The Upcoming 6Li Isotope Requirements Might Be Supplied by a Microalgal Enrichment Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae Strains and Growth Conditions
2.2. CAFE Strain Identification
2.3. Lithium Uptake and Isotope Fractionation Trials
2.4. Analytical Procedure
2.4.1. Sample Dissolution and Lithium Separation
2.4.2. Chromatography
2.4.3. Mass Spectrometry
3. Results
3.1. CAFE Strain Identification
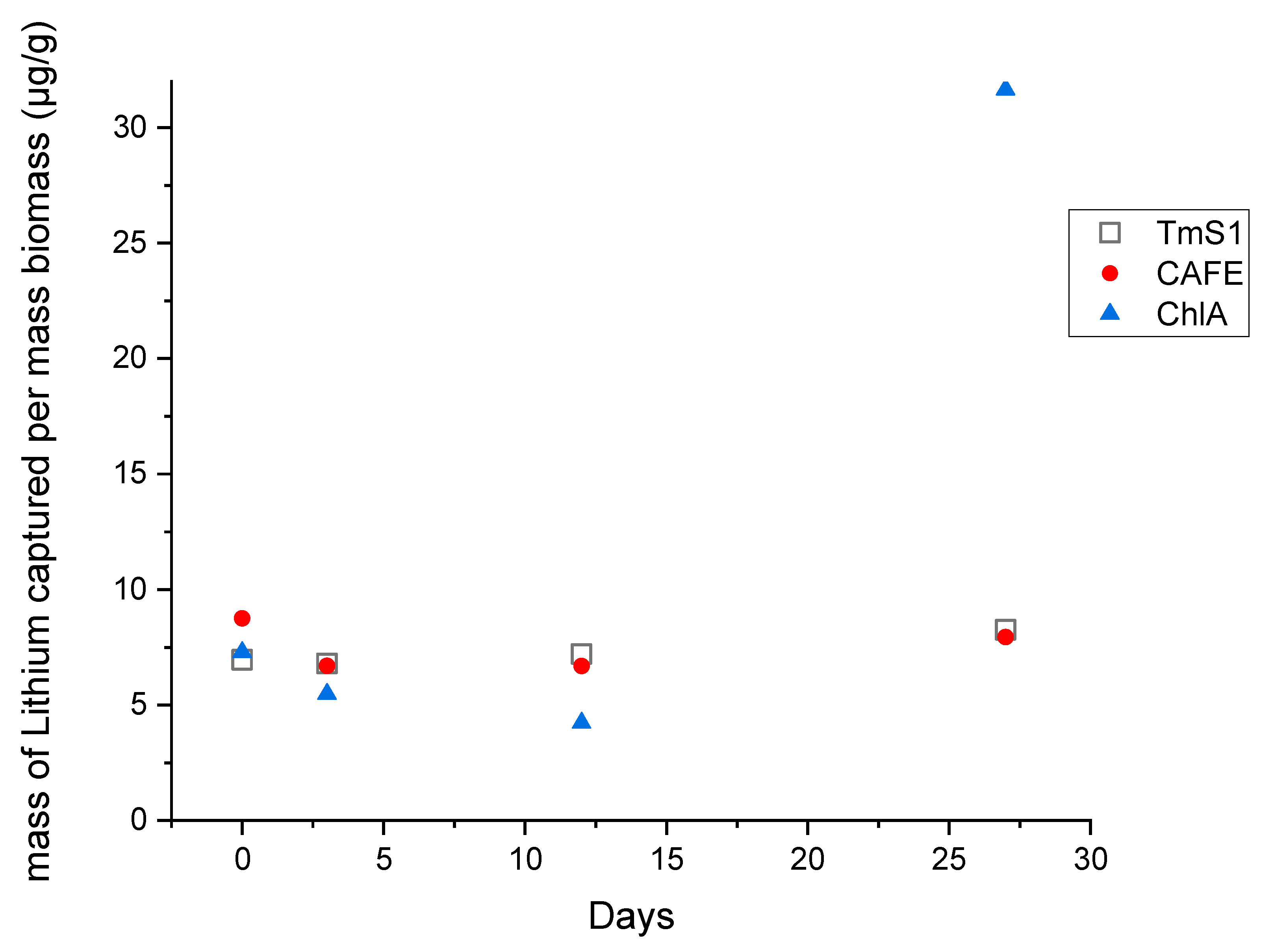
3.2. Lithium Uptake
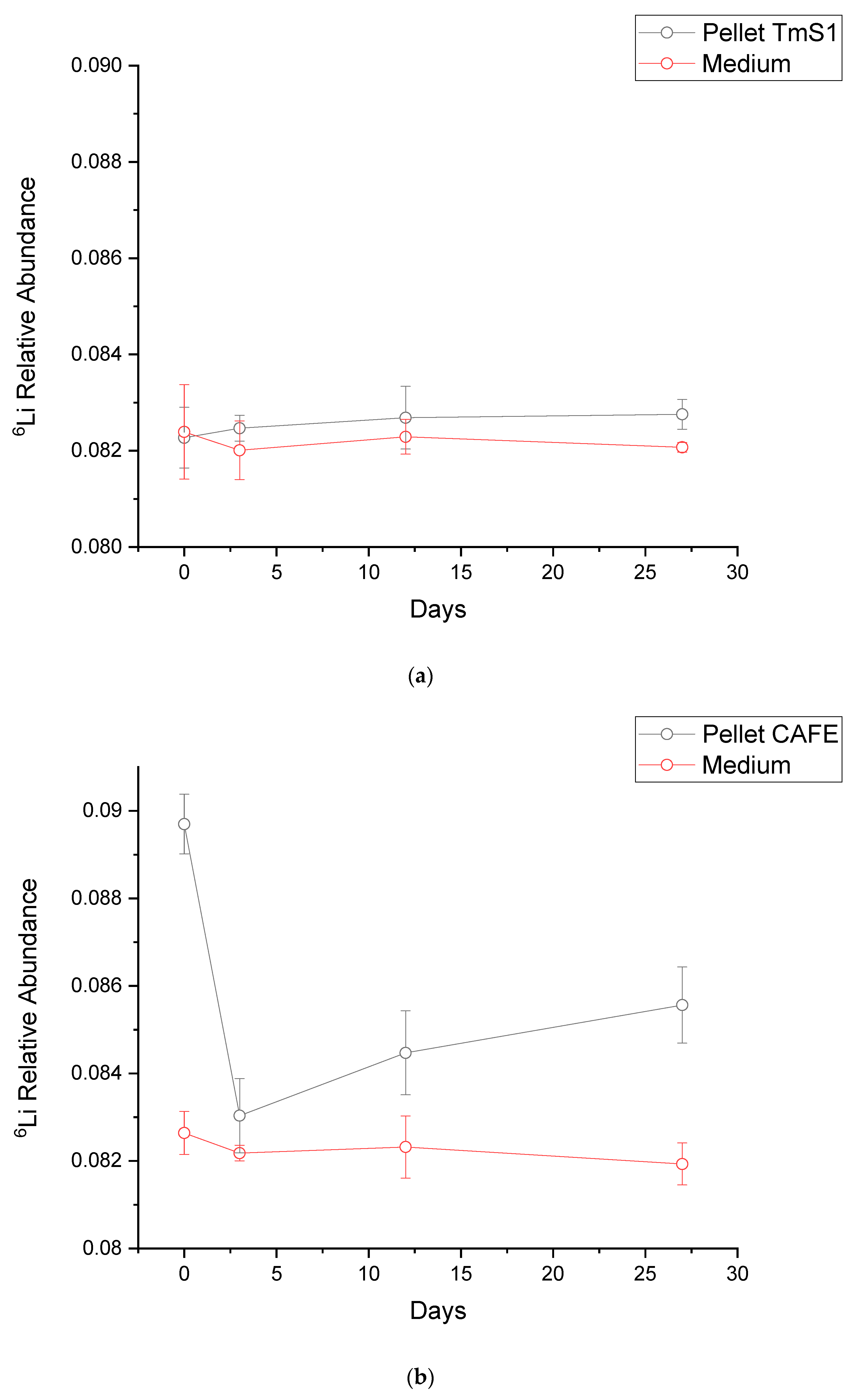
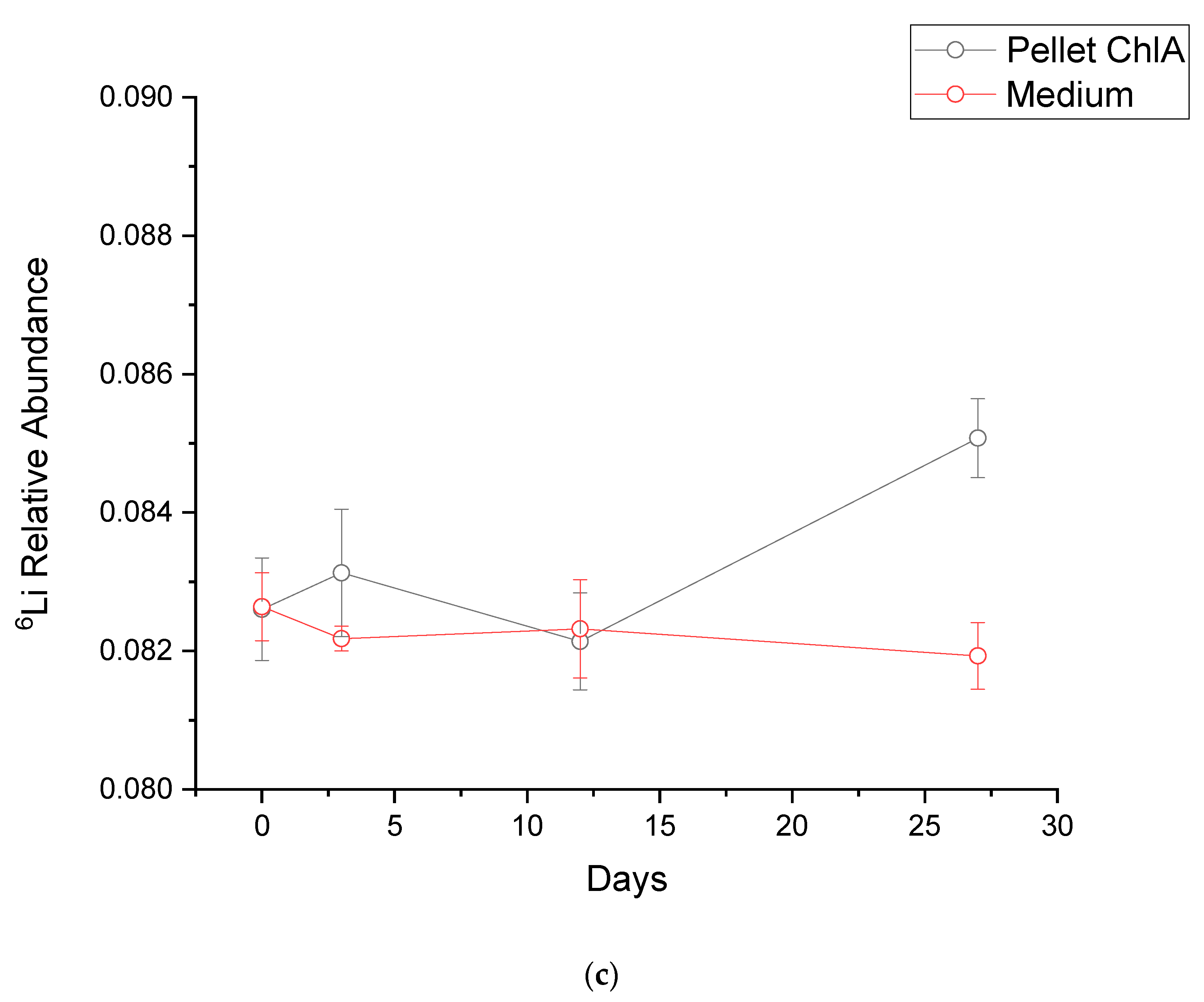
3.3. Isotope Fractionation Trials
4. Discussion
4.1. Lithium Capture
4.2. Fractionation
4.3. Biotechnological Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- International Energy Agency. World Energy Outlook 2020; International Energy Agency: Paris, France, 2020. [Google Scholar]
- Ongena, J.; Van Oost, G. Energy for future centuries: Prospects for fusion power as a future energy source. Fusion Sci. Tech. 2012, 61, 3–16. [Google Scholar] [CrossRef] [Green Version]
- ITER-The Way to New Energy. Available online: https://www.iter.org/ (accessed on 3 March 2020).
