Smells Like Teen Spirit—A Model to Generate Laundry-Associated Malodour In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Swatches
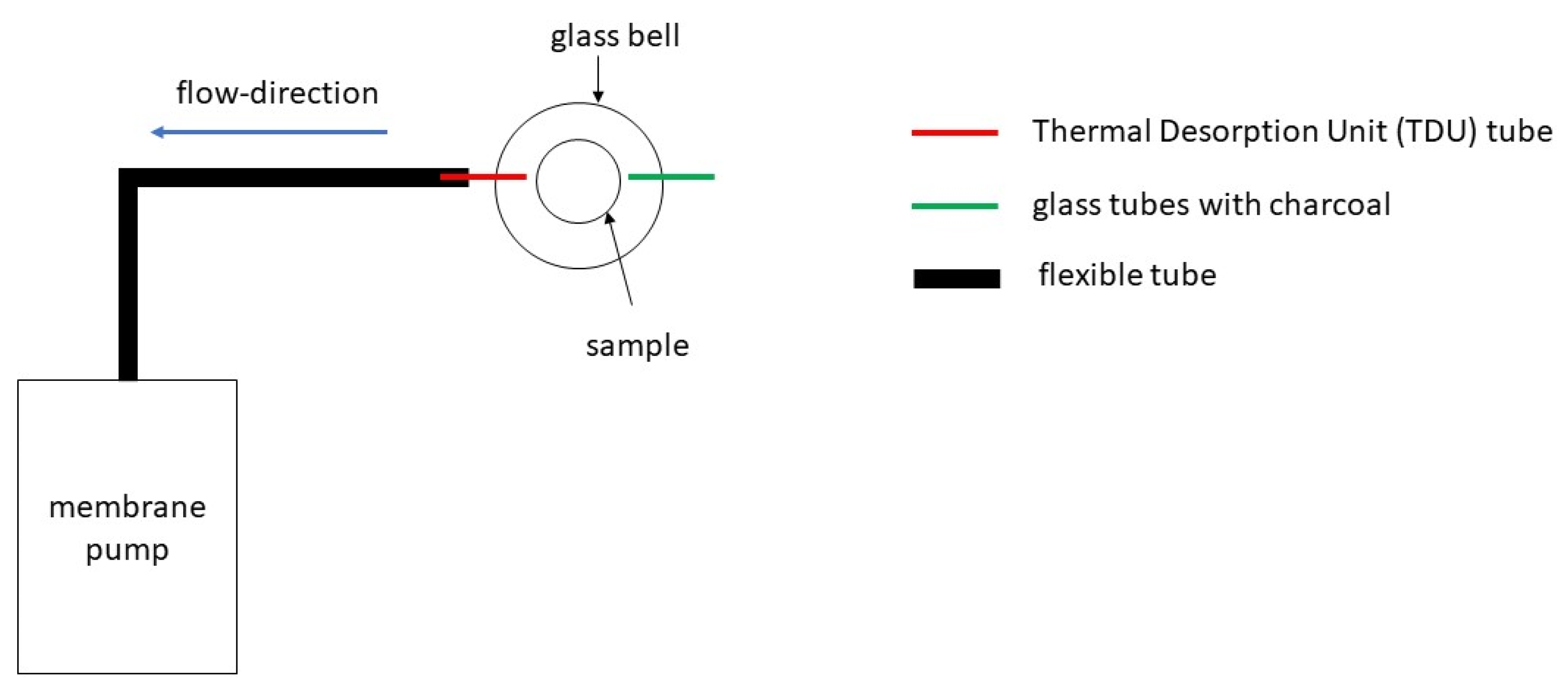
2.2. Gas Chromatography
2.3. Sensory Analysis
2.4. Statistical Analysis
2.5. Evaluation of the Reduction of Microbial Counts after a Simulated Wash Cycle (Rotawash)
3. Results
3.1. Gas Chromatograpy–Olfactometry (GC/O)
3.2. Sensory Analysis—Free Choice Profiling
3.3. Bacterial Growth and Effects of Biocides
4. Discussion
4.1. Gas Chromatograpy–Olfactometry (GC/O)
4.2. Laundry Malodour Bacteria
4.3. Sensory Analysis—Free Choice Profiling
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bockmühl, D.P.; Schages, J.; Rehberg, L. Laundry and textile hygiene in healthcare and beyond. Microb. Cell 2019, 6, 299–306. [Google Scholar] [CrossRef]
- Bockmühl, D. Laundry hygiene-how to get more than clean. J. Appl. Microbiol. 2017, 122, 1124–1133. [Google Scholar] [CrossRef]
- Munk, S.; Johansen, C.; Stahnke, L.H.; Adler-Nissen, J. Microbial survival and odor in laundry. J. Surfactants Deterg. 2001, 4, 385–394. [Google Scholar] [CrossRef]
- Callewaert, C.; Van Nevel, S.; Kerckhof, F.-M.; Granitsiotis, M.S.; Boon, N. Bacterial Exchange in Household Washing Machines. Front. Microbiol. 2015, 6, 1381. [Google Scholar] [CrossRef]
- Van Herreweghen, F.; Amberg, C.; Marques, R.; Callewaert, C. Biological and Chemical Processes that Lead to Textile Malodour Development. Microorganism 2020, 8, 1709. [Google Scholar] [CrossRef]
- Nagoh, Y.; Tobe, S.; Watanabe, T.; Mukaiyama, T. Analysis of Odorants Produced from Indoor Drying Laundries and Effects of Enzyme for Preventing Malodor Generation. Tenside Surfactants Deterg. 2005, 42, 7–12. [Google Scholar] [CrossRef]
- Takeuchi, K.; Hasegawa, Y.; Ishida, H.; Kashiwagi, M. Identification of novel malodour compounds in laundry. Flavour Fragr. J. 2011, 27, 89–94. [Google Scholar] [CrossRef]
- Kubota, H.; Mitani, A.; Niwano, Y.; Takeuchi, K.; Tanaka, A.; Yamaguchi, N.; Kawamura, Y.; Hitomi, J. Moraxella Species Are Primarily Responsible for Generating Malodor in Laundry. Appl. Environ. Microbiol. 2012, 78, 3317–3324. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial Community Variation in Human Body Habitats Across Space and Time. Science 2009, 326, 1694–1697. [Google Scholar] [CrossRef]
