Infective Endocarditis: A Focus on Oral Microbiota
Abstract
1. Introduction
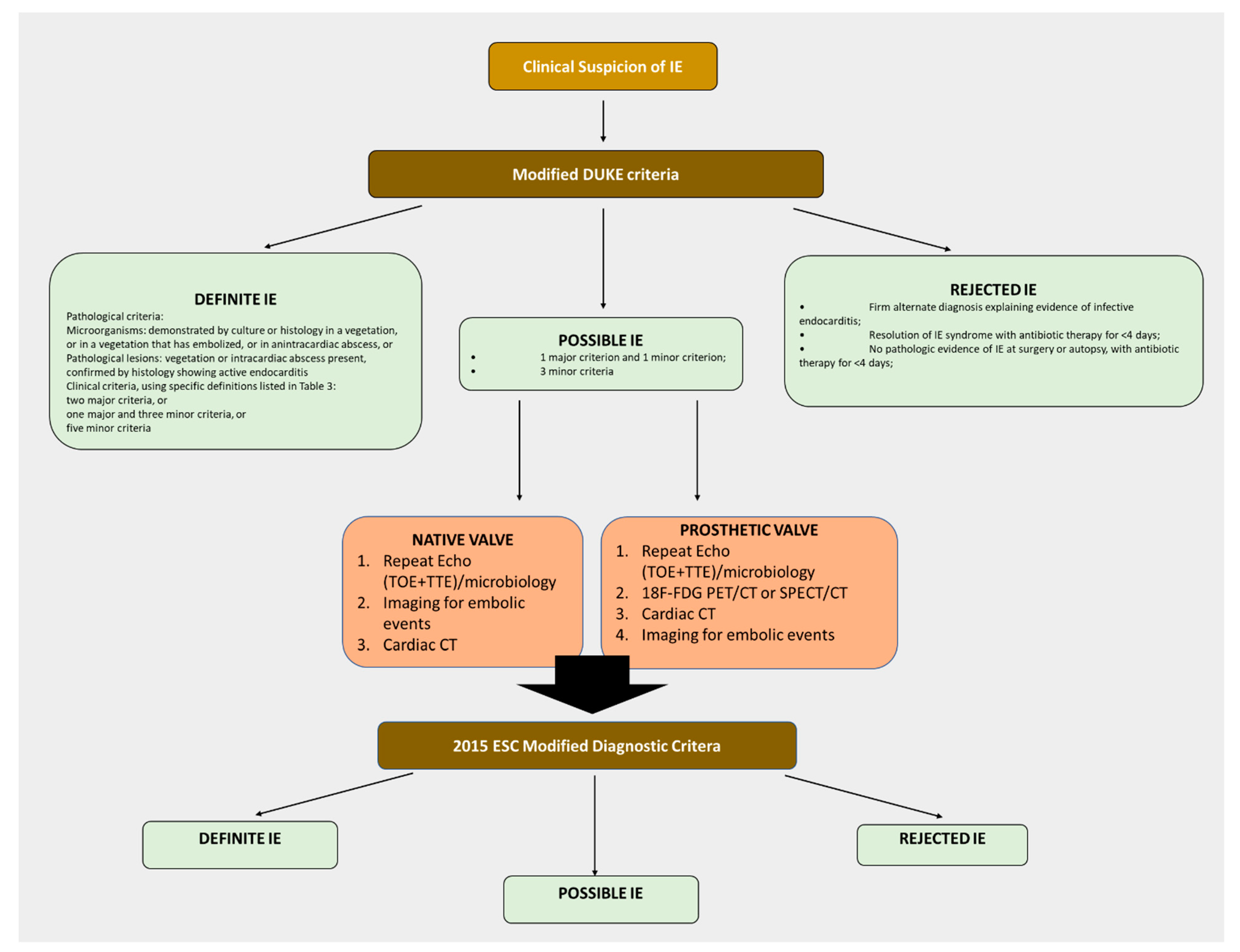
2. Infective Endocarditis: Epidemiology, Diagnosis, and Pathogenesis
3. Oral Dysbiosis
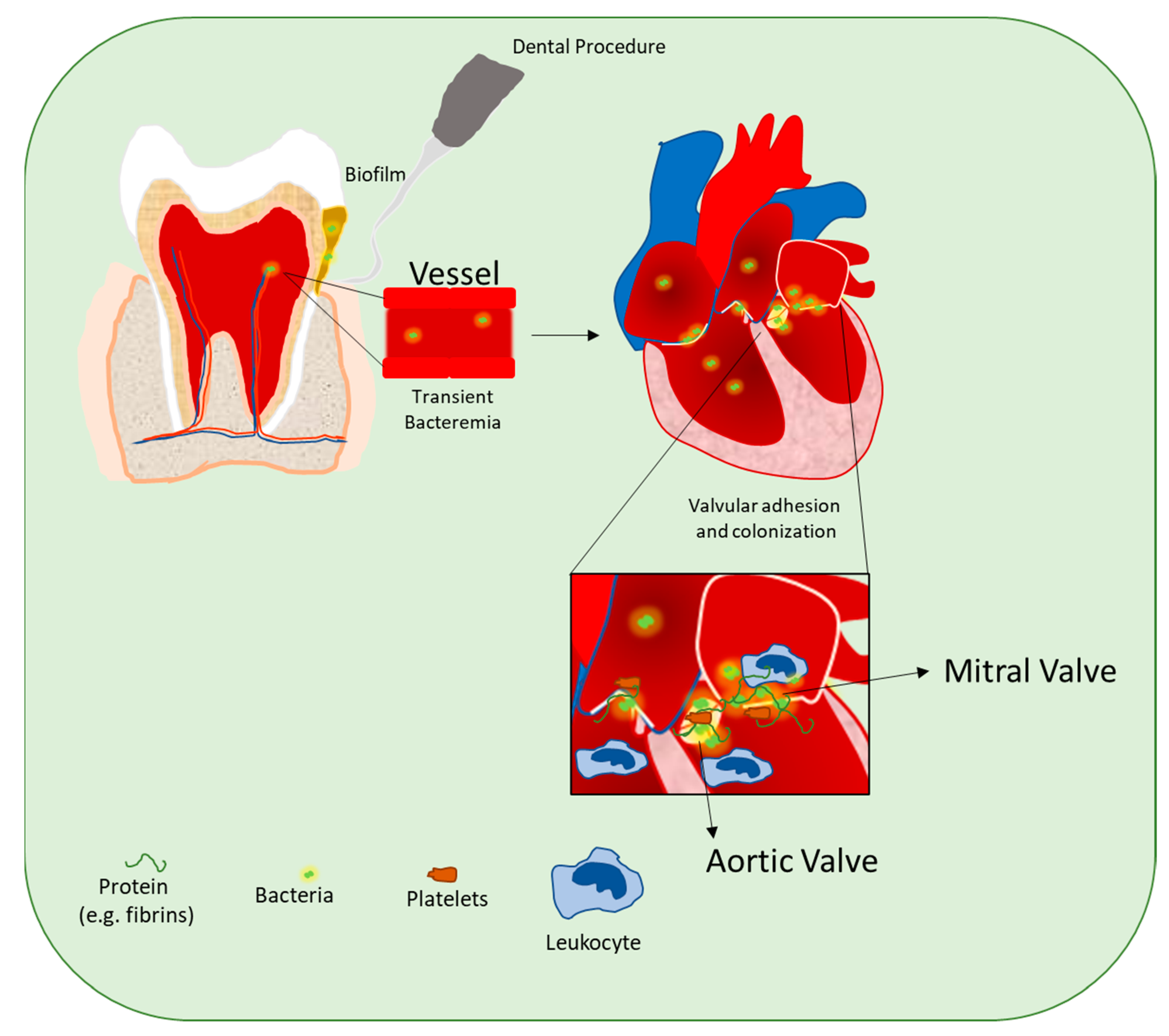
4. Oral Microbiota and the Pathogenesis of Infective Endocarditis (IE)
- Staphylococcus aureus (S. aureus): S. aureus is a Gram-positive bacterium and the most predominant pathogen causing IE throughout the world [88,89,90]. S. aureus is found in the environment and normal human flora of the skin and mucous membranes like the nasal area [91]. Moreover, several reports have identified this bacterium as a transient component of the oral microbiota and involved in the pathogenesis of periodontitis. This microorganism does not typically cause infection on healthy tissues; however, it may cause various potentially serious illnesses if it enters the bloodstream [91]. Notably, S. aureus can interact with platelets, inducing their aggregation with the thrombotic vegetation’s proliferation [92,93]. Infections are common in community- and hospital-acquired settings and treatment remains challenging to manage due to the emergence of drug resistant strains such as MRSA [94,95]. For instance, Garcia et al. recently demonstrated that MRSA can be isolated from human periodontal lesions. Importantly, these authors observed that these bacteria express high levels of virulence genes that complicate the successful treatment and resolution of periodontitis [96]. Interestingly, as recently supported by Liesenborghs et al. [97], developing a vaccine to prevent S. aureus IE might be more complicated than previously thought. These authors reported two distinct mechanisms that predispose cardiac valve to S. aureus adhesion and infection. In this regard, they demonstrated that most S. aureus vaccines are obsolete since they target factors like Clumping factor A (ClfA), which is not always involved in adhesion mechanisms of this pathogen. In particular, these authors used a murine model of IE and demonstrated that S. aureus, through the adhesins ClfA and von Willebrand factor (vWF)-binding protein can bind to local fibrin and vWF on the injured valve. In contrast, upon cardiac valve inflammation that predominates in subjects with structurally normal heart valves but who develop IE, extensive endothelial activation induces vWF release. The endothelial cell-bound vWF recruits platelets that in turn, are used as a bridge by S. aureus. Besides, S. aureus itself can induce inflammation and endothelial cell activation by releasing toxins, facilitating its adhesion.
