Irreplaceable Role of Amendment-Based Strategies to Enhance Soil Health and Disease Suppression in Potato Production
Abstract
1. Introduction
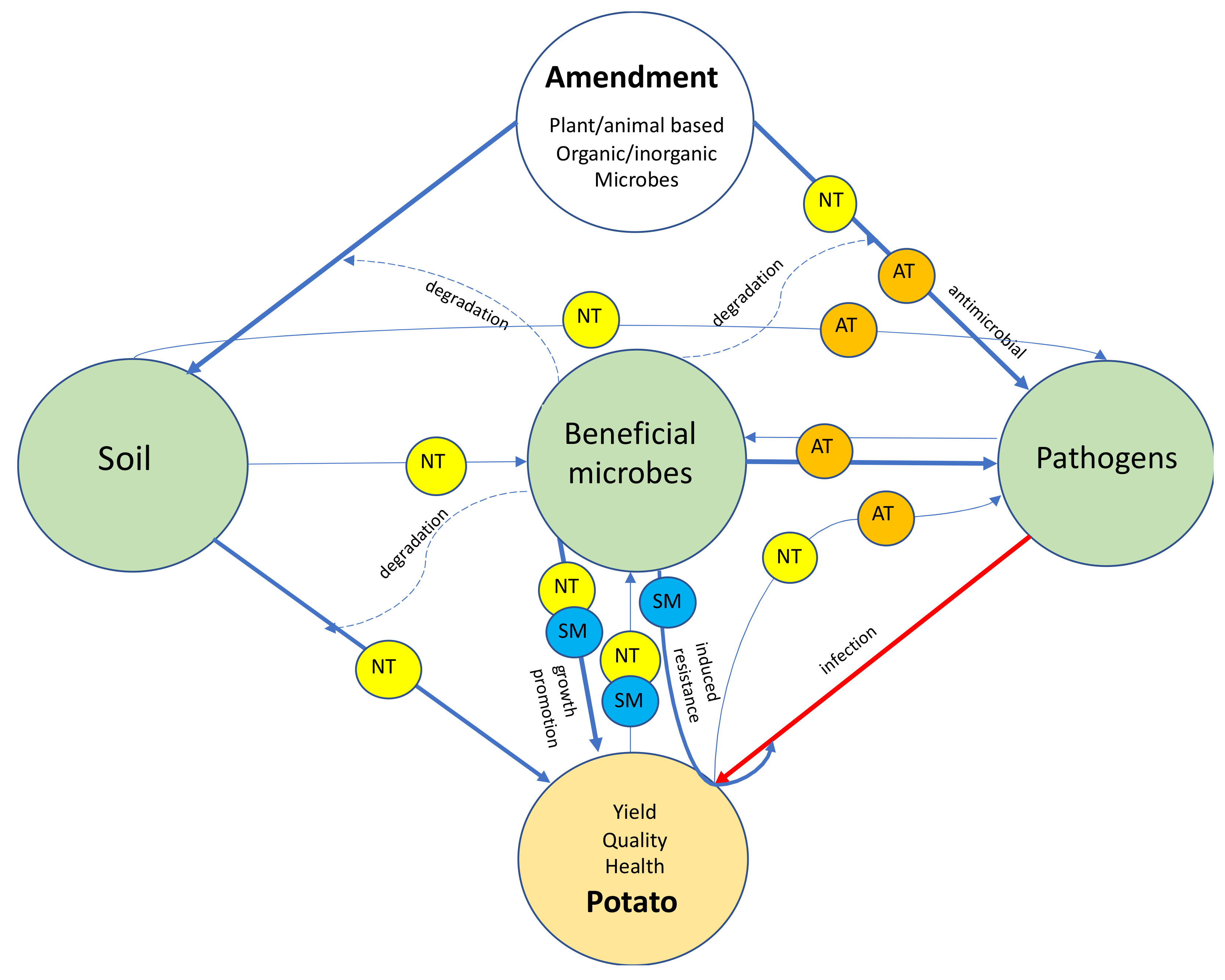
2. Soil Health and Plant Health—A Holistic Approach
3. Soil Amendment for Disease Management—From Practice to Promise
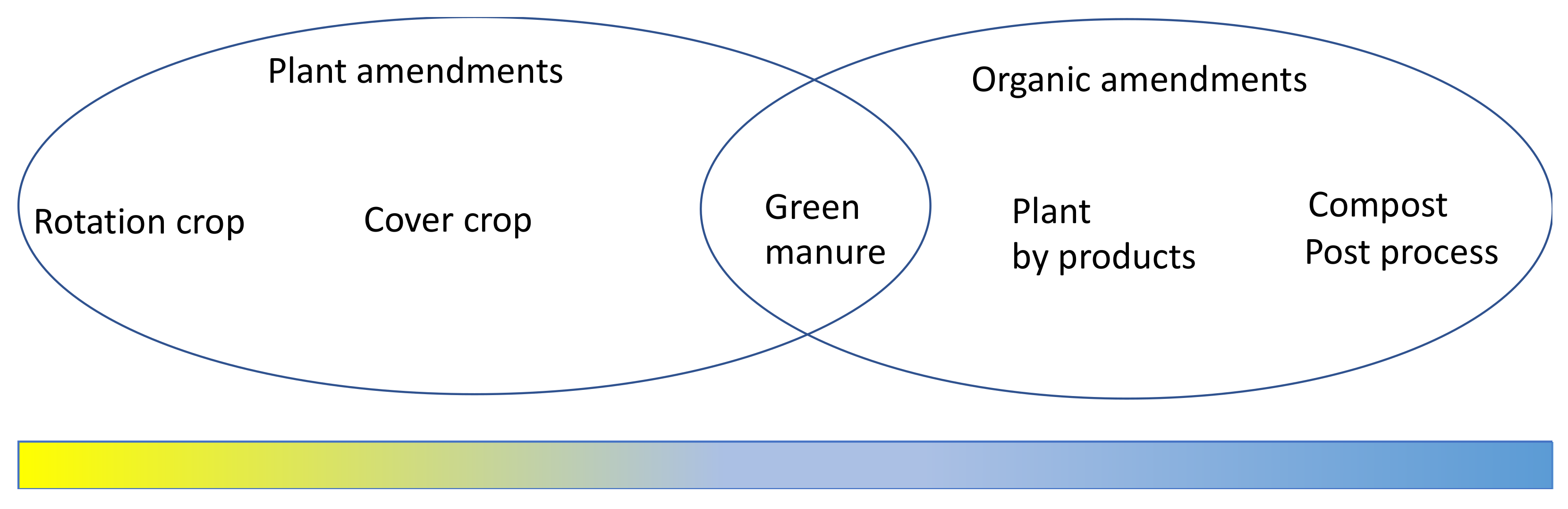
3.1. Plant-Based Soil Amendments—A Microbial Recruiter
3.2. Organic and Inorganic Amendments—The Fuel of The “Microbial Engine”
3.3. Microbial Amendments—A Biological Booster of Soil Health
4. Measuring Soil Health
5. Challenges of Applying Soil Amendment
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Johnson, D.A.; Powelson, M.L. (Eds.) Potato Health Management, 2nd ed.; American Phytopathological Society: Saint Paul, MN, USA, 2008; ISBN 978-0-89054-353-5. [Google Scholar]
- Thornton, M. Potato Growth and Development. In Potato Production Systems; Stark, J., Thornton, M., Nolte, P., Eds.; Springer: Berlin, Germany, 2020; pp. 19–33. [Google Scholar] [CrossRef]
- Stevenson, W.R.; Loria, R.; Franc, G.D.; Weingartner, D.P. Compendium of Potato Diseases; APS Press: Saint Paul, MN, USA, 2001; ISBN 978-0890542750. [Google Scholar]
- Fiers, M.; Edel-Hermann, V.; Chatot, C.; Le Hingrat, Y.; Alabouvette, C.; Steinberg, C. Potato soil-borne diseases. A review. Agron. Sustain. Dev. 2012, 32, 93–132. [Google Scholar] [CrossRef]
- Salas, B.; Stack, R.W.; Secor, G.A.; Gudmestad, N.C. The Effect of wounding, temperature, and inoculum on the development of pink rot of potatoes caused by Phytophthora erythroseptica. Plant Dis. 2000, 84, 1327–1333. [Google Scholar] [CrossRef]
- Fry, W.E.; McGrath, M.T.; Seaman, A.; Zitter, T.A.; McLeod, A.; Danies, G.; Small, I.M.; Myers, K.; Everts, K.; Gevens, A.J.; et al. The 2009 late blight pandemic in the eastern United States-Causes and results. Plant Dis. 2013, 97, 296–306. [Google Scholar] [CrossRef]
- Larkin, R.P.; Griffin, T.S. Control of soilborne potato diseases using Brassica green manures. Crop Prot. 2007, 26, 1067–1077. [Google Scholar] [CrossRef]
- Meng, Q.; Hanson, L.E.; Douches, D.; Hao, J.J. Managing scab diseases of potato and radish caused by Streptomyces spp. using Bacillus amyloliquefaciens BAC03 and other biomaterials. Biol. Control 2013, 67, 373–379. [Google Scholar] [CrossRef]
- Dees, M.W.; Wanner, L.A. In search of better management of potato common scab. Potato Res. 2012, 55, 249–268. [Google Scholar] [CrossRef]
- Peters, R.D.; MacLeod, C.; Seifert, K.A.; Martin, R.A.; Hale, L.R.; Grau, C.R.; MacInnis, S. Pathogenicity to potato tubers of Fusarium spp. isolated from potato, cereal and forage crops. Am. J. Potato Res. 2008, 85, 367–374. [Google Scholar] [CrossRef]
- Czajkowski, R.; Pérombelon, M.C.M.; Jafra, S.; Lojkowska, E.; Potrykus, M.; Van Der Wolf, J.M.; Sledz, W. Detection, identification and differentiation of Pectobacterium and Dickeya species causing potato blackleg and tuber soft rot: A review. Ann. Appl. Biol. 2015, 166, 18–38. [Google Scholar] [CrossRef] [PubMed]
- Hudec, C.; Novinscak, A.; Filion, M. Diversity and virulence of Streptomyces spp. causing potato common scab in Prince Edward Island, Canada. Phytopathology 2020, 111, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.C.; Powelson, M.L. Potato early dying: Management challenges in a changing production environment. Plant Dis. 2002, 86, 1184–1193. [Google Scholar] [CrossRef]
- Merz, U. Powdery scab of potato-Occurrence, life cycle and epidemiology. Am. J. Potato Res. 2008, 85, 241–246. [Google Scholar] [CrossRef]
- Ge, T.; Fatemeh, E.; Johnson, S.; Larkin, R.P.; Hao, J. Interaction between Dickeya dianthicola and Pectobacterium parmentieri in potato infection under field conditions. Microorganisms 2021, 9, 316. [Google Scholar] [CrossRef]
- Davis, J.R.; Huisman, O.C.; Everson, D.O.; Schneider, A.T. Verticillium wilt of potato: A model of key factors related to disease severity and tuber yield in southeastern Idaho. Am. J. Potato Res. 2001, 78, 291–300. [Google Scholar] [CrossRef]
- Menzies, J.D. Survival of microbial plant pathogens in soil. Bot. Rev. 1963, 29, 79–122. [Google Scholar] [CrossRef]
- Neilson, J.A.D.; Robertson, C.J.; Snowdon, E.W.; Yevtushenko, D.P. Impact of fumigation on soil microbial communities under potato cultivation in Southern Alberta. Am J Potato Res. 2020, 97, 115–126. [Google Scholar] [CrossRef]
- Hills, K.; Collins, H.; Yorgey, G.; Mcguire, A.; Kruger, C. Improving soil health in pacific northwest potato production: A review. Am. J. Potato Res. 2020, 97, 1–22. [Google Scholar] [CrossRef]
