Limonin Enhances the Antifungal Activity of Eugenol Nanoemulsion against Penicillium Italicum In Vitro and In Vivo Tests
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanoemulsion Preparation
2.3. Characterization of Nanoemulsions
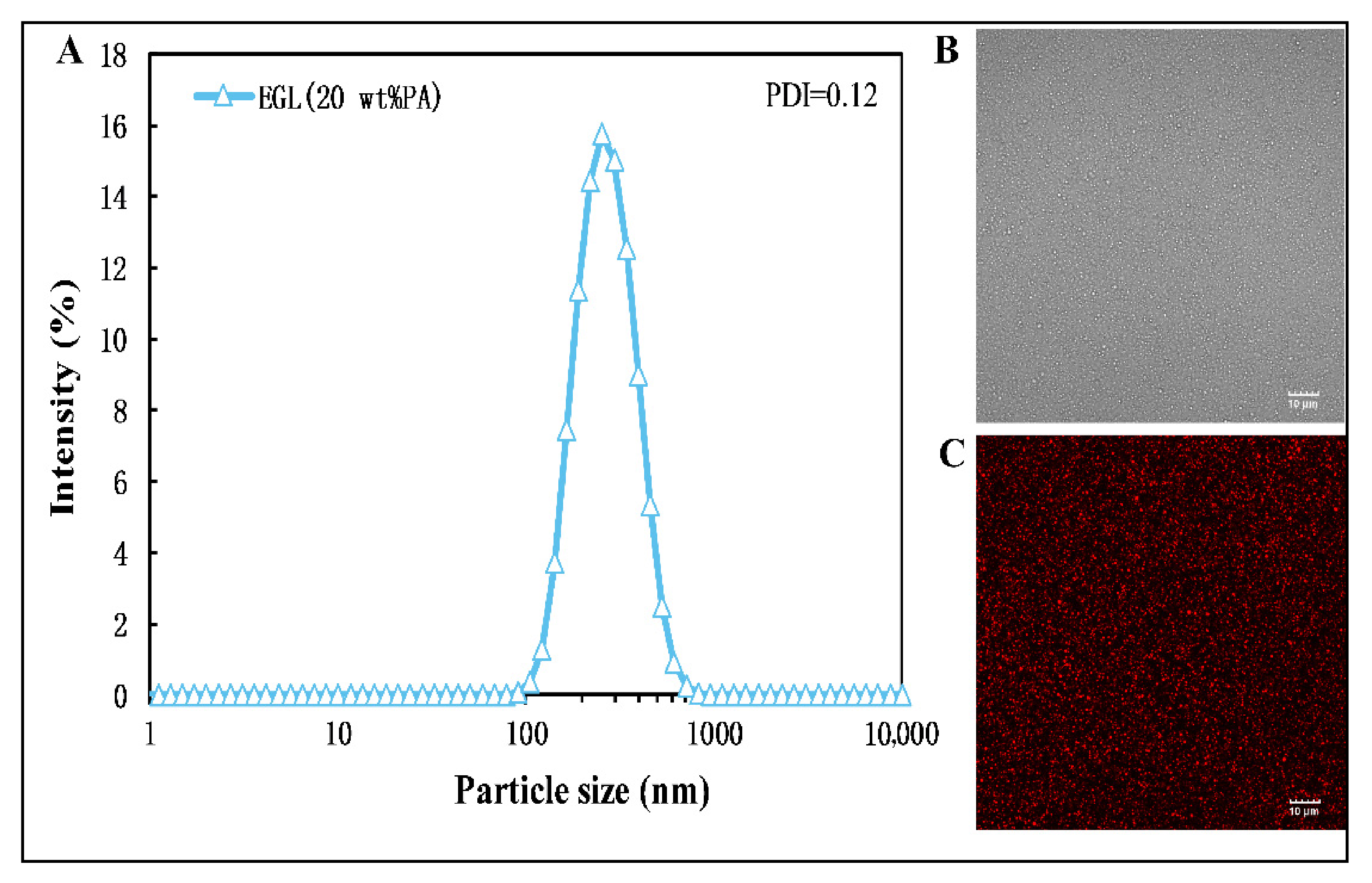
2.3.1. Particle Size Determination
2.3.2. Optical Microscopy and Confocal Laser Scanning Microscopy
2.4. Antifungal Activity of the Nanoemulsions
2.4.1. Fungal Strains
2.4.2. Determination of MIC and MFC
2.4.3. Influence of the Emulsions on Conidial Germination of P. italicum
2.4.4. Inhibitory Effect of the Nanoemulsion on Mycelial Growth of P. italicum
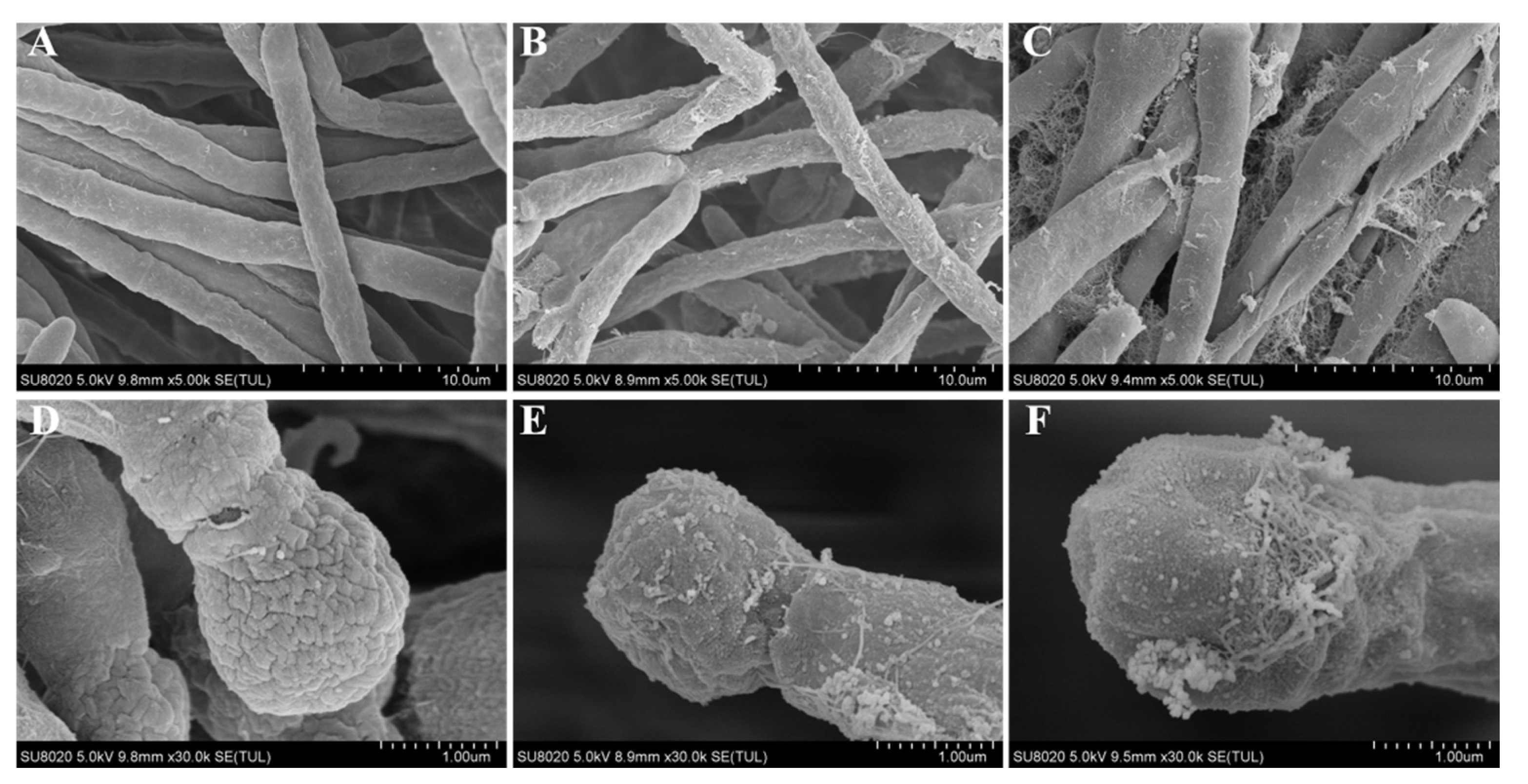
2.5. Morphological Observation
2.6. Determination of Microbial Cell Membrane Permeability
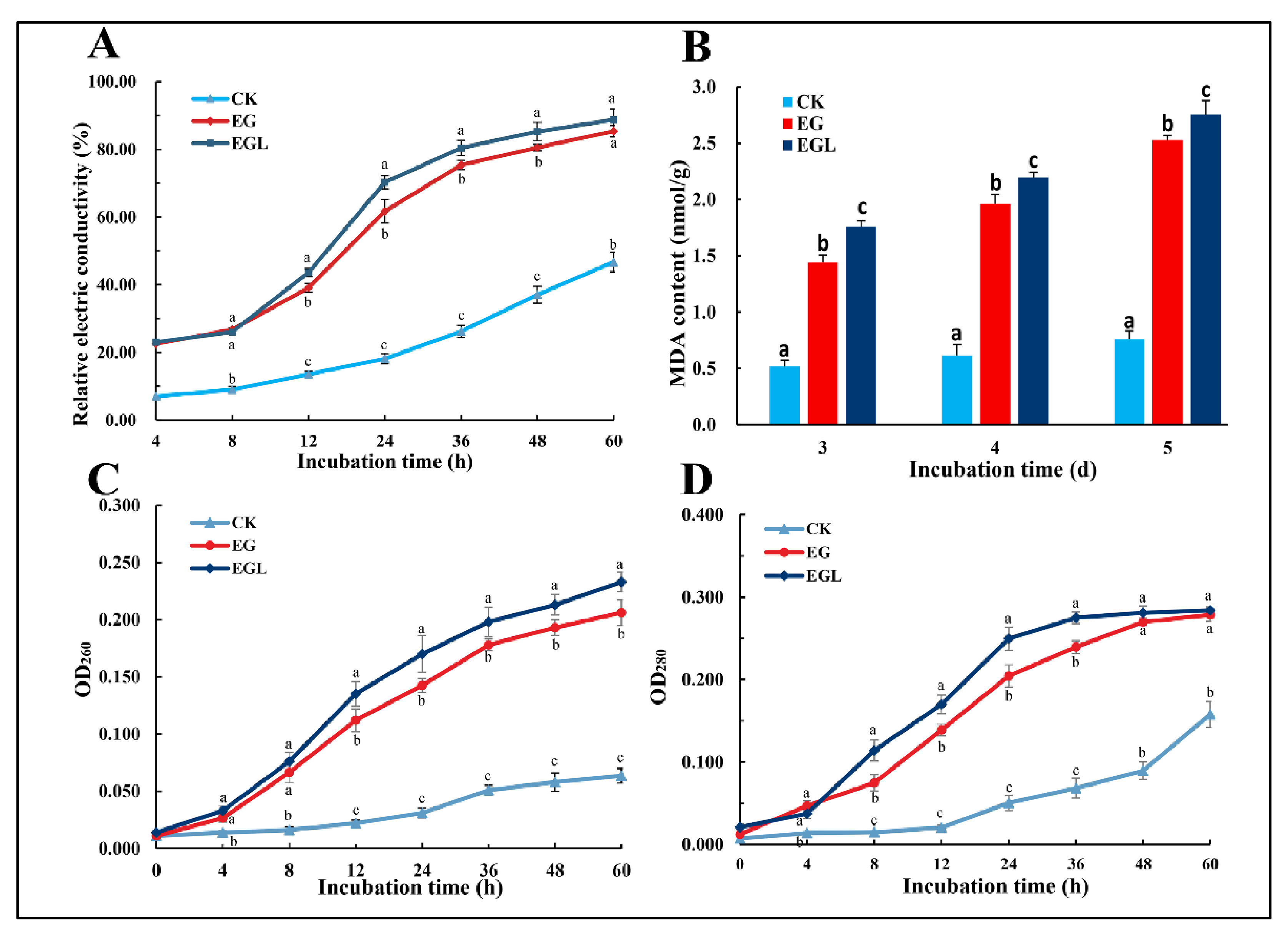
2.6.1. Extracellular Electric Conductivity Measurement
2.6.2. Leakage of Protein and Nucleic Acid
2.7. Lipid Peroxidation Assay
2.8. Challenge Test of Citrus Fruits
2.9. Statistical Analyses
3. Results
3.1. Characterization of Nanoemulsions
3.2. Antifungal Activity
3.2.1. MICs and MFCs Determination
3.2.2. Inhibition of Spore Germination
3.2.3. Inhibition of Mycelial Growth
3.3. Micromorphological Analysis by SEM
3.4. Influence of Nanoemulsions on the Cell Membrane Permeability
