Early Immune Initiation by Porcine Cells following Toxoplasma gondii Infection versus TLR Ligation
Abstract
:1. Introduction
2. Materials and Methods
2.1. PBMC Isolation
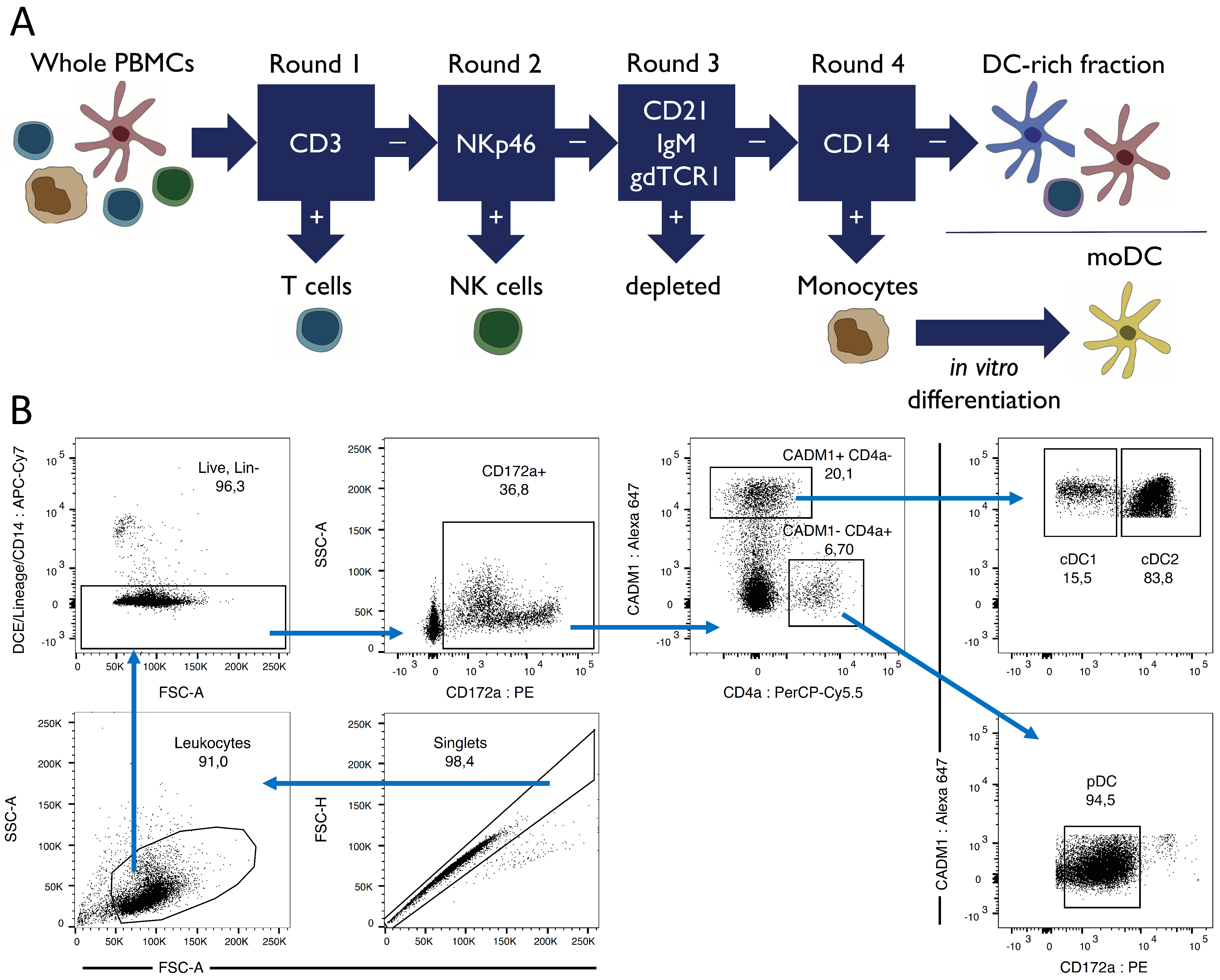
2.2. Cell Sorting Pre-Enrichment via Magnetic Cell Separation (MACS)
2.3. Fluorescence-activated Cell Sorting (FACS)
2.4. Generation of moDC from CD14+ Sorted Cells
2.5. T. Gondii Tachyzoite Culture
2.6. In Vitro TLR-ligand Stimulations and T. gondii Infections of Monocytes, moDC, cDC, pDC, T Cells, and CD3– NKp46+ Cells
2.7. FACS Analysis of GFP+ T. gondii Infection
2.8. Cytokine Measurements in Supernatants
2.9. FACS-analysis of Myeloid Cells after TLR-ligand Stimulation or T. gondii Infection
2.10. PBMC Stimulation with Recombinant IL-12 and IL-18 Stimulation, and IFNγ ELISA
2.11. Determining the Cellular Source of IFNγ following IL-12/IL-18 Stimulation
3. Results
3.1. Porcine Dendritic Cell Sorting
3.2. Porcine PMBC Subset Responses to TLR Ligation
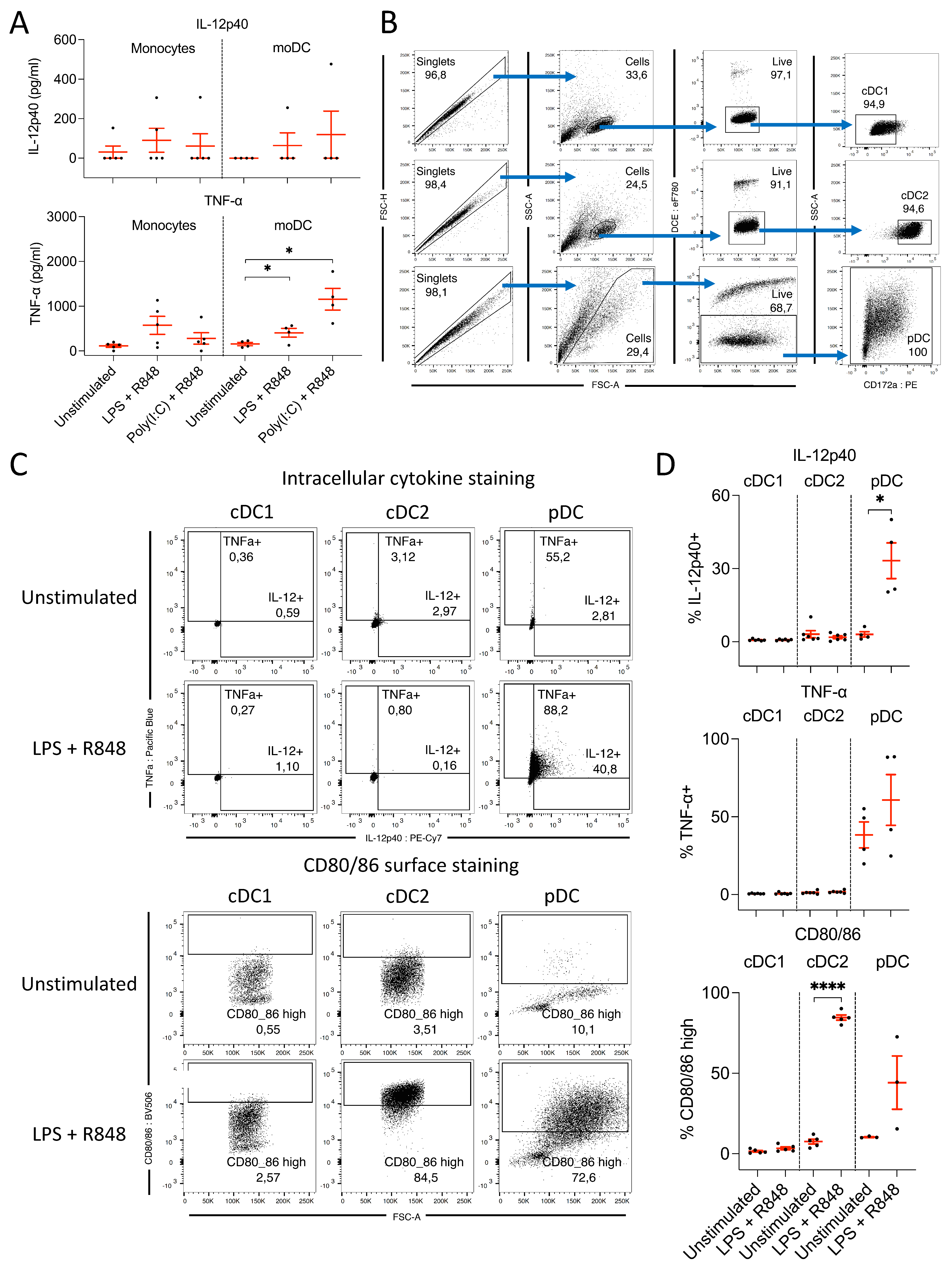
3.2.1. Monocytes and moDC Do Not Produce IL-12 following TLR Ligation
3.2.2. pDC, but Not cDC, Produce IL-12 following Ligation of TLR 4, 7, and 8
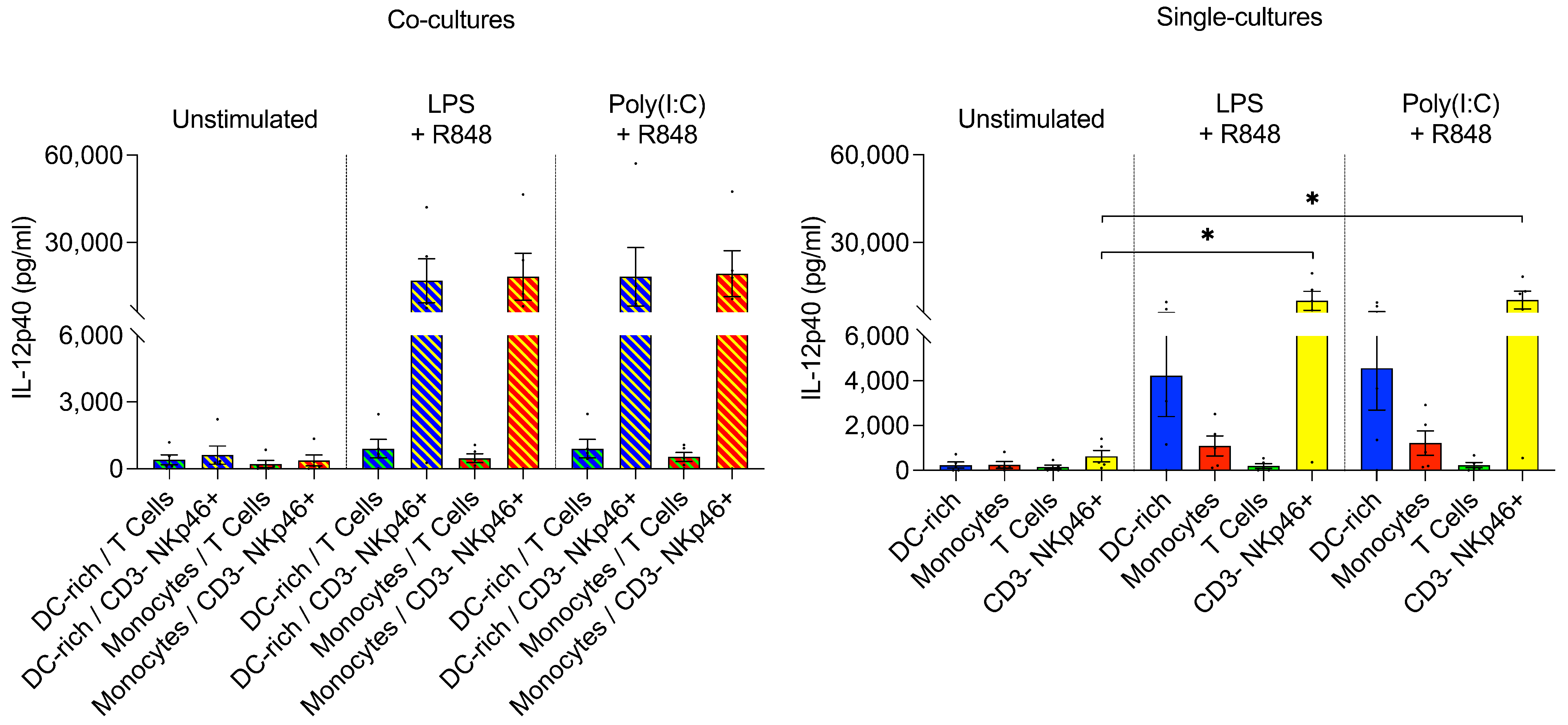
3.2.3. CD3– NKp46+ Cells Produce IL-12 following TLR Ligation
3.3. Porcine PMBC Subset Responses to Toxoplasma gondii Infection
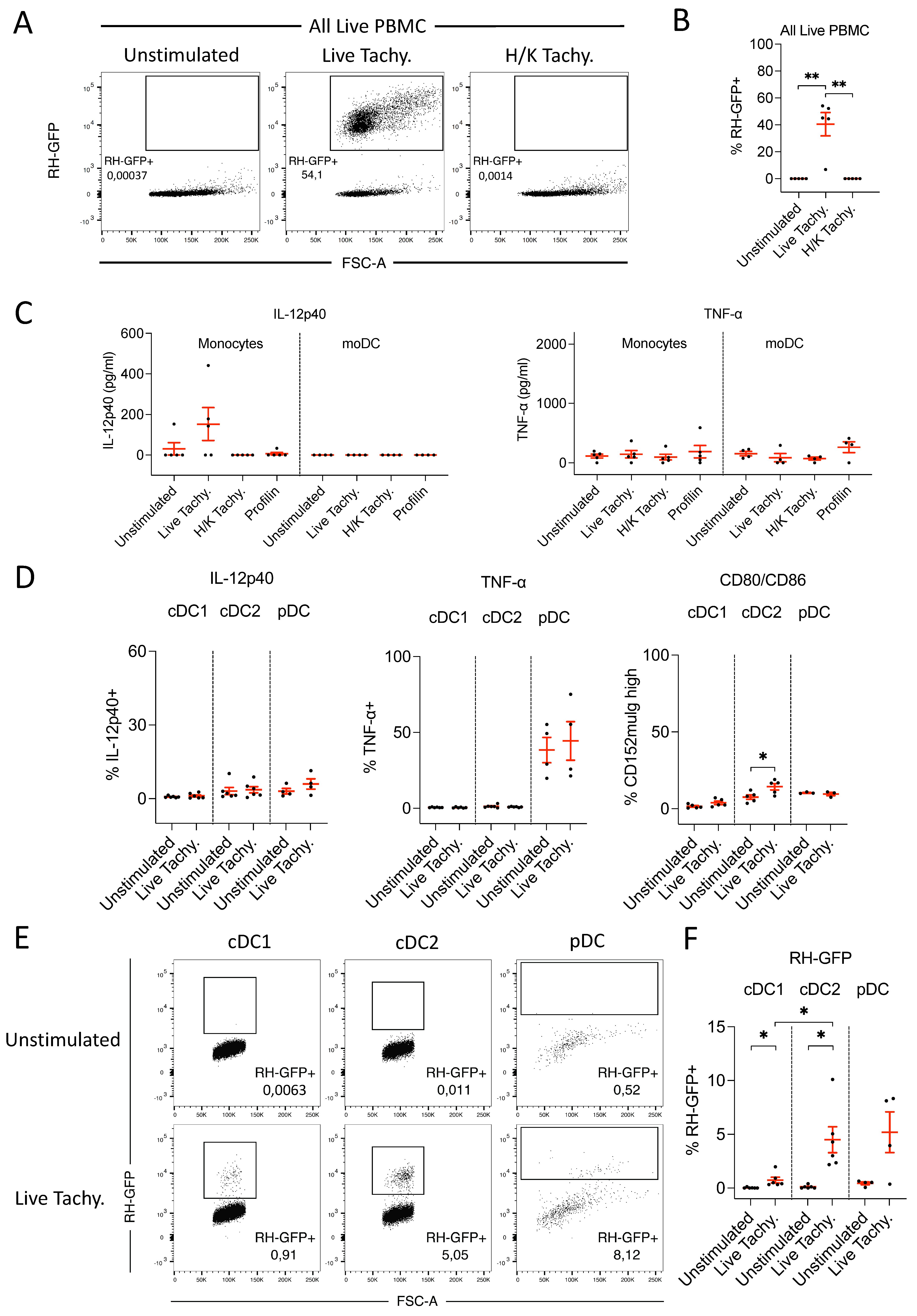
3.3.1. GFP-labelled T. gondii Tachyzoites Are Infective and Detectable by FACS
3.3.2. In Response to T. gondii Infection, Monocytes Produce Low Levels of IL-12p40
3.3.3. Neither cDC nor pDC Produce IL-12 following T. gondii Infection
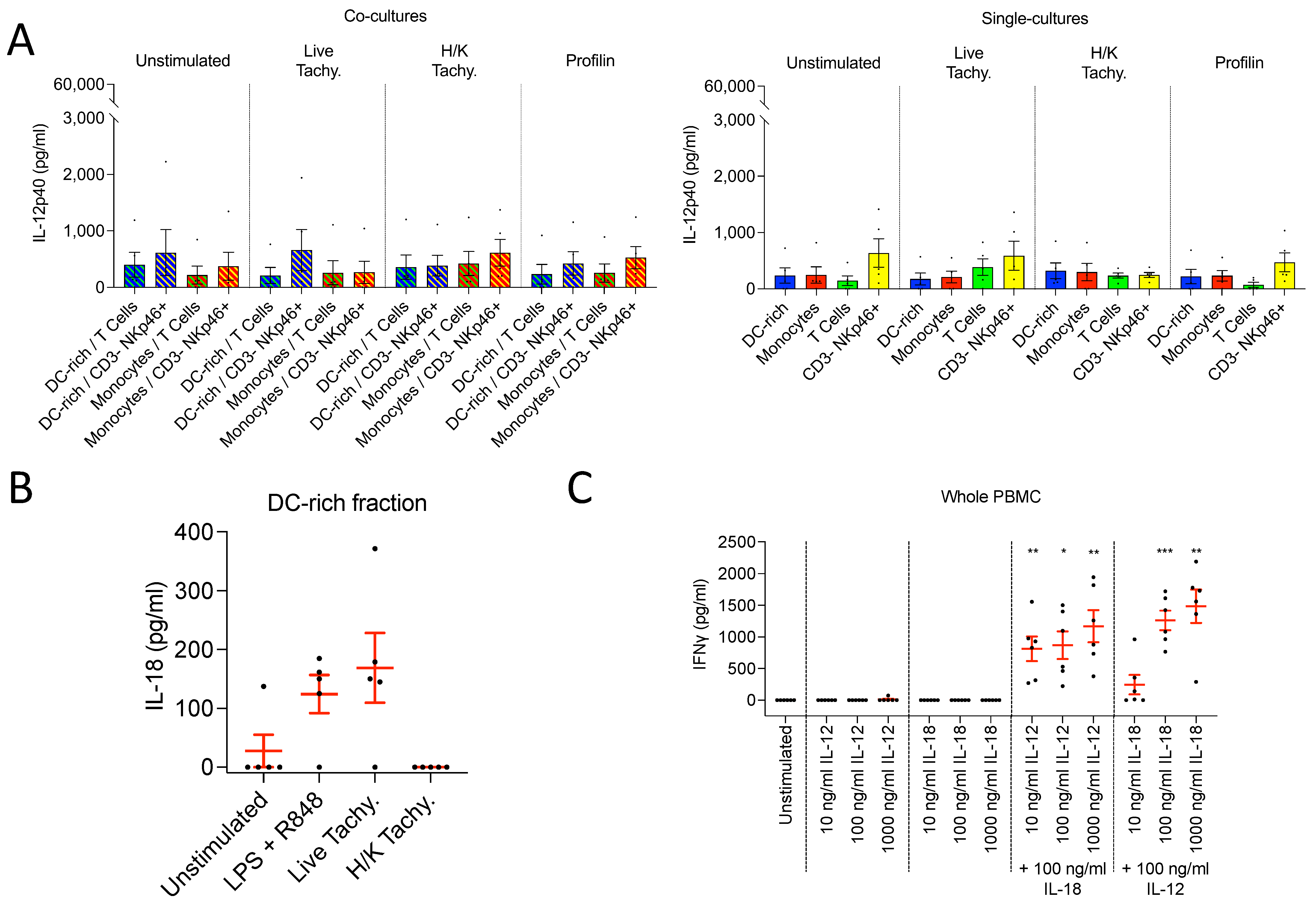
3.3.4. CD3– NKp46+ Cells Do Not Produce IL-12 following T. gondii Infection
3.3.5. DC-enriched PBMC Produce IL-18 following T. gondii Infection