- Cabrera, L.C.; Talamini, E.; Dewes, H. Potato breeding by many hands? Measuring the germplasm exchange based on a Cultivated Potatoes Database. Int J Food Syst Dyn. 2019, 10, 114–129. [Google Scholar]
- Jansky, S. Breeding, genetics, and cultivar development. In Advances in Potato Chemistry and Technology; Academic Press: Cambridge, MA, USA, 2009; pp. 27–62. [Google Scholar]
- Wu, C.; Liu, X.W.; Zhang, W.; Wang, Q.; Guo, H.C. Control effects of different potato varieties (lines) and rice-potato rotation system on root-knot nematode. Acta Agron. Sin. 2020, 46, 1456–1463. [Google Scholar]
- Tsror (Lahkim), L.; Erlich, O.; Hazanovsky, M.; Marshak, G.; Segev, G.; Zig, U. Fungicide field treatments to control potato leak caused by Pythium ultimum. Am J Potato Res. 2021, 98, 115–121. [Google Scholar] [CrossRef]
- Axel, C.; Zannini, E.; Coffey, A.; Guo, J.; Waters, D.M.; Arendt, E.K. Ecofriendly control of potato late blight causative agent and the potential role of lactic acid bacteria: A review. Appl Microbiol Biotechnol. 2012, 96, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.M.; McPhee, J.E.; Dean, G.; Hinton, S.; Sparrow, L.A.; Wilson, C.R.; Tegg, R.S. Managing soil health and crop productivity in potato: A challenging test system. Soil Res. 2020, 58, 697–712. [Google Scholar] [CrossRef]
- Soltani, N.; Conn, K.L.; Abbasi, P.A.; Lazarovits, G. Reduction of potato scab and verticillium wilt with ammonium lignosulfonate soil amendment in four Ontario potato fields. Can. J. Plant Pathol. 2002, 24, 332–339. [Google Scholar] [CrossRef]
- Bailey, K.L.; Lazarovits, G. Suppressing soil-borne diseases with residue management and organic amendments. Soil Tillage Res. 2003, 72, 169–180. [Google Scholar] [CrossRef]
- Brown, J.; Morra, M.J. Glucosinolate-Containing Seed Meal as a Soil Amendment to Control Plant Pests, 2000-2002; National Renewable Energy Laboratory: Golden, CO, USA, 2005; 99p. [Google Scholar]
- Ferris, H.; Tuomisto, H. Unearthing the role of biological diversity in soil health. Soil Biol. Biochem. 2015, 85, 101–109. [Google Scholar] [CrossRef]
- Larkin, R.P.; Tavantzis, S. Use of biocontrol organisms and compost amendments for improved control of soilborne diseases and increased potato production. Am J Potato Res. 2013, 90, 261–270. [Google Scholar] [CrossRef]
- Lazarovits, G.; Tenuta, M.; Conn, K.L. Organic amendments as a disease control strategy for soilborne diseases of high-value agricultural crops. Australas. Plant Pathol. 2001, 30, 111–117. [Google Scholar] [CrossRef]
- Ninh, H.T.; Grandy, A.S.; Wickings, K.; Snapp, S.S.; Kirk, W.; Hao, J. Organic amendment effects on potato productivity and quality are related to soil microbial activity. Plant Soil 2015, 386, 223–236. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef]
- Larkin, R.P. Characterization of soil microbial communities under different potato cropping systems by microbial population dynamics, substrate utilization, and fatty acid profiles. Soil Biol Biochem. 2003, 35, 1451–1466. [Google Scholar] [CrossRef]
- Larkin, R.P.; Honeycutt, C.W.; Griffin, T.S.; Olanya, O.M.; He, Z.; Halloran, J.M. Cumulative and residual effects of different potato cropping system management strategies on soilborne diseases and soil microbial communities over time. Plant Pathol. 2017, 66, 437–449. [Google Scholar] [CrossRef]
- Mazzola, M.; Freilich, S. Prospects for biological soilborne disease control: Application of indigenous versus synthetic microbiomes. Phytopathology 2017, 107, 256–263. [Google Scholar] [CrossRef]
- Tegg, R. Navigating the Wealth of Soil Health Information & Identification of Opportunities (PT16003); Hort Innovation: Sydney, Australia, 2018; 73, ISBN 978 0 7341 4401 0. [Google Scholar]
- Pereira, A.P.A.; de Souza, A.J.; de Chaves, M.G.; Fracetto, G.G.M.; Garcia, K.G.V.; Filho, P.F.M.; Cardoso, E.J.B.N. Mechanisms of the phytomicrobiome for enhancing soil fertility and health. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, Netherlands, 2021; pp. 1–14. [Google Scholar] [CrossRef]
- Janvier, C.; Villeneuve, F.; Alabouvette, C.; Edel-Hermann, V.; Mateille, T.; Steinberg, C. Soil health through soil disease suppression: Which strategy from descriptors to indicators? Soil Biol. Biochem. 2007, 39, 1–23. [Google Scholar] [CrossRef]