- Cundell, A.M. Microbial Ecology of the Human Skin. Microb. Ecol. 2018, 76, 113–120. [Google Scholar] [CrossRef]
- Troccaz, M.C.; Gaïa, N.; Beccucci, S.; Schrenzel, J.; Cayeux, I.; Starkenmann, C.; Lazarevic, V. Mapping axillary microbiota responsible for body odours using a culture-independent approach. Microbiome 2015, 3, 1–15. [Google Scholar] [CrossRef]
- James, A.G.; Austin, C.J.; Cox, D.S.; Taylor, D.; Calvert, R. Microbiological and biochemical origins of human axillary odour. FEMS Microbiol. Ecol. 2012, 83, 527–540. [Google Scholar] [CrossRef]
- Fredrich, E.; Barzantny, H.; Brune, I.; Tauch, A. Daily battle against body odor: Towards the activity of the axillary microbiota. Trends Microbiol. 2013, 21, 305–312. [Google Scholar] [CrossRef]
- Natsch, A.; Gfeller, H.; Gygax, P.; Schmid, J.; Acuna, G. A Specific Bacterial Aminoacylase Cleaves Odorant Precursors Secreted in the Human Axilla. J. Biol. Chem. 2003, 278, 5718–5727. [Google Scholar] [CrossRef]
- Chung, H.; Seok, H.J. Populations of malodor-forming bacteria and identification of volatile components in triolein-soiled cotton fabric. Fibers Polym. 2012, 13, 740–747. [Google Scholar] [CrossRef]
- Teufel, L.; Pipal, A.; Schuster, K.; Staudinger, T.; Redl, B. Material-dependent growth of human skin bacteria on textiles investigated using challenge tests and DNA genotyping. J. Appl. Microbiol. 2010, 108, 450–461. [Google Scholar] [CrossRef]
- Hammond, C.J. Chemical composition of household malodours—An overview. Flavour Fragr. J. 2013, 28, 251–261. [Google Scholar] [CrossRef]
- Denawaka, C.J.; Fowlis, I.A.; Dean, J.R. Source, impact and removal of malodour from soiled clothing. J. Chromatogr. A 2016, 1438, 216–225. [Google Scholar] [CrossRef]
- Stapleton, K.; Dean, J.R. A preliminary identification and determination of characteristic volatile organic compounds from cotton, polyester and terry-towel by headspace solid phase microextraction gas chromatography–mass spectrometry. J. Chromatogr. A 2013, 1295, 147–151. [Google Scholar] [CrossRef]
- Callewaert, C.; De Maeseneire, E.; Kerckhof, F.-M.; Verliefde, A.; Van De Wiele, T.; Boon, N. Microbial Odor Profile of Polyester and Cotton Clothes after a Fitness Session. Appl. Environ. Microbiol. 2014, 80, 6611–6619. [Google Scholar] [CrossRef] [PubMed]
