Multi-Drug Resistance Bacterial Infections in Critically Ill Patients Admitted with COVID-19
Abstract
:1. Introduction
Pathophysiology and Main Clinical Pattern of Superinfections
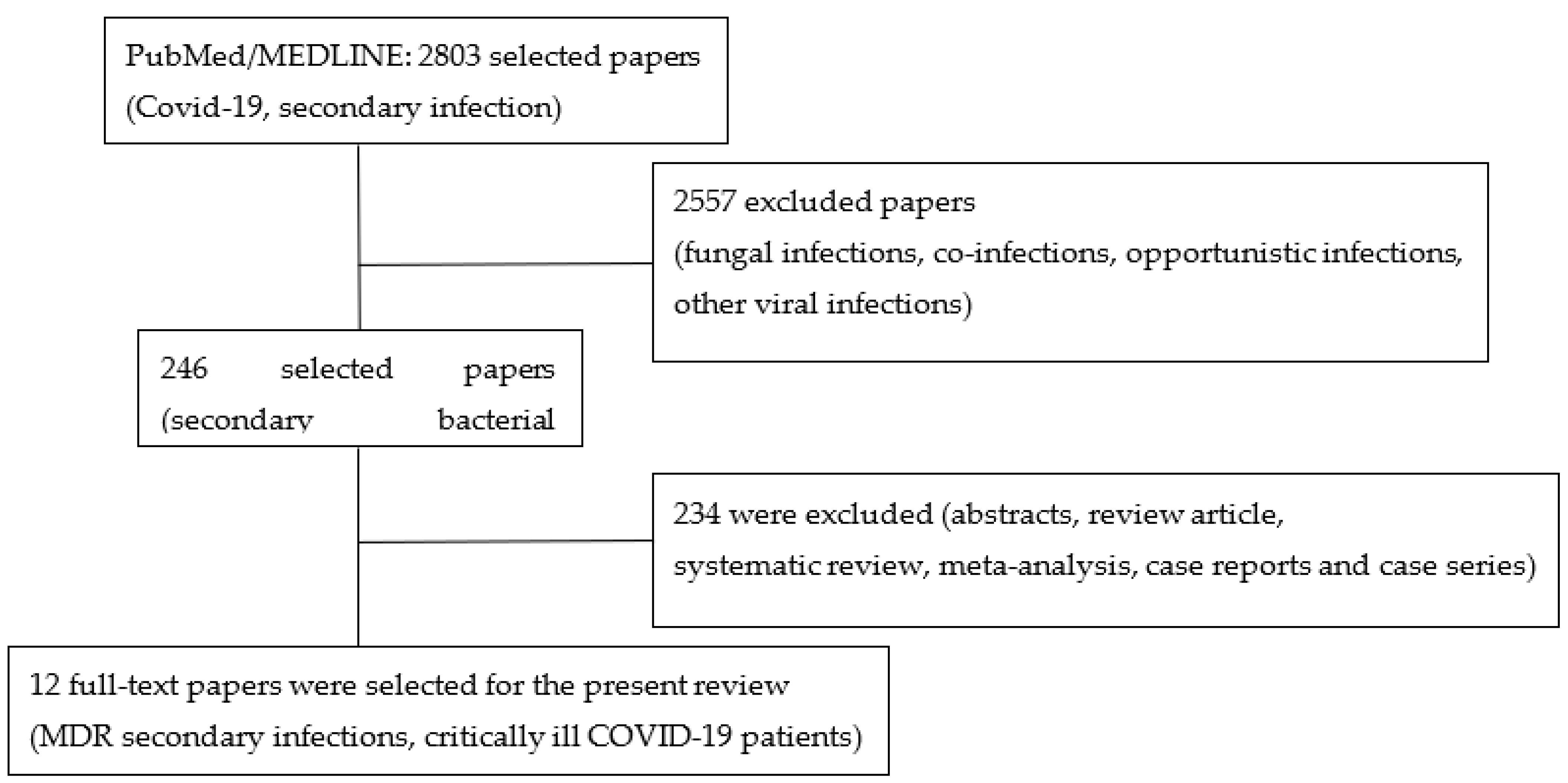
2. Methods
3. Results
4. Discussion
4.1. Multidrug Resistance Organisms: Development and Spread during the COVID-19 Outbreak
4.2. MDR Secondary Infections and Risk Factors
4.3. Limits of the Study
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Klein, E.Y.; Monteforte, B.; Gupta, A.; Jiang, W.; May, L.; Hsieh, Y.H.; Dugas, A. The frequency of influenza and bacterial coinfection: A systematic review and meta-analysis. Influenza Other Respir. Viruses 2016, 10, 394–403. [Google Scholar] [CrossRef]
- Rice, T.W.; Rubinson, L.; Uyeki, T.M.; Vaughn, F.L.; John, B.B.; Miller, R.R., 3rd; Higgs, E.; Randolph, A.G.; Smoot, B.E.; Thompson, B.T.; et al. Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit. Care Med. 2012, 40, 1487–1498. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.S.; Greenberg, J.A.; McNulty, M.C.; Gregg, K.S.; Riddell, J.t.; Mangino, J.E.; Weber, D.M.; Hebert, C.L.; Marzec, N.S.; Barron, M.A.; et al. Bacterial and viral co-infections complicating severe influenza: Incidence and impact among 507 U.S. patients, 2013–2014. J. Clin. Virol. 2016, 80, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Martin-Loeches, I.; Sanchez-Corral, A.; Diaz, E.; Granada, R.M.; Zaragoza, R.; Villavicencio, C.; Albaya, A.; Cerda, E.; Catalan, R.M.; Luque, P.; et al. Community-acquired respiratory coinfection in critically ill patients with pandemic 2009 influenza A(H1N1) virus. Chest 2011, 139, 555–562. [Google Scholar] [CrossRef]
- Alhazzani, W.; Moller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020, 46, 854–887. [Google Scholar] [CrossRef] [Green Version]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-Acquired Infections in Critically Ill Patients with COVID-19. Chest 2021, 160, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.E.; Cox, C.E.; Hope, A.A.; Carson, S.S. Chronic critical illness. Am. J. Respir. Crit. Care Med. 2010, 182, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Wang, W.; Liu, Z.; Liang, C.; Wang, W.; Ye, F.; Huang, B.; Zhao, L.; Wang, H.; Zhou, W.; et al. Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells. Nat. Commun. 2020, 11, 3910. [Google Scholar] [CrossRef] [PubMed]
- Chertow, D.S.; Memoli, M.J. Bacterial coinfection in influenza: A grand rounds review. JAMA 2013, 309, 275–282. [Google Scholar] [CrossRef] [PubMed]