- Abawi, G.S.; Widmer, T.L. Impact of soil health management practices on soilborne pathogens, nematodes and root diseases of vegetable crops. Appl. Soil Ecol. 2000, 15, 37–47. [Google Scholar] [CrossRef]
- Döring, T.F.; Pautasso, M.; Finckh, M.R.; Wolfe, M.S. Concepts of plant health-reviewing and challenging the foundations of plant protection. Plant Pathol. 2012, 61, 1–15. [Google Scholar] [CrossRef]
- Ray, P.; Lakshmanan, V.; Labbé, J.L.; Craven, K.D. Microbe to microbiome: A paradigm shift in the application of microorganisms for sustainable agriculture. Front. Microbiol. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Wiggins, B.E.; Kinkel, L.L. Green manures and crop sequences influence alfalfa root rot and pathogen inhibitory activity among soil-borne streptomycetes. Plant Soil 2005, 268, 271–283. [Google Scholar] [CrossRef]
- Mathre, D.E.; Cook, R.J.; Callan, N.W. From discovery to use: Traversing the world of commercializing biocontrol agents for plant disease control. Plant Dis. 1999, 83, 972–983. [Google Scholar] [CrossRef][Green Version]
- Larkin, R.P.; Honeycutt, C.W.; Griffin, T.S.; Olanya, O.M.; He, Z. Potato growth and yield characteristics under different cropping system management strategies in northeastern U.S. Agronomy 2021, 11, 165. [Google Scholar] [CrossRef]
- Larkin, R.P.; Griffin, T.S.; Honeycutt, C.W. Rotation and cover crop effects on soilborne potato diseases, tuber yield, and soil microbial communities. Plant Dis. 2010, 94, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Bernard, E.; Larkin, R.P.; Tavantzis, S.; Erich, M.S.; Alyokhin, A.; Sewell, G.; Lannan, A.; Gross, S.D. Compost, rapeseed rotation, and biocontrol agents significantly impact soil microbial communities in organic and conventional potato production systems. Appl. Soil Ecol. 2012, 52, 29–41. [Google Scholar] [CrossRef]
- Lazarovits, G. Management of soil-borne plant pathogens with organic soil amendments: A disease control. Agriculture 2001, 23, 1–7. [Google Scholar]
- Lazarovits, G. Managing soilborne disease of potatoes using ecologically based approaches. Am. J. Potato Res. 2010, 87, 401–411. [Google Scholar] [CrossRef]
- Larkin, R.P.; Lynch, R.P. Use and effects of different brassica and other rotation crops on soilborne diseases and yield of Potato. Horticulturae 2018, 4, 37. [Google Scholar] [CrossRef]
- Johnson, D.A.; Cummings, T.F. Effect of extended crop rotations on incidence of black dot, silver scurf, and Verticillium wilt of potato. Plant Dis. 2015, 99, 257–262. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carter, M.R.; Peters, R.D.; Noronha, C.; Kimpinski, J. Influence of 10 years of conservation tillage on some biological properties of a fine sandy loam in the potato phase of two crop rotations in Atlantic Canada. Can. J. Soil Sci. 2009, 89, 391–402. [Google Scholar] [CrossRef]
- Larkin, R.P.; Brewer, M.T. Effects of crop rotation and biocontrol amendments on rhizoctonia disease of potato and soil microbial communities. Agriculture 2020, 10, 128. [Google Scholar] [CrossRef]
- Carter, M.R.; Sanderson, J.B. Influence of conservation tillage and rotation length on potato productivity, tuber disease and soil quality parameters on a fine sandy loam in eastern Canada. Soil Tillage Res. 2001, 63, 1–13. [Google Scholar] [CrossRef]
- Hunjan, M.S.; Sabhikhi, H.S. Designing a crop rotation strategy to manage Streptomyces scabies causing potato scab in north India. J. Phytopathol. 2020, 168, 469–477. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, Y.; Qin, S.; Zhang, W.; Shi, M.F.; Fan, Y.; Yang, X. Ridge–mulch tillage and rotation with broad bean affect soil microbial community, diversity and crop yield in a long-term potato continuous cropping field. Soil Use Manag. 2020, 1–12. [Google Scholar] [CrossRef]
- Carter, M.R.; Kunelius, H.T.; Sanderson, J.B.; Kimpinski, J.; Platt, H.W.; Bolinder, M.A. Productivity parameters and soil health dynamics under long-term 2-year potato rotations in Atlantic Canada. Soil Tillage Res. 2003, 72, 153–168. [Google Scholar] [CrossRef]
- Cohen, M.F.; Yamasaki, H.; Mazzola, M. Brassica napus seed meal soil amendment modifies microbial community structure, nitric oxide production and incidence of Rhizoctonia root rot. Soil Biol. Biochem. 2005, 37, 1215–1227. [Google Scholar] [CrossRef]
