Cariogenic Biofilm: Pathology-Related Phenotypes and Targeted Therapy
Abstract
1. Introduction
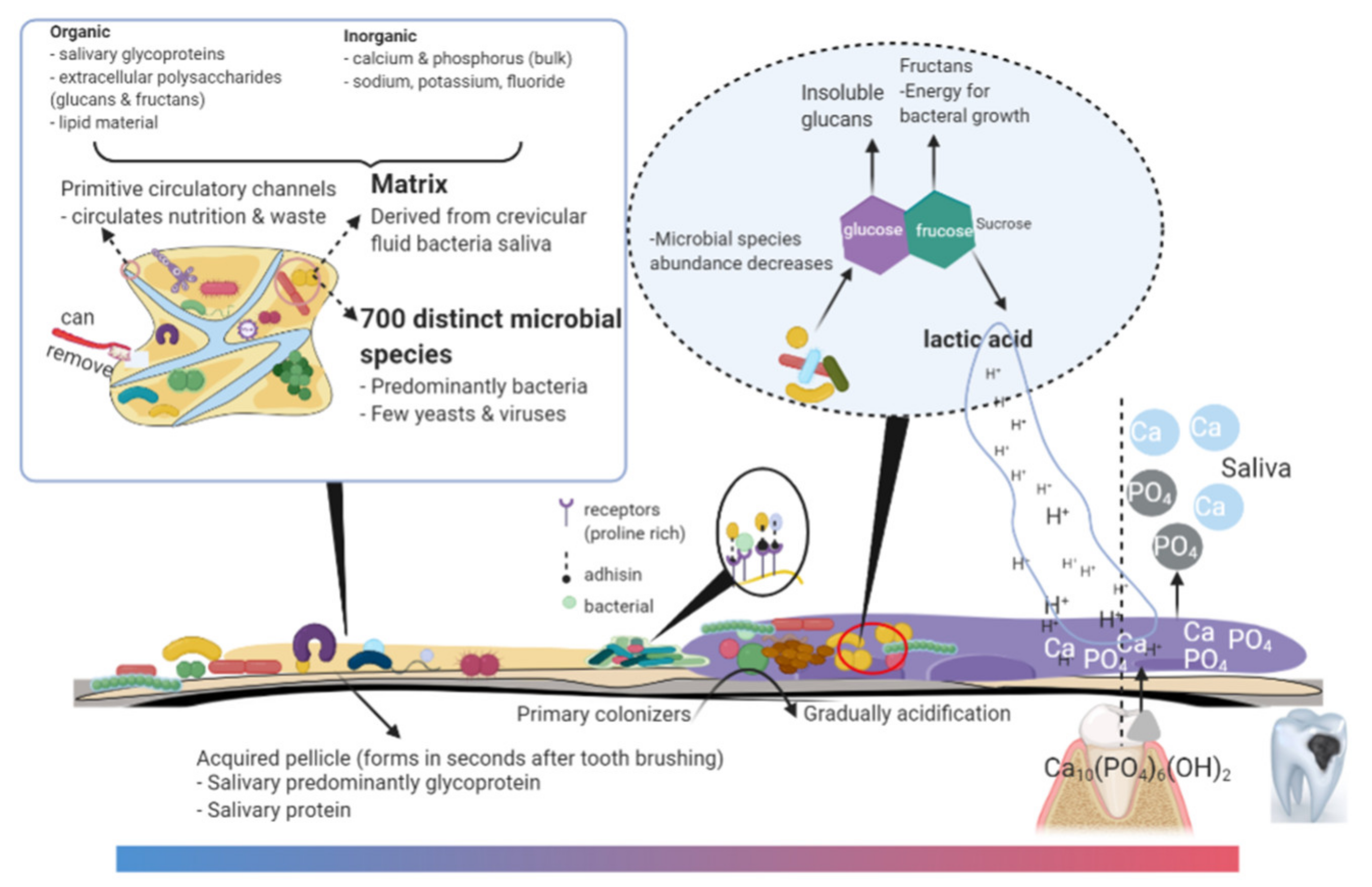
2. Microbes, Quorum Sensing, and Matrix in Cariogenic Biofilm
2.1. Biofilm Microbiota: Opportunistic Pathogens and Commensals
2.1.1. Streptococcus Species
2.1.2. Bifidobacterium Species
2.1.3. Lactobacillus Species
2.1.4. Scardovia Species
2.1.5. Other Species
2.2. Biofilm Microbiota: Interspecies Interactions
2.3. Matrixome in Cariogenic Biofilms
3. Diagnosis of Cariogenic Biofilm
3.1. Biomarker of Cariogenicity
3.2. Diagnosis of Biofilm Acidification
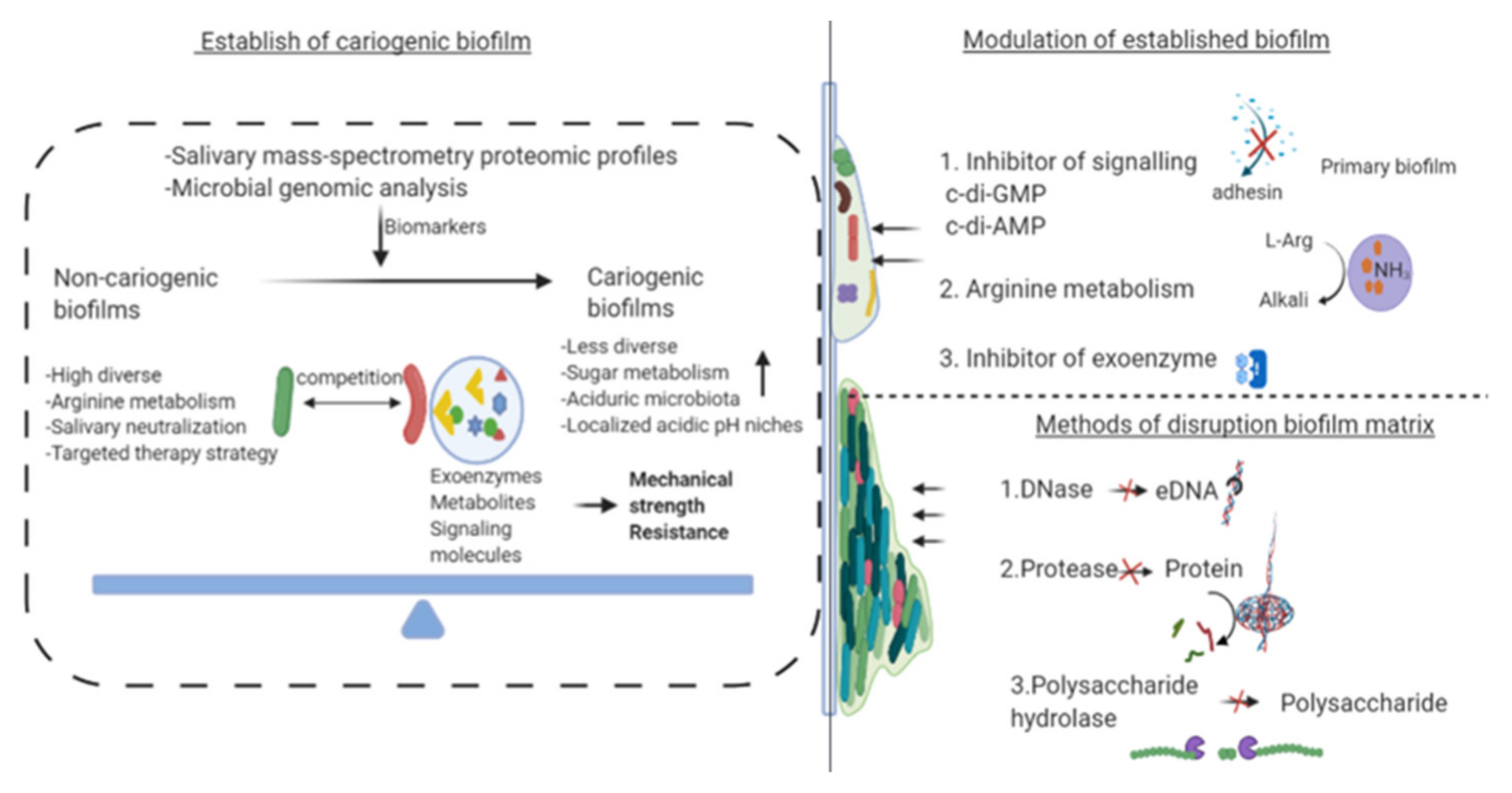
4. Recent Advances in Inhibition of Cariogenic Biofilms
4.1. Effect on Bacterial Diversity of Cariogenic Biofilm
4.2. Modulating Virulence and Effects on Active Attachment System of Biofilm
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Yang, Q.; Xi, Y.; Liu, H.; Luo, J.; Ouyang, Y.; Sun, M.; Yong, C.; Xiang, C.; Lin, Q. Free sugars intake among chinese adolescents and its association with dental caries: A cross-sectional study. Nutrients 2021, 13, 765. [Google Scholar] [CrossRef]
- Marsh, P.D. In sickness and in health—what does the oral microbiome mean to us? An ecological perspective. Adv. Dent. Res. 2018, 29, 60–65. [Google Scholar] [CrossRef]
- Mira, A. Oral microbiome studies: Potential diagnostic and therapeutic implications. Adv. Dent. Res. 2018, 29, 71–77. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, Z.; Hwang, G.; Koo, H. Therapeutic strategies targeting cariogenic biofilm microenvironment. Adv. Dent. Res. 2018, 29, 86–92. [Google Scholar] [CrossRef]
- Kidd, E.A.M.; Fejerskov, O. What constitutes dental caries? Histopathology of carious enamel and dentin related to the action of cariogenic biofilms. J. Dent. Res. 2004, 83, 35–38. [Google Scholar]
- Bhadila, G.; Dai, Q.; Melo, M.A.S. Anti-caries nanostructured dental adhesive reduces biofilm pathogenicity and raises biofilm pH to protect tooth structures. J. Mater. Res. Technol. 2021, 36, 1–14. [Google Scholar]
- Hajishengallis, E.; Parsaei, Y.; Klein, M.; Koo, H. Advances in the microbial etiology and pathogenesis of early childhood caries. Mol. Oral Microbiol. 2017, 32, 24–34. [Google Scholar] [CrossRef]
- Banas, J.A. Virulence properties of Streptococcus mutans. Front. Biosci. 2004, 9, 1267–1277. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Fakhruddin, K.S.; Ngo, H.C.; Samaranayake, L.P. Cariogenic microbiome and microbiota of the early primary dentition: A contemporary overview. Oral Dis. 2019, 25, 982–995. [Google Scholar] [CrossRef]
- Ito, S.; Misaki, T.; Naka, S.; Wato, K.; Nagasawa, Y.; Nomura, R.; Otsugu, M.; Matsumoto-Nakano, M.; Nakano, K.; Kumagai, H.; et al. Specific strains of Streptococcus mutans, a pathogen of dental caries, in the tonsils, are associated with IgA nephropathy. Sci. Rep. 2019, 9, 20130. [Google Scholar] [CrossRef]
- Gross, E.L.; Beall, C.J.; Kutsch, S.R.; Firestone, N.D.; Leys, E.J.; Griffen, A.L. Beyond Streptococcus mutans: Dental Caries Onset Linked to Multiple Species by 16S rRNA Community Analysis. PLoS ONE 2012, 7, 11. [Google Scholar] [CrossRef]