3.4. IFNγ Response of Porcine PBMC following IL-12 and IL-18 Stimulation
3.4.1. IL-12 and IL-18 Stimulation in Combination, but Not Alone, Induces IFNγ Production
3.4.2. IFNγ Produced following IL-12 and IL-18 Stimulation Is Derived from NK Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rostami, A.; Riahi, S.M.; Gamble, H.R.; Fakhri, Y.; Nourollahpour Shiadeh, M.; Danesh, M.; Behniafar, H.; Paktinat, S.; Foroutan, M.; Mokdad, A.H.; et al. Global prevalence of latent toxoplasmosis in pregnant women: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 673–683. [Google Scholar] [CrossRef]
- Almeria, S.; Dubey, J.P. Foodborne transmission of Toxoplasma gondii infection in the last decade. An overview. Res. Vet. Sci. 2021, 135, 371–385. [Google Scholar] [CrossRef]
- Belluco, S.; Mancin, M.; Conficoni, D.; Simonato, G.; Pietrobelli, M.; Ricci, A. Investigating the determinants of Toxoplasma gondii prevalence in meat: A systematic review and meta-regression. PLoS ONE 2016, 11, e0153856. [Google Scholar] [CrossRef] [Green Version]
- Batz, M.B.; Hoffmann, S.; Morris, J.G. Ranking the disease burden of 14 pathogens in food sources in the United States using attribution data from outbreak investigations and expert elicitation. J. Food Prot. 2012, 75, 1278–1291. [Google Scholar] [CrossRef] [Green Version]
- Hunter, C.A.; Sibley, L.D. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat. Rev. Microbiol. 2012, 10, 766–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sher, A.; Tosh, K.; Jankovic, D. Innate recognition of Toxoplasma gondii in humans involves a mechanism distinct from that utilized by rodents. Cell. Mol. Immunol. 2017, 14, 36–42. [Google Scholar] [CrossRef]