- de Medeiros, E.V.; Lima, N.T.; de Sousa Lima, J.R.; Pinto, K.M.S.; da Costa, D.P.; Franco Junior, C.L.; Souza, R.M.S.; Hammecker, C. Biochar as a strategy to manage plant diseases caused by pathogens inhabiting the soil: A critical review. Phytoparasitica 2021, 14. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Yang, K.; Wang, P.; Wang, H.; Guo, L.; Zhu, S.; Zhu, Y.; He, X. Biochar application alleviated negative plant-soil feedback by modifying soil microbiome. Front. Microbiol. 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lazarovits, G.; Tenuta, M.; Conn, K.L. Utilization of high nitrogen and swine manure amendments for control of soil-borne diseases: Efficacy and mode of action. Acta Hortic. 2000, 532, 59–64. [Google Scholar] [CrossRef]
- Abbasi, P.A. Establishing suppressive conditions against soilborne potato diseases with low rates of fish emulsion applied serially as a pre-plant soil amendment. Can. J. Plant Pathol. 2013, 35, 10–19. [Google Scholar] [CrossRef]
- Peters, R.D.; Sturz, A.V.; Carter, M.R.; Sanderson, J.B. Crop rotation can confer resistance to potatoes from Phytophthora erythroseptica attack. Can. J. Plant Sci. 2005, 85, 523–528. [Google Scholar] [CrossRef]
- Larkin, R.P.; Honeycutt, C.W.; Olanya, O.M. Management of Verticillium Wilt of Potato with Disease-Suppressive Green Manures and as Affected by Previous Cropping History. Plant Dis. 2011, 95, 568–576. [Google Scholar] [CrossRef]
- Wiggins, B.E.; Kinkel, L.L. Green manures and crop sequences influence potato diseases and pathogen inhibitory activity of indigenous streptomycetes. Phytopathology 2005, 95, 178–185. [Google Scholar] [CrossRef]
- Weinhold, A.R.; Osward, J.W.; Bowman, T.; Bishop, J.; Wright, D. Influence of green manures and crop rotation. Am. Potato J. 1964, 41, 265–273. [Google Scholar] [CrossRef]
- Meng, Q.; Jiang, H.H.; Hanson, L.E.; Hao, J.J. Characterizing a novel strain of Bacillus amyloliquefaciens BAC03 for potential biological control application. J. Appl. Microbiol. 2012, 113, 1165–1175. [Google Scholar] [CrossRef]
- Arseneault, T.; Goyer, C.; Filion, M. Pseudomonas fluorescens LBUM223 increases potato yield and reduces common scab symptoms in the field. Phytopathology 2015, 105, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Buxdorf, K.; Rahat, I.; Gafni, A.; Levy, M. The epiphytic fungus Pseudozyma aphidis induces jasmonic acid-and salicylic acid/nonexpressor of PR1-independent local and systemic resistance. Plant Physiol. 2013, 161, 2014–2022. [Google Scholar] [CrossRef] [PubMed]
- Griffin, T.S.; Larkin, R.P.; Honeycutt, C.W. Delayed tillage and cover crop effects in potato systems. Am. J. Potato Res. 2009, 86, 79–87. [Google Scholar] [CrossRef]
- Larkin, R.P.; Honeycutt, C.W. Effects of different 3-year cropping systems on soil microbial communities and rhizoctonia diseases of potato. Phytopathology 2006, 96, 68–79. [Google Scholar] [CrossRef]
- Pieters, A.J. Green Manuring: Principles and Practices; Agrobios: Jodhpur, India, 2013; ISBN 8177541889. [Google Scholar]
- Bakker, M.G.; Glover, J.D.; Mai, J.G.; Kinkel, L.L. Plant community effects on the diversity and pathogen suppressive activity of soil streptomycetes. Appl. Soil Ecol. 2010, 46, 35–42. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Rice, W.A.; Clayton, G.W. Soil microbial diversity and community structure under wheat as influenced by tillage and crop rotation. Soil Biol. Biochem. 1998, 30, 1733–1741. [Google Scholar] [CrossRef]
- Molina, O.I.; Tenuta, M.; El Hadrami, A.; Buckley, K.; Cavers, C.; Daayf, F. Potato early dying and yield responses to compost, green manures, seed meal and chemical treatments. Am. J. Potato Res. 2014, 91, 414–428. [Google Scholar] [CrossRef]
- Sparrow, L.A. Six years of results from a potato rotation and green manure trial in Tasmania, Australia. Acta Hortic. 2015, 1076, 29–36. [Google Scholar] [CrossRef]
- Pankhurst, C.E.; Lynch, J.M. Biocontrol of soil-borne plant diseases. In Encyclopedia of Soils in the Environment; Elsevier: Amsterdam, The Netherlands, 2005; pp. 129–136. ISBN 9780080547954. [Google Scholar]
- Davis, J.R.; Huisman, O.C.; Westermann, D.T.; Everson, D.O.; Schneider, A.; Sorensen, L.H. Some unique benefits with sudangrass for improved U.S. #1 yields and size of Russet Burbank potato. Am. J. Potato Res. 2004, 81, 403–413. [Google Scholar]
- Walker, T.S.; Bais, H.P.; Grotewold, E.; Vivanco, J.M. Root exudation and rhizosphere biology. Plant Physiol. 2003, 132, 44–51. [Google Scholar] [CrossRef]
- Jiang, H.; Hwang, H.W.; Ge, T.; Cole, B.; Perkins, B.; Hao, J. Leucine regulates zoosporic germination and infection by Phytophthora erythroseptica. Front. Microbiol. 2019, 10, 131. [Google Scholar] [CrossRef]