- Meija, J.; Coplen, T.B.; Berglund, M.; Brand, W.A.; De Bièvre, P.; Gröning, M.; Holden, N.E.; Irrgeher, J.; Loss, R.D.; Walczyk, T.; et al. Isotopic compositions of the elements 2013 (IUPAC Technical Report). Pure Appl. Chem. 2016, 88, 293–306. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, A.M.; Hamacher, T.; Fischer, U. Is nuclear fusion a sustainable energy form? Fusion Eng. Des. 2010, 86, 2770. [Google Scholar] [CrossRef] [Green Version]
- Nishio, S.; Ohmori, J.; Kuroda, T.; Tobita, K.; Enoeda, M.; Tsuru, D.; Hirose, S.; Sato, Y.; Kawamura, H.; Nakamura, M.; et al. Consideration on blanket structure for fusion DEMO plant at JAERI. Fusion Eng. Des. 2006, 81, 1271–1276. [Google Scholar] [CrossRef]
- Tobita, K.; Nishio, S.; Tanigawa, H.; Enoeda, M.; Isono, T.; Nakamura, H.; Tsuru, D.; Suzuki, S.; Hayashi, T.; Tsuchiya, K.; et al. Torus configuration and materials selection on a fusion DEMO reactor, SlimCS. J. Nucl. Mater. 2009, 386–388, 888–892. [Google Scholar] [CrossRef]
- Herranz García, J.L. Viabilidad Técnica para la Explotación y Separación Isotópica de Li y Prospectiva en el Mercado de Baterías de ION-7Li y de 6Li como Material Base de Diseño en Reactores de Fusión Nuclear; E.T.S.I. Minas (UPM): Madrid, Spain, 2013. [Google Scholar]
- Giegerich, T.; Battes, K.; Schwenzer, J.C.; Day, C. Development of a viable route for lithium-6 supply of DEMO and future fusion power plants. Fusion Eng. Des. 2019, 149, 111339. [Google Scholar] [CrossRef]
- Cui, L.; Yang, X.; Wang, J.; He, H.; Guo, Y.; Cheng, F.; Zhang, S. Theoretical prediction of 6 Li/ 7 Li separation in solvent extraction system using Urey model. Chem. Eng. J. 2019, 358, 435–445. [Google Scholar] [CrossRef]
- Brooks, S.C.; Southworth, G.R. History of mercury use and environmental contamination at the Oak Ridge Y-12 Plant. Environ. Pollut. 2011, 159, 219–228. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, M.; Sun, J.; Shao, F.; Jia, Y.; Jing, Y. Lithium Isotope Green Separation Using Water Scrubbing. Chem. Lett. 2019, 48, 1541–1543. [Google Scholar] [CrossRef]
- Burton, K.W.; Vigier, N. Lithium Isotopes as Tracers in Marine and Terrestrial Environments. In Handbook of Environmental Isotope Geochemistry. Advances in Isotope Geochemistry; Springer: Berlin/Heidelberg, Germany, 2012; pp. 41–59. [Google Scholar] [CrossRef]
- Taylor, T.I.; Urey, H.C. Fractionation of the Lithium and Potassium Isotopes by Chemical Exchange with Zeolites. J. Chem. Phys. 1938, 6, 429. [Google Scholar] [CrossRef]
- Pistiner, J.S.; Henderson, G.M. Lithium-isotope fractionation during continental weathering processes. Earth Planet. Sci. Lett. 2003, 214, 327–339. [Google Scholar] [CrossRef]
- Huh, Y.; Chan, L.H.; Edmond, J.M. Lithium isotopes as a probe of weathering processes: Orinoco River. Earth Planet. Sci. Lett. 2001, 194, 189–199. [Google Scholar] [CrossRef]
- Vigier, N.; Gislason, S.R.; Burton, K.W.; Millot, R.; Mokadem, F. The relationship between riverine lithium isotope composition and silicate weathering rates in Iceland. Earth Planet. Sci. Lett. 2009, 287, 434–441. [Google Scholar] [CrossRef]
- Pogge von Strandmann, P.A.E.; Kasemann, S.A.; Wimpenny, J.B. Lithium and Lithium Isotopes in Earth’s Surface Cycles. Elements 2020, 16, 253–258. [Google Scholar] [CrossRef]
- Hoefs, J.; Sywall, M. Lithium isotope composition of Quaternary and Tertiary biogene carbonates and a global lithium isotope balance. Geochim. Cosmochim. Acta 1997, 61, 2679–2690. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Tomascak, P.B.; Njo, H.B.; Gardner, L.R. Extreme lithium isotopic fractionation during continental weathering revealed in saprolites from South Carolina. Chem. Geol. 2004, 212, 45–57. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Nakamura, E. Preface to “Lithium isotope geochemistry”. Chem. Geol. 2004, 212, 1–4. [Google Scholar] [CrossRef]
- Balter, V.; Vigier, N. Natural variations of lithium isotopes in a mammalian model. Metallomics 2014, 6, 582–586. [Google Scholar] [CrossRef]