- Inquimbert, C.; Bourgeois, D.; Bravo, M.; Viennot, S.; Tramini, P.; Llodra, J.C.; Molinari, N.; Dussart, C.; Giraudeau, N.; Carrouel, F. The oral bacterial microbiome of interdental surfaces in adolescents according to carious risk. Microorganisms 2019, 7, 319. [Google Scholar] [CrossRef] [PubMed]
- De Matos, B.M.; Brighenti, F.L.; Do, T.; Beighton, D.; Koga-Ito, C.Y. Acidogenicity of dual-species biofilms of bifidobacteria and Streptococcus mutans. Clin. Oral Investig. 2017, 21, 1769–1776. [Google Scholar] [CrossRef]
- Bourgeois, D.; David, A.; Inquimbert, C.; Tramini, P.; Molinari, N.; Carrouel, F.J. Quantification of carious pathogens in the interdental microbiota of young caries-free adults. PLoS ONE 2017, 12, e0185804. [Google Scholar] [CrossRef]
- Johansson, I.; Witkowska, E.; Kaveh, B.; Holgerson, P.L.; Tanner, A.C.R. The microbiome in populations with a low and high prevalence of caries. J. Dent. Res. 2016, 95, 80–86. [Google Scholar] [CrossRef]
- Kameda, M.; Abiko, Y.; Washio, J.; Tanner, A.C.R.; Kressirer, C.A.; Mizoguchi, I.; Takahashi, N. Sugar metabolism of scardovia wiggsiae, a novel caries-associated bacterium. Front. Microbiol. 2020, 11, 479. [Google Scholar] [CrossRef]
- Matondkar, S.P.; Yavagal, C.; Kugaji, M.; Bhat, K.G. Quantitative assessment of Scardovia wiggsiae from dental plaque samples of children suffering from severe early childhood caries and caries free children. Anaerobe 2020, 62, 102110. [Google Scholar] [CrossRef]
- Pereira, D.F.A.; Seneviratne, C.J.; Koga-Ito, C.Y.; Samaranayake, L.P. Is the oral fungal pathogen Candida albicans a cariogen? Oral Dis. 2018, 24, 518–526. [Google Scholar] [CrossRef]
- Xiao, J.; Huang, X.; Alkhers, N.; Alzamil, H.; Alzoubi, S.; Wu, T.T.; Castillo, D.A.; Campbell, F.; Davis, J.; Herzog, K.; et al. Candida albicans and early childhood caries: A systematic review and meta-analysis. Caries Res. 2018, 52, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Li, W.; He, Y.; Wu, J.; Ren, B.; Zou, L. Cross-kingdom interaction of Candida albicans and Actinomyces viscosus elevated cariogenic virulence. Arch. Oral biol. 2019, 100, 106–112. [Google Scholar] [CrossRef]
- Xu, H.; Tian, J.; Hao, W.; Zhang, Q.; Zhou, Q.; Shi, W.; Qin, M.; He, X.; Chen, F. Oral microbiome shifts from caries-free to caries-affected status in 3-year-old chinese children: A longitudinal study. Front. Microbiol. 2018, 9, 2009. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.; Zaura, E. Dental biofilm: Ecological interactions in health and disease. J. Clin. Periodontol. 2017, 44, S12–S22. [Google Scholar] [CrossRef]
- Wu, J.; Li, M.; Huang, R. The effect of smoking on caries-related microorganisms. Tob. Induc. Dis. 2019, 17, 32. [Google Scholar] [CrossRef]
- Periasamy, S.; Kolenbrander, P.E. Central role of the early colonizer Veillonella sp. in establishing multispecies biofilm communities with initial, middle, and late colonizers of enamel. J. Bacteriol. 2010, 192, 2965–2972. [Google Scholar] [CrossRef]
- Chen, L.; Walker, A.R.; Burne, R.A.; Zeng, L. Amino Sugars Reshape Interactions between Streptococcus mutans and Streptococcus gordonii. AEM 2020, 87, e01459-20. [Google Scholar] [CrossRef]