- Meng, Q.; Yin, J.; Rosenzweig, N.; Douches, D.; Hao, J.J. Culture-based assessment of microbial communities in soil suppressive to potato common scab. Plant Dis. 2012, 96, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Suo, B.; Chen, Q.; Wu, W.; Wu, D.; Tian, M.; Jie, Y.; Zhang, B.; Wen, J. Chemotactic responses of Phytophthora sojae zoospores to amino acids and sugars in root exudates. J. Gen. Plant. Pathol. 2016, 82, 142–148. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.; Silk, W.K.; Passioura, J.B. Rates of root and organism growth, soil conditions, and temporal and spatial development of the rhizosphere. Ann. Bot. 2006, 97, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Xu, X.; Cheng, Z.; Xiong, J.; Ma, R.; Zhang, L.; Yang, X.; Zhu, Y.; Zhang, B.; Tian, B. Analysis of the community composition and bacterial diversity of the rhizosphere microbiome across different plant taxa. Microbiologyopen 2019, 8, e762. [Google Scholar] [CrossRef]
- Van Horn, C.; Somera, T.; Mazzola, M. Comparative analysis of the rhizosphere and endophytic microbiomes across apple rootstock genotypes in replant orchard soils. Phytobiomes J. 2021, 5, 231–243. [Google Scholar] [CrossRef]
- Turner, T.R.; James, E.K.; Poole, P.S. The Plant Microbiome. Genome Biol. 2013, 14, 209. [Google Scholar] [CrossRef]
- Deliopoulos, T.; Kettlewell, P.S.; Hare, M.C. Fungal disease suppression by inorganic salts: A review. Crop Prot. 2010, 29, 1059–1075. [Google Scholar] [CrossRef]
- Goss, M.J.; Tubeileh, A.; Goorahoo, D. A Review of the use of organic amendments and the risk to human health. Adv. Agron. 2013, 120, 275–379. [Google Scholar]
- Cole, E.; Pu, J.; Chung, H.; Quintanilla, M. Impacts of manures and manure-based composts on root lesion nematodes and Verticillium dahliae in Michigan potatoes. Phytopathology 2020, 110, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Tenuta, M.; Lazarovits, G. Soil properties associated with the variable effectiveness of meat and bone meal to kill microsclerotia of Verticillium dahliae. Appl. Soil Ecol. 2004, 25, 219–236. [Google Scholar] [CrossRef]
- Wilhelm, S. Effect of various soil amendments on the inoculum potential of the Verticillium wilt fungus. Phytopathology 1951, 41, 684–690. [Google Scholar]
- Tenuta, M.; Conn, K.L.; Lazarovits, G. Volatile fatty acids in liquid swine manure can kill microsclerotia of Verticillium dahliae. Phytopathology 2002, 92, 548–552. [Google Scholar] [CrossRef][Green Version]
- Khiareddine, H.J. Effect of fodder radish (Raphanus sativus L.) green manure on potato wilt, growth and yield parameters. Adv. Crop Sci. Technol. 2016, 4, 211. [Google Scholar] [CrossRef]
- Shetty, K.G.; Subbarao, K.V.; Huisman, O.C.; Hubbard, J.C. Mechanism of broccoli-mediated Verticillium wilt reduction in cauliflower. Phytopathology 2000, 90, 305–310. [Google Scholar] [CrossRef]
- Hao, J.; Subbarao, K.V.; Koike, S.T. Effects of broccoli rotation on lettuce drop caused by Sclerotinia minor and on the population density of sclerotia in soil. Plant Dis. 2003, 87, 159–166. [Google Scholar] [CrossRef]
- Gouws-Meyer, R.; McLeod, A.; Mazzola, M. Potato scab management with Brassica biofumigation and effect of volatiles on Streptomyces growth. Acta Hortic. 2020, 1269, 25–32. [Google Scholar] [CrossRef]
- Matthiessen, J.; Kirkegaard, J. Biofumigation and enhanced biodegradation: Opportunity and challenge in soilborne pest and disease management. Crit. Rev. in Plant Sci. 2006, 235–265. [Google Scholar] [CrossRef]
- Sarwar, M.; Kirkegaard, J.A.; Wong, P.T.W.; Desmarchelier, J.M. Biofumigation potential of brassicas III. In vitro toxicity of isothiocyanates to soil-borne fungal pathogens. Plant Soil 1998, 201, 103–112. [Google Scholar] [CrossRef]
- Mazzola, M.; Zhao, X. Brassica juncea seed meal particle size influences chemistry but not soil biology-based suppression of individual agents inciting apple replant disease. Plant Soil 2010, 337, 313–324. [Google Scholar] [CrossRef]
- Somera, T.S.; Freilich, S.; Mazzola, M. Comprehensive analysis of the apple rhizobiome as influenced by different Brassica seed meals and rootstocks in the same soil/plant system. Appl. Soil Ecol. 2021, 157, 103766. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Giordano, S.; Ricciardi, L.; Ferrara, S.; Montesano, D.; Castaldo Cobianchi, R.; Vuotto, M.L.; Ferrara, L. Antibacterial and allelopathic activity of extract from Castanea sativa leaves. Fitoterapia 2000, 71, 110–116. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant potential of chestnut (Castanea sativa L.) and almond (Prunus dulcis L.) by-products. Food Sci. Technol. Int. 2010, 16, 209–216. [Google Scholar] [CrossRef]