- Streptococcus sanguis or sanguinis (S. sanguinis): S. sanguinis is a Gram-positive non-spore-forming facultative anaerobe with a singular status in the history of the study of IE. Indeed, it was first isolated in 1946 by White and colleagues [98] from the blood of a patient with IE. Subsequently, in 1948 Alture-Werber and Loewe [99] demonstrated that antibiotic prophylaxis prevented S. sanguinis re-infection and recurrence of IE in 56 patients. To date, S. sanguinis has been recognized as one of the top three causal agents of IE, together with staphylococci and enterococci [68]. S. sanguinis is a pioneering colonizer and commensal bacterium that plays an essential role in the establishment of the oral biofilm. However, once it invades the bloodstream, this bacterium adheres to circulating platelets or to submucosal proteins such as collagen at the site of valve damage [30,100,101]. Although many factors may contribute to its pathogenicity, the platelet aggregation-associated protein (PAAP) of S. sanguinis is one of the first bacterial glycoprotein identified and has shown to contribute directly to experimental IE development [102,103]. Importantly, PAAP binding to the platelet α2β1 integrin induces platelet aggregation and activation with subsequent fibrinogen production and clotting factors V and VII. Activated platelets release dense and alpha granules, which, combined with thromboxane production, play a role in the later aggregation response. Alpha granules include platelet microbicidal proteins (PMP) that kill bacteria; they also induce fibrinogen production and clotting factors V and VII. The latter activates thrombin, which starts fibrinogen’s polymerization to fibrin [103]. The resulting fibrin-platelet network grows up in mass as cells colonize it and expand layer upon layer of vegetation [104]. Recently, Martini and colleagues [101] identified two novel virulence factors in S. sanguinis as essential in IE’s pathogenesis. These authors demonstrated that mutants for the SSA_1099 gene, which encodes for repeat-in-toxin (RTX) proteins, allowing adhesion to the platelets and mur2 encoding a peptidoglycan hydrolase, produced either no cardiac vegetation or vegetations of small size.
- Enterococcus faecalis (E. faecalis): In 1906 Andrews and Horder first described an association between Streptococcus faecalis infection and the presence of “malignant endocarditis” [105]. However, it is well-recognized that these bacteria represent the third most common cause of IE, following streptococci and S. aureus. Enterococci are Gram-positive cocci in the gastrointestinal tract and the vagina in humans [106]. Moreover, as reported by Souto et al., these microorganisms also inhabit the oral cavity of healthy subjects in a percentage between 14 and 17% [107]. Notably, under a pathological condition such as periodontitis, the levels of E. faecalis are significantly increased to 40–50% in the saliva and subgingival tissue. Thus, it is plausible that bacteremia due to E. faecalis is a significant risk factor for IE. Indeed, in a study by Dahl and colleagues [108], it has been shown that in patients with E. faecalis bacteremia, a high IE prevalence of 26% can be observed. At the molecular level, enterococcal endocarditis involves the establishment of a biofilm and vegetations on heart valves. Several adhesins or proteins known to function in biofilm formation have been identified as major contributors to E. faecalis endocarditis virulence like gelatinase [109], the protease Eep [110], the Ebp pili [111], the aggregation substance [112,113], and Ace [114].
- Actinobacillus actinomycetemcomitans (A. actinomycetemcomitans): Among the HACEK group bacteria, A. actinomycetemcomitans is the organism involved most in IE [115]. Klinger first described this small Gram-negative coccobacillus in 1912 [116]. However, it was only in 1953 that Vallée and Gaillard [117] mentioned the isolation of this microorganism in patients’ blood cultures with IE. Subsequently, in line with this report, in 1964 Mitchell and Gillepsie identified IE’s first case caused by A. actinomycetemcomitans [118]. A. actinomycetemcomitans is a constituent of the oral microbiota (it frequently colonizes the oropharynx), and its pathogenic role in periodontitis is well established [119]. Importantly, this pathogen presents fimbrial and nonfimbrial adhesins (Aae) [120] and Omp100 (ApiA) [121], that are crucially involved in the initial recognition of the host tissue. Moreover, A. actinomycetemcomitans via the extracellular matrix (ECM) adhesin A (EmaA) binds to acid-solubilized type I, III, and V collagen in vitro [122], the most important collagen isoform present in the periodontium [123], arteries [124], and cardiac valves [125].
- Porphyromonas Gingivalis (P. Gingivalis): Periodontitis is mostly caused by bacteria of the “Red complex” such as P. gingivalis, Prevotella Intermedia, and Tannerella forsythia. Among these, P. gingivalis is the most prominent and frequently observed (it has been found in 85.75% of subgingival plaque samples from patients with periodontitis) [126] Importantly, this periopathogen has been isolated in several non-oral tissues and organs including the aorta [127]. For this reason, infection of P. gingivalis is considered a high-risk event for IE. In this regard, in a recent case report, Isoshima and colleagues reported in a patient with severe periodontitis and IE a remarkably high IgG titer against P. gingivalis [85]. In addition, the infection by P. gingivalis was confirmed by PCR performed on DNA extracted by cardiac valve specimens. In line with this report, Oliveira and coworkers observed P. gingivalis DNA, even at low levels, in valve tissue and oral samples of patients undergoing cardiac valve replacement [128]. However, since PCR cannot distinguish live from dead bacteria, there is a great debate regarding the role of P. gingivalis in the pathogenesis of IE [128]. For this reason, further studies are needed to confirm the role of this pathogen in IE development.
5. Prevention of Infective Endocarditis
- Antibiotic Prophylaxis: Transient bacteremia has always been considered associated with IE incidence, especially in high-risk patients, even if no published data demonstrate a correlation between a greater and lower magnitude of bacteremia and the incidence of IE in humans. Thus, the American Heart Association in 1955 recommended the use of antibiotics to reduce the risk of IE in patients with underlying cardiac conditions undergoing bacteremia-producing procedures [14]. The recommendations were based on IE animal models and in vitro susceptibilities of microorganisms known to cause endocarditis. Amoxicillin has been shown to significantly impact the incidence and duration of bacteremia after dental procedures [129]. Since then, several updates of the guidelines have taken place [14,34,49,130,131,132,133,134,135,136,137,138]. From 2007 the American Heart Association limited prophylaxis to high-risk patients, including those with conditions like congenital heart defects, prosthetic heart valves, previous IE, and cardiac transplants with successive valvulopathies [138]. Reasons for the variation in recommendations included lack of randomized controlled trial data showing benefit from antibiotic prophylaxis and the absence of observational data demonstrating consistent associations between procedures and development of IE. Moreover, the absence of evidence supporting antibiotic prophylaxis’s cost-effectiveness and recognition of antibiotic management’s importance in the era of increasing antibiotic resistance contributed to the more conservative position about antibiotic prophylaxis [139]. Finally, the estimation of the risk of developing IE after daily tooth brushing and mastication, which is higher than from single tooth extraction, represented a more than valid reason for a change in the guidelines [46,138]. Subsequently, in 2008 antibiotic prophylaxis was completely abolished for all patients in the UK [140], posing the basis for a revision of the guidelines in other countries including Europe [141,142] with a reduction of types of cardiac conditions requiring prophylaxis. Despite these changes in antibiotic prophylaxis guidelines, large epidemiological studies demonstrated that in Europe and United States the incidence of IE remained stable [143,144]. However, these variations often cause confusion among clinicians and do not ameliorate IE patients’ clinical outcomes [145,146]. For this reason, there is an urgent need for global agreement among physicians, cardiologists, and dentists for the generation of more informative guidelines for the use of antibiotics before invasive dental procedures. Once generated, these guidelines will be central for healthcare workers globally and will provide significant health benefits.