- McCullers, J.A. Insights into the interaction between influenza virus and pneumococcus. Clin. Microbiol. Rev. 2006, 19, 571–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corsonello, A.; Lattanzio, F.; Bustacchini, S.; Garasto, S.; Cozza, A.; Schepisi, R.; Lenci, F.; Luciani, F.; Maggio, M.G.; Ticinesi, A.; et al. Adverse Events of Proton Pump Inhibitors: Potential Mechanisms. Curr. Drug Metab. 2018, 19, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Luxenburger, H.; Sturm, L.; Biever, P.; Rieg, S.; Duerschmied, D.; Schultheiss, M.; Neumann-Haefelin, C.; Thimme, R.; Bettinger, D. Treatment with proton pump inhibitors increases the risk of secondary infections and ARDS in hospitalized patients with COVID-19: Coincidence or underestimated risk factor? J. Intern. Med. 2021, 289, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; Beumer, J.; van der Vaart, J.; Knoops, K.; Puschhof, J.; Breugem, T.I.; Ravelli, R.B.G.; Paul van Schayck, J.; Mykytyn, A.Z.; Duimel, H.Q.; et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020, 369, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; He, L.; Zhang, Q.; Huang, Z.; Che, X.; Hou, J.; Wang, H.; Shen, H.; Qiu, L.; Li, Z.; et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: Implications for pathogenesis and virus transmission pathways. J. Pathol. 2004, 203, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Koehler, P.; Bassetti, M.; Kochanek, M.; Shimabukuro-Vornhagen, A.; Cornely, O.A. Intensive care management of influenza-associated pulmonary aspergillosis. Clin. Microbiol. Infect. 2019, 25, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Schauwvlieghe, A.; Rijnders, B.J.A.; Philips, N.; Verwijs, R.; Vanderbeke, L.; Van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van de Veerdonk, F.L.; Gommers, D.; et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir. Med. 2018, 6, 782–792. [Google Scholar] [CrossRef]
- D’Haese, J.; Theunissen, K.; Vermeulen, E.; Schoemans, H.; De Vlieger, G.; Lammertijn, L.; Meersseman, P.; Meersseman, W.; Lagrou, K.; Maertens, J. Detection of galactomannan in bronchoalveolar lavage fluid samples of patients at risk for invasive pulmonary aspergillosis: Analytical and clinical validity. J. Clin. Microbiol. 2012, 50, 1258–1263. [Google Scholar] [CrossRef] [Green Version]
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Executive Summary: Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, 433–442. [Google Scholar] [CrossRef] [Green Version]
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef]
- Cadena, J.; Thompson, G.R., 3rd; Patterson, T.F. Invasive Aspergillosis: Current Strategies for Diagnosis and Management. Infect. Dis. Clin. N. Am. 2016, 30, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, H.; Zhang, Y.; Huang, M.; He, Q.; Li, P.; Zhang, F.; Shi, Y.; Su, X. Diagnostic Value of Galactomannan Antigen Test in Serum and Bronchoalveolar Lavage Fluid Samples from Patients with Nonneutropenic Invasive Pulmonary Aspergillosis. J. Clin. Microbiol. 