Phytase-Producing Rahnella aquatilis JZ-GX1 Promotes Seed Germination and Growth in Corn (Zea mays L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Inoculum Preparation
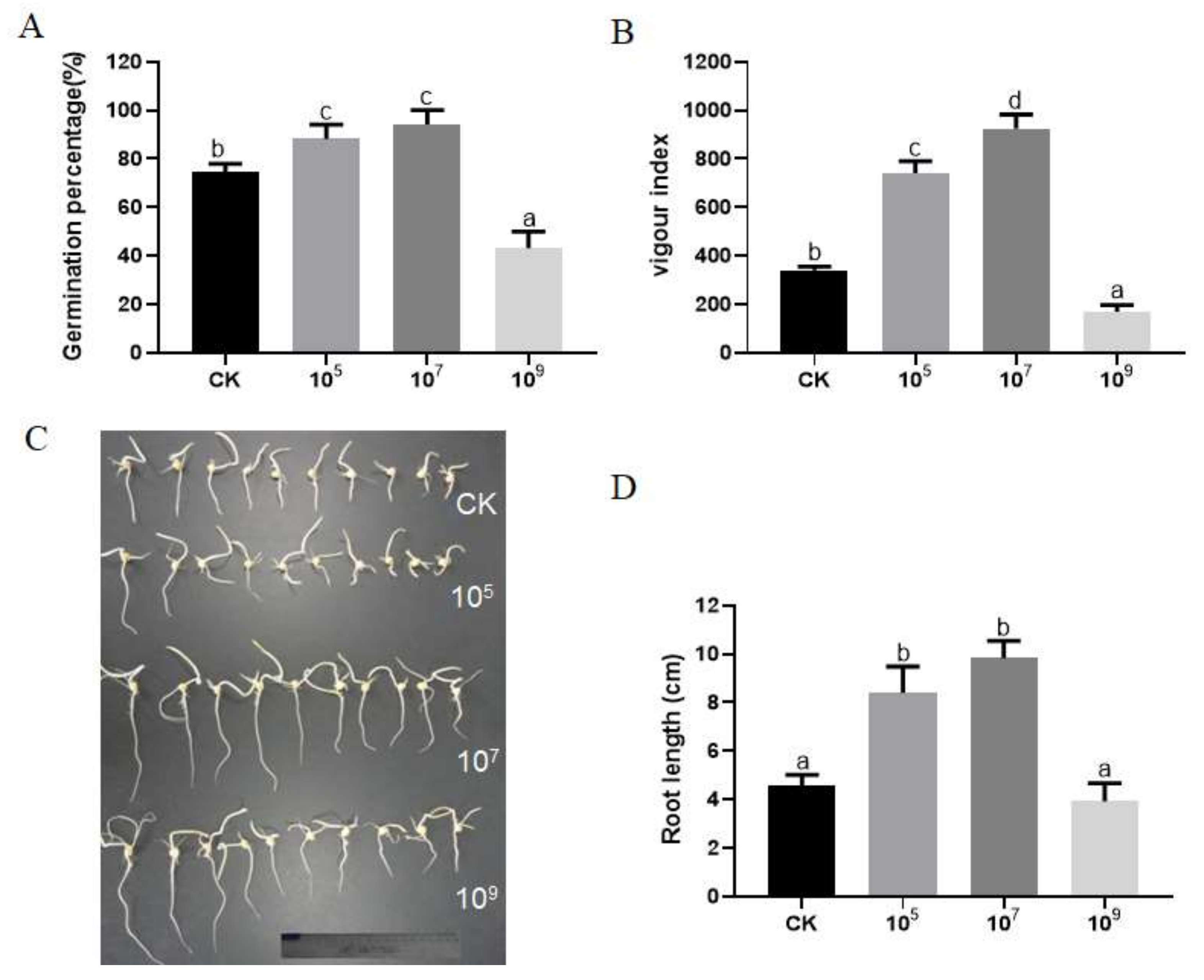
2.2. Seed Germination Assay
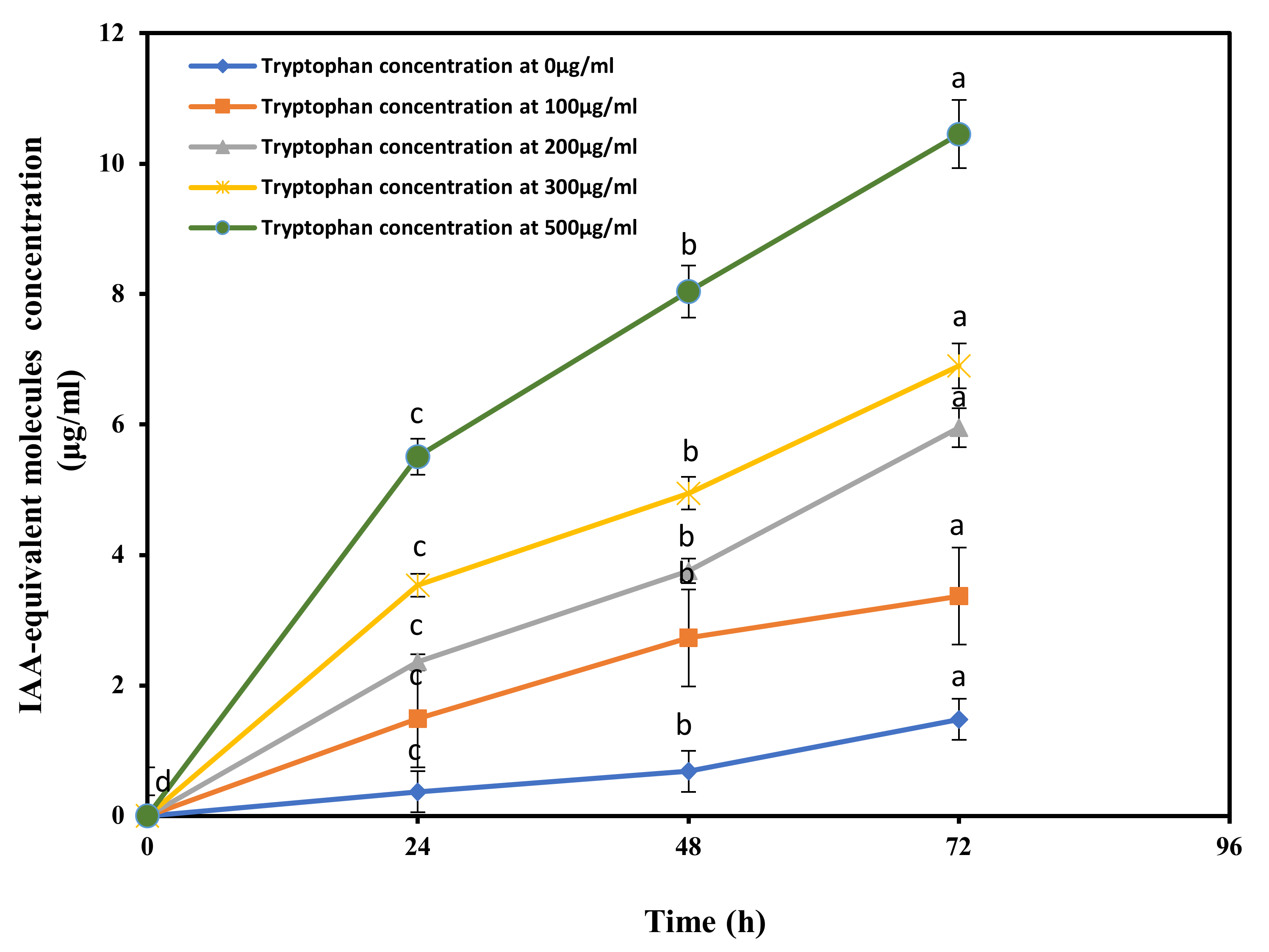
2.3. Quantitative Analysis of Indole Acetic Acid
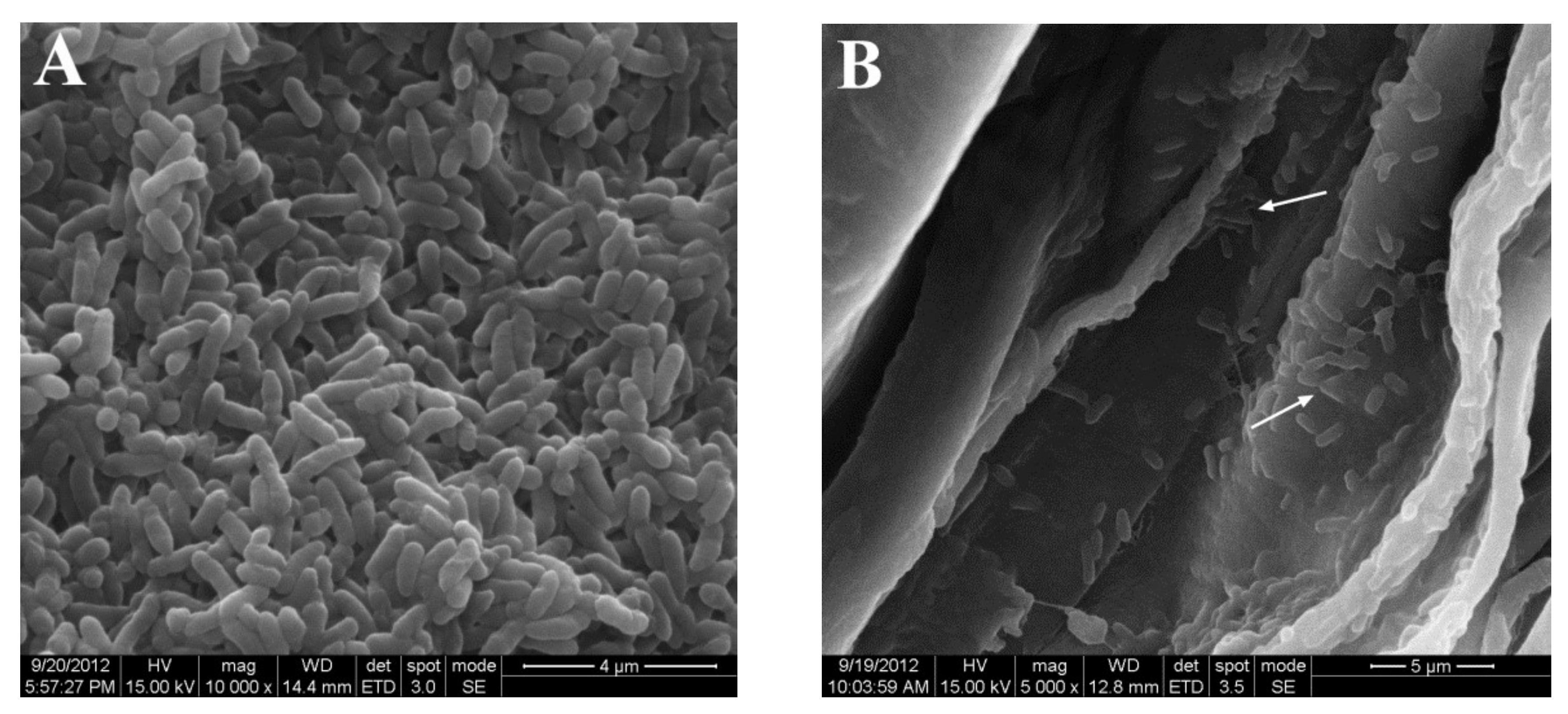
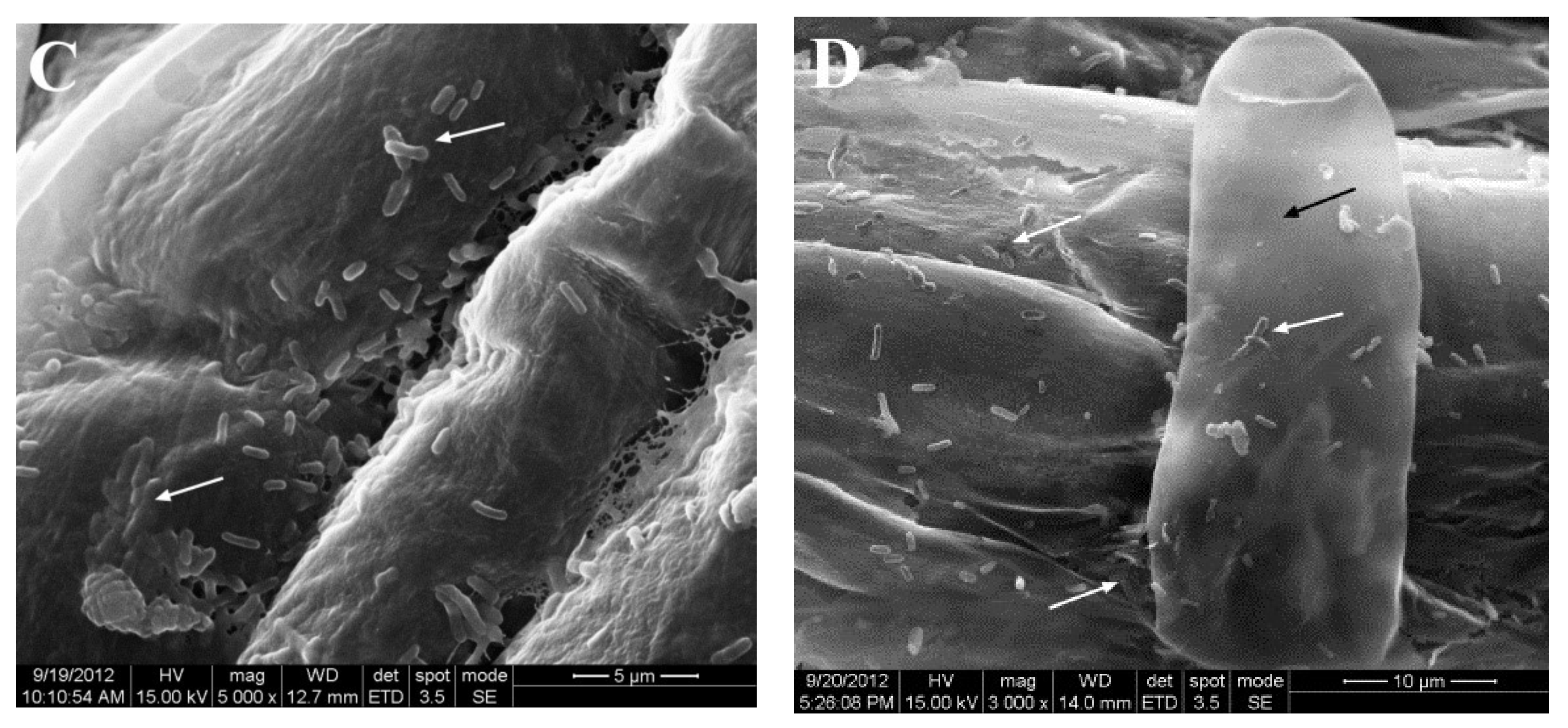
2.4. Colonization of Corn by JZ-GX1 In Vitro
2.5. Soil-Plant Experiment
2.6. Determination of Photosynthetic Parameters
2.7. Determination of Chlorophyll Content
2.8. Measurement of Phytase Activity in the Root
2.9. Measurement of Plant Total P Concentration
2.10. Biomass Determination
2.11. Statistical Analysis
3. Results
3.1. Effects of R. aquatilis JZ-GX1 on Seed Germination and Root Elongation in Corn
3.2. Indole Acetic Acid Produced by R. aquatilis JZ-GX1
3.3. Colonization of Corn Seedlings by R. aquatilis JZ-GX1 In Vitro
3.4. Effects of R. aquatilis JZ-GX1 on Seedlings in the Soil-Plant Experiment
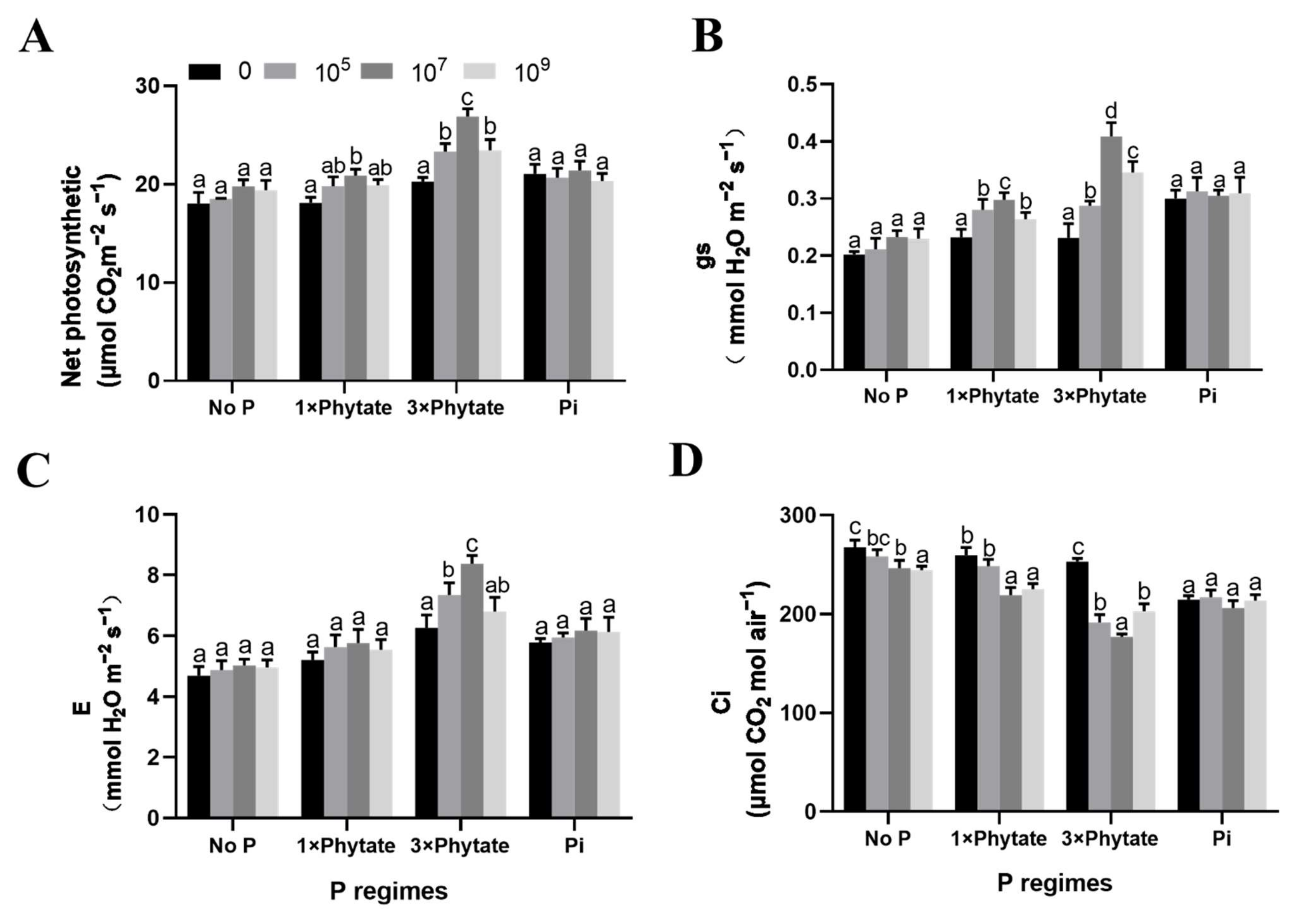
3.5. Photosynthetic Parameters and Chlorophyll Content of Corn Seedlings
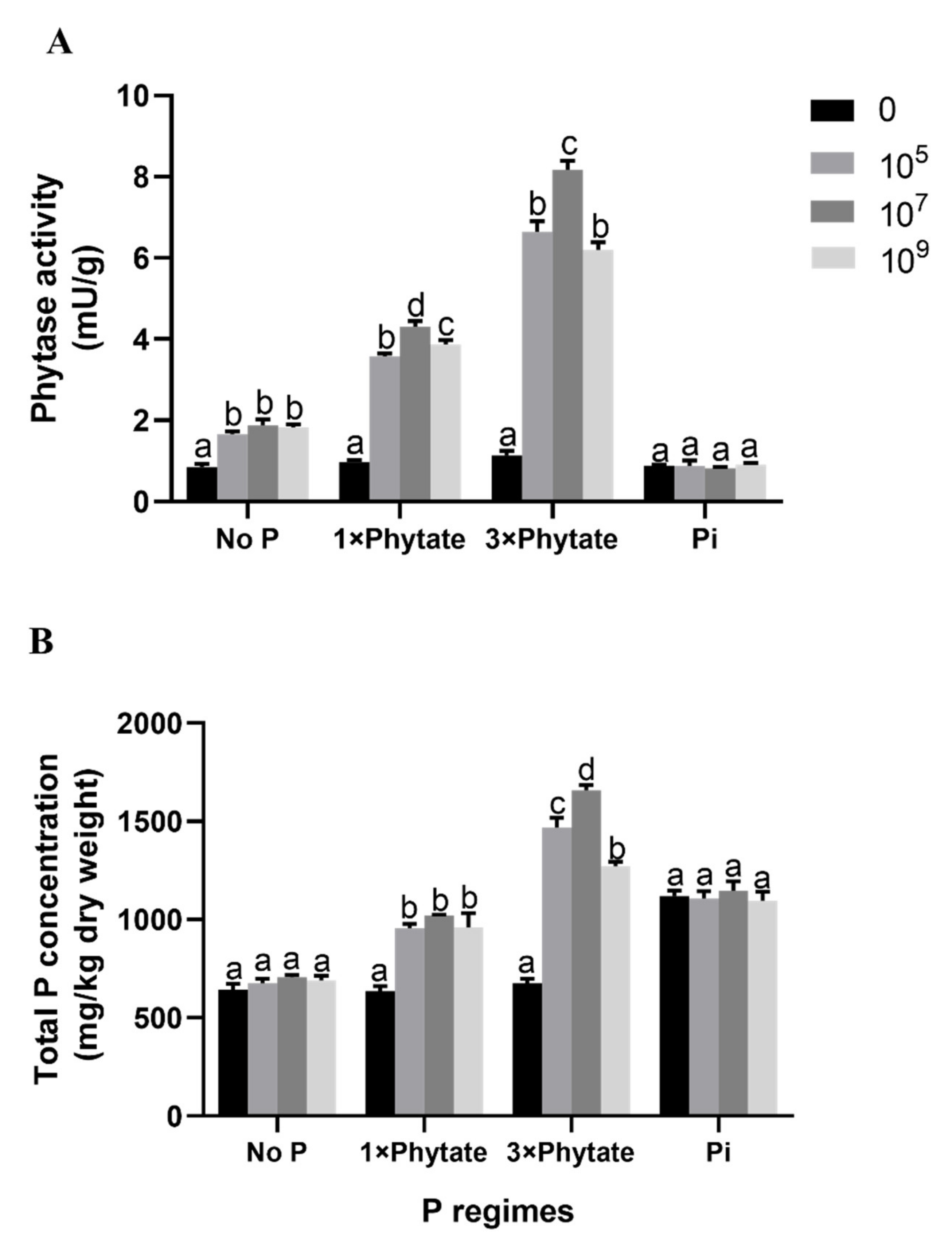
3.6. Phytase Activity in the Root and Total P Concentration of Plants
3.7. Effect of R. aquatilis JZ-GX1 on Biomass Accumulation in Corn Seedlings
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chauhan, H.; Bagyaraj, D.J.; Selvakumar, G.; Sundaram, S. Novel plant growth promoting rhizobacteria—Prospects and potential. Appl. Soil Ecol. 2015, 95, 38–53. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-Growth-Promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, B.; Wang, C.; Song, X.F.; Ding, X.L.; Wu, L.M.; Wu, H.J.; Gao, X.W.; Borriss, R. Bacillus velezensis FZB42 in 2018: The Gram-Positive Model Strain for Plant Growth Promotion and Biocontrol. Front. Microbiol. 2018, 9, 2491. