Replication Variance of African and Asian Lineage Zika Virus Strains in Different Cell Lines, Mosquitoes and Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Viruses
2.3. Mosquito Infection
2.4. Mice
2.5. Real-Time RT-qPCR
2.6. Focus Forming Assay
2.7. Cell Infection
2.8. Ethics Statement
2.9. Statistical Analysis
3. Results
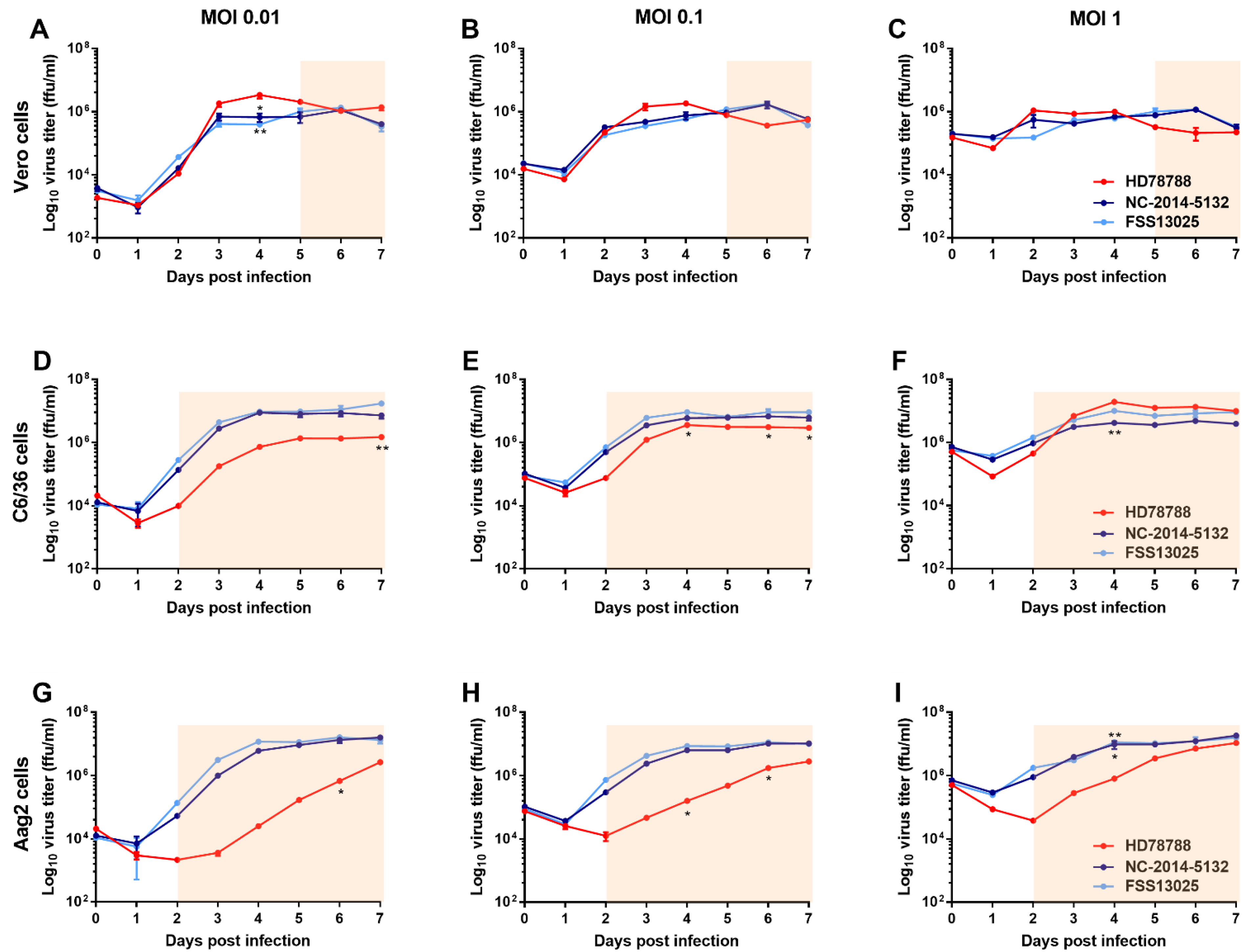
3.1. ZIKV Growth Kinetic in Mammalian and Mosquito Cell Lines
3.2. Vector Competence of Cambodian Ae. aegypti Mosquitoes
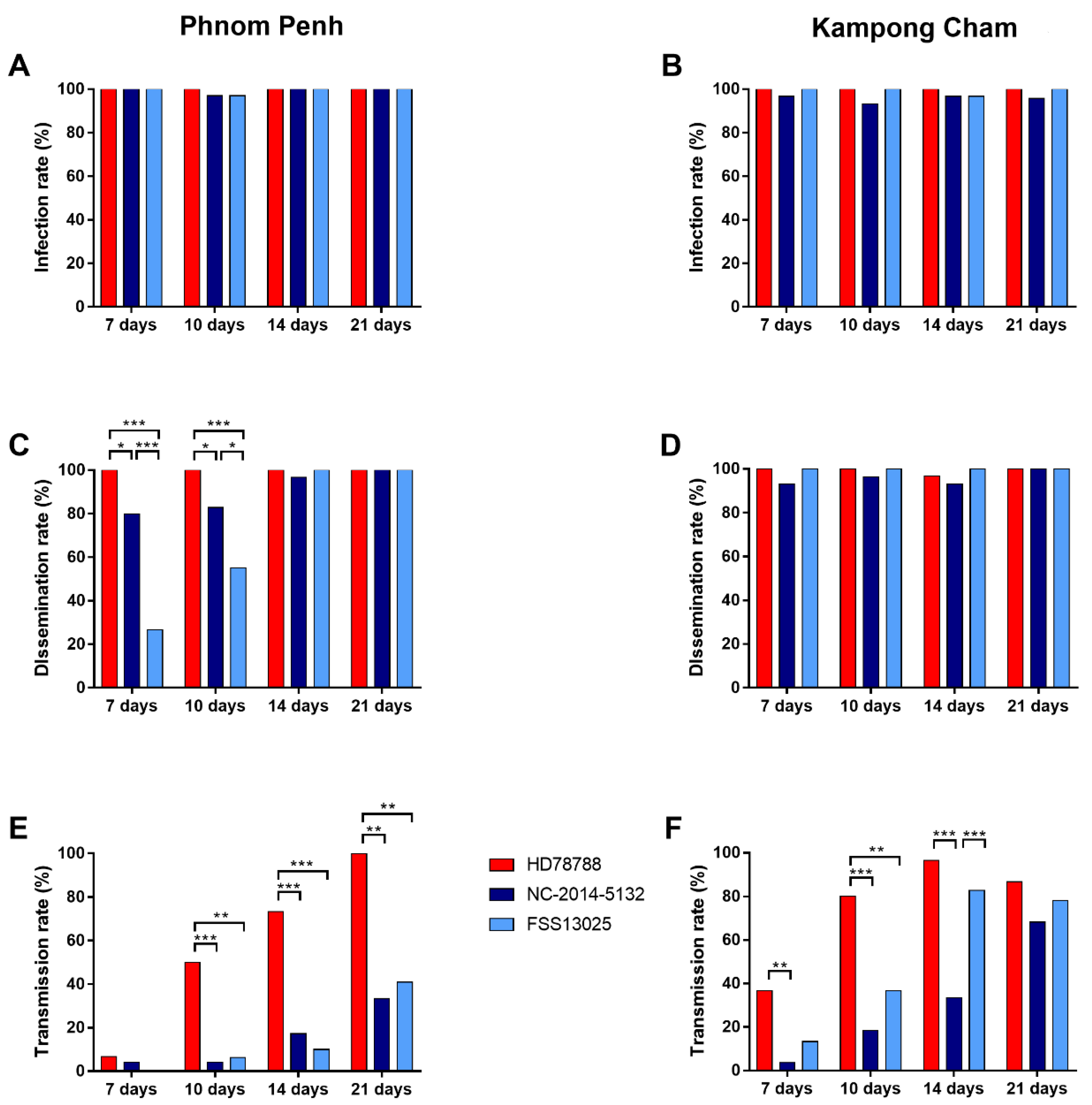