2017, 55, 2153–2161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahidi, M.M.; Lamb, C.; Murgu, S.; Musani, A.; Shojaee, S.; Sachdeva, A.; Maldonado, F.; Mahmood, K.; Kinsey, M.; Sethi, S.; et al. American Association for Bronchology and Interventional Pulmonology (AABIP) Statement on the Use of Bronchoscopy and Respiratory Specimen Collection in Patients With Suspected or Confirmed COVID-19 Infection. J. Bronchol. Interv. Pulmonol. 2020, 27, e52–e64. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and Fungal Coinfection in Individuals With Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef]
- Rawson, T.M.; Wilson, R.C.; Holmes, A. Understanding the role of bacterial and fungal infection in COVID-19. Clin. Microbiol. Infect. 2021, 27, 9–11. [Google Scholar] [CrossRef]
- Berry, L.; Lansbury, L.; Gale, L.; Carroll, A.M.; Lim, W.S. Point of care testing of Influenza A/B and RSV in an adult respiratory assessment unit is associated with improvement in isolation practices and reduction in hospital length of stay. J. Med. Microbiol. 2020, 69, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Lansbury, L.E.; Rodrigo, C.; Leonardi-Bee, J.; Nguyen-Van-Tam, J.; Shen Lim, W. Corticosteroids as Adjunctive Therapy in the Treatment of Influenza: An Updated Cochrane Systematic Review and Meta-analysis. Crit. Care Med. 2020, 48, e98–e106. [Google Scholar] [CrossRef]
- Alosaimi, B.; Naeem, A.; Hamed, M.E.; Alkadi, H.S.; Alanazi, T.; Al Rehily, S.S.; Almutairi, A.Z.; Zafar, A. Influenza co-infection associated with severity and mortality in COVID-19 patients. Virol. J. 2021, 18, 127. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Gregory, A.T.; Denniss, A.R. An Introduction to Writing Narrative and Systematic Reviews-Tasks, Tips and Traps for Aspiring Authors. Heart Lung Circ. 2018, 27, 893–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karruli, A.; Boccia, F.; Gagliardi, M.; Patauner, F.; Ursi, M.P.; Sommese, P.; De Rosa, R.; Murino, P.; Ruocco, G.; Corcione, A.; et al. Multidrug-Resistant Infections and Outcome of Critically Ill Patients with Coronavirus Disease 2019: A Single Center Experience. Microb. Drug Resist. 2021. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Emerick, M.; Cabunoc, M.K.; Williams, M.H.; Preas, M.A.; Schrank, G.; Rabinowitz, R.; Luethy, P.; Johnson, J.K.; Leekha, S. Rapid Spread and Control of Multidrug-Resistant Gram-Negative Bacteria in COVID-19 Patient Care Units. Emerg. Infect. Dis. 2021, 27, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Baiou, A.; Elbuzidi, A.A.; Bakdach, D.; Zaqout, A.; Alarbi, K.M.; Bintaher, A.A.; Ali, M.M.B.; Elarabi, A.M.; Ali, G.A.M.; Daghfal, J.; et al. Clinical characteristics and risk factors for the isolation of multi-drug-resistant Gram-negative bacteria from critically ill patients with COVID-19. J. Hosp. Infect. 2021, 110, 165–171. [Google Scholar] [CrossRef]