- Coagulation targeting: The great debate raised around antibiotic prophylaxis asks for new innovative therapies. In this scenario, the coagulation system appears as a novel potential and attractive therapeutic target. Indeed, IE is one of the best-characterized clinical models, where infection, inflammation, and coagulation are strongly interconnected in a bidirectional relationship which is often referred to as immunothrombosis [43,147]. The interactions between pathogens and platelets and the activation of the coagulation system are critical to initiation and growth of vegetation [148]. Moreover, the presence of systemic or cardiac inflammation, sepsis, and organ dysfunction accelerate the shift of the haemostatic system towards a thrombophilic state [148]. Thus, preventing this procoagulant imbalance with antiplatelet and anticoagulant strategies would represent a valid cornerstone of IE management [142]. Unfortunately, subjects with IE form a heterogeneous group, ranging from those who are successfully treated with no adverse events, to those with severe complications and a high mortality. Therefore, high-quality clinical trials in patients with IE are difficult to perform and the evidence currently available is conflicting [149,150]. Additionally, as demonstrated by Duval and coworkers, ~60% of IE patients suffer intracranial hemorrhagic lesions when assessed with MRI [151]. For this reason, IE patients are at high risk of developing intracranial bleeding. For instance, in 1986 Dewar et al. demonstrated that streptokinase-plasminogen complex administration to dogs with S. sanguis-induced endocarditis reduced the size of the vegetations but increased the risk of cerebral embolism [152]. In line with these preclinical results, Asaithambi and colleagues in 2013 demonstrated that in patients with IE, the rates of post-thrombolytic intracerebral hemorrhage were significantly higher than the non-IE group [153]. Hence, there is a difficult balancing among the risks associated with antithrombotic therapy and its potential beneficial effects. Notably, most of the evidence currently provided mainly consists of either preclinical models (cells and animals), or retrospective cohort trials [43,147,152,154,155,156]. Importantly, in 1995, Meyer and colleagues demonstrated in an experimental model of IE in rabbits that treatment with recombinant tissue plasminogen activator (rt-PA) and penicillin was more efficient than penicillin or rt-PA alone, decreasing the mass of vegetations and clinical signs to that of controls [157]. Of note, this observation has been reinforced by Anavekar et al., who in a retrospective study compared patients taking long-term Antiplatelet therapy (defined as aspirin, dipyridamole, clopidogrel, ticlopidine, or any combination of these agents) prior and after to the onset of IE versus controls with IE who did not receive these agents before or after the diagnosis of IE [149]. Interestingly, these authors demonstrated that the risk of symptomatic emboli associated with IE was markedly reduced in those patients who have received continuous daily antiplatelet therapy before the onset of IE [149]. Altogether, these findings provide a sound reason to recommend the prophylactic prescription of antiplatelet agents in addition to antibiotics to patients at high risk of IE. Of note, while there is no indication for the initiation of anticoagulant or antiplatelet therapies and thrombolytic drugs in patients with IE, the continuation of this treatment is believed safe, in the absence of hemorrhagic complications, in those patients who have already other indications for antithrombotic drug treatment [156,157].
- Oral hygiene: Maintenance of oral health is usually based on regular oral hygiene measures, i.e., flossing and brushing of teeth, topical use of fluoride, routine dental care, and low cariogenic nutrition [158]. However, data acquisition shows that the risk of developing IE after daily tooth brushing and chewing is higher than from single tooth extraction should lead to an analysis of the possible risk deriving from oral health conditions [47,136]. The risk of bacteremia in patients with a high mean plaque and calculus scores significantly increase by three–four times the risk of bacteremia following toothbrushing [46]. Thus, the improvement to high levels of oral hygiene and their maintenance should be considered from an oral health standpoint and possibly reduce the risk of IE. However, for a proper reduction of dental plaque, regular oral hygiene procedures should be completed with interdental cleaning devices [159]. While flossing was previously considered the gold standard, inter-dental brushes seem to be more effective [160]. Each clinician should customize device prescriptions about patient and site characteristics [160].
- Biofilm disruption: Biofilm-associated bacteria are less susceptible to antibiotics than planktonic cells [161,162]. Moreover, the variations of antibiotic concentration throughout the biofilm allow bacteria to be exposed to levels below the inhibitory concentrations and then develop resistance [163]. For this reason, the irresponsible use of antibiotics leads to the selection of pathogens that are difficult to eradicate. On the other hand, biofilms comprising multicellular, surface-adherent communities help microorganisms survive in various stress conditions, including antibiotics, heat shock, immune response, and lack of nutrients. For this reason, there will be an extended need for novel agents and strategies to treat biofilm-related infections because of the increment in the number of patients who require artificial medical devices. Indeed, the material matrix and biomaterials of these devices, provide a perfect site for bacterial adhesion, promoting mature biofilm formation [164]. Some strategies have been described recently, which appear to play an essential role in future antibiofilm therapies. For instance, one of the most common methods for preventing bacterial adhesion is modifying the surface, either directly or with a coating aid, to produce an uninhabitable barrier to bacteria [165]. These strategies have shown significant promise for preventing biofilm-related infections [164]. Finally, the use of biofilm eradication agents that comprise a variety of promising molecules (i.e., antimicrobial Peptides, Quaternary ammonium compounds, Antimicrobial lipids) offers exciting prospects for the future of biofilm therapeutics, especially for those infections that are refractory to conventional antibiotics.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Carinci, F.; Martinelli, M.; Contaldo, M.; Santoro, R.; Pezzetti, F.; Lauritano, D.; Candotto, V.; Mucchi, D.; Palmieri, A.; Tagliabue, A.; et al. Focus on periodontal disease and development of endocarditis. Biol. Regul. Homeost. Agents 2018, 32, 143–147. [Google Scholar]
- Lung, B.; Duval, X. Infective endocarditis: Innovations in the management of an old disease. Nat. Rev. Cardiol. 2019, 16, 623–635. [Google Scholar]
- Elliot, S.D. Bacteremia and oral sepsis. Proc. R. Soc. Med. 1939, 31, 747–754. [Google Scholar]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef]
- Eke, P.I.; Borgnakke, W.S.; Genco, R.J. Recent epidemiologic trends in periodontitis in the USA. Periodontology 2000 2020, 82, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.; Filioreanu, A.M.; Stelea, C.; Maftei, G.A.; Popescu, E. Prevalence of oral lesions modulated by patient’s age: The young versus the elderly. Rom. J. Oral Rehabil. 2018, 10, 50–56. [Google Scholar]