- Rahman, M.; Devriendt, B.; Jennes, M.; Algaba, I.G.; Dorny, P.; Dierick, K.; De Craeye, S.; Cox, E. Early kinetics of intestinal infection and immune responses to two Toxoplasma gondii strains in pigs. Front. Cell. Infect. Microbiol. 2020, 10, e161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scanga, C.A.; Aliberti, J.; Jankovic, D.; Tilloy, F.; Bennouna, S.; Denkers, E.Y.; Medzhitov, R.; Sher, A. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 2002, 168, 5997–6001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarovinsky, F.; Zhang, D.; Andersen, J.F.; Bannerberg, G.L.; Serhan, C.N.; Hayden, M.S.; Hieny, S.; Sutterwala, F.S.; Flavell, R.A.; Ghosh, S.; et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 2005, 308, 1626–1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koblansky, A.; Jankovic, D.; Oh, H.; Hieny, S.; Sungnak, W.; Mathur, M.; Hayden, M.S.; Akira, S.; Sher, A.; Ghosh, S. Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii. Immunity 2013, 38, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Tosh, K.W.; Mittereder, L.; Bonne-Annee, S.; Hieny, S.; Nutman, T.B.; Singer, S.M.; Sher, A.; Jankovic, D. The IL-12 response of primary human DC and monocytes to Toxoplasma gondii is stimulated by live phagocytosis of live parasites rather than host cell invasion. J. Immunol. 2016, 196, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.; French, T.; Rausch, S.; Kühl, A.; Hemminger, K.; Dunay, I.R.; Steinfelder, S.; Hartmann, S. Toxoplasma co-infection prevents Th2 differentiation and leads to a helminth-specific Th1 response. Front. Cell. Infect. Microbiol. 2017, 7, e341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bliss, S.K.; Butcher, B.A.; Denkers, E.Y. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. J. Immunol. 