Immunohistochemical Detection of Propionibacterium acnes in Granulomas for Differentiating Sarcoidosis from Other Granulomatous Diseases Utilizing an Automated System with a Commercially Available PAB Antibody
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Baseline Characteristics of Sarcoidosis and Control Patients
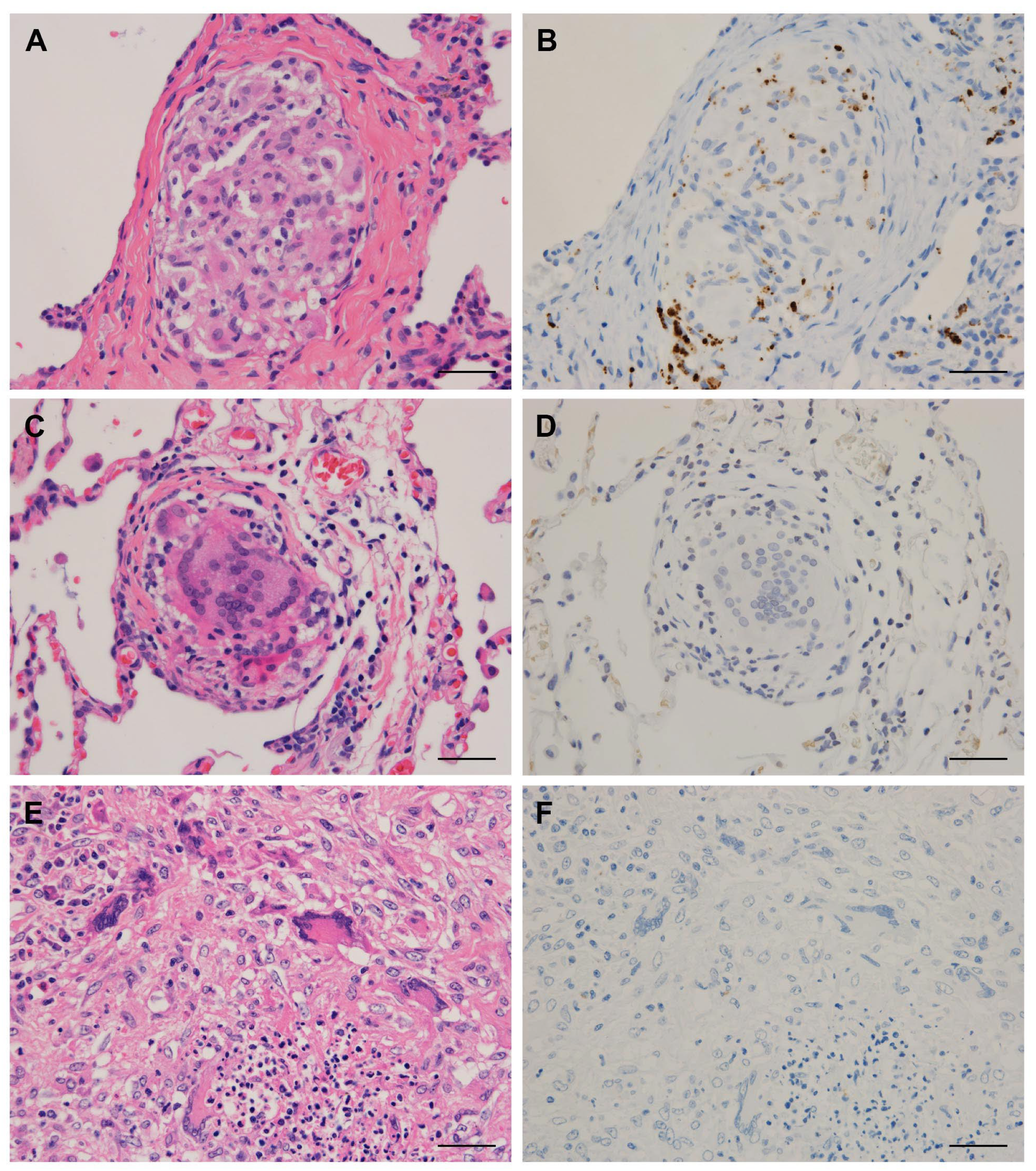
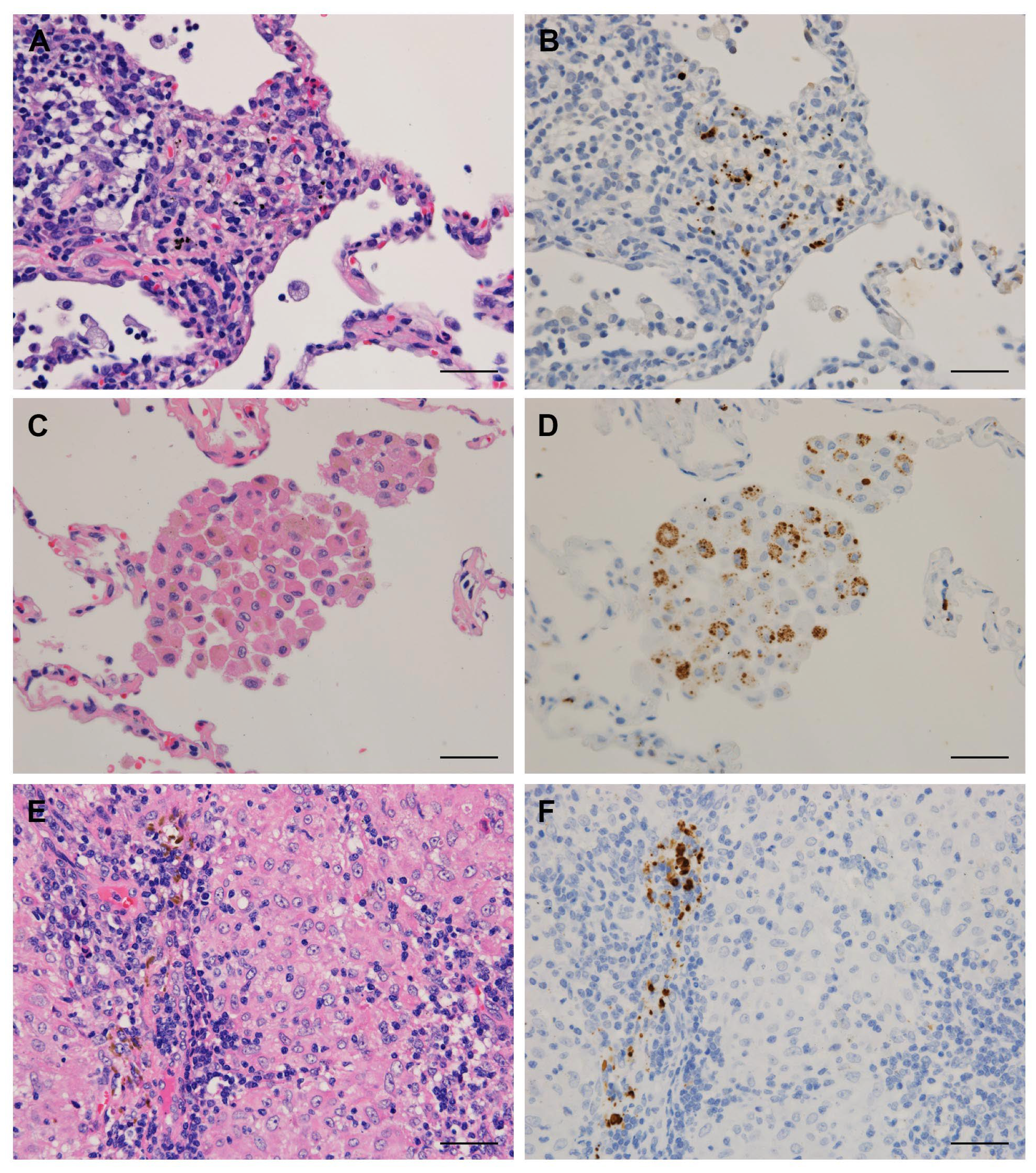
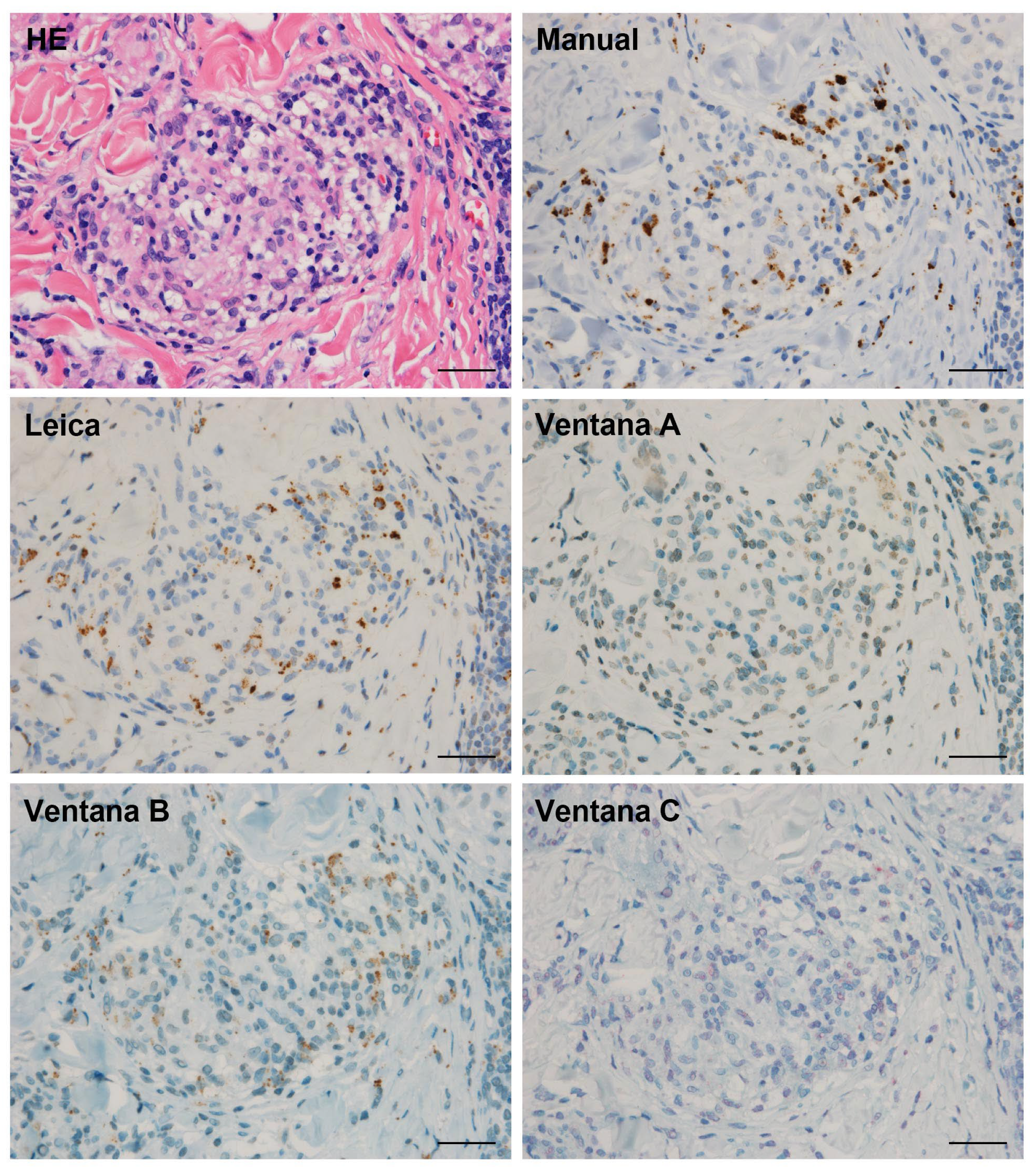
2.3. Immunohistochemistry
2.4. Data Analysis
3. Results
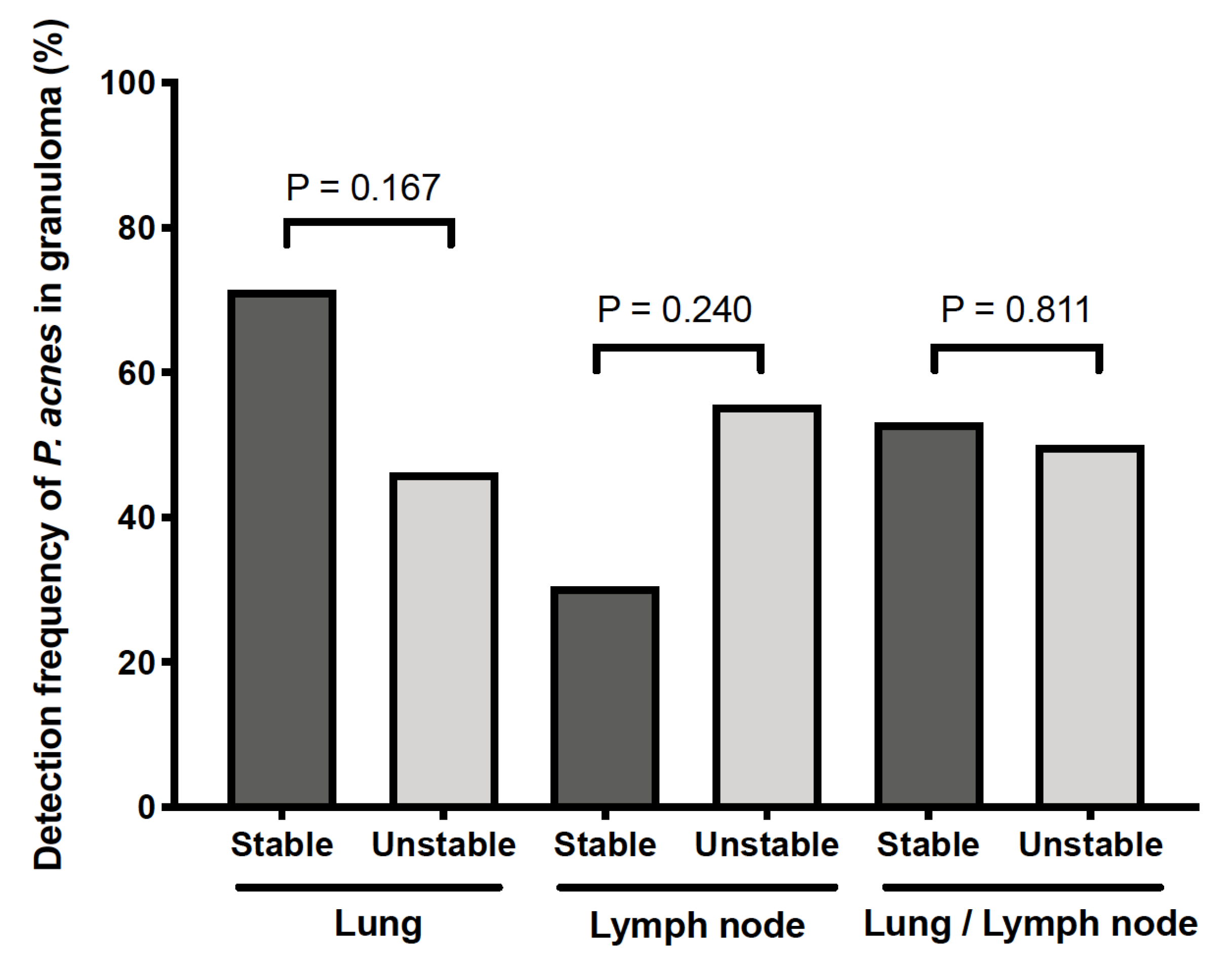
3.1. Detection Frequency of P. acnes in Tissues by Location
3.2. Clinical Data and Detection Status of P. acnes in Granulomas
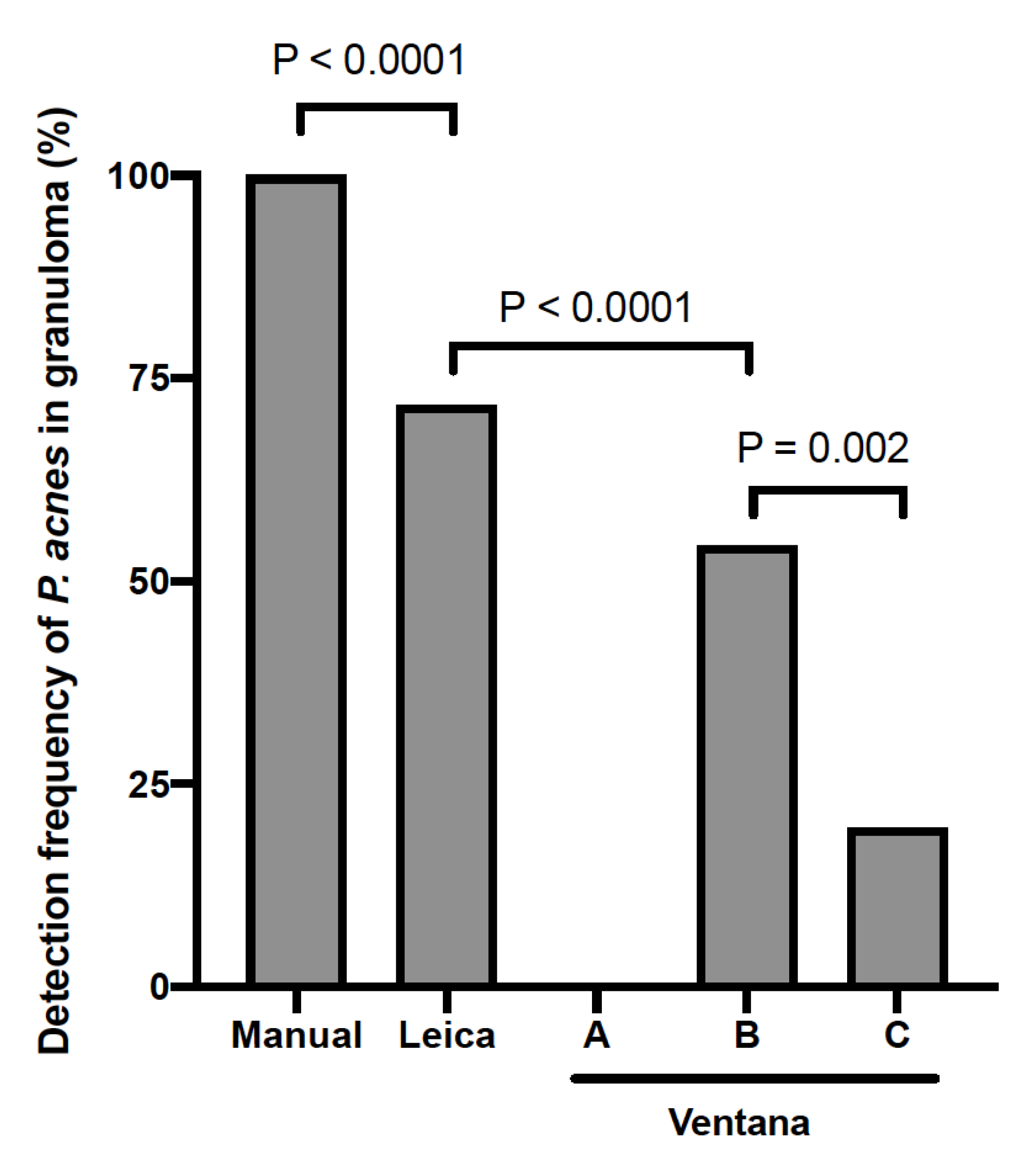
3.3. Detection Sensitivity of P. acnes in Granulomas by Automated IHC Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Valeyre, D.; Prasse, A.; Nunes, H.; Uzunhan, Y.; Brillet, P.Y.; Müller-Quernheim, J. Sarcoidosis. Lancet 2014, 383, 1155–1167. [Google Scholar] [CrossRef]
- Hunninghake, G.W.; Costabel, U.; Ando, M.; Baughman, R.; Cordier, J.F.; du Bois, R.; Eklund, A.; Kitaichi, M.; Lynch, J.; Rizzato, G.; et al. ATS/ERS/WASOG Statement on Sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and Other Granulomatous Disorders. Sarcoidosis Vasc. Diffus. Lung Dis. 1999, 16, 149–173. [Google Scholar]
- Alexeyev, O.A.; Dekio, I.; Layton, A.M.; Li, H.; Hughes, H.; Morris, T.; Zouboulis, C.C.; Patrick, S. Why We Continue to Use the Name Propionibacterium Acnes. Br. J. Dermatol. 2018, 179, 1227. [Google Scholar] [CrossRef] [PubMed]
