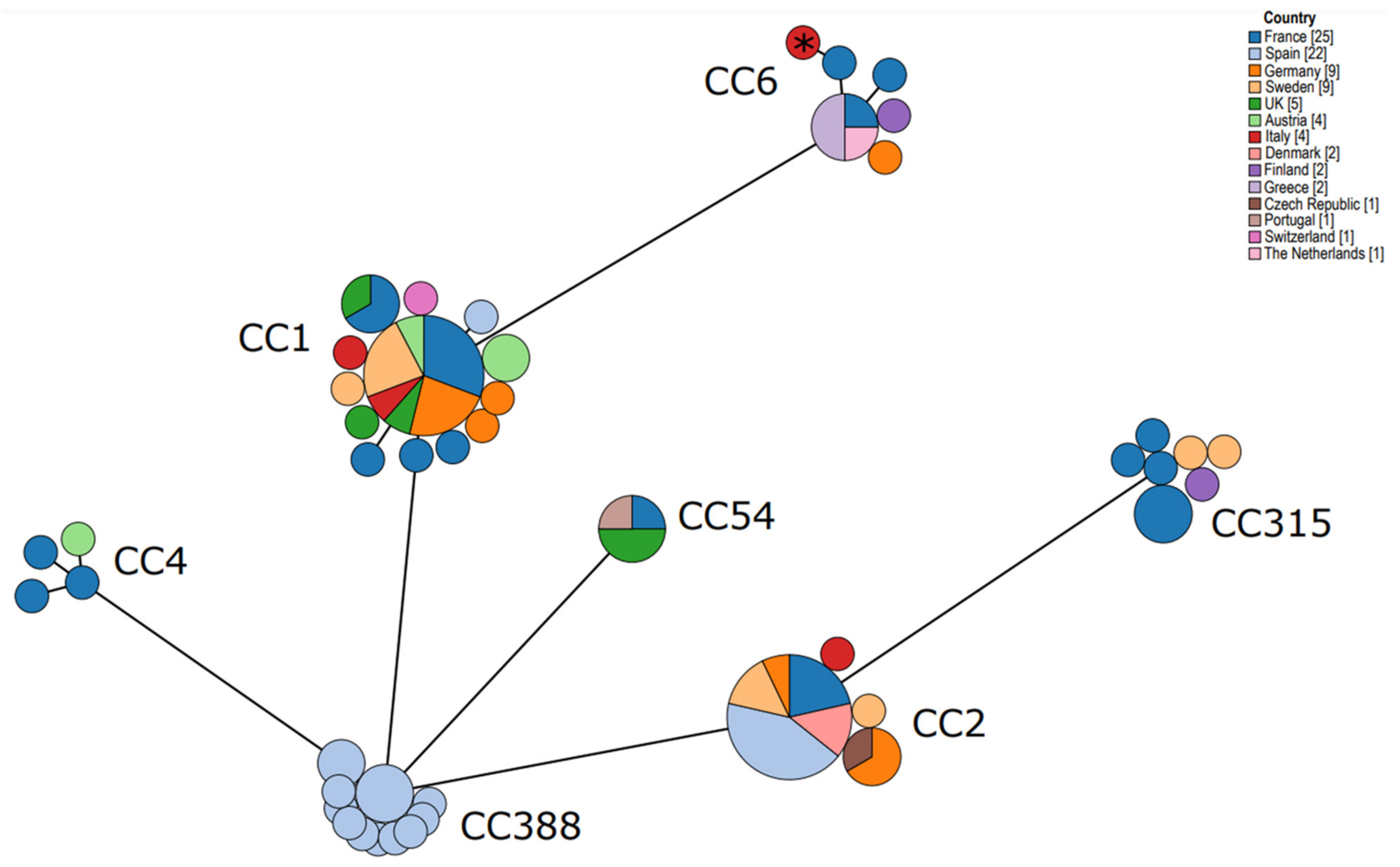
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Radoshevich, L.; Cossart, P. Listeria monocytogenes: Towards a complete picture of its physiology and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Papić, B.; Pate, M.; Félix, B.; Kušar, D. Genetic diversity of Listeria monocytogenes strains in ruminant abortion and rhombencephalitis cases in comparison with the natural environment. BMC Microbiol. 2019, 19, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walland, J.; Lauper, J.; Frey, J.; Imhof, R.; Stephan, R.; Seuberlich, T.; Oevermann, A. Listeria monocytogenes infection in ruminants: Is there a link to the environment, food and human health? A review. Schweiz. Arch. Tierheilkd. 2015, 157, 319–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, J.C.; Donachie, W. A review of Listeria monocytogenes and listeriosis. Vet. J. 1997, 153, 9–29. [Google Scholar] [CrossRef]
- Pell, A.N. Manure and microbes: Public and animal health problem? J. Dairy Sci. 1997, 80, 2673–2681. [Google Scholar] [CrossRef]
- Nightingale, K.K.; Schukken, Y.H.; Nightingale, C.R.; Fortes, E.D.; Ho, A.J.; Her, Z.; Grohn, Y.T.; McDonough, P.L.; Wiedmann, M. Ecology and transmission of Listeria monocytogenes infecting ruminants and in the farm environment. Appl. Environ. Microbiol. 2004, 70, 4458–4467. [Google Scholar] [CrossRef] [Green Version]