- Martu, M.A.; Maftei, G.A.; Luchian, I.; Popa, C.; Filioreanu, A.M.; Tatarciuc, D.; Nichitean, G.; Hurjui, L.L.; Foia, L.G. Wound healing of periodontal and oral tissues: Part II—Patho-phisiological conditions and metabolic diseases. Rom. J. Oral Rehab. 2020, 12, 30–40. [Google Scholar]
- Cecchi, E.; Chirillo, F.; Castiglione, A.; Faggiano, P.; Cecconi, M.; Moreo, A.; Cialfi, A.; Rinaldi, M.; Del Ponte, S.; Squeri, A.; et al. Clinical epidemiology in Italian Registry of Infective Endocarditis (RIEI): Focus on age, intravascular devices and enterococci. Int. J. Cardiol. 2015, 190, 151–156. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, C.; Rengo, C.; Rengo, G. Periodontal disease: A risk factor for diabetes and cardiovascular disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.O. Infective endocarditis and dental procedures: Evidences, pathogenesis, and prevention. J. Med. Investig. 2006, 53, 189–198. [Google Scholar] [CrossRef]
- Liccardo, D.; Marzano, F.; Carraturo, F.; Guida, M.; Femminella, G.D.; Bencivenga, L.; Agrimi, J.; Addonizio, A.; Melino, I.; Valletta, A.; et al. Potential bidirectional relationship between periodontitis and Alzheimer’s Disease. Front. Physiol. 2020, 11, 683–695. [Google Scholar] [CrossRef]
- Jones, T.D.; Baumgartner, L.; Bellows, M.T.; Breese, B.B.; Kuttner, A.G.; McCarty, M.; Rammelkamp, C.H. Prevention of rheumatic fever and bacterial endocarditis through control of streptococcal infections. Circulation 1955, 11, 317–320. [Google Scholar]
- Van der Meer, J.T.; Van Wijk, W.; Thompson, J.; Vandenbroucke, J.P.; Valkenburg, H.A.; Michel, M.F. Efficacy of antibiotic prophylaxis for prevention of native-valve endocarditis. Lancet 1992, 339, 135–139. [Google Scholar] [CrossRef]
- Lacassin, F.; Hoen, B.; Leport, C.; Selton-Suty, C.; Delahaye, F.; Goulet, V.; Etienne, J.; Briançon, S. Procedures associated with infective endocarditis in adults. A case control study. Eur. Heart J. 1995, 16, 1968–1974. [Google Scholar] [CrossRef]
- Forner, L.; Larsen, T.; Kilian, M.; Holmstrup, P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J. Clin. Periodontol. 2006, 33, 401–407. [Google Scholar] [CrossRef]
- Patini, R.; Gallenzi, P.; Spagnuolo, G.; Cordaro, M.; Cantiani, M.; Amalfitano, A.; Arcovito, A.; Callà, C.; Mingrone, G.; Nocca, G. Correlation Between Metabolic Syndrome, Periodontitis and Reactive Oxygen Species Production. A Pilot Study. Open Dent. J. 2017, 11, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Bin Abdulhak, A.A.; Baddour, L.M.; Erwin, P.J.; Hoen, B.; Cvhu, V.H.; Mensah, G.A.; Tleyjeh, I.M. Global and Regional Burden of Infective Endocarditis, 1990–2010. A Systematic Review of the Literature. Glob. Heart 2014, 9, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Correa de Sa, D.D.; Tleyjeh, I.M.; Anavekar, N.S.; Schultz, J.C.; Thomas, J.M.; Lahr, B.D.; Bachuwar, A.; Pazdernik, M.; Steckelberg, J.M.; Wilson, W.R.; et al. Epidemiological trends of infective endocarditis: A population-based study in Olmsted County, Minnesota. Mayo Clin. Proc. 2010, 85, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.C.; Anguita, M.P.; Torres, F.; Siles, J.R.; Mesa, D.; Vallés, F. Risk factors associated with endocarditis without underlying heart disease. Rev. Esp. Cardiol. 2002, 55, 304–307. [Google Scholar] [CrossRef]
- Bannay, A.; Hoen, B.; Duval, X.; Obadia, J.F.; Selton-Suty, C.; Le Moing, V.; Tattevin, P.; Iung, B.; Delahaye, F.; Alla, F.; et al. The impact of valve surgery on short- and long-term mortality in left-sided infective endocarditis: Do differences in methodological approaches explain previous conflicting results? Eur. Heart J. 2011, 32, 2003–2015. [Google Scholar] [CrossRef] [PubMed]
- Mocchegiani, R.; Nataloni, M. Complications of infective endocarditis. Cardiovasc. Hematol. Disord. Drug Targets 2009, 9, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Durack, D.T.; Lukes, A.S.; Bright, D.K.; Duke Endocarditis Service. New criteria for diagnosis of infective endocarditis: Utilization of specific echocardiographic findings. Am. J. Med. 1994, 96, 200–209. [Google Scholar] [CrossRef]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler Jr, T.R.; Bashore, T.; Corey, G.R. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 2000, 30, 633–638. [Google Scholar] [CrossRef]
- Cecci, E.; Parrini, I.; Chinaglia, A.; Pomari, F.; Brusasco, G.; Bobbio, M.; Trinchero, R.; Brusca, A. New diagnostic criteria for infective endocarditis, a study of sensitivity and specificity. Eur. Heart J. 1997, 18, 1149–1156. [Google Scholar] [CrossRef][Green Version]
- Sekeres, M.A.; Abrutyn, E.; Berlin, J.A.; Kaye, D.; Kinman, J.L.; Korzeniowski, O.M.; Levison, M.E.; Feldman, R.S.; Strom, B.L. An assessment of the usefulness of the Duke criteria for diagnosing active infective endocarditis. Clin. Infect. Dis. 1997, 24, 1185–1190. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dodds, G.A.; Sexton, D.J.; Durack, D.T.; Bashore, T.M.; Corey, G.R.; Kisslo, J. Negative predictive value of the Duke criteria for infective endocarditis. Am. J. Cardiol. 1996, 77, 403–407. [Google Scholar] [CrossRef]
- Nagano, Y.; Nakagawa, M.; Teshima, Y.; Takahashi, N. Infective Endocarditis--Blood Culture and Echocardiography. Rinsho Byori 2015, 63, 949–955. [Google Scholar]
- Holland, T.L.; Baddour, L.M.; Bayer, A.S.; Hoen, B.; Miro, J.M.; Fowler, V.G., Jr. Infective endocarditis. Nat. Rev. Dis. Primers 2016, 1, 16059. [Google Scholar] [CrossRef]
- Lepidi, H.; Durack, D.T.; Raoult, D. Diagnostic methods: Current best practices and guidelines for histologic evaluation in Infective endocarditis. Infect. Dis. Clin. N. Am. 2002, 16, 339–361. [Google Scholar] [CrossRef]
- Lepidi, H.; Fenollar, F.; Dumler, J.S.; Gauduchon, V.; Chalabreysse, L.; Bammert, A.; Bonzi, M.F.; Thivolet-Béjui, F.; Vandenesch, F.; Raoult, D. Cardiac valves in patients with Whipple endocarditis: Microbiological, molecular, quantitative histologic, and immunohistochemical studies of 5 patients. J. Infect. Dis. 2004, 190, 935–945. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC) Endorsed by: European Association for CardioThoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [PubMed]
- Bruun, N.E.; Habib, G.; Thuny, F.; Sogaard, P. Cardiac imaging in infectious endocarditis. Eur. Heart J. 2014, 35, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: Diagnosis, antimicrobial therapy, and management of complications: A scientific statement for healthcare professionals from the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [PubMed]
- Widmer, E.; Que, Y.A.; Entenza, J.M.; Moreillon, P. New concepts in the pathophysiology of infective endocarditis. Curr. Infect. Dis. Rep. 2006, 8, 271–279. [Google Scholar] [CrossRef]
- Cahill, T.J.; Prendergast, B.D. Infective endocarditis. Lancet 2016, 387, 882–893. [Google Scholar] [CrossRef]
- Que, Y.A.; Haefliger, J.A.; Piroth, L.; François, P.; Widmer, E.; Entenza, J.M.; Sinha, B.; Herrmann, M.; Francioli, P.; Vaudaux, P.; et al. Fibrinogen and fibronectin binding cooperate for valve infection and invasion in staphylococcus aureus experimental endocarditis. J. Exp. Med. 2005, 201, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Maftei, G.A.; Martu, C.M.; Popa, C.; Geletu, G.; Danila, V.; Jelihovschi, I.; Foia, L. The Biomechanical Properties of Suture Materials and Their Relationship to Bacterial Adherence. Mater. Plast. 2019, 56, 980–985. [Google Scholar] [CrossRef]