- Bikash, C.R.; Hamry, S.R.; Tal-Gan, Y. Structure–activity relationships of the competence stimulating peptide in streptococcus mutans reveal motifs critical for membrane protease sepm recognition and comd receptor activation. ACS Infect. Dis. 2018, 4, 1385–1394. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Ling, J. Streptococcus gordonii LuxS/autoinducer-2 quorum-sensing system modulates the dual-species biofilm formation with Streptococcus mutans. J. Basic Microbiol. 2017, 57, 605–616. [Google Scholar] [CrossRef]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7, 18. [Google Scholar] [CrossRef]
- Chi, F.; Nolte, O.; Bergmann, C.; Ip, M.; Hakenbeck, R. Crossing the barrier: Evolution and spread of a major class of mosaic pbp2x in Streptococcus pneumoniae, S. mitis and S. oralis. Int. J. Med. Microbiol. 2007, 297, 503–512. [Google Scholar] [CrossRef]
- Koo, H.; Yamada, K.M. Dynamic cell–matrix interactions modulate microbial biofilm and tissue 3D microenvironments. Curr. Opin. Cell Biol. 2016, 42, 102–112. [Google Scholar] [CrossRef]
- Hwang, G.; Liu, Y.; Kim, D.; Sun, V.; Aviles-Reyes, A.; Kajfasz, J.K.; Lemos, J.A.; Koo, H. Simultaneous spatiotemporal mapping of in situ pH and bacterial activity within an intact 3D microcolony structure. Sci. Rep. 2016, 6, 32841. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm matrixome: Extracellular components in structured microbial communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.G.; Vila, T.; Romo, J.A.; Montelongo-Jauregui, D.; Wall, G.; Ramasubramanian, A.; Lopez-Ribot, J.L. The Candida albicans biofilm matrix: Composition, structure and function. J. Fungi 2017, 3, 14. [Google Scholar] [CrossRef]
- Zijnge, V.; van Leeuwen, M.B.M.; Degener, J.E.; Abbas, F.; Thurnheer, T.; Gmür, R.; Harmsen, H.J.M. Oral biofilm architecture on natural teeth. PLoS ONE. 2010, 5, e9321. [Google Scholar] [CrossRef]
- Jiang, W.; Ling, Z.; Lin, X.; Chen, Y.; Zhang, J.; Yu, J.; Xiang, C.; Chen, H.J. Pyrosequencing analysis of oral microbiota shifting in various caries states in childhood. Microb. Ecol. 2014, 67, 962–969. [Google Scholar] [CrossRef]
- Takahashi, N.; Nyvad, B.J.C.R. Caries ecology revisited: Microbial dynamics and the caries process. Caries Res. 2008, 42, 409–418. [Google Scholar] [CrossRef]
- Hart, T.C.; Corby, P.M.; Hauskrecht, M.; Ryu, O.H.; Pelikan, R.; Valko, M.; Oliveira, M.B.; Hoehn, G.T.; Bretz, W.A. Identification of microbial and proteomic biomarkers in early childhood caries. Int. J. Dent. 2011, 2011, 196721. [Google Scholar] [CrossRef]
- Hemadi, A.S.; Huang, R.; Zhou, Y.; Zou, J. Salivary proteins and microbiota as biomarkers for early childhood caries risk assessment. Int. J. Oral Sci. 2017, 9, e1. [Google Scholar] [CrossRef]
- Marsh, P.D. The role of microbiology in models of dental caries. Adv. Dent. Res. 1995, 9, 244–254. [Google Scholar] [CrossRef]