3.2.1. ZIKV Infection Rate
3.2.2. ZIKV Dissemination Rate
3.2.3. ZIKV Transmission Rate
3.2.4. ZIKV Transmission Efficiency
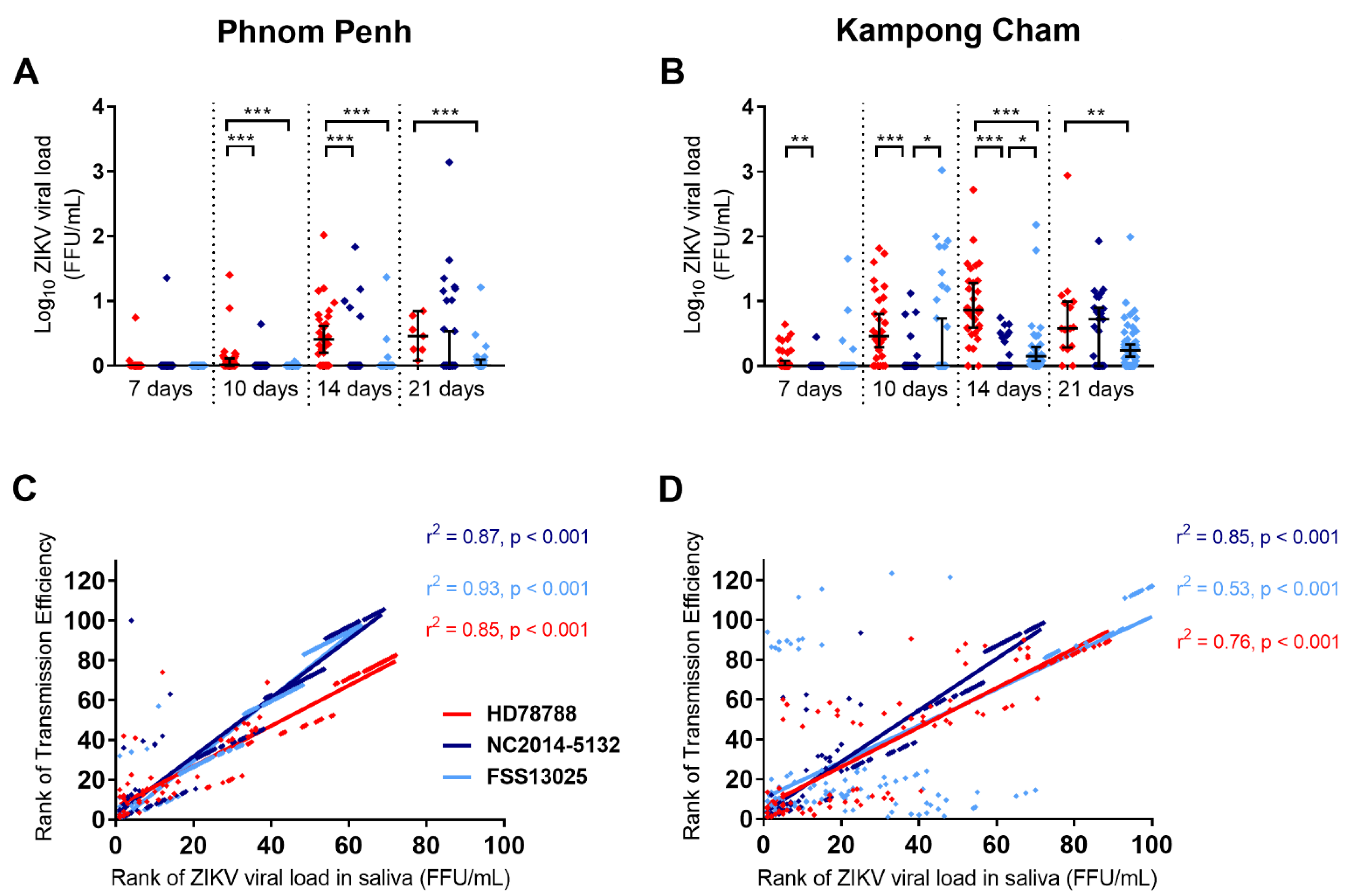
3.3. ZIKV Viral Load in Infected Mosquitoes
3.4. Survival Rate of ZIKV-Infected Mosquitoes
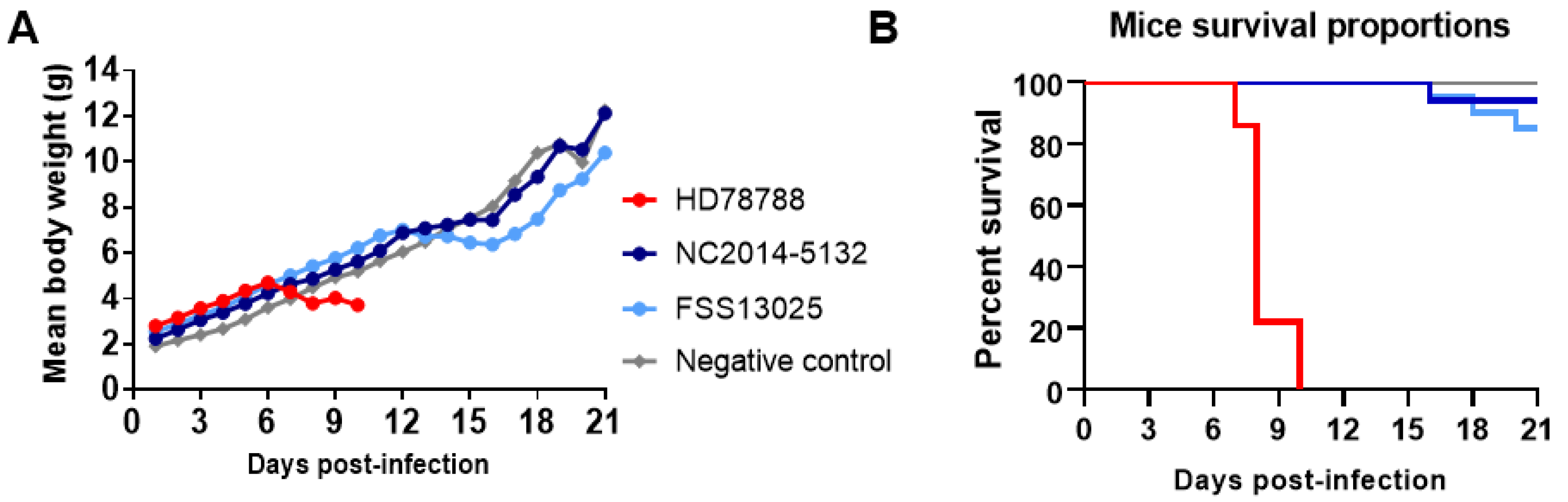
3.5. ZIKV Replication in Neonatal Swiss Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dick, G.W.A.; Kitchen, S.F.; Haddow, A.J. Zika Virus (I). Isolations and Serological Specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Marchette, N.J.; Garcia, R.; Rudnick, A. Isolation of Zika Virus from Aedes Aegypti Mosquitoes in Malaysia. Am. J. Trop. Med. Hyg. 1969, 18, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.G.; Ksiazek, T.G. Suhandiman; Triwibowo Zika Virus, a Cause of Fever in Central Java, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 1981, 75, 389–393. [Google Scholar] [CrossRef]
- Duong, V.; Dussart, P.; Buchy, P. Zika Virus in Asia. Int. J. Infect. Dis. 2017, 54, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.R.; Chen, T.-H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef]
- Heang, V.; Yasuda, C.Y.; Sovann, L.; Haddow, A.D.; Travassos da Rosa, A.P.; Tesh, R.B.; Kasper, M.R. Zika Virus Infection, Cambodia, 2010. Emerg. Infect. Dis. 2012, 18, 349–351. [Google Scholar] [CrossRef]
- Sanchez, J.D. PAHO/WHO | Regional Zika Epidemiological Update (Americas). 25 August 2017. Available online: https://www.paho.org/hq/index.php?option=com_content&view=article&id=11599:regional-zika-epidemiological-update-americas&Itemid=41691&lang=en (accessed on 11 October 2019).