- Sherman, W.R.; Munsell, L.Y.; Wong, Y.-H.H. Differential Uptake of Lithium Isotopes by Rat Cerebral Cortex and Its Effect on Inositol Phosphate Metabolism. J. Neurochem. 1984, 42, 880–882. [Google Scholar] [CrossRef]
- Lieberman, K.; Alexander, G.J.; Sechzer, J.A. Stable isotopes of lithium: Dissimilar biochemical and behavioral effects. Experientia 1986, 42, 985–987. [Google Scholar] [CrossRef]
- Stoll, P.M.; Stokes, P.E.; Okamoto, M. Lithium isotopes: Differential effects on renal function and histology. Bipolar Disord. 2001, 3, 174–180. [Google Scholar] [CrossRef]
- Lieberman, K.W.; Alexander, G.J.; Stokes, P. Dissimilar effects of lithium isotopes on motility in rats. Pharmacol. Biochem. Behav. 1979, 10, 933–935. [Google Scholar] [CrossRef]
- Jakobsson, E.; Argüello-Miranda, O.; Chiu, S.W.; Fazal, Z.; Kruczek, J.; Nunez-Corrales, S.; Pandit, S.; Pritchet, L. Towards a Unified Understanding of Lithium Action in Basic Biology and its Significance for Applied Biology. J. Membr. Biol. 2017, 250, 587–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahzad, B.; Mughal, M.N.; Tanveer, M.; Gupta, D.; Abbas, G. Is lithium biologically an important or toxic element to living organisms? An overview. Environ. Sci. Pollut. Res. 2017, 24, 103–115. [Google Scholar] [CrossRef]
- Dolara, P. Occurrence, exposure, effects, recommended intake and possible dietary use of selected trace compounds (aluminium, bismuth, cobalt, gold, lithium, nickel, silver). Int. J. Food Sci. Nutr. 2014, 65, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, M.; Hasanuzzaman, M.; Wang, L. Lithium in Environment and Potential Targets to Reduce Lithium Toxicity in Plants. J. Plant. Growth Regul. 2019, 38, 1574–1586. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Hassan, W.; Shah, A.N.; Anjum, S.A.; Cheema, S.A.; Ali, I. Lithium toxicity in plants: Reasons, mechanisms and remediation possibilities–A review. Plant Physiol. Biochem. 2016, 107, 104–115. [Google Scholar] [CrossRef]
- Lopilato, J.; Tsuchiya, T.; Wilson, T.H. Role of Na+ and Li+ in thiomethylgalactoside transport by the melibiose transport system of Escherichia coli. J. Bacteriol. 1978, 134, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Kayama-Gonda, Y.; Kawasaki, T. Role of lithium ions in proline transport in Escherichia coli. J. Bacteriol. 1979, 139, 560–564. [Google Scholar] [CrossRef] [Green Version]
- Granger, J.; Sigman, D.M.; Lehmann, M.F.; Tortell, P.D. Nitrogen and oxygen isotope fractionation during dissimilatory nitrate reduction by denitrifying bacteria. Limnol. Oceanogr. 2008, 53, 2533–2545. [Google Scholar] [CrossRef]
- Kritee, K.; Blum, J.D.; Johnson, M.W.; Bergquist, B.A.; Barkay, T. Mercury stable isotope fractionation during reduction of Hg(II) to Hg(0) by Mercury resistant microorganisms. Environ. Sci. Technol. 2007, 41, 1889–1895. [Google Scholar] [CrossRef]
- Macko, S.A.; Fogel, M.L.; Hare, P.E.; Hoering, T.C. Isotopic fractionation of nitrogen and carbon in the synthesis of amino acids by microorganisms. Chem. Geol. Isot. Geosci. Sect. 1987, 65, 79–92. [Google Scholar] [CrossRef]
- Penger, J.; Conrad, R.; Blaser, M. Stable carbon isotope fractionation of six strongly fractionating microorganisms is not affected by growth temperature under laboratory conditions. Geochim. Cosmochim. Acta 2014, 140, 95–105. [Google Scholar] [CrossRef]
- Chambers, L.A.; Trudinger, P.A. Microbiological fractionation of stable sulfur isotopes: A review and critique. Geomicrobiol. J. 1979, 1, 249–293. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Viers, J.; Emnova, E.E.; Kompantseva, E.I.; Freydier, R. Copper isotope fractionation during its interaction with soil and aquatic microorganisms and metal oxy(hydr)oxides: Possible structural control. Geochim. Cosmochim. Acta 2008, 72, 1742–1757. [Google Scholar] [CrossRef]