- McQueen, R.H.; Laing, R.M.; Brooks, H.J.L.; Niven, B.E. Odor Intensity in Apparel Fabrics and the Link with Bacterial Populations. Text. Res. J. 2007, 77, 449–456. [Google Scholar] [CrossRef]
- Honisch, M.; Stamminger, R.; Bockmühl, D. Impact of wash cycle time, temperature and detergent formulation on the hygiene effectiveness of domestic laundering. J. Appl. Microbiol. 2014, 117, 1787–1797. [Google Scholar] [CrossRef]
- Honisch, M.; Brands, B.; Weide, M.; Speckmann, H.-D.; Stamminger, R.; Bockmühl, D.P. Antimicrobial Efficacy of Laundry Detergents with Regard to Time and Temperature in Domestic Washing Machines. Tenside Surfactants Deterg. 2016, 53, 547–552. [Google Scholar] [CrossRef]
- Honisch, M.; Stamminger, R.; Bockmühl, D. Impact of Time and Temperature on the Inactivation of Microorganisms in Domestic Washing Machines. J. Appl. Microbiol. 2016, 117, 2016. [Google Scholar]
- Lucassen, R.; Merettig, N.; Bockmühl, D.P. Antimicrobial Efficacy of Hygiene Rinsers under Consumer-Related Conditions. Tenside Surfactants Deterg. 2013, 50, 259–262. [Google Scholar] [CrossRef]
- Bloomfield, S.F.; Exner, M.; Signorelli, C.; Scott, E.A. Effectiveness of Laundering Processes Used in Domestic (Home) Settings; International Scientific Forum on Home Hygiene: Somerset, UK, 2013; pp. 1–62. [Google Scholar]
- Nix, I.D.; Frontzek, A.; Bockmühl, D.P. Characterization of Microbial Communities in Household Washing Machines. Tenside Surfactants Deterg. 2015, 52, 432–440. [Google Scholar] [CrossRef]
- Payne, J.; Kudner, D. A Durable Antoodor Finish for Cotton Textiles. Text. Chem. Color. 1996, 28, 28–30. [Google Scholar]
- Linley, J.R. Laboratory tests of the effects of p-cresol and 4-methylcyclohexanol on oviposition by three species of Toxorhynchites mosquitoes. Med. Veter. Èntomol. 1989, 3, 347–352. [Google Scholar] [CrossRef]
- Hallem, E.A.; Fox, A.N.; Zwiebel, L.J.; Carlson, J.R. Mosquito receptor for human-sweat odorant. Nature 2004, 427, 212–213. [Google Scholar] [CrossRef] [PubMed]
- D-NOSES_EU. Gas Chromatography Olfactometry. 2021. Available online: https://odourobservatory.org/measuring-odour/gas-chromatography-olfactometry/ (accessed on 22 February 2021).