- Pettersson, J.H.-O.; Bohlin, J.; Dupont-Rouzeyrol, M.; Brynildsrud, O.B.; Alfsnes, K.; Cao-Lormeau, V.-M.; Gaunt, M.W.; Falconar, A.K.; de Lamballerie, X.; Eldholm, V.; et al. Re-Visiting the Evolution, Dispersal and Epidemiology of Zika Virus in Asia. Emerg. Microbes Infect. 2018, 7, 79. [Google Scholar] [CrossRef]
- Wongsurawat, T.; Athipanyasilp, N.; Jenjaroenpun, P.; Jun, S.-R.; Kaewnapan, B.; Wassenaar, T.M.; Leelahakorn, N.; Angkasekwinai, N.; Kantakamalakul, W.; Ussery, D.W.; et al. Case of Microcephaly after Congenital Infection with Asian Lineage Zika Virus, Thailand. Emerg. Infect. Dis. J. 2018, 24, 1758–1761. [Google Scholar] [CrossRef]
- Boyer, S.; Calvez, E.; Chouin-Carneiro, T.; Diallo, D.; Failloux, A.-B. An Overview of Mosquito Vectors of Zika Virus. Microbes Infect. 2018, 20, 646–660. [Google Scholar] [CrossRef]
- Weger-Lucarelli, J.; Rückert, C.; Chotiwan, N.; Nguyen, C.; Luna, S.M.G.; Fauver, J.R.; Foy, B.D.; Perera, R.; Black, W.C.; Kading, R.C.; et al. Vector Competence of American Mosquitoes for Three Strains of Zika Virus. PLoS Negl. Trop. Dis. 2016, 10, e0005101. [Google Scholar] [CrossRef]
- Roundy, C.M.; Azar, S.R.; Rossi, S.L.; Huang, J.H.; Leal, G.; Yun, R.; Fernandez-Salas, I.; Vitek, C.J.; Paploski, I.A.D.; Kitron, U.; et al. Variation in Aedes Aegypti Mosquito Competence for Zika Virus Transmission. Emerg. Infect. Dis. 2017, 23, 625–632. [Google Scholar] [CrossRef]
- Ciota, A.T.; Bialosuknia, S.M.; Zink, S.D.; Brecher, M.; Ehrbar, D.J.; Morrissette, M.N.; Kramer, L.D. Effects of Zika Virus Strain and Aedes Mosquito Species on Vector Competence. Emerg. Infect. Dis. 2017, 23, 1110–1117. [Google Scholar] [CrossRef]
- Pena, L.J.; Miranda Guarines, K.; Duarte Silva, A.J.; Sales Leal, L.R.; Mendes Félix, D.; Silva, A.; de Oliveira, S.A.; Junqueira Ayres, C.F.; Júnior, A.S.; de Freitas, A.C. In Vitro and in Vivo Models for Studying Zika Virus Biology. J. Gen. Virol. 2018, 99, 1529–1550. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.P.; Nagamine, C.M. Animal Models of Zika Virus. Comp. Med. 2017, 67, 242–252. [Google Scholar] [PubMed]
- Duong, V.; Ong, S.; Leang, R.; Huy, R.; Ly, S.; Mounier, U.; Ou, T.; In, S.; Peng, B.; Ken, S.; et al. Low Circulation of Zika Virus, Cambodia, 2007–2016. Emerg. Infect. Dis. 2017, 23, 296–299. [Google Scholar] [CrossRef]
- Huy, R.; Buchy, P.; Conan, A.; Ngan, C.; Ong, S.; Ali, R.; Duong, V.; Yit, S.; Ung, S.; Te, V.; et al. National Dengue Surveillance in Cambodia 1980–2008: Epidemiological and Virological Trends and the Impact of Vector Control. Bull. World Health Organ. 2010, 88, 650–657. [Google Scholar] [CrossRef]
- Faye, O.; Freire, C.C.M.; Iamarino, A.; Faye, O.; de Oliveira, J.V.C.; Diallo, M.; Zanotto, P.M.A.; Sall, A.A. Molecular Evolution of Zika Virus during Its Emergence in the 20th Century. PLoS Negl. Trop. Dis. 2014, 8, e2636. [Google Scholar] [CrossRef] [PubMed]
- Stojanovich, C.J.; Scott, H.G.; Stojanovich, C.J.; Communicable Disease Center (U.S.) ; United States Army; Special Epidemiologic Team. Illustrated Key to Aedes Mosquitoes of Vietnam; U.S. Department of Health, Education, and Welfare, Public Health Service, Communicable Disease Center: Atlanta, GA, USA, 1965.
- Reuben, R.; Hiriyan, J.; Akiyama, J. Illustrated Keys to Species of Culex (Culex) Associated with Japanese Encephalitis in Southeast Asia (Diptera: Culicidae). Mosq. Syst. 1994, 26, 75–96. [Google Scholar]
- Rattanarithikul, R.; Harbach, R.; Harrison, B.; Panthusiri, P.; Jones, J.; Coleman, R. Illustrated Keys to the Mosquitoes of Thailand. II. Genera Culex and Lutzia. Southeast Asian J. Trop. Med. Public Health 2005, 36, 1–97. [Google Scholar]
- Kumar, M.; Kumar, P. Euthanasia Procedure Used in Experimental Laboratory. In Animal Models of Neurological Disorders: Principle and Working Procedure for Animal Models of Neurological Disorders; Springer: Singapore, 2017; pp. 307–314. ISBN 978-981-10-5980-3. [Google Scholar]
- Ou, T.P.; Yun, C.; Auerswald, H.; In, S.; Leang, R.; Huy, R.; Choeung, R.; Dussart, P.; Duong, V. Improved Detection of Dengue and Zika Viruses Using Multiplex RT-QPCR Assays. J. Virol. Methods 2020, 282, 113862. [Google Scholar] [CrossRef] [PubMed]
- OIE-World Organisation for Animal Health. Available online: https://www.oie.int/index.php?id=169&L=0&htmfile=chapitre_aw_research_education.htm (accessed on 27 January 2020).