- Wiederhold, J.G. Metal stable isotope signatures as tracers in environmental geochemistry. Environ. Sci. Technol. 2015, 49, 2606–2624. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Tomita, O. Bioseparation of Lithium Isotopes by Using Microorganisms. Resour. Environ. Biotechnol. 2000, 3, 173–182. [Google Scholar]
- García-Balboa, C.; Baselga-Cervera, B.; García-Sanchez, A.; Igual, J.M.; Lopez-Rodas, V.; Costas, E. Rapid adaptation of microalgae to bodies of water with extreme pollution from uranium mining: An explanation of how mesophilic organisms can rapidly colonise extremely toxic environments. Aquat. Toxicol. 2013, 144–145, 116–123. [Google Scholar] [CrossRef] [PubMed]
- López-Rodas, V.; Marvá, F.; Rouco, M.; Costas, E.; Flores-Moya, A. Adaptation of the chlorophycean Dictyosphaerium chlorelloides to stressful acidic, mine metal-rich waters as result of pre-selective mutations. Chemosphere 2008, 72, 703–707. [Google Scholar] [CrossRef]
- Vadlamani, A.; Viamajala, S.; Pendyala, B.; Varanasi, S. Cultivation of Microalgae at Extreme Alkaline pH Conditions: A Novel Approach for Biofuel Production. ACS Sustain. Chem. Eng. 2017, 5, 7284–7294. [Google Scholar] [CrossRef]
- Abinandan, S.; Subashchandrabose, S.R.; Cole, N.; Dharmarajan, R.; Venkateswarlu, K.; Megharaj, M. Sustainable production of biomass and biodiesel by acclimation of non-acidophilic microalgae to acidic conditions. Bioresour. Technol. 2019, 271, 316–324. [Google Scholar] [CrossRef]
- Baselga-Cervera, B.; García-Balboa, C.; Díaz-Alejo, H.M.; Costas, E.; López-Rodas, V. Rapid Colonization of Uranium Mining-Impacted Waters, the Biodiversity of Successful Lineages of Phytoplankton Extremophiles. Microb. Ecol. 2020, 79, 576–587. [Google Scholar] [CrossRef]
- MeGraw, V.E.; Brown, A.R.; Boothman, C.; Goodacre, R.; Morris, K.; Sigee, D.; Anderson, L.; Lloyd, J.R. A novel adaptation mechanism underpinning algal colonization of a nuclear fuel storage pond. mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kottuparambil, S.; Jin, P.; Agusti, S. Adaptation of Red Sea Phytoplankton to Experimental Warming Increases Their Tolerance to Toxic Metal Exposure. Front. Environ. Sci. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Alvarez, E.L.; González-Ledezma, G.; Bolaños Prats, J.A.; Stephano-Hornedo, J.L.; Hildebrand, M. Evaluating Marinichlorella kaistiae KAS603 cell size variation, growth and TAG accumulation resulting from rapid adaptation to highly diverse trophic and salinity cultivation regimes. Algal Res. 2017, 25, 12–24. [Google Scholar] [CrossRef]
- Suresh Kumar, K.; Dahms, H.U.; Won, E.J.; Lee, J.S.; Shin, K.H. Microalgae- A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Gaur, J.P. Use of Algae for Removing Heavy Metal Ions From Wastewater: Progress and Prospects. Crit. Rev. Biotechnol. 2005, 25, 113–152. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Castro, P.M.L.; Malcata, F.X. Metal uptake by microalgae: Underlying mechanisms and practical applications. Biotechnol. Prog. 2012, 28, 299–311. [Google Scholar] [CrossRef]
- Sandau, E.; Sandau, P.; Pulz, O. Heavy metal sorption by microalgae. Acta Biotechnol. 1996, 16, 227–235. [Google Scholar] [CrossRef]
- Kaštánek, P.; Kronusová, O.; Kaštánek, F.; Brányiková, I.; Prochazková, G.; Jandová, J.; Brányik, T.; Bišová, K. Selective bioaccumulation of rubidium by microalgae from industrial wastewater containing rubidium and lithium. J. Appl. Phycol. 2018, 30, 461–467. [Google Scholar] [CrossRef]
- Levy, J.L.; Angel, B.M.; Stauber, J.L.; Poon, W.L.; Simpson, S.L.; Cheng, S.H.; Jolley, D.F. Uptake and internalisation of copper by three marine microalgae: Comparison of copper-sensitive and copper-tolerant species. Aquat. Toxicol. 2008, 89, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Saavedra, R.; Muñoz, R.; Taboada, M.E.; Vega, M.; Bolado, S. Comparative uptake study of arsenic, boron, copper, manganese and zinc from water by different green microalgae. Bioresour. Technol. 2018, 263, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terry, P.A.; Stone, W. Biosorption of cadmium and copper contaminated water by Scenedesmus abundans. Chemosphere 2002, 47, 249–255. [Google Scholar] [CrossRef]