- Hao, J.J.; Liu, H.; Donis-Gonzalez, I.R.; Lu, X.H.; Jones, D.; Fulbright, D.W. Antimicrobial activity of chestnut extracts for potential use in managing soilborne plant pathogens. Plant Dis. 2012, 96, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Inderjit. Soil microorganisms: An important determinant of allelopathic activity. Plant Soil 2005, 274, 227–236. [Google Scholar] [CrossRef]
- Coffin, R.H.; Borza, T.; Alam, M.Z.; Liu, Y.; Desai, F.; Xi, Y.; Zhang, Z.; Beaton, B.; Goyer, C.; Coffin, J.; et al. Assessing the suppressive effects of biopesticides and phosphite on common scab development in potatoes. Biocontrol Sci. Technol. 2020, 30, 1133–1149. [Google Scholar] [CrossRef]
- Farooque, A.A.; Zaman, Q.; Abbas, F.; Hammad, H.M.; Acharya, B.; Easu, T. How can potatoes be smartly cultivated with biochar as a soil nutrient amendment technique in Atlantic Canada? Arab. J. Geosci. 2020, 13, 336. [Google Scholar] [CrossRef]
- Meilin, A.; Rubiana, R.; Hendri, J.; Primilestari, S.; Handoko, S. Rustam Study of tricho-compost and rice husk biochar applications to development of Phytophthora late blight diseases and yields of potato plants. IOP Conf. Ser. Earth Environ. Sci. 2020, 458, 012023. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Kinkel, L. Soil health: Managing the soil microflora to enhance potato health. In Potato Health Management; Johnson, D.A., Ed.; APS Press: St. Paul, MN, USA, 2008; pp. 11–14. [Google Scholar]
- Yan, K.; Wang, H.; Lou, J.; Jianming, X.U. Bibliometric analysis of status quo and trend of the research on soil-borne diseases based on the web of science database. Acta Pedol. Sin. 2020, 57, 680–690. [Google Scholar]
- Mazzola, M. Assessment and management of soil microbial community structure for disease suppression. Annu. Rev. Phytopathol. 2004, 42, 35–59. [Google Scholar] [CrossRef]
- Mazzola, M.; Brown, J.; Izzo, A.D.; Cohen, M.F. Mechanism of action and efficacy of seed meal-induced pathogen suppression differ in a brassicaceae species and time-dependent manner. Phytopathology 2007, 97, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Singhai, P.K.; Sarma, B.K.; Srivastava, J.S. Biological management of common scab of potato through Pseudomonas species and vermicompost. Biol. Control 2011, 57, 150–157. [Google Scholar] [CrossRef]
- Bernard, E.; Larkin, R.P.; Tavantzis, S.; Erich, M.S.; Alyokhin, A.; Gross, S.D. Rapeseed rotation, compost and biocontrol amendments reduce soilborne diseases and increase tuber yield in organic and conventional potato production systems. Plant Soil 2014, 374, 611–627. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, C.; Hao, J.J.; Li, Y.C.; Li, D.Z.; Zhang, D.M.; Xing, X.; Liang, Y. A novel streptomyces sp. strain PBSH9 for controlling potato common scab caused by streptomyces galilaeus. Plant Dis. 2020, 104, 1986–1993. [Google Scholar] [CrossRef] [PubMed]
- Yamunarani, K.; Sundaram, A.K.; Pandiyan, M. Streptomycetes as a potential biocontrol agent. J. Entomol. Zool. Stud. 2019, 7, 637–644. [Google Scholar]
- Cui, L.; Yang, C.; Wei, L.; Li, T.; Chen, X. Isolation and identification of an endophytic bacteria Bacillus velezensis 8-4 exhibiting biocontrol activity against potato scab. Biol. Control 2020, 141, e104156. [Google Scholar] [CrossRef]
- Liu, Q.; Shen, Y.R.; Yin, K. De The degradation fragments of gamma-glutamyl transpeptidase from Bacillus subtilis BU108 have antimicrobial activity against Streptomyces scabiei. J. Plant Dis. Prot. 2020, 128, 279–285. [Google Scholar] [CrossRef]
- Liu, Q.; Shen, Y.; Yin, K. The antimicrobial activity of protein elicitor AMEP412 against Streptomyces scabiei. World J. Microbiol. Biotechnol. 2020, 36, 18. [Google Scholar] [CrossRef]
- Weller, D.M.; Landa, B.B.; Mavrodi, O.V.; Schroeder, K.L.; De La Fuente, L.; Blouin Bankhead, S.; Allende Molar, R.; Bonsall, R.F.; Mavrodi, D.V.; Thomashow, L.S. Role of 2,4-diacetylphloroglucinol-producing fluorescent Pseudomonas spp. in the defense of plant roots. Plant Biol. 2007, 9, 4–20. [Google Scholar] [CrossRef]
- Bailly, A.; Weisskopf, L. Mining the volatilomes of plant-associated microbiota for new biocontrol solutions. Front. Microbiol. 2017, 8, 1683. [Google Scholar] [CrossRef]