- Kay, B.H.; Prakash, G.; Andre, R.G. Aedes Albopictus and Other Aedes (Stegomyia) Species in Fiji. J. Am. Mosq. Control. Assoc. 1995, 11, 230–234. [Google Scholar]
- Vicenti, I.; Boccuto, A.; Giannini, A.; Dragoni, F.; Saladini, F.; Zazzi, M. Comparative Analysis of Different Cell Systems for Zika Virus (ZIKV) Propagation and Evaluation of Anti-ZIKV Compounds in Vitro. Virus Res. 2018, 244, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Fukasawa, M.; Shirasago, Y.; Suzuki, R.; Osada, N.; Yamaji, T.; Wakita, T.; Konishi, E.; Hanada, K. Comparative Characterization of Flavivirus Production in Two Cell Lines: Human Hepatoma-Derived Huh7.5.1-8 and African Green Monkey Kidney-Derived Vero. PLoS ONE 2020, 15, e0232274. [Google Scholar] [CrossRef]
- Haddow, A.D.; Schuh, A.J.; Yasuda, C.Y.; Kasper, M.R.; Heang, V.; Huy, R.; Guzman, H.; Tesh, R.B.; Weaver, S.C. Genetic Characterization of Zika Virus Strains: Geographic Expansion of the Asian Lineage. PLoS Negl. Trop. Dis. 2012, 6, e1477. [Google Scholar] [CrossRef] [PubMed]
- Anfasa, F.; Siegers, J.Y.; van der Kroeg, M.; Mumtaz, N.; Stalin Raj, V.; de Vrij, F.M.S.; Widagdo, W.; Gabriel, G.; Salinas, S.; Simonin, Y.; et al. Phenotypic Differences between Asian and African Lineage Zika Viruses in Human Neural Progenitor Cells. mSphere 2017, 2, e00292-17. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.R.; Quicke, K.M.; Maddur, M.S.; O’Neal, J.T.; McDonald, C.E.; Fedorova, N.B.; Puri, V.; Shabman, R.S.; Pulendran, B.; Suthar, M.S. Zika Virus Antagonizes Type I Interferon Responses during Infection of Human Dendritic Cells. PLoS Pathog. 2017, 13, e1006164. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, E.; Ramani, A.; Karow, U.; Gottardo, M.; Natarajan, K.; Gooi, L.M.; Goranci-Buzhala, G.; Krut, O.; Peters, F.; Nikolic, M.; et al. Recent Zika Virus Isolates Induce Premature Differentiation of Neural Progenitors in Human Brain Organoids. Cell Stem Cell 2017, 20, 397–406.e5. [Google Scholar] [CrossRef]
- Sheridan, M.A.; Yunusov, D.; Balaraman, V.; Alexenko, A.P.; Yabe, S.; Verjovski-Almeida, S.; Schust, D.J.; Franz, A.W.; Sadovsky, Y.; Ezashi, T.; et al. Vulnerability of Primitive Human Placental Trophoblast to Zika Virus. Proc. Natl. Acad. Sci. USA 2017, 114, e1587–e1596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Hammack, C.; Ogden, S.C.; Cheng, Y.; Lee, E.M.; Wen, Z.; Qian, X.; Nguyen, H.N.; Li, Y.; Yao, B.; et al. Molecular Signatures Associated with ZIKV Exposure in Human Cortical Neural Progenitors. Nucleic Acids Res. 2016, 44, 8610–8620. [Google Scholar] [CrossRef]
- McGrath, E.L.; Rossi, S.L.; Gao, J.; Widen, S.G.; Grant, A.C.; Dunn, T.J.; Azar, S.R.; Roundy, C.M.; Xiong, Y.; Prusak, D.J.; et al. Differential Responses of Human Fetal Brain Neural Stem Cells to Zika Virus Infection. Stem Cell Rep. 2017, 8, 715–727. [Google Scholar] [CrossRef]
- Weaver, S.C.; Costa, F.; Garcia-Blanco, M.A.; Ko, A.I.; Ribeiro, G.S.; Saade, G.; Shi, P.-Y.; Vasilakis, N. Zika Virus: History, Emergence, Biology, and Prospects for Control. Antivir. Res. 2016, 130, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; DeLalio, L.J.; Isakson, B.E.; Wang, T.T. AXL-Mediated Productive Infection of Human Endothelial Cells by Zika Virus. Circ. Res. 2016, 119, 1183–1189. [Google Scholar] [CrossRef]