- Esteves, T.; Aparicio, G.; Garcia-Patos, V. Is There Any Association between Sarcoidosis and Infectious Agents? A Systematic Review and Meta-Analysis. BMC Pulm. Med. 2016, 16, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, T.; Costabel, U.; McDowell, A.; Guzman, J.; Uchida, K.; Ohashi, K.; Eishi, Y. Immunohistochemical Detection of Potential Microbial Antigens in Granulomas in the Diagnosis of Sarcoidosis. J. Clin. Med. 2021, 10, 983. [Google Scholar] [CrossRef]
- Sell, S. Granulomatous Reactions. In Immunology Immunopathology and Immunity; Elsevier Science Publishing Company, Inc.: Washington, DC, USA, 1987; pp. 529–544. [Google Scholar]
- Song, Z.; Marzilli, L.; Greenlee, B.M.; Chen, E.S.; Silver, R.F.; Askin, F.B.; Teirstein, A.S.; Zhang, Y.; Cotter, R.J.; Moller, D.R. Mycobacterial Catalase-Peroxidase is a Tissue Antigen and Target of the Adaptive Immune Response in Systemic Sarcoidosis. J. Exp. Med. 2005, 201, 755–767. [Google Scholar] [CrossRef]
- Kyra, A.; Oswald-Richter, W.P.D. Dual Analysis for Mycobacteria and Propionibacteria in Sarcoidosis BAL. J Clin. Immunol. 2012, 32, 1129–1140. [Google Scholar] [CrossRef] [Green Version]