- Suzuki, M.; Satoh, N.; Nakamura, M.; Horita, S.; Seki, G.; Moriya, K. Bacteremia in hemodialysis patients. World J. Nephrol. 2016, 5, 489–496. [Google Scholar] [CrossRef]
- Moreillon, P.; Que, Y.A.; Bayer, A.S. Pathogenesis of streptococcal and staphylococcal endocarditis. Infect. Dis. Clin. N. Am. 2002, 16, 297–318. [Google Scholar] [CrossRef]
- Ghasemian, A.; Peerayeh, S.N.; Bakhshi, B.; Mirzaee, M. The Microbial Surface Components Recognizing Adhesive Matrix Molecules (MSCRAMMs) Genes among Clinical Isolates of Staphylococcus aureus from Hospitalized Children. Iran. J. Pathol. Fall 2015, 10, 258–264. [Google Scholar]
- Liesenborghs, L.; Meyers, S.; Vanassche, T.; Verhamme, P. Coagulation: At the heart of infective endocarditis. J. Thromb. Haemost. 2020, 18, 995–1008. [Google Scholar] [CrossRef]
- Davis, J.A.; Weisman, M.H.; Dail, D.H. Vascular disease in infective endocarditis. Report of immune-mediated events in skin and brain. Arch. Intern. Med. 1978, 138, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Lockhart, P.B.; Brennan, M.T.; Thornhill, M.; Michalowicz, B.S.; Noll, J.; Bahrani-Mougeot, F.K.; Sasser, H.C. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J. Am. Den. Assoc. 2009, 140, 1238–1244. [Google Scholar] [CrossRef]
- Lockhart, P.B.; Brennan, M.T.; Sasser, H.C.; Fox, P.C.; Paster, B.J.; Bahrani-Mougeot, F.K. Bacteremia associated with toothbrushing and dental extraction. Circulation 2008, 117, 3118–3125. [Google Scholar] [CrossRef] [PubMed]
- Cotti, E.; Mercuro, G. Apical periodontitis and cardiovascular diseases: Previous findings and ongoing research. Int. Endod. J. 2015, 48, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Dajani, A.S.; Taubert, K.A.; Wilson, W.; Bolger, A.F.; Bayer, A.; Ferrieri, P.; Gewitz, M.H.; Shulman, S.T.; Nouri, S.; Newburger, J.W.; et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA 1997, 277, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Okell, C.C.; Elliott, S.D. Bacteraemia and oral sepsis: With special reference to the aetiology of subacute endocarditis. Lancet 1935, 2, 869–872. [Google Scholar] [CrossRef]
- Delahaye, F.; M’Hammedi, A.; Guerpillon, B.; de Gevigney, G.; Boibieux, A.; Dauwalder, O.; Bouchiat, C.; Vandenesch, F. Systematic Search for Present and Potential Portals of Entry for Infective Endocarditis. J. Am. Coll. Cardiol. 2016, 67, 151–158. [Google Scholar] [CrossRef]
- Horder, T.J. Infective endocarditis: With an analysis of 150 cases and with special reference to the chronic form of the disease. Q. J. Med. 1909, 2, 289–324. [Google Scholar]
- Jia, G.; Zhi, A.; Lai, P.F.H.; Wang, G.; Xia, Y.; Xiong, Z.; Zhang, H.; Che, N.; Ai, L. The oral microbiota—A mechanistic role for systemic diseases. Br. Dent. J. 2018, 224, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Abusleme, L.; Dupuy, A.K.; Dutzan, N.; Silva, N.; Burleson, J.A.; Strausbaugh, L.D.; Gamonal, J.; Diaz, P.I. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013, 7, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.Y.; Furtado Araujo, M.V.; Strausbaugh, L.D.; Terzi, E.; Ioannidou, E.; Diaz, P. Microbiome profiles in periodontitis in relation to host and disease characteristics. PLoS ONE 2015, 10, e0127077. [Google Scholar] [CrossRef] [PubMed]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Li, H.; Ni, C.; Du, Z.; Yan, F. Human oral microbiota and its modulation for oral health. Biomed. Pharmacother. 2018, 99, 883–893. [Google Scholar] [CrossRef]
- Buduneli, N. Environmental factors and periodontal microbiome. Periodontol 2000 2020, 85, 1–12. [Google Scholar]
- Madison, A.; Kiecolt-Glaser, J.K. Stress, depression, diet, and the gut microbiota: Human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019, 28, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Wade, W. Oral Microbial Ecology: Current Research and New Perspective; Caister Academic Press: Pool, UK, 2013. [Google Scholar]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.J., Jr. Composition and development of oral bacterial communities. Periodontology 2000 2014, 64, 20–39. [Google Scholar] [CrossRef]
- Gholizadeh, P.; Eslami, H.; Yousefi, M.; Asgharzadeh, M.; Aghazadeh, M.; Kafil, H.S. Role of oral microbiome on oral cancers, a review. Biomed. Pharmacother. 2016, 84, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Sallberg, M. Oral viral infections of children. Periodontology 2000 2009, 49, 87–95. [Google Scholar] [CrossRef]
- Presti, R.M.; Handley, S.A.; Droit, L.; Ghannoum, M.; Jacobson, M.; Shiboski, C.H.; Webster-Cyriaque, J.; Brown, T.; Yin, M.T.; Overton, E.T. Alterations in the oral microbiome in HIV-infected participants after antiretroviral therapy administration are influenced by immune status. AIDS 2018, 32, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Kreth, J.; Giacaman, R.A.; Raghavan, R.; Merritt, J. The road less travele—Defining molecular commensalism with Streptococcus sanguinis. Mol. Oral Microbiol. 2017, 32, 181–196. [Google Scholar] [CrossRef]
- Griffen, A.L.; Beall, C.J.; Campbell, J.H.; Firestone, N.D.; Kumar, P.S.; Yang, Z.K.; Podar, M.; Leys, E.J. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2012, 6, 1176–1185. [Google Scholar] [CrossRef]
- Zhu, B.; Macleod, L.C.; Kitten, T.; Xu, P. Streptococcus sanguinis biofilm formation & interaction with oral pahogens. Future Microbiol. 2018, 13, 915–932. [Google Scholar] [PubMed]
- Lu, M.; Xuan, S.; Wang, Z. Oral microbiota: A new view of body health. Food Sci. Hum. Well 2019, 8, 8–15. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Palmer, R.J., Jr.; Periasamy, S.; Jakubovics, N.S. oral multispecies biofilm development and the key role of cell-cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef]
- Theilade, E.; Wright, W.H.; Jensen, S.B.; Löe, H. Experimental gingivitis in man. II. A longitudinal clinical and bacteriological investigation. J. Periodontal. Res. 1966, 1, 1–13. [Google Scholar] [CrossRef]
- Ritz, H.L. Microbial population shifts in developing human dental plaque. Arch. Oral Biol. 1967, 12, 1561–1568. [Google Scholar] [CrossRef]
- Sønju, C.A.B.; Hannig, M.; Skjørland, K.; Sønju, T. Analytical and ultrastructural studies of pellicle on primary teeth. Acta. Dontol. Scad. 1997, 55, 339–343. [Google Scholar]
- Hannig, M. Transmission electron microscopic study of in vivo pellicle formation on dental restorative materials. Eur. J. Oral Sci. 1997, 105, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Rönström, A.; Edwardsson, S.; Atiström, R. Streptococcus sanguinis and streptococcus salivarius in early plaque formation on plastic films. J. Periodontal. Res. 1977, 12, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; London, J. Adhere today, here tomorrow: Oral bacterial adherence. J. Bact. 1993, 175, 3247–3252. [Google Scholar] [CrossRef]
- Haffajee, A.D.; Socransky, S.S.; Feres, M.; Ximenez-Fyvie, L.A. Plaque Microbiology in Health and Disease. In Dental Plaque Revisited. Oral Biofilms in Health and Disease, 1st ed.; Newman, H.N., Wilson, M., Eds.; BioLine: Cardiff, UK, 1999; Volume 1, pp. 255–282. [Google Scholar]
- Kolenbrander, P.E.; Andersen, R.N.; Moore, L.V. Coaggregation of Fusobacterium nucleatum, Selenomonas flueggei, Selenomonas infelix, Selenomonas noxia, and Selenomonas sputigena with strains from 11 genera of oral bacteria. Infect. Immun. 1989, 57, 3194–3203. [Google Scholar] [CrossRef]