- Cugola, F.R.; Fernandes, I.R.; Russo, F.B.; Freitas, B.C.; Dias, J.L.M.; Guimarães, K.P.; Benazzato, C.; Almeida, N.; Pignatari, G.C.; Romero, S.; et al. The Brazilian Zika Virus Strain Causes Birth Defects in Experimental Models. Nature 2016, 534, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Moghadas, S.M.; Shoukat, A.; Espindola, A.L.; Pereira, R.S.; Abdirizak, F.; Laskowski, M.; Viboud, C.; Chowell, G. Asymptomatic Transmission and the Dynamics of Zika Infection. Sci. Rep. 2017, 7, 51–829. [Google Scholar] [CrossRef] [PubMed]
- Heinz, F.X.; Stiasny, K. The Antigenic Structure of Zika Virus and Its Relation to Other Flaviviruses: Implications for Infection and Immunoprophylaxis. Microbiol. Mol. Biol. Rev. 2017, 81, e00055-16. [Google Scholar] [CrossRef] [PubMed]
- Calvez, E.; O’Connor, O.; Pol, M.; Rousset, D.; Faye, O.; Richard, V.; Tarantola, A.; Dupont-Rouzeyrol, M. Differential Transmission of Asian and African Zika Virus Lineages by Aedes Aegypti from New Caledonia. Emerg. Microbes Infect. 2018, 7, 159. [Google Scholar] [CrossRef]
- Franz, A.W.E.; Kantor, A.M.; Passarelli, A.L.; Clem, R.J. Tissue Barriers to Arbovirus Infection in Mosquitoes. Viruses 2015, 7, 3741–3767. [Google Scholar] [CrossRef]
- Blair, C.D. Mosquito RNAi Is the Major Innate Immune Pathway Controlling Arbovirus Infection and Transmission. Future Microbiol. 2011, 6, 265–277. [Google Scholar] [CrossRef]
- Sim, S.; Jupatanakul, N.; Dimopoulos, G. Mosquito Immunity against Arboviruses. Viruses 2014, 6, 4479–4504. [Google Scholar] [CrossRef]
- Pongsiri, A.; Ponlawat, A.; Thaisomboonsuk, B.; Jarman, R.G.; Scott, T.W.; Lambrechts, L. Differential Susceptibility of Two Field Aedes Aegypti Populations to a Low Infectious Dose of Dengue Virus. PLoS ONE 2014, 9, e92971. [Google Scholar] [CrossRef]
- Fernandes, N.C.C.A.; Nogueira, J.S.; Réssio, R.A.; Cirqueira, C.S.; Kimura, L.M.; Fernandes, K.R.; Cunha, M.S.; Souza, R.P.; Guerra, J.M. Experimental Zika Virus Infection Induces Spinal Cord Injury and Encephalitis in Newborn Swiss Mice. Exp. Toxicol. Pathol. 2017, 69, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Aliota, M.T.; Caine, E.A.; Walker, E.C.; Larkin, K.E.; Camacho, E.; Osorio, J.E. Characterization of Lethal Zika Virus Infection in AG129 Mice. PLoS Negl. Trop. Dis. 2016, 10, e0004682. [Google Scholar] [CrossRef] [PubMed]
- Simonin, Y.; Loustalot, F.; Desmetz, C.; Foulongne, V.; Constant, O.; Fournier-Wirth, C.; Leon, F.; Molès, J.-P.; Goubaud, A.; Lemaitre, J.-M.; et al. Zika Virus Strains Potentially Display Different Infectious Profiles in Human Neural Cells. EBioMedicine 2016, 12, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Udenze, D.; Trus, I.; Berube, N.; Gerdts, V.; Karniychuk, U. The African Strain of Zika Virus Causes More Severe in Utero Infection than Asian Strain in a Porcine Fetal Transmission Model. Emerg. Microbes Infect. 2019, 8, 1098–1107. [Google Scholar] [CrossRef]