- Baselga-Cervera, B.; García-Balboa, C.; López-Rodas, V.; Fernández Díaz, M.; Costas, E. Evidence of microalgal isotopic fractionation through enrichment of depleted uranium. Sci. Rep. 2019, 9, 1973. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.A.; Hamouda, R.A.; Rabei, N.H.; Mousa, I.E.; Abdel-Hamid, M.S. Phycoremediation of lithium ions from aqueous solutions using free and immobilized freshwater green alga Oocystis solitaria: Mathematical modeling for bioprocess optimization. Environ. Sci. Pollut. Res. 2019, 26, 19335–19351. [Google Scholar] [CrossRef]
- Scaife, M.A.; Nguyen, G.T.D.T.; Rico, J.; Lambert, D.; Helliwell, K.E.; Smith, A.G. Establishing Chlamydomonas reinhardtii as an industrial biotechnology host. Plant J. 2015, 82, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, C.L.; Muñoz, C.; San Martín, M.; Cadoret, J.P.; Henríquez, V. Chloroplast Dual Divergent Promoter Plasmid for Heterologous Protein Expression in Tetraselmis suecica (Chlorophyceae, Chlorodendrales). J. Phycol. 2020, 56, 1066–1076. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, R.M.; Gao, Z.M.; Bougouffa, S.; Qian, P.-Y. Optimal Eukaryotic 18S and Universal 16S/18S Ribosomal RNA Primers and Their Application in a Study of Symbiosis. PLoS ONE 2014, 9, e90053. [Google Scholar] [CrossRef] [Green Version]
- Cheng, T.; Xu, C.; Lei, L.; Li, C.; Zhang, Y.; Zhou, S. Barcoding the kingdom Plantae: New PCR primers for ITS regions of plants with improved universality and specificity. Mol. Ecol. Resour. 2016, 16, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Baselga-Cervera, B.; Romero-López, J.; García-Balboa, C.; Costas, E.; López-Rodas, V. Improvement of the uranium sequestration ability of a Chlamydomonas sp. (ChlSP strain) isolated from extreme uranium mine tailings through selection for potential bioremediation application. Front. Microbiol. 2018, 9, 523. [Google Scholar] [CrossRef]
- Zhao, Z.; Rasool, M.A.; Chen, C.; Ma, S.; Wang, L.; Huang, G. Identification and screening of multiple tropical microalgal strains for antioxidant activity in vitro. Food Biosci. 2020, 36, 100649. [Google Scholar] [CrossRef]
- Jeong, D.; Jang, A. Exploration of microalgal species for simultaneous wastewater treatment and biofuel production. Environ. Res. 2020, 188, 109772. [Google Scholar] [CrossRef]
- Litio y Sodio-Propiedades. Available online: https://elementos.org.es/litio-y-sodio (accessed on 20 January 2021).
- Hagemann, M. Coping with High and Variable Salinity: Molecular Aspects of Compatible Solute Accumulation. In The Physiology of Microalgae; Borowitzka, M.A., Beardall, J., Raven, J.A., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 359–372. [Google Scholar] [CrossRef]
- Clergue, C.; Dellinger, M.; Buss, H.L.; Gaillardet, J.; Benedetti, M.F.; Dessert, C. Influence of atmospheric deposits and secondary minerals on Li isotopes budget in a highly weathered catchment, Guadeloupe (Lesser Antilles). Chem. Geol. 2015, 414, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Pogge von Strandmann, P.A.E.; Burton, K.W.; Opfergelt, S.; Eiríksdóttir, E.S.; Murphy, M.J.; Einarsson, A.; Gislason, S. The effect of hydrothermal spring weathering processes and primary productivity on lithium isotopes: Lake Myvatn, Iceland. Chem. Geol. 2016, 445, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Liu, X.M.; Chadwick, O.A. Lithium isotope behavior in Hawaiian regoliths: Soil-atmosphere-biosphere exchanges. Geochim. Cosmochim. Acta 2020, 285, 175–192. [Google Scholar] [CrossRef]
- Li, W. Vital effects of K isotope fractionation in organisms: Observations and a hypothesis. Acta Geochim. 2017, 36, 374–378. [Google Scholar] [CrossRef]
- Alhattab, M.; Kermanshahi-Pour, A.; Brooks, M.S.L. Microalgae disruption techniques for product recovery: Influence of cell wall composition. J. Appl. Phycol. 2019, 31, 61–88. [Google Scholar] [CrossRef]