- Okwumabua, O.; O’Connor, M.; Shull, E.; Strelow, K.; Hamacher, M.; Kurzynski, T.; Warshauer, D. Characterization of Listeria monocytogenes isolates from food animal clinical cases: PFGE pattern similarity to strains from human listeriosis cases. FEMS Microbiol. Lett. 2005, 249, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Gianfranceschi, M.V.; D’Ottavio, M.C.; Gattuso, A.; Bella, A.; Aureli, P. Distribution of serotypes and pulsotypes of Listeria monocytogenes from human, food and environmental isolates (Italy 2002–2005). Food Microbiol. 2009, 26, 520–526. [Google Scholar] [CrossRef]
- Lopez-Valladares, G.; Tham, W.; Parihar, V.S.; Helmersson, S.; Andersson, B.; Ivarsson, S.; Johansson, C.; Ringberg, H.; Tjernberg, I.; Henriques-Normark, B.; et al. Human isolates of Listeria monocytogenes in Sweden during half a century (1958–2010). Epidemiol. Infect. 2014, 142, 2251–2260. [Google Scholar] [CrossRef] [Green Version]
- Esposito, C.; Cardillo, L.; Borriello, G.; Ascione, G.; Valvini, O.; Galiero, G.; Fusco, G. First Detection of Listeria monocytogenes in a Buffalo Aborted Foetus in Campania Region (Southern Italy). Front. Vet. Sci. 2021, 7, 571654. [Google Scholar] [CrossRef]
- OIE World Organization for Animal Health. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 8th ed.; Chapter 3.9.6; OIE World Organization for Animal Health: Paris, France, 2018; pp. 1705–1722. Available online: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.09.06_LISTERIA_MONO.pdf (accessed on 15 July 2019).