- Gorman, M.J.; Caine, E.A.; Zaitsev, K.; Begley, M.C.; Weger-Lucarelli, J.; Uccellini, M.B.; Tripathi, S.; Morrison, J.; Yount, B.L.; Dinnon, K.H.; et al. An Immunocompetent Mouse Model of Zika Virus Infection. Cell Host. Microbe 2018, 23, 672–685. [Google Scholar] [CrossRef]
- Hu, T.; Li, J.; Carr, M.J.; Duchêne, S.; Shi, W. The Asian Lineage of Zika Virus: Transmission and Evolution in Asia and the Americas. Virol Sin 2019, 34, 1–8. [Google Scholar] [CrossRef]
- Pompon, J.; Morales-Vargas, R.; Manuel, M.; Huat Tan, C.; Vial, T.; Hao Tan, J.; Sessions, O.M.; da Vasconcelos, P.C.; Ng, L.C.; Missé, D.A. Zika Virus from America Is More Efficiently Transmitted than an Asian Virus by Aedes Aegypti Mosquitoes from Asia. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
| Days after Infection | Mosquito Population (Origin) | HD78788 | NC-2014-5132 | FSS13025 | |||
|---|---|---|---|---|---|---|---|
| Transmission Efficiency * | p Value # | Transmission Efficiency * | p Value # | Transmission Efficiency * | p Value # | ||
| 7 | urban-PP | 6.7% (2/30) | 0.0102 | 3.3% (1/30) | 1.000 | 0% (0/30) | 0.1124 |
| rural-KC | 36.7% (11/30) | 3.3% (1/30) | 13.3% (4/30) | ||||
| 10 | urban-PP | 50.0% (15/30) | 0.0292 | 3.3% (1/30) | 0.1945 | 3.3% (1/30) | 0.0025 |
| rural-KC | 80.0% (24/30) | 16.7% (5/30) | 36.7% (11/30) | ||||
| 14 | urban-PP | 73.3% (22/30) | 0.0797 | 16.7% (5/30) | 0.3604 | 10.0% (3/30) | <0.0001 |
| rural-KC | 93.3% (28/30) | 30.0% (9/30) | 80.0% (24/30) | ||||
| 21 | urban-PP | 100% (7/7) | 1.000 | 33.3% (10/30) | 0.0283 | 40.9% (9/22) | 0.0031 |
| rural-KC | 86.7% (13/15) | 68.2% (15/23) | 78.0% (39/50) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, T.P.; Auerswald, H.; In, S.; Peng, B.; Pang, S.; Boyer, S.; Choeung, R.; Dupont-Rouzeyrol, M.; Dussart, P.; Duong, V. Replication Variance of African and Asian Lineage Zika Virus Strains in Different Cell Lines, Mosquitoes and Mice. Microorganisms 2021, 9, 1250. https://doi.org/10.3390/microorganisms9061250
Ou TP, Auerswald H, In S, Peng B, Pang S, Boyer S, Choeung R, Dupont-Rouzeyrol M, Dussart P, Duong V. Replication Variance of African and Asian Lineage Zika Virus Strains in Different Cell Lines, Mosquitoes and Mice. Microorganisms. 2021; 9(6):1250. https://doi.org/10.3390/microorganisms9061250
Chicago/Turabian StyleOu, Tey Putita, Heidi Auerswald, Saraden In, Borin Peng, Senglong Pang, Sébastien Boyer, Rithy Choeung, Myrielle Dupont-Rouzeyrol, Philippe Dussart, and Veasna Duong. 2021. "Replication Variance of African and Asian Lineage Zika Virus Strains in Different Cell Lines, Mosquitoes and Mice" Microorganisms 9, no. 6: 1250. https://doi.org/10.3390/microorganisms9061250
APA StyleOu, T. P., Auerswald, H., In, S., Peng, B., Pang, S., Boyer, S., Choeung, R., Dupont-Rouzeyrol, M., Dussart, P., & Duong, V. (2021). Replication Variance of African and Asian Lineage Zika Virus Strains in Different Cell Lines, Mosquitoes and Mice. Microorganisms, 9(6), 1250. https://doi.org/10.3390/microorganisms9061250