- Cultrera, R.; Barozzi, A.; Libanore, M.; Marangoni, E.; Pora, R.; Quarta, B.; Spadaro, S.; Ragazzi, R.; Marra, A.; Segala, D.; et al. Co-Infections in Critically Ill Patients with or without COVID-19: A Comparison of Clinical Microbial Culture Findings. Int. J. Environ. Res. Public Health 2021, 18, 4358. [Google Scholar] [CrossRef]
- Bogossian, E.G.; Taccone, F.S.; Izzi, A.; Yin, N.; Garufi, A.; Hublet, S.; Njimi, H.; Ego, A.; Gorham, J.; Byl, B.; et al. The Acquisition of Multidrug-Resistant Bacteria in Patients Admitted to COVID-19 Intensive Care Units: A Monocentric Retrospective Case Control Study. Microorganisms 2020, 8, 1821. [Google Scholar] [CrossRef]
- Nasir, N.; Rehman, F.; Omair, S.F. Risk factors for bacterial infections in patients with moderate to severe COVID-19: A case-control study. J. Med. Virol. 2021, 93, 4564–4569. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; Battaglini, D.; Ball, L.; Brunetti, I.; Bruzzone, B.; Codda, G.; Crea, F.; De Maria, A.; Dentone, C.; Di Biagio, A.; et al. Bloodstream infections in critically ill patients with COVID-19. Eur. J. Clin. Investig. 2020, 50, e13319. [Google Scholar] [CrossRef] [PubMed]
- Bonazzetti, C.; Morena, V.; Giacomelli, A.; Oreni, L.; Casalini, G.; Galimberti, L.R.; Bolis, M.; Rimoldi, M.; Ballone, E.; Colombo, R.; et al. Unexpectedly High Frequency of Enterococcal Bloodstream Infections in Coronavirus Disease 2019 Patients Admitted to an Italian ICU: An Observational Study. Crit. Care Med. 2021, 49, e31–e40. [Google Scholar] [CrossRef]
- Ripa, M.; Galli, L.; Poli, A.; Oltolini, C.; Spagnuolo, V.; Mastrangelo, A.; Muccini, C.; Monti, G.; De Luca, G.; Landoni, G.; et al. Secondary infections in patients hospitalized with COVID-19: Incidence and predictive factors. Clin. Microbiol. Infect. 2021, 27, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; Battaglini, D.; Enrile, E.M.; Dentone, C.; Vena, A.; Robba, C.; Ball, L.; Bartoletti, M.; Coloretti, I.; Di Bella, S.; et al. Incidence and Prognosis of Ventilator-Associated Pneumonia in Critically Ill Patients with COVID-19: A Multicenter Study. J. Clin. Med. 2021, 10, 555. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Yang, Y.; Cai, P.; Cao, J.; Cai, X.; Zhang, Y. Etiology and antimicrobial resistance of secondary bacterial infections in patients hospitalized with COVID-19 in Wuhan, China: A retrospective analysis. Antimicrob. Resist. Infect. Control 2020, 9, 153. [Google Scholar] [CrossRef]
- Bassetti, M.; Kollef, M.H.; Timsit, J.F. Bacterial and fungal superinfections in critically ill patients with COVID-19. Intensive Care Med. 2020, 46, 2071–2074. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-Garcia, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef]
- Tiri, B.; Sensi, E.; Marsiliani, V.; Cantarini, M.; Priante, G.; Vernelli, C.; Martella, L.A.; Costantini, M.; Mariottini, A.; Andreani, P.; et al. Antimicrobial Stewardship Program, COVID-19, and Infection Control: Spread of Carbapenem-Resistant Klebsiella Pneumoniae Colonization in ICU COVID-19 Patients. What Did Not Work? J. Clin. Med. 2020, 9, 2744. [Google Scholar] [CrossRef]