2000, 165, 4515–4521. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, M.; Sandau, M.M.; Dunay, I.R.; Frickel, E.M.; Khan, A.; Goldszmid, R.S.; Sher, A.; Ploegh, H.L.; Murphy, T.L.; Sibley, L.D.; et al. CD8a(+) dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity 2011, 35, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Dunay, I.R.; Fuchs, A.; Sibley, L.D. Inflammatory monocytes but not neutrophils are necessary to control infection with Toxoplasma gondii in mice. Infect. Immun. 2010, 78, 1564–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boothroyd, J.C.; Dubremetz, J.-F. Kiss and spit: The dual roles of Toxoplasma rhoptries. Nat. Rev. Microbiol. 2008, 6, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Meurens, F.; Summerfield, A.; Nauwynck, H.; Saif, L.; Gerdts, V. The pig: A model for human infectious diseases. Trends Microbiol. 2012, 20, 50–57. [Google Scholar] [CrossRef]
- Mair, K.H.; Sedlak, C.; Käser, T.; Pasternak, A.; Levast, B.; Gerner, W.; Saalmüller, A.; Summerfield, A.; Gerdts, V.; Wilson, H.L.; et al. The porcine innate immune system: An update. Dev. Comp. Immunol. 2014, 45, 321–343. [Google Scholar] [CrossRef]
- Dubey, J.P. Comparative infectivity of oocysts and bradyzoites of Toxoplasma gondii for intermediate (mice) and definitive (cats) hosts. Vet. Parasitol. 2006, 140, 69–75. [Google Scholar] [CrossRef]
- Jungersen, G.; Bille-Hansen, V.; Jensen, L.; Lind, P. Transplacental transmission of Toxoplasma gondii in minipigs infected with strains of different virulence. J. Parasitol. 2001, 87, 108–113. [Google Scholar] [CrossRef]
- Shiono, Y.; Mun, H.-S.; He, N.; Nakazaki, Y.; Fang, H.; Furuya, M.; Aosai, F.; Yano, A. Maternal-fetal transmission of Toxoplasma gondii in interferon-g deficient pregnant mice. Parasitol. Int. 2007, 56, 141–148. [Google Scholar] [CrossRef]