- Deutsches Institut für Normung. Sensorische Prüfverfahren—Einfach Beschreibende Prüfung; Deutsches Institut für Normung: Berlin, Germany, 2014. [Google Scholar]
- Lawless, H.; Klein, B. Sensory Science Theory and Applications in Foods; Dekker, M., Ed.; CRC Press: New York, NY, USA, 1991. [Google Scholar]
- Oreskovich, D.; Klein, B.; Sutherland, J. Procrustes Analysis and it‘s Application to Free-Choice and other sensory profiling. In Sensory Science Theory and Applications in Foods; Dekker, M., Ed.; CRC Press: New York, NY, USA, 1991. [Google Scholar]
- Dijksterhuis, G. Multivariate data analysis in sensory and consumer science: An overview of developments. Trends Food Sci. Technol. 1995, 6, 206–211. [Google Scholar] [CrossRef]
- Lawless, H.; Heymann, H. Sensory Evaluation of Food. Principles and Practices, 2nd ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Narain, C.; Paterson, A.; Reid, E. Free choice and conventional profiling of commercial black filter coffees to explore consumer perceptions of character. Food Qual. Prefer. 2004, 15, 31–41. [Google Scholar] [CrossRef]
- Addinsoft. XLSTAT Statistical and Data Analysis Solution; Addinsoft: Paris, France, 2019. [Google Scholar]
- Clarke, J.; Oakes, L.; Miller, L.; Hindley, P.; McGeechan, P.; Petkov, J.; Bockmühl, D. Towards a Lab-Scale Efficacy Test Method for the Evaluation of Hygienic Laundry Rinse-Stage Disinfectants. Tenside Surfactants Deterg. 2018, 55, 410–416. [Google Scholar] [CrossRef]
- Schages, J.; Stamminger, R.; Bockmühl, D.P. A New Method to Evaluate the Antimicrobial Efficacy of Domestic Laundry Detergents. J. Surfactants Deterg. 2020, 23, 629–639. [Google Scholar] [CrossRef]
- Gattlen, J.; Amberg, C.; Zinn, M.; Mauclaire, L. Biofilms isolated from washing machines from three continents and their tolerance to a standard detergent. Biofouling 2010, 26, 873–882. [Google Scholar] [CrossRef]
- Microbiology S for G. Bacterial Genetic Pathway Involved in Body Odor Production Discovered. ScienceDaily. 2015. Available online: https://www.sciencedaily.com/releases/2015/03/150330213947.htm (accessed on 12 May 2019).
- Symrise, A.G.; (Holzminden, Germany). Persönliche Kommunikation, 2019.
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Legrum, W. Riechstoffe, Zwischen Gestank und Duft: Vorkommen, Eigenschaften und Anwendung von Riechstoffen und Deren Gemischen; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Bundeszentrale für Gesundheit und Verbraucherschutz. Bundeslebensmittelschlüssel; Bundeszentrale für Gesundheit und Verbraucherschutz: Bonn, Germany, 2019. [Google Scholar]
- Garrido-Delgado, R.; Arce, L.; Guamán, A.; Pardo, A.; Marco, S.; Valcarcel, M. Direct coupling of a gas-liquid separator to an ion mobility spectrometer for the classification of different white wines using chemometrics tools. Talanta 2011, 84, 471–479. [Google Scholar] [CrossRef]
- Zamora, D.; Alcalà, M.; Blanco, M. Determination of trace impurities in cosmetic intermediates by ion mobility spectrometry. Anal. Chim. Acta 2011, 708, 69–74. [Google Scholar] [CrossRef]
- Stapleton, K.; Hill, K.; Day, K.; Perry, J.; Dean, J. The potential impact of washing machines on laundry malodour generation. Lett. Appl. Microbiol. 2013, 56, 299–306. [Google Scholar] [CrossRef]
- Borrel, B. Why Study Pig Odor? 2009. Available online: https://www.scientificamerican.com/article/why-study-pig-odor/ (accessed on 29 July 2019).