- van Palenstein Helderman, W.H. Microbial etiology of periodontal disease. J. Clin. Periodontol. 1981, 8, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef]
- Cafiero, C.; Spagnuolo, G.; Marenzi, G.; Martuscelli, R.; Colamaio, M.; Leuci, S. Predictive Periodontitis: The Most Promising Salivary Biomarkers for Early Diagnosis of Periodontitis. J. Clin. Med. 2021, 10, 1488. [Google Scholar] [CrossRef]
- Khor, B.; Snow, M.; Herrman, E.; Ray, N.; Mansukhani, K.; Patel, K.A.; Said-Al-Naief, N.; Maier, T.; Machida, C.A. Interconnections Between the Oral and Gut Microbiomes: Reversal of Microbial Dysbiosis and the Balance Between Systemic Health and Disease. Microorganisms 2021, 9, 496. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.Y.; Lin, S.K.; Kok, S.H.; Cheng, S.J.; Lee, M.S.; Wang, T.T.; Chen, C.S.; Lin, L.D.; Wang, J.S. The role of lipopolysaccharide in infectious bone resorption of periapical lesion. J. Oral Pathol. Med. 2004, 33, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Dahlen, G.; Basic, A.; Bylund, J. Importance of Virulence Factors for the Persistence of Oral Bacteria in the Inflamed Gingival Crevice and in the Pathogenesis of Periodontal Disease. J. Clin. Med. 2019, 8, 1339. [Google Scholar] [CrossRef]
- Isoshima, D.; Yamashiro, K.; Matsunaga, K.; Shinobe, M.; Nakanishi, N.; Nakanishi, I.; Omori, K.; Yamamoto, Y.; Takashiba, S. Assessment of pathogenesis of infective endocarditis by plasma IgG antibody titer test against periodontal bacteria. Clin. Case Rep. 2017, 5, 1580–1586. [Google Scholar] [CrossRef]
- Megran, D.W. Enterococcal endocarditis. Clin. Infect. Dis. 1992, 15, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Kupferwasser, L.I.; Bayer, A.S. Update on culture-negative endocarditis. Curr. Clin. Top. Infect. Dis. 2000, 20, 113–133. [Google Scholar]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miró, J.M.; Fowler, V.G.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Bolger, A.F.; Levison, M.E.; Ferrieri, P.; Gerber, M.A.; Tani, L.Y.; Gewitz, M.H.; et al. Infective endocarditis: Diagnosis, antimicrobial therapy, and management of complications: A statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: Endorsed by the Infectious Diseases Society of America. Circulation 2005, 111, 394–434. [Google Scholar]
- Selton-Suty, C.; Célard, M.; Le Moing, V.; Doco-Lecompte, T.; Chirouze, C.; Iung, B.; Strady, C.; Revest, M.; Vandenesch, F.; Bouvet, A.; et al. Preeminence of Staphylococcus aureus in infective endocarditis: A 1-year population-based survey. Clin. Infect. Dis. 2012, 54, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Ford, I.; Douglas, C.W. The role of platelets in infective endocarditis. Platelets 1997, 8, 285–294. [Google Scholar] [PubMed]
- Yeaman, M.R. Platelets in defense against bacterial pathogens. Cell Mol. Life Sci. 2010, 67, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections—Los Angeles County, California, 2002–2003. MMWR Morb. Mortal Wkly. Rep. 2003, 52, 88. [Google Scholar]
- Boucher, H.W.; Corey, G.R. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 2008, 46, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Uribe-García, A.; Paniagua-Contreras, G.L.; Monroy-Pérez, E.; Bustos-Martínez, J.; Hamdan-Partida, A.; Garzón, J.; Alanís, J.; Quezada, R.; Vaca-Paniagua, F.; Vaca, S. Frequency and expression of genes involved in adhesion and biofilm formation in Staphylococcus aureus strains isolated from periodontal lesions. J. Microbiol. Immunol. Infect. 2021, 54, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Liesenborghs, L.; Meyers, S.; Lox, M.; Criel, M.; Claes, J.; Peetermans, M.; Trenson, S.; Vande Velde, G.; Vanden Berghe, P.; Baatsen, P.; et al. Staphylococcus aureus endocarditis: Distinct mechanisms of bacterial adhesion to damaged and inflamed heart valves. Eur. Heart J. 2019, 40, 3248–3259. [Google Scholar] [CrossRef] [PubMed]
- White, J.C.; Niven, C.F. Streprococcus, S.B.E: A Streptococcus associated with subacute bacterial endocarditis. J. Bacteriol. 1946, 51, 717–722. [Google Scholar] [CrossRef]
- Alture-Werber, E.; Loewe, L. Prophylactic Immunization against Streptococcus sanguis. J. Bacteriol. 1948, 56, 391–395. [Google Scholar] [CrossRef]
- Keynan, Y.; Rubinstein, E. Pathophysiology of infective endocarditis. Curr. Infect. Dis. Rep. 2013, 15, 342–346. [Google Scholar] [CrossRef]
- Martini, A.M.; Moricz, B.S.; Ripperger, A.K.; Tran, P.M.; Sharp, M.-E.-; Forsythe, A.N.; Kulhankova, K.; Salgado-Pabón, W.; Jones, B.D. Association of Novel Streptococcus sanguinis Virulence Factors with Pathogenesis in a Native Valve Infective Endocarditis Model. Front. Microbiol. 2020, 31, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Erickson, P.R.; Herzberg, M.C. The Streptococcus sanguis platelet aggregation-associated protein. Identification and characterization of the minimal platelet-interactive domain. J. Biol. Chem. 1993, 268, 1646–1649. [Google Scholar] [CrossRef]
- Herzberg, M.C. Platelet-streptococcal interactions in endocarditis. Crit. Rev. Oral Biol. Med. 1996, 7, 222–236. [Google Scholar] [CrossRef]
- Mandell, G.L.; Korzeniowski, O.M. Atlas of Infectious Diseases: Cardiovascular Infections, 1st ed.; Current Medicine: London, UK, 1997; pp. 1–202. [Google Scholar]
- Andrews, F.W.; Horder, T.J. A study of the streptococci pathogenic for man. Lancet 1906, 2, 775. [Google Scholar] [CrossRef]
- Jett, B.D.; Huycke, M.M.; Gilmore, M.S. Virulence of enterococci. Clin. Microbiol. Rev. 1994, 7, 462–478. [Google Scholar] [CrossRef] [PubMed]
- Souto, R.; Vieira Colombo, A.P. Prevalence of Enterococcus faecalis in subgingival biofilm and saliva of subjects with chronic periodontal infection. Arch. Oral Biol. 2008, 53, 155–160. [Google Scholar] [CrossRef]
- Dahl, A.; Iversen, K.; Tonder, N.; Hoest, N.; Arpi, M.; Dalsgaard, M.; Chehri, M.; Soerensen, L.L.; Fanoe, S.; Junge, S.; et al. Prevalence of Infective Endocarditis in Enterococcus faecalis Bacteremia. J. Am. Coll. Cardiol. 2019, 74, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Thurlow, L.R. Chittezham Thomas, V.; Narayanan, S.; Olson, S.; Fleming, S.D.; Hancock, L.E. Gelatinase contributes to the pathogenesis of endocarditis caused by Enterococcus faecalis. Infect. Immun. 2010, 78, 4936–4943. [Google Scholar] [CrossRef] [PubMed]
- Frank, K.L.; Barnes, A.M.T.; Grindle, S.M.; Manias, D.A.; Schlievert, P.M.; Dunny, G.M. Use of Recombinase-Based In Vivo Expression Technology to Characterize Enterococcus faecalis Gene Expression during Infection Identifies In Vivo-Expressed Antisense RNAs and Implicates the Protease Eep in Pathogenesis. Infect. Immun. 2012, 80, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Nallapareddy, S.R.; Singh, K.V.; Sillanpää, J.; Garsin, D.A.; Höök, M.; Erlandsen, S.L.; Murray, B.E. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J. Clin. Investig. 2006, 116, 2799–2807. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.W.; Thal, L.A.; Perri, M.B.; Vazquez, J.A.; Donabedian, S.M.; Clewell, D.B.; Zervos, M.J. Plasmid-associated hemolysin and aggregation substance production contribute to virulence in experimental enterococcal endocarditis. Antimicrob. Agents Chemother. 1993, 37, 2474–2477. [Google Scholar] [CrossRef]
- Chuang, O.N.; Schlievert, P.M.; Wells, C.L.; Manias, D.A.; Tripp, T.J.; Dunny, G.M. Multiple Functional Domains of Enterococcus faecalis Aggregation Substance Asc10 Contribute to Endocarditis Virulence. Infect. Immun. 2009, 77, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.V.; Nallapareddy, S.R.; Sillanpää, J.; Murray, B.E. Importance of the collagen adhesin ace in pathogenesis and protection against Enterococcus faecalis experimental endocarditis. PLoS Pathog. 2010, 6, e1000716. [Google Scholar] [CrossRef]