- Andreote, F.D.; De Araújo, W.L.; De Azevedo, J.L.; Van Elsas, J.D.; Da Rocha, U.N.; Van Overbeek, L.S. Endophytic colonization of potato (Solanum tuberosum L.) by a novel competent bacterial endophyte, Pseudomonas putida strain P9, and its effect on associated bacterial communities. Appl. Environ. Microbiol. 2009, 75, 3396–3406. [Google Scholar] [CrossRef] [PubMed]
- Lahlali, R.; Hijri, M. Screening, identification and evaluation of potential biocontrol fungal endophytes against Rhizoctonia solani AG3 on potato plants. FEMS Microbiol. Lett. 2010, 311, 152–159. [Google Scholar] [CrossRef]
- Bandy, B.R.; Tavantzis, S.M. Effect of hypovirulent rhizoctonia solani on rhizoctonia disease, growth, and development of potato plants. Am. Potato J. 1990, 67, 189–199. [Google Scholar] [CrossRef]
- Aguk, J.A.; Karanja, N.; Schulte-Geldermann, E.; Bruns, C.; Kinyua, Z.; Parker, M. Control of bacterial wilt (Ralstonia solanacearum) in potato (Solanum tuberosum) using rhizobacteria and arbuscular mycorrhiza fungi. Afr. J. Food Agric. Nutr. Dev. 2018, 18, 13371–13387. [Google Scholar]
- Alaux, P.L.; César, V.; Naveau, F.; Cranenbrouck, S.; Declerck, S. Impact of Rhizophagus irregularis MUCL 41833 on disease symptoms caused by Phytophthora infestans in potato grown under field conditions. Crop Prot. 2018, 107, 26–33. [Google Scholar] [CrossRef]
- Chifetete, V.W.; Dames, J.F. Mycorrhizal Interventions for Sustainable Potato Production in Africa. Front. Sustain. Food Syst. 2020, 4, 1–17. [Google Scholar] [CrossRef]
- Park, K.; Paul, D.; Yeh, W.H. Bacillus vallismortis EXTN-1-mediated growth promotion and disease suppression in rice. Plant Pathol. J. 2006, 22, 278–282. [Google Scholar] [CrossRef]
- Pageni, B.B.; Lupwayi, N.Z.; Akter, Z.; Larney, F.J.; Kawchuk, L.M.; Gan, Y.T. Plant growth-promoting and phytopathogen-antagonistic properties of bacterial endophytes from potato (Solanum tuberosum L.) cropping systems. Can. J. Plant Sci. 2014, 94, 835–844. [Google Scholar] [CrossRef]
- Frommel, M.I.; Nowak, J.; Lazarovits, G. Treatment of potato tubers with a growth promoting Pseudomonas sp.: Plant growth responses and bacterium distribution in the rhizosphere. Plant Soil 1993, 150, 51–60. [Google Scholar] [CrossRef]
- Meng, Q.; Jiang, H.; Hao, J.J. Effects of Bacillus velezensis strain BAC03 in promoting plant growth. Biol. Control 2016, 98, 18–26. [Google Scholar] [CrossRef]
- Sturz, A.V.; Christie, B.R.; Matheson, B.G.C. Associations of bacterial endophyte populations from red clover and potato crops with potential for beneficial allelopathy. Can. J. Microbiol. 1998, 44, 162–167. [Google Scholar] [CrossRef]
- Menzies, J.D. Occurrence and transfer of a biological factor in soil that suppresses potato scab. Phytopathology 1959, 49, 648–652. [Google Scholar]
- Semenov, M.V.; Krasnov, G.S.; Semenov, V.M.; van Bruggen, A.H.C. Long-term fertilization rather than plant species shapes rhizosphere and bulk soil prokaryotic communities in agroecosystems. Appl. Soil Ecol. 2020, 154, 1036412. [Google Scholar] [CrossRef]
- Chatterton, S.; Yang, H.E.; Ortega Polo, R.; McAllister, T.A.; Safarieskandari, S.; Lupwayi, N. Bacterial and fungal communities, but not physicochemical properties, of soil differ according to root rot status of pea. Pedobiologia 2021, 84, 1–12. [Google Scholar] [CrossRef]
- Sederholm, M.R.; Schmitz, B.W.; Barberán, A.; Pepper, I.L. Effects of metam sodium fumigation on the abundance, activity, and diversity of soil bacterial communities. Appl. Soil Ecol. 2018, 124, 27–33. [Google Scholar] [CrossRef]
- Jeanne, T.; Parent, S.É.; Hogue, R. Using a soil bacterial species balance index to estimate potato crop productivity. PLoS ONE 2019, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Manici, L.M.; Caputo, F. Fungal community diversity and soil health in intensive potato cropping systems of the east Po valley, northern Italy. Ann. Appl. Biol. 2009, 155, 245–258. [Google Scholar] [CrossRef]
- Rosenzweig, N.; Tiedje, J.M.; Quensen, J.F.; Meng, Q.; Hao, J.J. Microbial communities associated with potato common Scab-Suppressive soil determined by pyrosequencing analyses. Plant Dis. 2012, 96, 718–725. [Google Scholar] [CrossRef]
- Ushiki, J.; Tahara, S.; Hayakawa, Y.; Tadano, T. Medicinal plants for suppressing soil-borne plant diseases: II. Suppressive effect of geranium pratense l. On common scab of potato and identification of the active compound. Soil Sci. Plant Nutr. 1998, 44, 157–165. [Google Scholar] [CrossRef]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.F.; Verardo, V. Phenolic compounds in the potato and its byproducts: An overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.R.; Dessaux, Y.; Thomashow, L.S.; Weller, D.M. Rhizosphere engineering and management for sustainable agriculture. Plant Soil 2009, 321, 363–383. [Google Scholar] [CrossRef]