- Rotsinger, J.E.; Celada, L.J.; Polosukhin, V.V.; Atkinson, J.B.; Drake, W.P. Molecular Analysis of Sarcoidosis Granulomas Reveals Antimicrobial Targets. Am. J. Respir. Cell Mol. Biol. 2016, 55, 128–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, T.; Eishi, Y.; Ikeda, S.; Ishige, I.; Suzuki, T.; Takemura, T.; Takizawa, T.; Koike, M. In Situ Localization of Propionibacterium Acnes DNA in Lymph Nodes from Sarcoidosis Patients by Signal Amplification with Catalysed Reporter Deposition. J. Pathol. 2002, 198, 541–547. [Google Scholar] [CrossRef]
- Dubaniewicz, A.; Dubaniewicz-Wybieralska, M.; Sternau, A.; Zwolska, Z.; Izycka-Świeszewska, E.; Augustynowicz-Kopeć, E.; Skokowski, J.; Singh, M.; Zimnoch, L. Mycobacterium Tuberculosis Complex and Mycobacterial Heat Shock Proteins in Lymph Node Tissue from Patients with Pulmonary Sarcoidosis. J. Clin. Microbiol. 2006, 44, 3448–3451. [Google Scholar] [CrossRef] [Green Version]
- Isshiki, T.; Matsuyama, H.; Sakamoto, S.; Honma, N.; Mikami, T.; Shibuya, K.; Eishi, Y.; Homma, S. Development of Propionibacterium Acnes-Associated Sarcoidosis during Etanercept Therapy. Intern. Med. 2019, 58, 1473–1477. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Fujita, A. Implication of Immunohistochemistry for Propionibacterium Acnes in Differential Diagnosis of Necrotizing Granuloma. J. Pulm. Respir. Med. 2016, 6, 2–4. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Ishii, H.; Eishi, Y.; Uchida, K.; Yoshimura, M.; Iwasaki, A.; Fujita, M.; Nabeshima, K.; Watanabe, K. Histological Differences between Sarcoidosis and Lung Cancer-Related Sarcoid Reaction. Respir. Investig. 2020, 58, 421–424. [Google Scholar] [CrossRef]