- Mathus, T. Anaerobic biogenesis of phenol and p-cresol from ρ-tyrosine. Fuel 1995, 74, 1505–1508. [Google Scholar] [CrossRef]
- Saito, Y.; Sato, T.; Nomoto, K.; Tsuji, H. Identification of phenol- and p-cresol-producing intestinal bacteria by using media supplemented with tyrosine and its metabolites. FEMS Microbiol. Ecol. 2018, 94, fiy125. [Google Scholar] [CrossRef]
- Natsch, A.; Gfeller, H.; Gygax, P.; Schmid, J. Isolation of a bacterial enzyme releasing axillary malodor and its use as a screening target for novel deodorant formulations1. Int. J. Cosmet. Sci. 2005, 27, 115–122. [Google Scholar] [CrossRef]
- McQueen, R.H.; Laing, R.M.; Wilson, C.A.; Niven, B.E.; Delahunty, C.M. Odor Retention on Apparel Fabrics: Development of Test Methods for Sensory Detection. Text. Res. J. 2007, 77, 645–652. [Google Scholar] [CrossRef]
- Li, M.; Budding, A.E.; Van Der Lugt-Degen, M.; Du-Thumm, L.; Vandeven, M.; Fan, A. The influence of age, gender and race/ethnicity on the composition of the human axillary microbiome. Int. J. Cosmet. Sci. 2019, 41, 371–377. [Google Scholar] [CrossRef]
- Marmann, A.; Aly, A.H.; Lin, W.; Wang, B.; Proksch, P. Co-Cultivation—A Powerful Emerging Tool for Enhancing the Chemical Diversity of Microorganisms. Mar. Drugs 2014, 12, 1043–1065. [Google Scholar] [CrossRef]
- Knight, V.; Sanglier, J.-J.; DiTullio, D.; Braccili, S.; Bonner, P.; Waters, J.; Hughes, D.; Zhang, L. Diversifying microbial natural products for drug discovery. Appl. Microbiol. Biotechnol. 2003, 62, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Brakhage, A.A.; Schuemann, J.; Bergmann, S.; Scherlach, K.; Schroeckh, V.; Hertweck, C. Activation of fungal silent gene clusters: A new avenue to drug discovery. Nat. Compd. Drugs 2008, 66, 1–12. [Google Scholar]
- Brakhage, A.A.; Schroeckh, V. Fungal secondary metabolites—Strategies to activate silent gene clusters. Fungal Genet. Biol. 2011, 48, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Schroeckh, V.; Scherlach, K.; Nützmann, H.-W.; Shelest, E.; Schmidt-Heck, W.; Schuemann, J.; Martin, K.; Hertweck, C.; Brakhage, A.A. Intimate bacterial-fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 2009, 106, 14558–14563. [Google Scholar] [CrossRef] [PubMed]
- Ola, A.R.B.; Thomy, D.; Lai, D.; Brötz-Oesterhelt, H.; Proksch, P. Inducing Secondary Metabolite Production by the Endophytic Fungus Fusarium tricinctum through Coculture with Bacillus subtilis. J. Nat. Prod. 2013, 76, 2094–2099. [Google Scholar] [CrossRef] [PubMed]
- McQueen, R.H.; Vaezafshar, S. Odor in textiles: A review of evaluation methods, fabric characteristics, and odor control technologies. Text. Res. J. 2020, 90, 1157–1173. [Google Scholar] [CrossRef]