- De Farias, A.L.; de Carvalho, L.P.F.; Méndez, D.A.C.; Cruvinel, T.; Brighenti, F.L. Characterization of polymicrobial biofilms obtained from saliva or carious lesions in dentin. Biofouling 2020, 36, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Schoilew, K.; Ueffing, H.; Dalpke, A.; Wolff, B.; Frese, C.; Wolff, D.; Boutin, S.J. Bacterial biofilm composition in healthy subjects with and without caries experience. J. Oral Microbiol. 2019, 11, 1633194. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-F.; Hsu, C.-L.; Chen, L.-R. Correlation between salivary mutans streptococci, lactobacilli and the severity of early childhood caries. J. Dent. Sci. 2019, 14, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Naha, P.C.; Liu, Y.; Hwang, G.; Huang, Y.; Gubara, S.; Jonnakuti, V.; Simon-Soro, A.; Kim, D.; Gao, L.; Koo, H.J. Dextran-coated iron oxide nanoparticles as biomimetic catalysts for localized and pH-activated biofilm disruption. ACS Nano 2019, 13, 4960–4971. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Esboei, B.R.; Hodjat, M.; Bahador, A. Sonodynamic excitation of nanomicelle curcumin for eradication of Streptococcus mutans under sonodynamic antimicrobial chemotherapy: Enhanced anti-caries activity of nanomicelle curcumin. Photodiagn. Photodyn. Ther. 2020, 30, 101780. [Google Scholar] [CrossRef]
- Chen, Z.; Schlafer, S.; Göstemeyer, G.; Schwendicke, F.J.M. Probiotic effects on multispecies biofilm composition, architecture, and caries activity in vitro. Microorganisms 2020, 8, 1272. [Google Scholar] [CrossRef]
- Liang, J.; Liu, F.; Zou, J.; Xu, H.; Han, Q.; Wang, Z.; Li, B.; Yang, B.; Ren, B.; Li, M.J. pH-responsive antibacterial resin adhesives for secondary caries inhibition. J. Dent. Res. 2020, 99, 1368–1376. [Google Scholar] [CrossRef]
- Balhaddad, A.A.; AlQranei, M.S.; Ibrahim, M.S.; Weir, M.D.; Martinho, F.C.; Xu, H.H.; Melo, M.A. Light energy dose and photosensitizer concentration are determinants of effective photo-killing against caries-related biofilms. Int. J. Mol. Sci. 2020, 21, 7612. [Google Scholar] [CrossRef]
- Jiang, W.; Luo, J.; Wang, Y.; Chen, X.; Jiang, X.; Feng, Z.; Zhang, L. The ph-responsive property of antimicrobial peptide gh12 enhances its anticaries effects at acidic pH. Caries Res. 2020, 54, 1–11. [Google Scholar]
- Farias, J.M.; Stamford, T.C.M.; Resende, A.H.M.; Aguiar, J.S.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Mouthwash containing a biosurfactant and chitosan: An eco-sustainable option for the control of cariogenic microorganisms. Int. J. Biol. Macromol. 2019, 129, 853–860. [Google Scholar] [CrossRef]
- Legéňová, K.; Kovalčíková, M.; Černáková, L.; Bujdáková, H. The contribution of photodynamic inactivation vs. Corsodyl mouthwash to the control of streptococcus mutans biofilms. Curr. Microbiol. 2020, 77, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.-Y.; Kang, S.-M.; Lee, E.-S.; Kim, B.-I. Antimicrobial activity of Curcuma xanthorrhiza nanoemulsions on Streptococcus mutans biofilms. Biofouling 2020, 36, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Y.; Shah, S.; Kim, D.; Simon-Soro, A.; Ito, T.; Hajfathalian, M.; Li, Y.; Hsu, J.C.; Nieves, L.M.; et al. Precision targeting of bacterial pathogen via bi-functional nanozyme activated by biofilm microenvironment. Biomaterials 2021, 268, 120581. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Yang, T.; Zhang, C.; Peng, X.; Ju, Y.; Li, C.; Zhou, X.; Luo, Y.; Xu, X. Repurposing napabucasin as an antimicrobial agent against oral streptococcal biofilms. BioMed Res. Int. 2020, 2020, 8379526. [Google Scholar] [CrossRef]