- Asakawa, N.; Uchida, K.; Sakakibara, M.; Omote, K.; Noguchi, K.; Tokuda, Y.; Kamiya, K.; Hatanaka, K.C.; Matsuno, Y.; Yamada, S.; et al. Immunohistochemical Identification of Propionibacterium Acnes in Granuloma and Inflammatory Cells of Myocardial Tissues Obtained from Cardiac Sarcoidosis Patients. PLoS ONE 2017, 12, 1–15. [Google Scholar] [CrossRef]
- Goto, H.; Usui, Y.; Umazume, A.; Uchida, K.; Eishi, Y. Propionibacterium Acnes as a Possible Pathogen of Granuloma in Patients with Ocular Sarcoidosis. Br. J. Ophthalmol. 2017, 101, 1510–1513. [Google Scholar] [CrossRef]
- Nagata, K.; Eishi, Y.; Uchida, K.; Yoneda, K.; Hatanaka, H.; Yasuhara, T.; Nagata, M.; Sotozono, C.; Kinoshita, S. Immunohistochemical Detection of Propionibacterium Acnes in the Retinal Granulomas in Patients with Ocular Sarcoidosis. Sci. Rep. 2017, 7, 15226. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Eishi, Y.; Raza, A.; Rojas, H.; Achiriloaie, A.; de Los Reyes, K.; Raghavan, R. Propionibacterium Acnes-Associated Neurosarcoidosis: A Case Report with Review of the Literature. Neuropathology 2018, 38, 159–164. [Google Scholar] [CrossRef]
- Akimoto, J.; Nagai, K.; Ogasawara, D.; Tanaka, Y.; Izawa, H.; Kohno, M.; Uchida, K.; Eishi, Y. Solitary Tentorial Sarcoid Granuloma Associated with Propionibacterium Acnes Infection: Case Report. J. Neurosurg. 2017, 127, 687–690. [Google Scholar] [CrossRef] [Green Version]