- Tacconelli, E.; Cataldo, M.A.; Dancer, S.J.; De Angelis, G.; Falcone, M.; Frank, U.; Kahlmeter, G.; Pan, A.; Petrosillo, N.; Rodriguez-Bano, J.; et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin. Microbiol. Infect. 2014, 20 (Suppl. S1), 1–55. [Google Scholar] [CrossRef] [Green Version]
- Clancy, C.J.; Buehrle, D.J.; Nguyen, M.H. PRO: The COVID-19 pandemic will result in increased antimicrobial resistance rates. JAC Antimicrob. Resist. 2020, 2, dlaa049. [Google Scholar] [CrossRef]
- Ranney, M.L.; Griffeth, V.; Jha, A.K. Critical Supply Shortages—The Need for Ventilators and Personal Protective Equipment during the COVID-19 Pandemic. N. Engl. J. Med. 2020, 382, e41. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.K. The Novel Coronavirus COVID-19 Outbreak: Global Implications for Antimicrobial Resistance. Front. Microbiol. 2020, 11, 1020. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Lee, S.W.; Ha, E.K.; Yeniova, A.O.; Moon, S.Y.; Kim, S.Y.; Koh, H.Y.; Yang, J.M.; Jeong, S.J.; Moon, S.J.; Cho, J.Y.; et al. Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: A nationwide cohort study with propensity score matching. Gut 2021, 70, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Kow, C.S.; Hasan, S.S. Use of proton pump inhibitors and risk of adverse clinical outcomes from COVID-19: A meta-analysis. J. Intern. Med. 2021, 289, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Bonell, A.; Azarrafiy, R.; Huong, V.T.L.; Viet, T.L.; Phu, V.D.; Dat, V.Q.; Wertheim, H.; van Doorn, H.R.; Lewycka, S.; Nadjm, B. A Systematic Review and Meta-analysis of Ventilator-associated Pneumonia in Adults in Asia: An Analysis of National Income Level on Incidence and Etiology. Clin. Infect. Dis. 2019, 68, 511–518. [Google Scholar] [CrossRef]
- Dudeck, M.A.; Horan, T.C.; Peterson, K.D.; Allen-Bridson, K.; Morrell, G.; Anttila, A.; Pollock, D.A.; Edwards, J.R. National Healthcare Safety Network report, data summary for 2011, device-associated module. Am. J. Infect. Control 2013, 41, 286–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Study | Type of Study | Country of the Study | Sample Size | Age Median (IQR) | Empiric Antibiotic Therapy at Admission N (%) | Steroid N (%) | Any MDR Bacterial Infection N (%) | CPE Infections N (%) | Acinetobacter Baumannii Infections N (%) | Pseudomonas Aeruginosas Infections N (%) | MRSA N(%) | Enterococcus Species N (%) | ICU-LOS, Days Median (IQR) | ICU */In-hospital Mortality N (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Karulli A. et al. [32] | Retrosp, single-center | Italy | 32 | 68 (55–75) | 25 (78.1) | 17 (53.1) | 16 (50) | 5 (32) | 3 (19) | 3 (19) | 1 (6) | 2 (13) | 10.5 (5.7–17) | 23 (71.8) * |