- El-Allaky, H.S.; Wahba, N.A.; Talaat, D.M.; Zakaria, A.S. Antimicrobial effect of propolis administered through two different vehicles in high caries risk children: A randomized clinical trial. J. Clin. Pediatr. Dent. 2020, 44, 289–295. [Google Scholar] [CrossRef]
- Sorkhdini, P.; Gregory, R.L.; Crystal, Y.O.; Tang, Q.; Lippert, F. Effectiveness of in vitro primary coronal caries prevention with silver diamine fluoride-Chemical vs biofilm models. J. Dent. 2020, 99, 103418. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Pourhajibagher, M.; Chiniforush, N.; Aminian, M.; Bahador, A. Anti-biofilm and anti-metabolic effects of antimicrobial photodynamic therapy using chlorophyllin-phycocyanin mixture against Streptococcus mutans in experimental biofilm caries model on enamel slabs. Photodiagn. Photodyn. Ther. 2020, 29, 101620. [Google Scholar] [CrossRef]
- Bin, C.; Al-Dhabi, N.A.; Esmail, G.A.; Arokiyaraj, S.; Arasu, M.V. Potential effect of Allium sativum bulb for the treatment of biofilm forming clinical pathogens recovered from periodontal and dental caries. Saudi J. Biol. Sci. 2020, 27, 1428–1434. [Google Scholar] [CrossRef]
- Shafiei, Z.; Rahim, Z.H.A.; Philip, K.; Thurairajah, N.; Yaacob, H. Potential effects of Psidium sp., Mangifera sp., Mentha sp. and its mixture (PEM) in reducing bacterial populations in biofilms, adherence and acid production of S. sanguinis and S. mutans. Arch. Oral Biol. 2020, 109, 104554. [Google Scholar] [CrossRef]
- Lima, R.A.; de Souza, S.L.X.; Lima, L.A.; Batista, A.L.X.; de Araújo, J.T.C.; Sousa, F.F.O.; Rolim, J.P.M.L.; Bandeira, T.D.J.P.G. Antimicrobial effect of anacardic acid–loaded zein nanoparticles loaded on Streptococcus mutans biofilms. Braz. J. Microbiol. 2020, 51, 1623–1630. [Google Scholar] [CrossRef]
- Liang, J.; Liang, D.; Liang, Y.; He, J.; Zuo, S.; Zhao, W. Effects of a derivative of reutericin 6 and gassericin A on the biofilm of Streptococcus mutans in vitro and caries prevention in vivo. Odontology 2020, 109, 1–14. [Google Scholar] [CrossRef]
- Eckert, R.; He, J.; Yarbrough, D.K.; Qi, F.; Anderson, M.H.; Shi, W. Targeted killing of streptococcus mutans by a pheromone-guided “smart” antimicrobial peptide. Antimicrob. Agents Chemother. 2006, 50, 3651–3657. [Google Scholar] [CrossRef]
- Tian, X.; Chen, C.; Cyr, K.; Dong, G.; Salim, H.J.J.A.R. Targeted killing of Streptococcus mutans in biofilms by a pheromone guided antimicrobial peptide HP30. J. Antimicrob. Res. 2017, 1, 201. [Google Scholar]
- Xiang, S.W.; Shao, J.; He, J.; Wu, X.Y.; Xu, X.H.; Zhao, W.H. A membrane-targeted peptide inhibiting ptxa of phosphotransferase system blocks streptococcus mutans. Caries Res. 2019, 53, 176–193. [Google Scholar] [CrossRef]
- Baker, J.; He, X.; Shi, W. Precision reengineering of the oral microbiome for caries management. Adv. Dent. Res. 2019, 30, 34–39. [Google Scholar] [CrossRef] [PubMed]
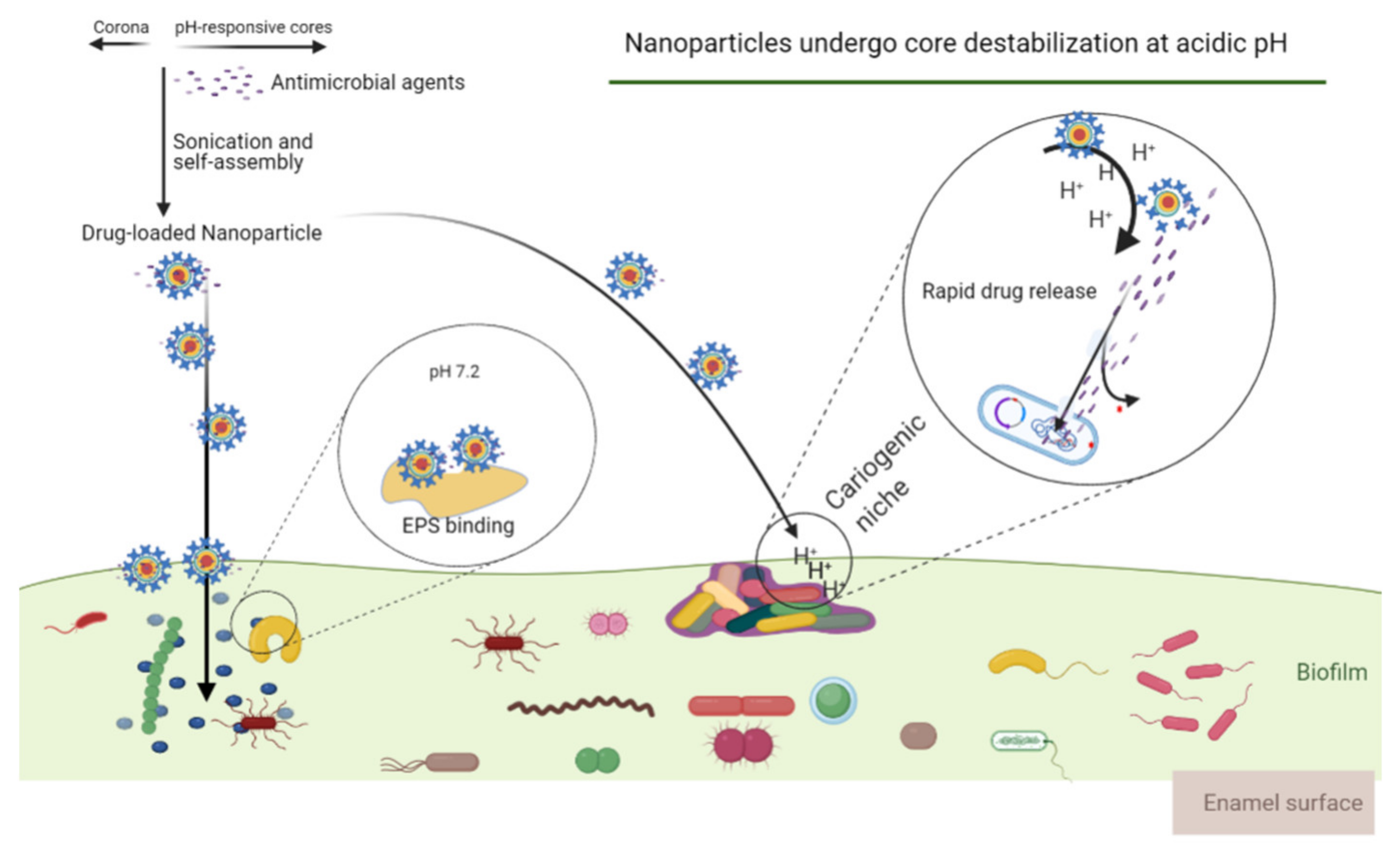
- Benoit, D.S.W.; Sims, K.R.; Fraser, D. Nanoparticles for oral biofilm treatments. ACS Nano 2019, 13, 4869–4875. [Google Scholar] [CrossRef]