3.4.1. Extracellular Conductivity
3.4.2. Leakage of Nucleic Acid and Protein
3.5. Lipid Peroxidation
3.6. Challenge Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.; Chen, J.; Wan, C. Pinocembrin-7-Glucoside (P7G) Reduced Postharvest Blue Mold of Navel Orange by Suppressing Penicillium Italicum Growth. Microorganisms 2020, 8, 536. [Google Scholar] [CrossRef] [PubMed]
- Caserta, R.; Teixeira-Silva, N.S.; Granato, L.M.; Dorta, S.O.; Rodrigues, C.M.; Mitre, L.K.; Yochikawa, J.T.H.; Fischer, E.R.; Nascimento, C.A.; Souza-Neto, R.R.; et al. Citrus Biotechnology: What Has Been Done to Improve Disease Resistance in Such an Important Crop? Biotechnol. Res. Innov. 2019, 3, 95–109. [Google Scholar] [CrossRef]
- Papoutsis, K.; Mathioudakis, M.M.; Hasperué, J.H.; Ziogas, V. Non-Chemical Treatments for Preventing the Postharvest Fungal Rotting of Citrus Caused by Penicillium Digitatum (Green Mold) and Penicillium Italicum (Blue Mold). Trends Food Sci. Technol. 2019, 86, 479–491. [Google Scholar] [CrossRef]
- Feng, J.; Hu, Y.; Grant, E.; Lu, X. Determination of Thiabendazole in Orange Juice Using an MISPE-SERS Chemosensor. Food Chem. 2018, 239, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Alarcan, J.; Waizenegger, J.; Solano, M.; de