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wu, C.F.; Xing, Z.; Gao, B.L.; Zhang, L.Q. Engineering the bacterial endophyte Burkholderia pyrrocinia JK-SH007 for the control of lepidoptera larvae by introducing the cry218 genes of Bacillus thuringiensis. Biotechnol. Biotechnol. Equip. 2017, 31, 1167–1172. [Google Scholar] [CrossRef] [Green Version]
- Kong, W.-L.; Wu, X.-Q.; Zhao, Y.-J. Effects of Rahnella aquatilis JZ-GX1 on Treat Chlorosis Induced by Iron Deficiency in Cinnamomum camphora. J. Plant Growth Regul. 2019, 39, 877–887. [Google Scholar] [CrossRef]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z. Current knowledge and perspectives of Paenibacillus: A review. Microb. Cell Factories 2016, 15, 203–221. [Google Scholar] [CrossRef] [Green Version]
- Van Loon, L.C. Plant responses to plant growth-promoting rhizobacteria. Eur. J. Plant Pathol. 2007, 119, 243–254. [Google Scholar] [CrossRef] [Green Version]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of Plant Growth-Promoting Bacteria for Biocontrol of Plant Diseases: Principles, Mechanisms of Action, and Future Prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, T.T.; Ren, J.H.; Liu, R.X. Identification, characterization and growth-promoting effects of an organophosphate-solubilizing bacterium from Taxus chinensis var. mairei rhizosphere. Acta Bot. Boreal–Occident. Sin. 2016, 36, 1819–1827. (In Chinese) [Google Scholar]
- Wan, B.B.; Liu, Y.; Wu, Y.; Zhang, D.Y.; Wang, G.W.; Jiang, Y. Screening and identification of maize growth-promoting rhizobacteria and its promoting effects on maize. Biotechnol. Bull. 2016, 32, 169–176. (In Chinese) [Google Scholar] [CrossRef]
- Richardson, J.; Stead, D.E.; Coutts, R.H.A. Incidence of the cblA major subunit pilin gene among Burkholderia species. FEMS Microbiol. Lett. 2001, 196, 61–66. [Google Scholar] [CrossRef] [Green Version]