- Negi, M.; Takemura, T.; Guzman, J.; Uchida, K.; Furukawa, A.; Suzuki, Y.; Iida, T.; Ishige, I.; Minami, J.; Yamada, T.; et al. Localization of Propionibacterium Acnes in Granulomas Supports a Possible Etiologic Link between Sarcoidosis and the Bacterium. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc 2012, 25, 1284–1297. [Google Scholar] [CrossRef]
- Beijer, E.; Seldenrijk, K.; Eishi, Y.; Uchida, K.; Damen, J.; Grutters, J.C.; Veltkamp, M. Presence of Propionibacterium Acnes in Granulomas Associates with a Chronic Disease Course in Dutch Sarcoidosis Patients. ERJ Open Res. 2021, 7, 00486–02020. [Google Scholar] [CrossRef]
- Costabel, U.; Hunninghake, G.W. ATS/ERS/WASOG Statement on Sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur. Respir. J. 1999, 14, 735–737. [Google Scholar] [CrossRef] [Green Version]
- Eishi, Y. Etiologic Link between Sarcoidosis and Propionibacterium Acnes. Respir. Investig. 2013, 51, 56–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishige, I.; Eishi, Y.; Takemura, T.; Kobayashi, I.; Nakata, K.; Tanaka, I.; Nagaoka, S.; Iwai, K.; Watanabe, K.; Takizawa, T.; et al. Propionibacterium Acnes Is the Most Common Bacterium Commensal in Peripheral Lung Tissue and Mediastinal Lymph Nodes from Subjects without Sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 2005, 22, 33–42. [Google Scholar] [PubMed]
- Fischer, N.; Mak, T.N.; Shinohara, D.B.; Sfanos, K.S.; Meyer, T.F.; Brüggemann, H. Deciphering the Intracellular Fate of Propionibacterium Acnes in Macrophages. BioMed. Res. Int. 2013, 2013, 603046. [Google Scholar] [CrossRef] [Green Version]