Lourdes MarzoSolano, M.; Luckert, C.; Peijnenburg, A.; Stoopen, G.; Sharma, R.P.; Kumar, V.; Marx-Stoelting, P.; et al. Hepatotoxicity of the Pesticides Imazalil, Thiacloprid and Clothianidin—Individual and Mixture Effects in a 28-Day Study in Female Wistar Rats. Food Chem. Toxicol. 2020, 140, 111306. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Torres, P.; Tuset, J.J. Molecular Insights into Fungicide Resistance in Sensitive and Resistant Penicillium Digitatum Strains Infecting Citrus. Postharvest Biol. Technol. 2011, 59, 159–165. [Google Scholar] [CrossRef]
- Samaras, A.; Ntasiou, P.; Myresiotis, C.; Karaoglanidis, G. Multidrug Resistance of Penicillium Expansum to Fungicides: Whole Transcriptome Analysis of MDR Strains Reveals Overexpression of Efflux Transporter Genes. Int. J. Food Microbiol. 2020, 335, 108896. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Qin, T.; Li, N.; Niu, Y.; Li, D.; Yuan, Y.; Geng, H.; Xiong, L.; Liu, D. Whole Transcriptome Analysis of Penicillium Digitatum Strains Treatmented with Prochloraz Reveals Their Drug-Resistant Mechanisms. BMC Genom. 2015, 16, 855. [Google Scholar] [CrossRef]
- Guan, J.; de Waard, M.A. Interaction of Microsomal Cytochrome P450 Isozymes Isolated from Wild-Type and DMI-Resistant Isolates of Penicillium Italicum with DMI Fungicides. Pestic. Biochem. Physiol. 1992, 43, 152–161. [Google Scholar] [CrossRef]