- Benkeblia, N. (Ed.) Omics Technologies and Crop Improvement; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Brimner, T.A.; Boland, G.J. A review of the non-target effects of fungi used to biologically control plant diseases. Agric. Ecosyst. Environ. 2003, 100, 3–16. [Google Scholar] [CrossRef]
- Lankau, E.W.; Xue, D.; Christensen, R.; Gevens, A.J.; Lankau, R.A. Management and soil conditions influence common scab severity on potato tubers via indirect effects on soil microbial communities. Phytopathology 2020, 110, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Armour, C.R.; Hu, C.; Mei, M.; Tian, C.; Sharpton, T.J.; Jiang, Y. Microbiome multi-omics network analysis: Statistical considerations, limitations, and opportunities. Front. Genet. 2019, 10, 995. [Google Scholar] [CrossRef] [PubMed]
| Type of Input | Target Disease | Potential Mechanism to Reduce Diseases | Source |
|---|---|---|---|
| Organic Amendment | |||
| Brassica napus seed meal | Rhizoctonia solani | Change in soil microbial communities that induce plant resistance | [58] |
| Biochar | Various pathogens | Induced plant resistance, improve soil properties and microbial growth, toxin immobilization and transformation | [59,60] |
| Blood meal | Verticillium dahliae | Ammonia, nitrous acid | [49] |
| Swine manure | Verticillium dahliae Streptomyces spp. | Volatile fatty acids—ammonium lignosulfonate | [61] |
| Ammonium lignosulfonate | Verticillium dahliae | Antifungal effect | [26] |
| Fish emulsion | Verticillium dahliae Streptomyces spp. | Organic acids, toxic compounds | [62] |
| Compost | Rhizoctonia solani | Increased utilization of complex substrates and increased levels of Gram-positive bacteria and fungi | [30,47] |
| Rotation | |||
| Barley/ryegrass | Rhizoctonia solani | Enhanced soil microbial activities in disease suppression | [7,53] |
| Red clover or Barley undersown with red clover | Rhizoctonia solani Phytophthora erythroseptica | Pathogen suppression | [54,63] |
| Mungbean and Sunn hemp | Streptomyces scabies Nematodes | Pathogen suppression and enhancing beneficial microorganisms | [38,55] |
| Broad bean | Non-specific | Enhancing soil microbial communities, diversity and crop yield | [56] |
| Green Manure/Cover Crop | |||
| Mustard | Verticillium dahliae and other soilborne diseases | Antimicrobial activities | [64] |
| Brassica | Rhizoctonia solani and other soilborne diseases | Antimicrobial activities | [7] |
| Sunn hemp | Common scab (Streptomyces spp.) | Pathogen suppression | [55] |
| Buckwheat | Verticillium wilt | Modifying antagonistic streptomycetes | [65] |
| Soybean | Common scab | Pathogen suppression | [65,66] |
| Microbial Amendment | |||
| Bacillus Velezensis | Common scab (Streptomyces spp.) | Plant resistance inducing, LCI protein and volatile Organic compounds for antimicrobial activity, hormones promoting plant growth | [8,67] |
| Pseudomonas fluorescens | Common scab (Streptomyces scabies) | Produces Phenazine–1–Carboxylic (PCA) production as antimicrobial substance | [68] |
| Pseudozyma aphidis | Botrytis cinerea | Antimicrobial activity, induced plant resistance by inducing jasmonic acid and salicylic acid/NPR1 | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, J.; Ashley, K. Irreplaceable Role of Amendment-Based Strategies to Enhance Soil Health and Disease Suppression in Potato Production. Microorganisms 2021, 9, 1660. https://doi.org/10.3390/microorganisms9081660
Hao J, Ashley K. Irreplaceable Role of Amendment-Based Strategies to Enhance Soil Health and Disease Suppression in Potato Production. Microorganisms. 2021; 9(8):1660. https://doi.org/10.3390/microorganisms9081660
Chicago/Turabian StyleHao, Jianjun, and Katherine Ashley. 2021. "Irreplaceable Role of Amendment-Based Strategies to Enhance Soil Health and Disease Suppression in Potato Production" Microorganisms 9, no. 8: 1660. https://doi.org/10.3390/microorganisms9081660
APA StyleHao, J., & Ashley, K. (2021). Irreplaceable Role of Amendment-Based Strategies to Enhance Soil Health and Disease Suppression in Potato Production. Microorganisms, 9(8), 1660. https://doi.org/10.3390/microorganisms9081660