- Abdul-Bari, M.M.; McQueen, R.H.; De La Mata, A.P.; Batcheller, J.C.; Harynuk, J.J. Retention and release of odorants in cotton and polyester fabrics following multiple soil/wash procedures. Text. Res. J. 2020, 90, 2212–2222. [Google Scholar] [CrossRef]
- Munk, S.; Münch, P.; Stahnke, L.; Adler-Nissen, J.; Schieberle, P. Primary odorants of laundry soiled with sweat/sebum: Influence of lipase on the odor profile. J. Surfactants Deterg. 2000, 3, 505–515. [Google Scholar] [CrossRef]
- Pugliese, S.; Jespersen, M.F.; Pernov, J.B.; Shenolikar, J.; Nygaard, J.; Nielsen, O.J.; Johnson, M.S. Chemical analysis and origin of the smell of line-dried laundry. Environ. Chem. 2020, 17, 355. [Google Scholar] [CrossRef]
- Dijksterhuis, G. Procrustes Analysis in Sensory Research; Elsevie: Amsterdam, The Netherlands, 1996; Volume 16, pp. 185–219. [Google Scholar]
- Meilgaard, M.C.; Carr, B.T. Sensory Evaluation Techniques; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]




| Strains | Code |
|---|---|
| Corynebacterium jeikeium | DSM 7171, ATCC 43734 |
| Micrococcus luteus | DSM 1790, ATCC 10240 |
| Moraxella osloensis | DSM 6998, ATCC 19976 |
| Pseudomonas aeruginosa | DSM 939, ATCC 15442 |
| Staphylococcus epidermidis | DSM 1798, ATCC 12228 |
| Staphylococcus hominis | DSM 20329, ATCC 27845 |
| Program Step/Device Setting | Parameter |
|---|---|
| Oven program |
|
| TDU tube |
|
| Universal injector |
|
| Desorption of the TDU tube |
|
| Mass spectrometer |
|
| Combination of Microorganisms | Abbreviation | Evaluation after Three Days | Evaluation after Seven Days |
|---|---|---|---|
| M. luteus + S. epidermidis + M. osloensis | MlSeMo | 0 | 1 |
| M. luteus + S. epidermidis + S. hominis | MlSeSh | 0 | 3 |
| M. luteus + S. epidermidis + P. aeruginosa | MlSePa | 0 | 2 |
| M. luteus + S. epidermidis + C. jeikeium | MlSeCj | 0 | 2 |
| M. luteus + M. osloensis + S. hominis | MlMoSh | 2 | 0 |
| M. luteus + M. osloensis + P. aeruginosa | MlMoPa | 0 | 0 |
| M. luteus + S. hominis + C. jeikeium | MlShCj | 3 | 3 |
| M. luteus + P. aeruginosa + C. jeikeium | MlPaCj | 1 | 1 |
| S. epidermidis + M. osloensis + S. hominis | SeMoSh | 2 | 1 |
| S. epidermidis + M. osloensis + P. aeruginosa | SeMoPa | 2 | 2 |
| S. epidermidis + M. osloensis + C. jeikeium | SeMoCj | 2 | 2 |
| S. epidermidis + S. hominis + C. jeikeium | SeShCj | 1 | 2 |
| S. epidermidis + P. aeruginosa + C. jeikeium | SePaCj | 1 | 1 |
| M. osloensis + S. hominis + P. aeruginosa | MoShPa | 0 | 1 |
| M. osloensis + P. aeruginosa + C. jeikeium | MoPaCj | 0 | 0 |
| S. hominis + P. aeruginosa + C. jeikeium | ShPaCj | 0 | 0 |
| M. luteus | ML | 1 | 2 |
| M. osloensis | Mo | 1 | 2 |
| P. aeruginosa | Pa | 0 | 0 |
| C. jeikeium | Cj | 0 | 0 |