- Sims, K.R.; Maceren, J.P.; Liu, Y.; Rocha, G.R.; Koo, H.; Benoit, D.S.W. Dual antibacterial drug-loaded nanoparticles synergistically improve treatment of Streptococcus mutans biofilms. Acta Biomater. 2020, 115, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Shimotoyodome, A.; Kobayashi, H.; Tokimitsu, I.; Matsukubo, T.; Takaesu, Y.J. Statherin and histatin 1 reduce parotid saliva-promoted Streptococcus mutans strain MT8148 adhesion to hydroxyapatite surfaces. Caries Res. 2006, 40, 403–411. [Google Scholar] [CrossRef]
- Marx, P.; Sang, Y.; Qin, H.; Wang, Q.; Guo, R.; Pfeifer, C.; Kreth, J.; Merritt, J. Environmental stress perception activates structural remodeling of extant Streptococcus mutans biofilms. NPJ Biofilms Microbiomes 2020, 6, 17. [Google Scholar] [CrossRef]
- Rainey, K.; Michalek, S.M.; Wen, Z.T.; Wu, H. Glycosyltransferase-Mediated Biofilm Matrix Dynamics and Virulence of Streptococcus mutans. Appl. Environ. Microbiol. 2019, 85, e02247-18. [Google Scholar] [CrossRef]
- Horev, B.; Klein, M.I.; Hwang, G.; Li, Y.; Kim, D.; Koo, H.; Benoit, D.S.W. pH-Activated nanoparticles for controlled topical delivery of farnesol to disrupt oral biofilm virulence. ACS Nano 2015, 9, 2390–2404. [Google Scholar] [CrossRef]
- Garcia, S.S.; Blackledge, M.S.; Michalek, S.; Su, L.; Ptacek, T.; Eipers, P.; Morrow, C.; Lefkowitz, E.J.; Melander, C.; Wu, H. Targeting of Streptococcus mutans Biofilms by a Novel Small Molecule Prevents Dental Caries and Preserves the Oral Microbiome. J. Dent. Res. 2017, 96, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M. Role of oral microbiota in cancer development. Microorganisms 2019, 7, 20. [Google Scholar] [CrossRef]
- Li, B.Z.; Zhou, H.Y.; Guo, B.; Chen, W.J.; Tao, J.H.; Cao, N.W.; Chu, X.J.; Meng, X.J. Dysbiosis of oral microbiota is associated with systemic lupus erythematosus. Arch. Oral Biol. 2020, 113, 104708. [Google Scholar] [CrossRef]
- Sureda, A.; Daglia, M.; Castilla, S.A.; Sanadgol, N.; Nabavi, S.F.; Khan, H.; Belwal, T.; Jeandet, P.; Marchese, A.; Pistollato, F.J.P.R. Oral microbiota and Alzheimer’s disease: Do all roads lead to Rome? Pharmacol. Res. 2020, 151, 104582. [Google Scholar] [CrossRef] [PubMed]
- Vedam, V.; Ganapathy, S.J. Association of oral microbiota with obesity in children: Insight from dental physicians. Hong Kong Med. J. 2020, 26, 354. [Google Scholar] [CrossRef]
- Bourgeois, D.; Inquimbert, C.; Ottolenghi, L.; Carrouel, F. Periodontal pathogens as risk factors of cardiovascular diseases, diabetes, rheumatoid arthritis, cancer, and chronic obstructive pulmonary disease-is there cause for consideration? Microorganisms 2019, 7, 424. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, Z.; Wang, J.; Su, X.; Yang, J.; Zhang, Q.; Zhang, L.J.E. The oral microbiome profile and biomarker in Chinese type 2 diabetes mellitus patients. Endocrine 2020, 68, 564–572. [Google Scholar] [CrossRef]
- Cobb, C.M.; Kelly, P.J.; Williams, K.B.; Babbar, S.; Angolkar, M.; Derman, R.J. The oral microbiome and adverse pregnancy outcomes. Int. J. Womens Health 2017, 9, 551–559. [Google Scholar] [CrossRef]