- Paturel, L.; Casalta, J.P.; Habib, G.; Nezri, M.; Raoult, D. Actinobacillus actinomycetemcomitans endocarditis. Clin. Microbiol. Infect. 2004, 10, 98–118. [Google Scholar] [CrossRef]
- Klinger, R. Untersuchungen über menschliche Aktinomykose. Zentralbl. Bakteriol. 1912, 62, 191–200. [Google Scholar]
- Valée, A.; Gaillard, J.A. A contagious pyogenic infection of mice caused by Actinobacillus actinomycetemcomitans. Ann. Inst. Pasteur. 1953, 84, 647–649. [Google Scholar]
- Mitchell, R.G.; Gillespie, W.A. Bacterial endocarditis due to an Actinobacillus. J. Clin. Pathol. 1964, 17, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Brouqui, P.; Raoult, D. Endocarditis due to rare and fastidious bacteria. Clin. Microbiol. Rev. 2001, 14, 177–207. [Google Scholar] [CrossRef] [PubMed]
- Amano, A. Bacterial adhesins to host components in periodontitis. Periodontology 2000 2010, 52, 12–37. [Google Scholar] [CrossRef] [PubMed]
- Danforth, D.R.; Tang-Siegel, G.; Ruiz, T.; Mintz, K.P. A Nonfimbrial Adhesin of Aggregatibacter actinomycetemcomitans Mediates Biofilm Biogenesis. Infect. Immun. 2019, 87, e00704–e00718. [Google Scholar]
- Mintz, K.; Fives-Taylor, P. Binding of the periodontal pathogen Actinobacillus actinomycetemcomitans to extracellular matrix proteins. Oral Microbiol. Immunol. 1999, 14, 109–116. [Google Scholar] [CrossRef]
- Becker, J.; Schuppan, D.; Rabanus, J.; Rauch, R.; Niechoy, U.; Gelderblom, H. Immunoelectron microscopic localization of collagens type I, V, VI and of procollagen type III in human periodontal ligament and cementum. J. Histochem. Cytochem. 1991, 39, 103–110. [Google Scholar] [CrossRef]
- Weber, K. Monitoring vascular sclerosis in hypertension: A new window of opportunity. Circulation 1998, 98, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Kitten, T.; Munro, C.L.; Wellman, G.C.; Mintz, K.P. EmaA, a potential virulence determinant of Aggregatibacter actinomycetemcomitans in infective endocarditis. Infect. Immun. 2008, 76, 2316–2324. [Google Scholar] [CrossRef]
- Datta, H.K.; Ng, W.F.; Walker, J.A.; Tuck, S.P.; Varanasi, S.S. The cell biology of bone metabolism. J. Clin. Pathol. 2008, 61, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Hokamura, K.; Inaba, H.; Nakano, K.; Nomura, R.; Yoshioka, H.; Taniguchi, K.; Ooshima, T.; Wada, K.; Amano, A.; Umemura, K. Molecular analysis of aortic intimal hyperplasia caused by Porphyromonas gingivalis infection in mice with endothelial damage. J. Periodontal. Res. 2010, 45, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.A.F.; Forte, C.P.F.; Silva, P.G.B.; Lopes, C.B.; Montenegro, R.C.; Santos, Â.K.C.R.D.; Sobrinho, C.R.M.R. Mário Rogério Lima Mota, Sousa, F.B.; Alves, A.P.N.N. Molecular Analysis of Oral Bacteria in Heart Valve of Patients with Cardiovascular Disease by Real-Time Polymerase Chain Reaction. Medicine 2015, 94, e2067. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, P.B.; Brennan, M.T.; Kent, M.L.; Norton, H.J.; Weinrib, D.A. Impact of amoxicillin prophylaxis on the incidence, nature, and duration of bacteremia in children after intubation and dental procedures. Circulation 2004, 109, 2878–2884. [Google Scholar] [CrossRef]
- Rammelkamp, C.H., Jr.; Breese, B.B.; Griffeath, H.I.; Houser, H.B.; Kaplan, M.H.; Kuttner, A.G.; McCarty, M.; Stollerman, G.H.; Wannamaker, L.W. (Committee on Prevention of Rheumatic Fever and Bacterial Endocarditis, American Heart Association). Prevention of rheumatic fever and bacterial endocarditis through control of streptococcal infections. Circulation 1957, 15, 154–158. [Google Scholar] [CrossRef]
- Shulman, S.T.; Amren, D.P.; Bisno, A.L.; Dajani, A.S.; Durack, D.T.; Gerber, M.A.; Kaplan, E.L.; Millard, H.D.; Sanders, W.E.; Schwartz, R.H.; et al. Prevention of bacterial endocarditis. A statement for health professionals by the Committee on Rheumatic Fever and Bacterial Endocarditis of the Council on Cardiovascular Diseases in the Young of the American Heart Association. Am. J. Dis. Child. 1985, 139, 232–235. [Google Scholar] [CrossRef]
- Wannamaker, L.W.; Denny, F.W.; Diehl, A.; Jawetz, E.; Kirby, W.M.M.; Markowitz, M.; McCarty, M.; Mortimer, E.A.; Paterson, P.Y.; Perry, W.; et al. Prevention of bacterial endocarditis. Circulation 1965, 31, 953–954. [Google Scholar]
- Kaplan, E.L.; Anthony, B.F.; Bisno, A.; Durack, D.; Houser, H.; Millard, H.D.; Sanford, J.; Shulman, S.T.; Stollerman, M.; Taranta, A.; et al. Prevention of bacterial endocarditis. Circulation 1977, 56, 139–143. [Google Scholar]
- Shulman, S.T.; Amren, D.P.; Bisno, A.L.; Dajani, A.S.; Durack, D.T.; Gerber, M.A.; Kaplan, E.L.; Millard, H.D.; Sanders, W.E.; Schwartz, R.H.; et al. Prevention of bacterial endocarditis: A statement for health professionals by the Committee on Rheumatic Fever and Infective Endocarditis of the Council on Cardiovascular Disease in the Young. Circulation 1984, 70, 1123–1127. [Google Scholar]
- Dajani, A.S.; Bisno, A.L.; Chung, K.J.; Durack, D.T.; Freed, M.; Gerber, M.A.; Karchmer, A.W.; Millard, H.D.; Rahimtoola, S.; Shulman, S.T.; et al. Prevention of bacterial endocarditis: Recommendations by the American Heart Association. JAMA 1990, 264, 2919–2922. [Google Scholar] [CrossRef]
- Taubert, K.A.; Dajani, A.S. Optimisation of the prevention and treatment of bacterial endocarditis. Drugs Aging 2001, 18, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Danchin, N.; Duval, X.; Leport, C. Prophylaxis of infective endocarditis: French recommendations 2002. Heart 2005, 91, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.; Taubert, K.A.; Gewitz, M.; Lockhart, P.B.; Baddour, L.M.; Levison, M.; Bolger, A.; Cabell, C.H.; Takahashi, M.; Baltimore, R.S.; et al. Prevention of infective endocarditis: Guidelines from the American Heart Association: A guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007, 116, 1736–1754. [Google Scholar] [PubMed]
- Peterson, G.E.; Crowley, A.L. Antibiotic Prophylaxis for Infective Endocarditis. Circulation 2019, 140, 181–183. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Prophylaxis Against Infective Endocarditis: Antimicrobial Prophylaxis Against Infective Endocarditis in Adults and Children Undergoing Interventional Procedures; NICE: London, UK, 2016; pp. 1–377. [Google Scholar]
- Daly, C.G. Antibiotic prophylaxis for dental procedures. Aust. Prescr. 2017, 40, 184–188. [Google Scholar] [CrossRef]
- Habib, G.; Hoen, B.; Tornos, P.; Thuny, F.; Prendergast, B.; Vilacosta, I.; Moreillon, P.; de Jesus Antunes, M.; Thilen, U.; Lekakis, J.; et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): The Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur. Heart J. 2009, 30, 2369–2413. [Google Scholar]
- Shah, A.S.V. McAllister, D.A.; Gallacher, P.; Astengo, F.; Rodríguez Pérez, J.A.; Hall, J.; Lee, K.K.; Bing, R.; Anand, A.; Nathwani, D.; et al. Incidence, Microbiology, and Outcomes in Patients Hospitalized with Infective Endocarditis. Circulation 2020, 141, 2067–2077. [Google Scholar] [CrossRef]
- Toyoda, N.; Chikwe, J.; Itagaki, S.; Gelijns, A.C.; Adams, D.H.; Egorova, N.N. Trends in Infective Endocarditis in California and New York State, 1998–2013. JAMA 2017, 317, 1652–1660. [Google Scholar] [CrossRef]