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasebe, R.; Nakao, R.; Ohnuma, A.; Yamasaki, T.; Sawa, H.; Takai, S.; Horiuchi, M. Listeria monocytogenes serotype 4b strains replicate in monocytes/macrophages more than the other serotypes. J. Vet. Med. Sci. 2017, 79, 962–969. [Google Scholar] [CrossRef] [Green Version]
- Centorame, P.; Acciari, V.A.; Orsini, M.; Torresi, M.; Iannetti, L.; Angius, A.; Di Giammartino, D.; Prencipe, V.A.; Migliorati, G. Whole-Genome Sequence of Listeria monocytogenes Serovar 4b Strain IZSAM_Lm_hs2008, Isolated from a Human Infection in Italy. Genome Announc. 2015, 3, e00053-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaltriti, E.; Bolzoni, L.; Vocale, C.; Morganti, M.; Menozzi, I.; Re, M.C.; Pongolini, S. Population Structure of Listeria monocytogenes in Emilia-Romagna (Italy) and Implications on Whole Genome Sequencing Surveillance of Listeriosis. Front. Public Health 2020, 8, 519293. [Google Scholar] [CrossRef]
- Lee, S.; Chen, Y.; Gorski, L.; Ward, T.J.; Osborne, J.; Kathariou, S. Listeria monocytogenes Source Distribution Analysis Indicates Regional Heterogeneity and Ecological Niche Preference among Serotype 4b Clones. mBio 2018, 9, e00396-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maury, M.M.; Tsai, Y.H.; Charlier, C.; Touchon, M.; Chenal-Francisque, V.; Leclercq, A.; Criscuolo, A.; Gaultier, C.; Roussel, S.; Brisabois, A.; et al. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat. Genet. 2016, 48, 308–313. [Google Scholar] [CrossRef] [Green Version]
- Wagner, E.; Zaiser, A.; Leitner, R.; Quijada, N.M.; Pracser, N.; Pietzka, A.; Ruppitsch, W.; Schmitz-Esser, S.; Wagner, M.; Rychli, K. Virulence characterization and comparative genomics of Listeria monocytogenes sequence type 155 strains. BMC Genom. 2020, 21, 847. [Google Scholar] [CrossRef]
- Maury, M.M.; Bracq-Dieye, H.; Huang, L.; Vales, G.; Lavina, M.; Thouvenot, P.; Disson, O.; Leclercq, A.; Brisse, S.; Lecuit, M. Hypervirulent Listeria monocytogenes clones’ adaption to mammalian gut accounts for their association with dairy products. Nat.Commun. 2019, 10, 2488. [Google Scholar] [CrossRef] [Green Version]
- De las Heras, A.; Cain, R.J.; Bielecka, M.K.; Vázquez-Boland, J.A. Regulation of Listeria virulence: PrfA master and commander. Curr. Opin. Microbiol. 2011, 14, 118–127. [Google Scholar] [CrossRef]
- Hadjilouka, A.; Paramithiotis, S.; Drosinos, E.H. Genetic Analysis of the Listeria Pathogenicity Island 1 of Listeria monocytogenes 1/2a and 4b Isolates. Curr. Microbiol. 2018, 75, 857–865. [Google Scholar] [CrossRef]
- Cotter, P.D.; Draper, L.A.; Lawton, E.M.; Daly, K.M.; Groeger, D.S.; Casey, P.G.; Ross, R.P.; Hill, C. Listeriolysin S, a novel peptide haemolysin associated with a subset of lineage I Listeria monocytogenes. PLoS Pathog. 2008, 4, e1000144. [Google Scholar] [CrossRef] [Green Version]
- Lei, X.H.; Fiedler, F.; Lan, Z.; Kathariou, S. A novel serotype-specific gene cassette (gltA-gltB) is required for expression of teichoic acid-associated surface antigens in Listeria monocytogenes of serotype 4b. J. Bacteriol. 2001, 183, 1133–1139. [Google Scholar] [CrossRef] [Green Version]
- Promadej, N.; Fiedler, F.; Cossart, P.; Dramsi, S.; Kathariou, S. Cell wall teichoic acid glycosylation in Listeria monocytogenes serotype 4b requires gtcA, a novel, serogroup-specific gene. J. Bacteriol. 1999, 181, 418–425. [Google Scholar] [CrossRef] [Green Version]