- George, T.S.; Quiquampoix, H.; Simpson, R.J.; Richardson, A.E. Interactions between phytases and soil constituents: Implications for the hydrolisis of inositol phosphates. In Inositol Phosphates: Linking Agriculture and the Environment; Turner, B.L., Richardson, A.E., Mullaney, E.J., Eds.; CAB International: Oxfordshire, UK, 2007; pp. 221–241. [Google Scholar]
- Sanguin, H.; Wilson, N.L.; Kertesz, M.A. Assessment of functional diversity and structure of phytate-hydrolysing bacterial community in Lolium perenne rhizosphere. Plant Soil 2016, 401, 151–167. [Google Scholar] [CrossRef] [Green Version]
- Esitken, A.; Yildiz, H.E.; Ercisli, S.; Donmez, M.F.; Turan, M.; Gunes, A. Effects of plant growth promoting bacteria (PGPB) on yield, growth and nutrient contents of organically grown strawberry. Sci. Hortic. 2010, 124, 62–66. [Google Scholar] [CrossRef]
- Liu, L.; Du, W.; Luo, W.; Su, Y.; Hui, J.; Ma, S. Development of an Engineered Soil Bacterium Enabling to Convert Both Insoluble Inorganic and Organic Phosphate into Plant Available Phosphate and Its Use as a Biofertilizer. Mol. Biotechnol. 2015, 57, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Shulse, C.N.; Chovatia, M.; Agosto, C.; Wang, G.; Hamilton, M.; Deutsch, S.; Yoshikuni, Y.; Blow, M.J. Engineered Root Bacteria Release Plant-Available Phosphate from Phytate. Appl. Environ. Microbiol. 2019, 85, e01210–e01918. [Google Scholar] [CrossRef] [Green Version]
- Song, H.-Y.; El Sheikha, A.F.; Hu, D.-M. The positive impacts of microbial phytase on its nutritional applications. Trends Food Sci. Technol. 2019, 86, 553–562. [Google Scholar] [CrossRef]
- Hou, X.; Shen, Z.; Li, N.; Kong, X.; Sheng, K.; Wang, J.; Wang, Y. A novel fungal beta-propeller phytase from nematophagous Arthrobotrys oligospora: Characterization and potential application in phosphorus and mineral release for feed processing. Microb. Cell Factories 2020, 19, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, A.E.; Barea, J.M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- George, T.; Turner, B.; Gregory, P.J.; Cade-Menun, B.; Richardson, A.E. Depletion of organic phosphorus from Oxisols in relation to phosphatase activities in the rhizosphere. Eur. J. Soil Sci. 2006, 57, 47–57. [Google Scholar] [CrossRef]
- George, T.; Richardson, A.E.; Smith, J.B.; Hadobas, P.A.; Simpson, R.J. Limitations to the Potential of Transgenic Trifolium subterraneum L. Plants that Exude Phytase when Grown in Soils with a Range of Organic P Content. Plant Soil 2005, 278, 263–274. [Google Scholar] [CrossRef]
- Parray, J.A.; Jan, S.; Kamili, A.N.; Qadri, R.A.; Egamberdieva, D.; Ahmad, P. Current Perspectives on Plant Growth-Promoting Rhizobacteria. J. Plant Growth Regul. 2016, 35, 877–902. [Google Scholar] [CrossRef]
- Vurukonda, S.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, C.A.; Kloepper, J.W. Plant growth promotion by Bacillus amyloliquefaciens FZB45 depends on inoculum rate and P-related soil properties. Biol. Fertil. Soils 2010, 46, 835–844. [Google Scholar] [CrossRef]
- Hu, Q.; Ma, X.; Pan, X.; Binxiang, H. Climate Warming Changed the Planting Boundaries of Varieties of Summer Corn with Different Maturity Levels in the North China Plain. J. Appl. Meteorol. Clim. 2019, 58, 2605–2615. [Google Scholar] [CrossRef]
- Li, G.-E.; Wu, X.-Q.; Ye, J.-R.; Hou, L.; Zhou, A.-D.; Zhao, L. Isolation and identification of phytate-degrading rhizobacteria with activity of improving growth of poplar and Masson pine. World J. Microbiol. Biotechnol. 2013, 29, 2181–2193. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Singh, P.; Tiwari, R.; Meena, K.K.; Yandigeri, M.; Singh, D.P.; Arora, D.K. Salt-tolerant rhizobacteria-mediated induced tolerance in wheat (Triticum aestivum) and chemical diversity in rhizosphere enhance plant growth. Biol. Fertil. Soils 2011, 47, 907–916. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida indole acetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef] [Green Version]