- Eishi, Y. Etiologic Aspect of Sarcoidosis as an Allergic Endogenous Infection Caused by Propionibacterium Acnes. BioMed. Res. Int. 2013, 2013, 935289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beijer, E.; Seldenrijk, K.; Meek, B.; Damen, J.; Quanjel, M.J.R.; Grutters, J.C.; Veltkamp, M. Detection of C. Acnes in Granulomas of Patients with either Hypersensitivity Pneumonitis or Vasculitis Reveals that its Presence is Not Unique for Sarcoidosis. ERJ Open Res. 2021, 7, 00930–02020. [Google Scholar] [CrossRef]
| Sarcoidosis | |
|---|---|
| Number of patients | 94 |
| Age, years | 52 ± 14 |
| Sex, male, n (%) | 55 (59%) |
| Smoking status (current/former/never), n | 25/36/33 |
| Laboratory data | |
| ACE, U/L | 20.9 ± 7.9 |
| Lysozyme, μg/mL | 11.1 ± 4.8 |
| sIL-2R, U/mL | 1036 ± 792 |
| Pulmonary function testing | |
| %FVC, % | 107 ± 16 |
| FEV1%, % | 81 ± 12 |
| Chest XP stage (0/I/II/III/IV) | 1/41/36/15/1 |
| BALF findings | |
| CD4/8 | 6.2 ± 5.0 |
| Lymphocyte, % | 33 ± 23 |
| Control | |
|---|---|
| Number of patients | 30 |
| Age, years | 62 ± 15 |
| Sex, male, n (%) | 15 (50%) |
| Smoking status (current/former/never), n | 5/10/15 |
| Diseases, n | |
| Tuberculosis | 9 |
| Hypersensitivity pneumonia | 9 |
| Sarcoid reaction with cancer | 8 |
| Granulomatosis with polyangiitis | 4 |
| Tissue | in Granulomas | in Other Area outside Granulomas | ||||||