- Luque-Sastre, L.; Arroyo, C.; Fox, E.M.; McMahon, B.J.; Bai, L.; Li, F.; Fanning, S. Antimicrobial Resistance in Listeria Species. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poyart-Salmeron, C.; Carlier, C.; Trieu-Cuot, P.; Courvalin, P.; Courtieu, A.L. Transferable plasmid-mediated antibiotic resistance in Listeria monocytogenes. Lancet 1990, 335, 1422–1426. [Google Scholar] [CrossRef]
- Gomez, D.; Azon, E.; Marco, N.; Carraminana, J.J.; Rota, C.; Ariño, A.; Yangüela, J. Antimicrobial resistance of Listeria monocytogenes and Listeria innocua from meat products and meat-processing environment. Food Microbiol. 2014, 42, 61–65. [Google Scholar] [CrossRef]
- Şanlıbaba, P.; Tezel, B.U.; Çakmak, G.A. Prevalence and Antibiotic Resistance of Listeria monocytogenes Isolated from Ready-to-Eat Foods in Turkey. J. Food Qual. 2018, 2018, 7693782. [Google Scholar] [CrossRef] [Green Version]
| Molecular Characterization | Molecular Results |
|---|---|
| Taxonomy | Molecular serogroup IVb; phylogenetic lineage 1; ST6; CT3331; SL6; CC6 |
| Antimicrobial resistance | fosX, lmo0919(lin), norB, sul |
| Major virulence genes | gtcA, inlJ, fbpA, lap, actA, inlA, inlB, lplA1, prsA2, bsh, pdgA, oatA, inlC, inlK, intA, plcA, mpl, plcB |
| Listeria pathogenicity islands | LIPI-1 LIPI-3 |
| Other virulence genes | gltA, gltB, prfA, hly, orfX, dltA, aut_IVb, iap, lpeA, vip, hpt, purQ, svpA, agrA, agrC, cheA, cheY, fur, lisK, lisR, stp, virR, virS, lgt, lspA, srtA, srtB, mdrM, comK, codY, pdeE, LMON_RS01340, LMON_RS01345 |
| Clonal Complex | Source of Origin | Tot | ||||
|---|---|---|---|---|---|---|
| Food | Animal | Human | Environment | N.R.1 | ||
| CC1 | 3 (11%) | 4 (14%) | 19 (68%) | 1 (4%) | 1 (4%) | 28 |
| CC2 | 5 (26%) | 1 (5%) | 11 (58%) | 0 | 2 (11%) | 19 |
| CC4 | 1 (25%) | 0 | 2 (50%) | 0 | 1 (25%) | 4 |
| CC6 | 2 (22%) | 2 (10%) | 4 (44%) | 1 (11%) | 0 | 9 |
| CC54 | 0 | 0 | 4 (100%) | 0 | 0 | 4 |
| CC315 | 2 (22%) | 1 (11%) | 5 (56%) | 1 (11%) | 0 | 9 |
| CC388 | 0 | 15 (100%) | 0 | 0 | 0 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paradiso, R.; Riccardi, M.G.; Cecere, B.; Riccone, N.; Scialla, R.; Anzalone, A.; Cerrone, A.; De Carlo, E.; Borriello, G.; Fusco, G. Whole-Genome Sequencing-Based Characterization of a Listeria monocytogenes Strain from an Aborted Water Buffalo in Southern Italy. Microorganisms 2021, 9, 1875. https://doi.org/10.3390/microorganisms9091875
Paradiso R, Riccardi MG, Cecere B, Riccone N, Scialla R, Anzalone A, Cerrone A, De Carlo E, Borriello G, Fusco G. Whole-Genome Sequencing-Based Characterization of a Listeria monocytogenes Strain from an Aborted Water Buffalo in Southern Italy. Microorganisms. 2021; 9(9):1875. https://doi.org/10.3390/microorganisms9091875
Chicago/Turabian StyleParadiso, Rubina, Marita Georgia Riccardi, Bianca Cecere, Nunzia Riccone, Roberto Scialla, Antonietta Anzalone, Anna Cerrone, Esterina De Carlo, Giorgia Borriello, and Giovanna Fusco. 2021. "Whole-Genome Sequencing-Based Characterization of a Listeria monocytogenes Strain from an Aborted Water Buffalo in Southern Italy" Microorganisms 9, no. 9: 1875. https://doi.org/10.3390/microorganisms9091875
APA StyleParadiso, R., Riccardi, M. G., Cecere, B., Riccone, N., Scialla, R., Anzalone, A., Cerrone, A., De Carlo, E., Borriello, G., & Fusco, G. (2021). Whole-Genome Sequencing-Based Characterization of a Listeria monocytogenes Strain from an Aborted Water Buffalo in Southern Italy. Microorganisms, 9(9), 1875. https://doi.org/10.3390/microorganisms9091875