- Panhwar, Q.A.; Naher, U.A.; Radziah, O.; Shamshuddin, J.; Razi, I.M. Eliminating Aluminum Toxicity in an Acid Sulfate Soil for Rice Cultivation Using Plant Growth Promoting Bacteria. Molecules 2015, 20, 3628–3646. [Google Scholar] [CrossRef]
- Gamalero, E.; Fracchia, L.; Cavaletto, M.; Garbaye, J.; Frey-Klett, P.; Varese, G.; Martinotti, M. Characterization of functional traits of two fluorescent pseudomonads isolated from basidiomes of ectomycorrhizal fungi. Soil Biol. Biochem. 2003, 35, 55–65. [Google Scholar] [CrossRef]
- Ren, J.H.; Ye, J.R.; Liu, H.; Xu, X.L.; Wu, X.Q. Isolation and characterization of a new Burkholderia pyrrocinia strain JK-SH007 as a potential biocontrol agent. World J. Microb. Biot. 2011, 21, 2203–2215. [Google Scholar] [CrossRef]
- Idriss, E.E.; Makarewicz, O.; Farouk, A.; Rosner, K.; Greiner, R.; Bochow, H.; Richter, T.; Borriss, R. Extracellular phytase activity of Bacillus amyloliquefaciens FZB45 contributes to its plant-growth-promoting effect a aThe GenBank accession numbers for the sequences determined in this work are AY055219 to AY055226. Microbiology 2002, 148, 2097–2109. [Google Scholar] [CrossRef] [Green Version]
- Li, C.Y. Effects of Exogenous Orangic Acids and Foliar Spraying on Phosphorus Uptake and Development of Maize (Zea mays L.) Under P-Limiting Soil. Master’s Thesis, Northwest A&F University, Yangling, China, 2011. [Google Scholar]
- Tian, F.X.; Gong, J.F.; Wang, G.P.; Fan, Z.Y.; Wang, W. Improved drought resistance in a wheat stay-green mutant tasg1 under field conditions. Biol. Plant. 2012, 56, 509–515. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 10, 350–382. [Google Scholar] [CrossRef]