| Patel A. et al. [33] | Retrosp, single-center | Maryland, USA | 71 | N-A. | 69 (97) | 25 (35) | 71 (100) | 33 (46) | 27 (38) | 27 (38) | N.A. | N.A. | 11 (7–20) | 27 (38) * |
| Baiou A. et al. [34] | Retrosp, single-center | Qatar | 234 | 49 (40–60) | NA | 11 (4.7) | 78 (33) | 2 (2.5) | 0 | 6 (7.7) | N.A. | N.A. | 31 (20–48) | 12 (15) * |
| Cultrera R. et al. [35] | Retrosp, single-center, case-control study | Italy | 28 | N.A. | N.A. | N.A. | 9 (32) | 4 (44) | 17 (61) | 3 (33) | 5 (56) | 24 (86) | N.A. | N.A. |
| Bogossian E. G. et al. [36] | Retrosp, single-center, case-control study | Belgium | 72 | 61 (14) | 56 (78) | 7 (10) | 24 (33) | 3 (9) | 0 | 4 (13) | 0 | 3 (10) | 11 (3–28) | 22 (31) * |
| Nasir N. et al. [37] | Retrosp, single-center, case-control study | Pakistan | 100 | 60 (52–70) | 82 (82) | 77 (77) | 28 (56) | 0 | 16 (32) | 5 (10) | 5 (10) | 2 (4) | 9 (6–14) | 30 (30) |
| Giacobbe D.R. et al. [38] | Retrosp, single-center | Italy | 78 | 66 (57–70) | 75 (96) | 24 (31) | N.A. | 0 | 0 | 0 | 6 (13) | 8 (18) | N.A. | 20 (26) * |
| Bonazzetti C. et al. [39] | Retrosp, single-center | Italy | 89 | 61 (53–69) | N.A. | N.A. | 32 (36) | 10 (8.5) | 1 (0.8) | 1 (0.8) | 9 (7.6) | 53 (45) | 12 (8–18) | 44 (49.4) * |
| Ripa M. et al. [40] | Retrosp, single-center | Italy | 731 | 64 (55–76) | N.A. | 483 (66.1) | 21 (2.8) | 2 (10) | 9 (43) | 5 (24) | 1 (4.8) | 4 (19) | N.A. | 194 (26.5) |
| Grasselli G. et al. [8] | Retrosp, multicentric | Italy | 774 | 62 (54–68) | 534 (69) | 207 (27) | 272 (35) | 72 (26) | 19 (2.4) | 34 (4.4) | 83 (31) | 29 (4) | 14 (8–26) | 234 (30) * |
| Giacobbe D.R. et al. [41] | Retrosp, multicentric | Italy | 171 | 64 (57–71) | 162 (95) | 108 (63) | 60 (35) | 25 (15) | N.A. | 27 (16) | 8 (5) | N.A. | N.A. | 78 (46) * |
| Li J. et al. [42] | Retrosp, single-center | Wuhan, China | 102 | 66 (30–93) | 99 (97.1) | NA | 69(4.6) | 32 (31) | 50 (49) | 7 (4.4) | 3 (1.9) | 6 (3.8) | N.A | 50 (49) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasero, D.; Cossu, A.P.; Terragni, P. Multi-Drug Resistance Bacterial Infections in Critically Ill Patients Admitted with COVID-19. Microorganisms 2021, 9, 1773. https://doi.org/10.3390/microorganisms9081773
Pasero D, Cossu AP, Terragni P. Multi-Drug Resistance Bacterial Infections in Critically Ill Patients Admitted with COVID-19. Microorganisms. 2021; 9(8):1773. https://doi.org/10.3390/microorganisms9081773
Chicago/Turabian StylePasero, Daniela, Andrea Pasquale Cossu, and Pierpaolo Terragni. 2021. "Multi-Drug Resistance Bacterial Infections in Critically Ill Patients Admitted with COVID-19" Microorganisms 9, no. 8: 1773. https://doi.org/10.3390/microorganisms9081773
APA StylePasero, D., Cossu, A. P., & Terragni, P. (2021). Multi-Drug Resistance Bacterial Infections in Critically Ill Patients Admitted with COVID-19. Microorganisms, 9(8), 1773. https://doi.org/10.3390/microorganisms9081773