|---|---|---|---|---|---|---|---|---|
| Interstitial Macrophages | Alveolar Macrophages | Sinus Macrophages | ||||||
| Sarcoidosis | Control | Sarcoidosis | Control | Sarcoidosis | Control | Sarcoidosis | Control | |
| Lung | 27/42 (64) | 0/17 (0) | 36/42 (86) | 14/17 (82) | 18/42 (43) | 6/17 (35) | - | - |
| TBLB | 25/39 (64) | 0/6 (0) | 34/39 (87) | 5/6 (83) | 17/39 (44) | 2/6 (33) | - | - |
| VATS | 2/3 (67) | 0/11 (0) | 2/3 (67) | 9/11 (82) | 1/3 (33) | 4/11 (37) | - | - |
| Lymph node | 14/36 (39) | 0/10 (0) | - | - | - | - | 7/36 (19) | 5/10 (50) |
| EBUS-TBNA | 10/31 (32) | 0/5 (0) | - | - | - | - | 3/31 (10) | 1/5 (20) |
| Biopsy or resection | 4/5 (80) | 0/5 (0) | - | - | - | - | 4/5 (80) | 4/5 (80) |
| Skin | 16/20 (80) | 0/1 (0) | 17/20 (85) | 0/1 (0) | - | - | - | - |
| Tissue (Sampling Method) | Manual | Leica | Ventana | ||
|---|---|---|---|---|---|
| A | B | C | |||
| Lung (TBLB) | 18 | 10 | 0 | 9 | 5 |
| Lung (VATS) | 2 | 2 | 0 | 1 | 0 |
| Lymph node (EBUS-TBNA) | 6 | 5 | 0 | 3 | 0 |
| Lymph node (biopsy) | 4 | 3 | 0 | 2 | 1 |
| Skin (biopsy) | 16 | 13 | 0 | 10 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isshiki, T.; Homma, S.; Eishi, Y.; Yabe, M.; Koyama, K.; Nishioka, Y.; Yamaguchi, T.; Uchida, K.; Yamamoto, K.; Ohashi, K.; et al. Immunohistochemical Detection of Propionibacterium acnes in Granulomas for Differentiating Sarcoidosis from Other Granulomatous Diseases Utilizing an Automated System with a Commercially Available PAB Antibody. Microorganisms 2021, 9, 1668. https://doi.org/10.3390/microorganisms9081668