- Chenicheri, S.; Usha, R.; Ramachandran, R.; Thomas, V.; Wood, A. Insight into oral biofilm: Primary, secondary and residual caries and phyto-challenged solutions. Open Dent. J. 2017, 11, 312–333. [Google Scholar] [CrossRef] [PubMed]
| The Material Used for Biofilm Modulation | Targets | Mechanism | Reference |
|---|---|---|---|
| Dextran-coated iron oxide nanozymes; H2O2. | Acidogenic biofilm (bacterial killing and EPS-matrix breakdown). | Nanozymes catalytic H2O2 at acidic conditions. | Naha et al. [45] (2019) |
| Sonodynamic excitation of nanomicelle curcumin. | S. mutans (local therapy). | Curcumin activated by ultrasound waves irradiates and produces the ROS. | Pourhajibagher et al. [46] (2020) |
| Probiotic. | Composition of cariogenic biofilm. | Modification of inherent ADS activity; Production of antimicrobial agent (bacteriocin and hydrogen peroxide); Metabolism of lactic acid. | Chen et al. [47] (2020) |
| DMAEM and HMAEM [tertiary amine] | S. mutans biofilms; Microbial diversity of saliva-derived biofilms. | Materials have long-term reversible acid-activated properties that could quickly show an antibacterial effect via protonation. | Liang et al. [48] (2020) |
| TBO-mediated photodynamic therapy. | Cariogenic biofilms. | TBO can absorb light energy and catalyze the formation of ROS. | Balhaddad et al. [49] (2020) |
| Peptide GH12. | Acidogenic bacteria. | Net positive charge of GH12 increased and the tryptophan fluorescence intensity heightened with the peak shifting towards the short wavelength at pH 5.5, which demonstrated that GH12 could be more easily attracted to the anionic microbial cell membranes and that GH12 showed stronger interactions with the lipid membranes. | Jiang et al. [50] (2020) |
| Biosurfactant; Chitosan. | Cariogenic microorganisms. | The surfactant can associate strongly to the polymer, which generally leads to the occurrence of micellisation at lower concentrations of the tensioactive agent; Chitosan chain (NH3+) positive charges and the negatively charged cell wall and/or cytoplasm membrane of the microbial surface cause the breakdown of these structures and the loss of intracellular material. | Farias et al. [51] (2019) |
| Photodynamic inactivation employing methylene blue with irradiation from a red laser. | S. mutans biofilms. | High quantum yield (ΦΔ ≈ 0.5) and long absorption wavelength (λmax = 664 nm; red light), which allows better light penetration in live tissue. | Legéňová et al. [52] (2020) |
| Curcuma xanthorrhiza nanoemulsions. | S. mutans biofilms. | For nanoemulsions with nano-sized droplets stability can be maintained for a long period of time because their diffusion rate is higher than gravity settling or creaming due to Brownian motion; the antimicrobial activity is mainly attributed to the –OH group and the hydrocarbon chain of xanthorrhizol. | Cho et al. [53] (2020) |
| Bi-functional nanozyme. | Cariogenic biofilm microenvironment. | The nanohybrid contains glucose-oxidase that catalyzes glucose present in biofilms to increase intrinsic H2O2, which is converted by iron oxide nanoparticles with peroxidase-like activity into ROS in acidic pH. | Huang et al. [54] (2021) |