- Quan, C.-S.; Fan, S.-D.; Zhang, L.-H.; Wang, Y.-J.; Ohta, Y. Purification and properties of a phytase from Candida krusei WZ-001. J. Biosci. Bioeng. 2002, 94, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis; Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Huang, W.; Yu, X.L.; Lin, Y.M.; Wu, C.Z.; Li, J.; Chen, C.; Fan, H.L.; Hong, W. Effects of two strains of Bacillus sphaericus on growth and photosynthetic characteristics of Eucalyptus grandis seedlings. Chin. J. Appl. Environ. Biol. 2016, 22, 839–844. (In Chinese) [Google Scholar]
- Gong, W.X.; Cao, Y.Y.; Ni, H.T.; Sun, L.N.; Tang, X.Y. Screening of affinity PGPRs from tobacco root and their growth-promotion effects on tobacco. Acta Tabacaria Sinica 2016, 22, 55–63. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, L.; Xu, Y.; Chang, J.; Li, M.; Zhang, Y.; Dang, Y.; Wang, M.; Cheng, Y.; Zhang, B. Screening, resistance and growth-promoting effect of endophytic bacteria with ACC deaminase activity isolated from soybean nodules. Acta Microbiol. Sin. 2016, 56, 1009–1021. [Google Scholar]
- Unno, Y.; Okubo, K.; Wasaki, J.; Shinano, T.; Osaki, M. Plant growth promotion abilities and microscale bacterial dynamics in the rhizosphere of Lupin analysed by phytate utilization ability. Environ. Microbiol. 2005, 7, 396–404. [Google Scholar] [CrossRef] [PubMed]
- George, T.S.; E Richardson, A.; A Hadobas, P.; Simpson, R.J. Characterization of transgenic Trifolium subterraneum L. which expresses phyA and releases extracellular phytase: Growth and P nutrition in laboratory media and soil. Plant Cell Environ. 2004, 27, 1351–1361. [Google Scholar] [CrossRef]
- Prasad, K.; Bhatnagar-Mathur, P.; Waliyar, F.; Sharma, K.K. Overexpression of a chitinase gene in transgenic peanut confers enhanced resistance to major soil borne and foliar fungal pathogens. J. Plant Biochem. Biotechnol. 2012, 22, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Grichko, V.P.; Filby, B.; Glick, B.R. Increased ability of transgenic plants expressing the bacterial enzyme ACC deaminase to accumulate Cd, Co, Cu, Ni, Pb, and Zn. J. Biotechnol. 2000, 81, 45–53. [Google Scholar] [CrossRef]