Isshiki T, Homma S, Eishi Y, Yabe M, Koyama K, Nishioka Y, Yamaguchi T, Uchida K, Yamamoto K, Ohashi K, et al. Immunohistochemical Detection of Propionibacterium acnes in Granulomas for Differentiating Sarcoidosis from Other Granulomatous Diseases Utilizing an Automated System with a Commercially Available PAB Antibody. Microorganisms. 2021; 9(8):1668. https://doi.org/10.3390/microorganisms9081668
Chicago/Turabian StyleIsshiki, Takuma, Sakae Homma, Yoshinobu Eishi, Matsuko Yabe, Kazuya Koyama, Yasuhiko Nishioka, Tetsuo Yamaguchi, Keisuke Uchida, Kurara Yamamoto, Kenichi Ohashi, and et al. 2021. "Immunohistochemical Detection of Propionibacterium acnes in Granulomas for Differentiating Sarcoidosis from Other Granulomatous Diseases Utilizing an Automated System with a Commercially Available PAB Antibody" Microorganisms 9, no. 8: 1668. https://doi.org/10.3390/microorganisms9081668
APA StyleIsshiki, T., Homma, S., Eishi, Y., Yabe, M., Koyama, K., Nishioka, Y., Yamaguchi, T., Uchida, K., Yamamoto, K., Ohashi, K., Arakawa, A., Shibuya, K., Sakamoto, S., & Kishi, K. (2021). Immunohistochemical Detection of Propionibacterium acnes in Granulomas for Differentiating Sarcoidosis from Other Granulomatous Diseases Utilizing an Automated System with a Commercially Available PAB Antibody. Microorganisms, 9(8), 1668. https://doi.org/10.3390/microorganisms9081668