- Bakhsh, A.A.; Shabeeh, H.; Mannocci, F.; Niazi, S.M. A Review of Guidelines for Antibiotic Prophylaxis before Invasive Dental Treatments. Appl. Sci. 2021, 11, 311. [Google Scholar] [CrossRef]
- Coffey, S.; Cox, B.; Williams, M.J. Lack of progress in valvular heart disease in the pre-transcatheter aortic valve replacement era: Increasing deaths and minimal change in mortality rate over the past three decades. Am. Heart J. 2014, 167, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.A.; Moreillon, P. Infective endocarditis. Nat. Rev. Cardiol. 2011, 8, 322–336. [Google Scholar] [CrossRef]
- Durante-Mangoni, E.; Molaro, R.; Iossa, D. The role of hemostasis in infective endocarditis. Curr. Infect. Dis. Rep. 2014, 16, 435. [Google Scholar] [CrossRef] [PubMed]
- Anavekar, N.S.; Tleyjeh, I.M.; Anavekar, N.S.; Mirzoyev, Z.; Steckelberg, J.M.; Haddad, C.; Khandaker, M.H.; Wilson, W.R.; Chandrasekaran, K.; Baddour, L.M. Impact of prior antiplatelet therapy on risk of embolism in infective endocarditis. Clin. Infect. Dis. 2007, 44, 1180–1186. [Google Scholar] [CrossRef]
- Chan, K.L.; Tam, J.; Dumesnil, J.G.; Cujec, B.; Sanfilippo, A.J.; Jue, J.; Turek, M.; Robinson, T.; Williams, K. Effect of long-term aspirin use on embolic events in infective endocarditis. Clin. Infect. Dis. 2008, 46, 37–41. [Google Scholar] [CrossRef]
- Duval, X.; Iung, B.; Klein, I.; Brochet, E.; Thabut, G.; Arnoult, F.; Lepage, L.; Laissy, J.P.; Wolff, M.; Leport, C.; et al. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: A prospective study. Ann. Inter. Med. 2010, 152, 497–504. [Google Scholar] [CrossRef]
- Dewar, H.A.; Jones, M.R.; Barnes, W.S.; Griffin, S.G. Fibrinolytic therapy in bacterial endocarditis: Experimental studies in dogs. Eur. Heart J. 1986, 7, 520–527. [Google Scholar] [CrossRef]
- Asaithambi, G.; Adil, M.M.; Qureshi, A.I. Thrombolysis for ischemic stroke associated with infective endocarditis: Results from the nationwide inpatient sample. Stroke 2013, 44, 2917–2919. [Google Scholar] [CrossRef] [PubMed]
- Kupferwasser, L.I.; Yeaman, M.R.; Shapiro, S.M.; Nast, C.C.; Sullam, P.M.; Filler, S.G.; Bayer, A.B. Acetylsalicylic acid reduces vegetation bacterial density, hematogenous bacterial dissemination, and frequency of embolic events in experimental Staphylococcus aureus endocarditis through antiplatelet and antibacterial effect. Circulation 1999, 99, 2791–2797. [Google Scholar] [CrossRef]
- Meyer, M.W.; Witt, A.R.; Krishnan, L.K.; Yokota, M.; Roszkowski, M.J.; Rudney, J.D.; Herzberg, M.C. Therapeutic advantage of recombinant human plasminogen activator in endocarditis: Evidence from experiments in rabbits. Thromb. Haemost. 1995, 73, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Vanassche, T.; Peetermans, W.E.; Herregods, M.C.; Herijgers, P.; Verhamme, P. Anti-thrombotic therapy in infective endocarditis. Expert Rev. Cardiovasc. Ther. 2011, 9, 1203–1219. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; Simeone, P.; Davì, G. Coagulation and infective endocarditis: Sooner or later. Intern. Emerg. Med. 2015, 10, 539–541. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef]
- Crocombe, L.A.; Brennan, D.S.; Slade, G.D.; Loc, D.O. Is self interdental cleaning associated with dental plaque levels, dental calculus, gingivitis, and periodontal disease? J. Periodontal. Res. 2012, 47, 188–197. [Google Scholar] [CrossRef]
- Kotsakis, G.A.; Lian, Q.; Ioannou, A.L.; Michalowicz, B.S.; John, M.T.; Chu, H. A network meta-analysis of interproximal oral hygiene methods in the reduction of clinical indices of inflammation. J. Periodontol. 2018, 89, 558–570. [Google Scholar] [CrossRef]
- Verdosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial biofilm eradication agents: A current review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef]
- Olson, M.E.; Ceri, H.; Morck, D.W.; Buret, A.G.; Read, R.R. Biofilm bacteria: Formation and comparative susceptibility to antibiotics. Can. J. Vet. Res. 2002, 66, 86. [Google Scholar] [PubMed]
- Sharma, R.; Bajpai, P.; Sayyed, U.; Ahmad, I.Z. Approaches Towards Microbial Biofilm Disruption by Natural Bioactive Agents. In Biofilms in Human Diseases: Treatment and Control, 1st ed.; Kumar, S., Chandra, N., Singh, L., Hashmi, M.Z., Varma, A., Eds.; Springer: New York, NY, USA, 2019; Volume 1, pp. 233–261. [Google Scholar]
- Arciola, C.R.; Campoccia, D.; Speziale, P.; Montanaro, L.; Costerton, J.W. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 2012, 33, 5967–5982. [Google Scholar] [CrossRef] [PubMed]
- Bazaka, K.; Jacob, M.V.; Crawford, R.J.; Ivanova, E.P. Efficient surface modification of biomaterial to prevent biofilm formation and the attachment of microorganisms. Appl. Microbiol. Biotechnol. 2012, 95, 299–311. [Google Scholar] [CrossRef]
- Šutej, I.; Peroš, K.; Trkulja, V.; Rudež, I.; Barić, D.; Alajbeg, I.; Pintarić, H.; Stevanović, R.; Lepur, D. The epidemiological and clinical features of odontogenic infective endocarditis. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 637–645. [Google Scholar] [CrossRef]
| Major Criteria | Minor Criteria |
|---|---|
Blood Culture Positive for IE
|
|
| Bacteria | Characteristics |
|---|---|
| Actinobacillus actinomycetemcomitans | Gram-negative coccobacillus; facultative anaerobe; non motile; non spore forming. |
| Cardiobacterium hominis | Gram-negative bacillus; microaerophilic; non motile; non spore forming. |
| Clostridium septicum | Gram-positive; anaerobe; motile: spore forming. |
| Eikenella corrodens | Gram-negative bacillus; facultative anaerobe; non motile; non spore forming. |
| Enterococcus faecalis | Gram-positive; facultative anaerobe; non motile; non spore forming. |
| Haemophilus sp. | Gram-negative coccobacillus; facultative anaerobe; non motile; non spore forming. |
| Kingella kingae | Gram-negative coccobacillus; aerobe or facultative anaerobe; non motile; non spore forming. |
| Rothia dentocariosa | Gram-positive; aerobe; non motile; non spore forming. |
| Staphylococcus aureus | Gram-positive; aerobe; non motile; non spore forming. |
| Streptococcus bovis | Gram-positive; facultative anaerobe; non motile; non spore forming. |
| Streptococcus sanguinis (viridans group) | Gram-positive; facultative anaerobe; non motile; non spore forming. |
| Porphyromonas gingivalis | Gram-negative; obligate anaerobe; non motile; non spore forming. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Giudice, C.; Vaia, E.; Liccardo, D.; Marzano, F.; Valletta, A.; Spagnuolo, G.; Ferrara, N.; Rengo, C.; Cannavo, A.; Rengo, G. Infective Endocarditis: A Focus on Oral Microbiota. Microorganisms 2021, 9, 1218. https://doi.org/10.3390/microorganisms9061218
Del Giudice C, Vaia E, Liccardo D, Marzano F, Valletta A, Spagnuolo G, Ferrara N, Rengo C, Cannavo A, Rengo G. Infective Endocarditis: A Focus on Oral Microbiota. Microorganisms. 2021; 9(6):1218. https://doi.org/10.3390/microorganisms9061218
Chicago/Turabian StyleDel Giudice, Carmela, Emanuele Vaia, Daniela Liccardo, Federica Marzano, Alessandra Valletta, Gianrico Spagnuolo, Nicola Ferrara, Carlo Rengo, Alessandro Cannavo, and Giuseppe Rengo. 2021. "Infective Endocarditis: A Focus on Oral Microbiota" Microorganisms 9, no. 6: 1218. https://doi.org/10.3390/microorganisms9061218
APA StyleDel Giudice, C., Vaia, E., Liccardo, D., Marzano, F., Valletta, A., Spagnuolo, G., Ferrara, N., Rengo, C., Cannavo, A., & Rengo, G. (2021). Infective Endocarditis: A Focus on Oral Microbiota. Microorganisms, 9(6), 1218. https://doi.org/10.3390/microorganisms9061218