- Nau, J.; Eller, S.K.; Wenning, J.; Spekker-Bosker, K.H.; Schroten, H.; Schwerk, C.; Hotop, A.; Groß, U.; Däubener, W. Experimental porcine Toxoplasma gondii infection as a representative model for human toxoplasmosis. Mediat. Inflamm. 2017, 2017, e3260289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uenishi, H.; Shinkai, H. Porcine toll-like receptors: The front line of pathogen monitoring and possible implications for disease resistance. Dev. Comp. Immunol. 2009, 33, 353–361. [Google Scholar] [CrossRef]
- Macri, C.; Pang, E.S.; Patton, T.; O’Keeffe, M. Dendritic cell subsets. Semin. Cell Dev. Biol. 2018, 84, 11–21. [Google Scholar] [CrossRef]
- Auray, G.; Keller, I.; Python, S.; Gerber, M.; Bruggmann, R.; Ruggli, N.; Summerfield, A. Characterization and transcriptomic analysis of porcine blood conventional and plasmacytoid dendritic cells reveals striking species-specific differences. J. Immunol. 2016, 197, 4791–4806. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Grigg, M.E. Toxoplasma gondii: Laboratory maintenance and growth. Curr. Protoc. Microbiol. 2017, 44, 20C.1.1–20C.1.17. [Google Scholar] [CrossRef] [Green Version]
- Edwards, J.C.; Everett, H.E.; Pedrera, M.; Mokhtar, H.; Marchi, E.; Soldevila, F.; Kaveh, D.A.; Hogarth, P.J.; Johns, H.L.; Nunez-Garcia, J.; et al. CD1- and CD1+ porcine blood dentritic cells are enriched for the orthologues of the two major mammalian conventional subsets. Sci. Rep. 2017, 7, e40942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grouard, G.; Rissoan, M.C.; Filgueira, L.; Durand, I.; Banchereau, J.; Liu, Y.J. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 1997, 185, 1101–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in health and disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Shen, B. Transcriptomic analyses reveal distinct response of porcine macrophages to Toxoplasma gondii infection. Parasitol. Res. 2020, 119, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Channon, J.Y.; Seguin, R.M.; Kasper, L.H. Differential infectivity and division of Toxoplasma gondii in human peripheral blood leukocytes. Infect. Immun. 2000, 68, 4822–4826. [Google Scholar] [CrossRef] [Green Version]
- Walzer, T.; Blery, M.; Chaix, J.; Fuseri, N.; Chasson, L.; Robbins, S.H.; Jaeger, S.; Andre, P.; Gauthier, L.; Daniel, L.; et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc. Natl. Acad. Sci. USA 2007, 104, 3384–3389. [Google Scholar] [CrossRef] [Green Version]
- Westgaard, I.H.; Berg, S.F.; Vaage, J.T.; Wang, L.L.; Yokoyama, W.M.; Dissen, E.; Fossum, S. Rat NKp46 activates natural killer cell cytotoxicity and is associated with FcεRIγ and CD3ζ. J. Leukoc. Biol. 2004, 76, 1200–1206. [Google Scholar] [CrossRef]
- Storset, A.K.; Kulberg, S.; Berg, I.; Boysen, P.; Hope, J.C.; Dissen, E. NKp46 defines a subset of bovine leukocytes with natural killer cell characteristics. Eur. J. Immunol. 2004, 34, 669–676. [Google Scholar] [CrossRef]
- Connelley, T.; Storset, A.K.; Pemberton, A.; Machugh, N.; Brown, J.; Lund, H.; Morrison, I.W. NKp46 defines ovine cells that have characteristics corresponding to NK cells. Vet. Res. 2011, 42, e37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mair, K.H.; Essler, S.E.; Patzl, M.; Storset, A.K.; Saalmüller, A.; Gerner, W. NKp46 expression discriminates porcine NK cells with different functional properties. Eur. J. Immunol. 