| Napabucasin. | Oral streptococcal biofilms. | Napabucasin exhibited good antimicrobial activity against oral streptococcal planktonic cultures and biofilms but with lessened cytotoxicity as compared to chlorhexidine. | Kuang et al. [55] (2020) |
| Propolis. | Dental plaque in the mouth of high caries risk children. | Propolis as a natural product has high bactericidal effect and low toxicity. | El-Allaky et al. [56] (2020) |
| Silver diamine fluoride. | Cariogenic bacteria isolated from human saliva. | Electrostatic adhesion of silver ions with bacterial enzymes inactivates them and prevents metabolic activities of the bacterial enzymes via silver-induced protein coagulation; fluoride inhibits demineralization by being absorbed onto the hydroxyapatite crystals and are resilient to a repeated acid attack; silver and fluoride shows synergistic effects. | Sorkhdini et al. [57] (2020) |
| Chlorophyllin-phycocyanin mixture. | S. mutans biofilms. | The decrease in metabolic activity can be due to the 8-fold to 10-fold increase in the production of ROS in the photodynamic process that by reducing the membrane potential and intracellular adenosine triphosphate affects cell membranes. | Afrasiabi et al. [58] (2020) |
| Allium sativum bulb. | Cariogenic biofilm. | Allicin showed high antibacterial activity against the cariogenic bacteria due to protease inhibiting ability. | Bin et al. [59] (2020) |
| Psidium sp., Mangifera sp., Mentha sp., and its mixture, | Cell-surface hydrophobicity; initial pH change in the oral biofilm; S. mutans adhesins. | The phenolic content of the plant extracts may interfere with the adhesion of bacterial cells in the experimental pellicle; the plant extracts create a balance between the two bacterial species. | Shafiei et al. [60] (2020) |
| Zein nanoparticles containing anacardic acid. | S. mutans biofilms. | The activity of the inhibit bacterial proliferation of anacardic acid was associated with the ability to permeate the lipid bilayer of cell membranes and causes its disruption; nanoparticles from corn protein zein that are biodegradable and have a relatively low cost provides anacardic acid stabilization and enhanced its esthetic characteristics. | Lima et al. [61] (2020) |
| Antimicrobial peptides derived from eutericin 6 and gassericin A. | S. mutans biofilms. | Selective membrane disruption. | Liang et al. [62] (2020) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Daliri, E.B.-M.; Tyagi, A.; Oh, D.-H. Cariogenic Biofilm: Pathology-Related Phenotypes and Targeted Therapy. Microorganisms 2021, 9, 1311. https://doi.org/10.3390/microorganisms9061311
Chen X, Daliri EB-M, Tyagi A, Oh D-H. Cariogenic Biofilm: Pathology-Related Phenotypes and Targeted Therapy. Microorganisms. 2021; 9(6):1311. https://doi.org/10.3390/microorganisms9061311
Chicago/Turabian StyleChen, Xiuqin, Eric Banan-Mwine Daliri, Akanksha Tyagi, and Deog-Hwan Oh. 2021. "Cariogenic Biofilm: Pathology-Related Phenotypes and Targeted Therapy" Microorganisms 9, no. 6: 1311. https://doi.org/10.3390/microorganisms9061311
APA StyleChen, X., Daliri, E. B.-M., Tyagi, A., & Oh, D.-H. (2021). Cariogenic Biofilm: Pathology-Related Phenotypes and Targeted Therapy. Microorganisms, 9(6), 1311. https://doi.org/10.3390/microorganisms9061311