| S. hominis | Sh | 0 | 0 |
| S. epidermidis | Se | 0 | 0 |
| Control | 0 | 1 |
| Sample | Microbial Count (cfu/cm2) |
|---|---|
| MlShCj before incubation | 4.15 × 107 |
| MlShCj after incubation | 1.04 × 108 |
| Control (beef tallow only) | 0 |
| Retention Time (min) | Identified Substance | Intensity | Odour Description |
|---|---|---|---|
| 2.62 | n.A. | 1 | fatty, waxy |
| 3.67 | n.A. | 1 | mouldy |
| 4.07 | Dimethyl Disulphide | 1 | cabbage |
| 5.88 | n.A. | 1 | acidic, penetrative |
| 6.61 | n.A. | 1 | acidic, mouldy |
| 7.65 | n.A. | 1 | acidic, waxy |
| 8.19 | Dimethyl Trisulphide | 1 | mouldy, cabbage |
| 8.86 | n.A. | 3 | mouldy, fatty, rotten |
| 10.02 | n.A. | 2 | fatty, meaty, metalic |
| 10.48 | n.A. | 2 | fatty, green, cucumber, aldehyde |
| 12.89 | n.A. | 1 | fatty, meaty |
| 14.72 | n.A. | 1 | animalic, scratchy, urine |
| 15.12 | n.A. | 3 | acidic, fatty, wet fabric malodour |
| 17.07 | n.A. | 1 | roasted, caramel |
| 17.72 | p-Cresol | 2 | animalic, urine |
| 17.98 | n.A. | 1 | powdery, scratchy |
| 18.49 | n.A. | 1 | acidic, fatty |
| 18.90 | n.A. | 2 | technical, phenolic |
| 21.85 | Indole | 4 | technical Indole |
| 22.31 | n.A. | 3 | phenolic, technical, like Indole |
| 23.23 | n.A. | 2 | technical, acidic |
| 23.62 | n.A. | 1 | sweet, phenylic, honey, fruity |
| 26.78 | n.A. | 2 | phenolic, smokey, burnt |
| 26.96 | n.A. | 2 | phenolic, smokey, burnt |
| 28.60 | n.A. | 2 | mouldy, acidic |
| Sample | Dimethyl Disulphide (%) | Dimethyl Trisulfide (%) | Indole (%) |
|---|---|---|---|
| MlShCj | 0.36 | 0.10 | 0.24 |
| MlSeCj | 0.08 | 0.00 | 0.17 |
| SeMoPa | 0.07 | 0.00 | 0.56 |
| MlSeSh | 0.04 | 0.01 | 0.12 |
| SeShCj | 0.06 | 0.00 | 0.07 |
| MoShPa | 0.03 | 0.00 | 0.23 |
| Ml | 0.05 | 0.01 | 0.13 |
| Mo | 0.02 | 0.00 | 0.03 |
| SeMoCj | 0.04 | 0.02 | 0.15 |
| ShPaCj | 0.02 | 0.00 | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zinn, M.-K.; Singer, M.; Bockmühl, D. Smells Like Teen Spirit—A Model to Generate Laundry-Associated Malodour In Vitro. Microorganisms 2021, 9, 974. https://doi.org/10.3390/microorganisms9050974
Zinn M-K, Singer M, Bockmühl D. Smells Like Teen Spirit—A Model to Generate Laundry-Associated Malodour In Vitro. Microorganisms. 2021; 9(5):974. https://doi.org/10.3390/microorganisms9050974
Chicago/Turabian StyleZinn, Marc-Kevin, Marco Singer, and Dirk Bockmühl. 2021. "Smells Like Teen Spirit—A Model to Generate Laundry-Associated Malodour In Vitro" Microorganisms 9, no. 5: 974. https://doi.org/10.3390/microorganisms9050974
APA StyleZinn, M.-K., Singer, M., & Bockmühl, D. (2021). Smells Like Teen Spirit—A Model to Generate Laundry-Associated Malodour In Vitro. Microorganisms, 9(5), 974. https://doi.org/10.3390/microorganisms9050974