2012, 42, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, L.; Kapetanovic, R.; Beraldi, D.; Sester, D.P.; Tuggle, C.K.; Archibald, A.L.; Hume, D.A. Comparative analysis of monocyte subsets in the pig. J. Immunol. 2013, 190, 6389–6396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pelsmaeker, S.; Devriendt, B.; Leclercq, G.; Favoreel, H.W. Porcine NK cells display features associated with antigen-presenting cells. J. Leukoc. Biol. 2017, 103, 129–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, B.; Benson, A.; Kuzmich, L.; Defranco, A.L.; Yaroninsky, F. Critical coordination of innate immune defense against Toxoplasma gondii by dendritic cells responding via their toll-like receptors. Proc. Natl. Acad. Sci. USA 2011, 108, 278–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tominaga, K.; Yoshimoto, T.; Torigoe, K.; Kurimoto, M.; Matsui, K.; Hada, T.; Okamura, H.; Nakanishi, K. IL-12 synergizes with IL-18 or IL-1β for IFN-γ production from human T cells. Int. Immunol. 2000, 12, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Delgado Betancourt, E.; Hamid, B.; Fabian, B.T.; Klotz, C.; Hartmann, S.; Seeber, F. From entry to early dissemination–Toxoplasma gondii’s initial encounter with its host. Front. Cell. Infect. Microbiol. 2019, 9, e46. [Google Scholar] [CrossRef]
- Holthaus, D.; Delgado-Betancourt, E.; Aebischer, T.; Seeber, F.; Klotz, C. Harmonization of protocols for multi-species organoid platforms to study the intestinal biology of Toxoplasma gondii and other protozoan infections. Front. Cell. Infect. Microbiol. 2021, 10, e610368. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.; Pifer, R.; Behrendt, C.L.; Hooper, L.V.; Yarovinsky, F. Gut commensal bacteria direct a protective immune response against Toxoplasma gondii. Cell Host Microbe 2009, 6, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briceno, M.P.; Nascimento, L.A.C.; Nogueira, N.P.; Barenco, P.V.C.; Ferro, E.A.V.; Rezende-Oliveira, K.; Goulart, L.R.; Alves, P.T.; Barbosa, B.d.F.; Lima, W.R.; et al. Toxoplasma gondii infection promotes epithelial barrier dysfunction of Caco-2 cells. J. Histochem. Cytochem. 2016, 64, 459–469. [Google Scholar] [CrossRef] [Green Version]
- Ebner, F.; Schwiertz, P.; Steinfelder, S.; Pieper, R.; Zentek, J.; Schütze, N.; Baums, C.G.; Alber, G.; Geldhof, P.; Hartmann, S. Pathogen-reactive T helper cell analysis in the pig. Front. Immunol. 2017, 8, e565. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamid, B.; Schlosser-Brandenburg, J.; Bechtold, L.; Ebner, F.; Rausch, S.; Hartmann, S. Early Immune Initiation by Porcine Cells following Toxoplasma gondii Infection versus TLR Ligation. Microorganisms 2021, 9, 1828. https://doi.org/10.3390/microorganisms9091828
Hamid B, Schlosser-Brandenburg J, Bechtold L, Ebner F, Rausch S, Hartmann S. Early Immune Initiation by Porcine Cells following Toxoplasma gondii Infection versus TLR Ligation. Microorganisms. 2021; 9(9):1828. https://doi.org/10.3390/microorganisms9091828
Chicago/Turabian StyleHamid, Benjamin, Josephine Schlosser-Brandenburg, Lalita Bechtold, Friederike Ebner, Sebastian Rausch, and Susanne Hartmann. 2021. "Early Immune Initiation by Porcine Cells following Toxoplasma gondii Infection versus TLR Ligation" Microorganisms 9, no. 9: 1828. https://doi.org/10.3390/microorganisms9091828
APA StyleHamid, B., Schlosser-Brandenburg, J., Bechtold, L., Ebner, F., Rausch, S., & Hartmann, S. (2021). Early Immune Initiation by Porcine Cells following Toxoplasma gondii Infection versus TLR Ligation. Microorganisms, 9(9), 1828. https://doi.org/10.3390/microorganisms9091828