- Morozov, N.P. (Table 2d) Lithium and rubidium concentrations in waters of the Mediterranean Sea. PANGAEA 1968. [Google Scholar] [CrossRef]
- Hayashida, G.; Schneider, C.; Espíndola, L.; Arias, D.; Riquelme, C.; Wulff-Zottele, C.; Díaz-Palma, P.; Rivas, M. Characterization of a Chlorophyta microalga isolated from a microbial mat in Salar de Atacama (northern Chile) as a potential source of compounds for biotechnological applications. Phycol. Res. 2017, 65, 202–211. [Google Scholar] [CrossRef]
- Hernández, K.L.; Yannicelli, B.; Olsen, L.M.; Dorador, C.; Menschel, E.J.; Molina, V.; Remonsellez, F.; Hengst, M.B.; Jeffrey, W.H. Microbial Activity Response to Solar Radiation across Contrasting Environmental Conditions in Salar de Huasco, Northern Chilean Altiplano. Front. Microbiol. 2016, 7, 1857. [Google Scholar] [CrossRef] [Green Version]
- Martínez, M.; Leyton, Y.; Cisternas, L.; Riquelme, C. Metal Removal from Acid Waters by an Endemic Microalga from the Atacama Desert for Water Recovery. Minerals 2018, 8, 378. [Google Scholar] [CrossRef] [Green Version]
| Gene | Primer Name | Primer Sequence |
|---|---|---|
| 18S | 18SF1 | GGT TGA TYC TGC CAG TAG |
| 18SR1 | GMW ACC TTG TTA CGA CTT | |
| ITS | ITSu1 | GGA AGK ARA AGT CGT AAC AAG G |
| ITSu4 | RGT TTC TTT TCC TCC GCT TA |
| Strain | Day | Pellet Mass (g) | Li Mass in the Pellet (ng) | Li Mass/Biomass (µg/g) |
|---|---|---|---|---|
| TmS1 | 0 | 0.1273 | 887 | 6.96 |
| TmS1 | 3 | 0.2861 | 1946 | 6.8 |
| TmS1 | 12 | 0.1542 | 1113 | 7.21 |
| TmS1 | 27 | 0.4174 | 3447 | 8.26 |
| CAFE | 0 | 0.0971 | 850 | 8.76 |
| CAFE | 3 | 0.1888 | 1265 | 6.7 |
| CAFE | 12 | 0.2261 | 1512 | 6.69 |
| CAFE | 27 | 0.5786 | 4597 | 7.94 |
| ChlA | 0 | 0.1214 | 885 | 7.29 |
| ChlA | 3 | 0.1432 | 784 | 5.48 |
| ChlA | 12 | 0.3876 | 1641 | 4.23 |
| ChlA | 27 | 0.1926 | 6098 | 31.66 |
| 6Li/7Li Relative Abundance | ||||
|---|---|---|---|---|
| Strain | Time (Days) | Pellet (±Uncertainty) | Medium (±Uncertainty) | δ6 |
| TmS1 | 0 | 0.08227 ± 0.00063 | 0.08239 ± 0.00098 | −1.45 |
| 3 | 0.08247 ± 0.00027 | 0.08201 ± 0.00061 | 5.58 | |
| 12 | 0.08269 ± 0.00065 | 0.08229 ± 0.00036 | 4.80 | |
| 27 | 0.08276 ± 0.00031 | 0.08207 ± 0.00010 | 8.35 | |
| CAFE | 0 | 0.0897 ± 0.00068 | 008264 ± 0.00049 | 85.39 |
| 3 | 0.08303 ± 0.00085 | 0.08218 ± 0.00018 | 10.39 | |
| 12 | 0.08447 ± 0.00096 | 0.08232 ± 0.00071 | 26.12 | |
| 27 | 0.08556 ± 0.00087 | 0.08193 ± 0.00048 | 44.32 | |
| ChlA | 0 | 0.0826 ± 0.00074 | 0.08264 ± 0.00049 | −0.44 |
| 3 | 0.08313 ± 0.00092 | 0.08218 ± 0.00018 | 11.54 | |
| 12 | 0.08214 ± 0.00070 | 0.08232 ± 0.00071 | −2.19 | |
| 27 | 0.08508 ± 0.00057 | 0.08193 ± 0.00048 | 38.40 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Alejo, H.M.; López-Rodas, V.; García-Balboa, C.; Tarín, F.; Barrado, A.I.; Conde, E.; Costas, E. The Upcoming 6Li Isotope Requirements Might Be Supplied by a Microalgal Enrichment Process. Microorganisms 2021, 9, 1753. https://doi.org/10.3390/microorganisms9081753
Díaz-Alejo HM, López-Rodas V, García-Balboa C, Tarín F, Barrado AI, Conde E, Costas E. The Upcoming 6Li Isotope Requirements Might Be Supplied by a Microalgal Enrichment Process. Microorganisms. 2021; 9(8):1753. https://doi.org/10.3390/microorganisms9081753
Chicago/Turabian StyleDíaz-Alejo, Héctor M., Victoria López-Rodas, Camino García-Balboa, Francisco Tarín, Ana I. Barrado, Estefanía Conde, and Eduardo Costas. 2021. "The Upcoming 6Li Isotope Requirements Might Be Supplied by a Microalgal Enrichment Process" Microorganisms 9, no. 8: 1753. https://doi.org/10.3390/microorganisms9081753
APA StyleDíaz-Alejo, H. M., López-Rodas, V., García-Balboa, C., Tarín, F., Barrado, A. I., Conde, E., & Costas, E. (2021). The Upcoming 6Li Isotope Requirements Might Be Supplied by a Microalgal Enrichment Process. Microorganisms, 9(8), 1753. https://doi.org/10.3390/microorganisms9081753