- Distefano, G.; La Malfa, S.; Vitale, A.; Lorito, M.; Deng, Z.; Gentile, A. Defence-related gene expression in transgenic lemon plants producing an antimicrobial Trichoderma harzianum endochitinase during fungal infection. Transgenic Res. 2008, 17, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Li, G.E.; Wu, X.Q. Study on enzymology characteristics of phytase secreted by efficient phytate-degrading rhizobacteria Rahnella aquatilis JZ-GX1. J. Cent. South Univ. 2014, 34, 90–93. [Google Scholar]
- Kamilova, F.; Validov, S.; Azarova, T.; Mulders, I.; Lugtenberg, B. Enrichment for enhanced competitive plant root tip colonizers selects for a new class of biocontrol bacteria. Environ. Microbiol. 2005, 7, 1809–1817. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, B.J.J.; Dekkers, L.; Bloemberg, G.V. Molecular determinants of rhizospherecolonization by Pseudomonas. Annu. Rev. Phytopathol. 2001, 39, 461–490. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-H.; Chen, F.-F.; Wang, C.-E.; Fu, H.-H.; Fang, X.-Q.; Ye, J.-R.; Shi, J.-Y. Indole-3-Acetic Acid in Burkholderia pyrrocinia JK-SH007: Enzymatic Identification of the Indole-3-Acetamide Synthesis Pathway. Front. Microbiol. 2019, 10, 2559. [Google Scholar] [CrossRef] [Green Version]
- Dobbelaere, S.; Croonenborghs, A.; Thys, A.; Broek, A.V.; Vanderleyden, J. Phytostimulatory effect of Azospirillum brasilense wild type and mutant strains altered in IAA production on wheat. Plant Soil 1999, 212, 153–162. [Google Scholar] [CrossRef]
- Kammoun, R.; Farhat, A.; Chouayekh, H.; Bouchaala, K.; Bejar, S. Phytase production by Bacillus subtilis US417 in submerged and solid state fermentations. Ann. Microbiol. 2011, 62, 155–164. [Google Scholar] [CrossRef]
- Bhakta, J.N.; Munekage, Y.; Ohnishi, K.; Jana, B.B. Isolation and identification of cadmium- and lead-resistant lactic acid bacteria for application as metal removing probiotic. Int. J. Environ. Sci. Technol. 2012, 9, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.M.; Ladha, J.K. Optimizing phosphorus fertilization in an intensive vegetable-rice cropping system. Biol. Fertil. Soils 2004, 40, 277–283. [Google Scholar] [CrossRef]
- Jorquera, M.; Hernández, M.T.; Rengel, Z.; Marschner, P.; Mora, M.D.L.L. Isolation of culturable phosphobacteria with both phytate-mineralization and phosphate-solubilization activity from the rhizosphere of plants grown in a volcanic soil. Biol. Fertil. Soils 2008, 44, 1025–1034. [Google Scholar] [CrossRef]
- Shen, L.; Wu, X.-Q.; Zeng, Q.-W.; Liu, H.-B. Regulation of Soluble Phosphate on the Ability of Phytate Mineralization and β-Propeller Phytase Gene Expression of Pseudomonas fluorescens JZ-DZ1, a Phytate-Mineralizing Rhizobacterium. Curr. Microbiol. 2016, 73, 915–923. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.-E.; Kong, W.-L.; Wu, X.-Q.; Ma, S.-B. Phytase-Producing Rahnella aquatilis JZ-GX1 Promotes Seed Germination and Growth in Corn (Zea mays L.). Microorganisms 2021, 9, 1647. https://doi.org/10.3390/microorganisms9081647
Li G-E, Kong W-L, Wu X-Q, Ma S-B. Phytase-Producing Rahnella aquatilis JZ-GX1 Promotes Seed Germination and Growth in Corn (Zea mays L.). Microorganisms. 2021; 9(8):1647. https://doi.org/10.3390/microorganisms9081647
Chicago/Turabian StyleLi, Gui-E, Wei-Liang Kong, Xiao-Qin Wu, and Shi-Bo Ma. 2021. "Phytase-Producing Rahnella aquatilis JZ-GX1 Promotes Seed Germination and Growth in Corn (Zea mays L.)" Microorganisms 9, no. 8: 1647. https://doi.org/10.3390/microorganisms9081647
APA StyleLi, G.-E., Kong, W.-L., Wu, X.-Q., & Ma, S.-B. (2021). Phytase-Producing Rahnella aquatilis JZ-GX1 Promotes Seed Germination and Growth in Corn (Zea mays L.). Microorganisms, 9(8), 1647. https://doi.org/10.3390/microorganisms9081647