- Moussa, H.; Omari, B.E.; Chefchaou, H.; Tanghort, M.; Mzabi, A.; Chami, N.; Remmal, A. Action of Thymol, Carvacrol and Eugenol on Penicillium and Geotrichum Isolates Resistant to Commercial Fungicides and Causing Postharvest Citrus Decay. Can. J. Plant Pathol. 2021, 43, 26–34. [Google Scholar] [CrossRef]
- Tao, N.; Jia, L.; Zhou, H. Anti-Fungal Activity of Citrus Reticulata Blanco Essential Oil against Penicillium Italicum and Penicillium Digitatum. Food Chem. 2014, 153, 265–271. [Google Scholar] [CrossRef]
- Govindachari, T.R.; Suresh, G.; Gopalakrishnan, G.; Masilamani, S.; Banumathi, B. Antifungal Activity of Some Tetranortriterpenoids. Fitoterapia 2000, 71, 317–320. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Ji, S.; Chen, T.; Tian, S.; Qin, G. Enhancement of Biocontrol Efficacy of Cryptococcus Laurentii by Cinnamic Acid against Penicillium Italicum in Citrus Fruit. Postharvest Biol. Technol. 2019, 149, 42–49. [Google Scholar] [CrossRef]
- Hu, J.; Shi, X.; Chen, J.; Mao, X.; Zhu, L.; Yu, L.; Shi, J. Alkaloids from Toddalia Asiatica and Their Cytotoxic, Antimicrobial and Antifungal Activities. Food Chem. 2014, 148, 437–444. [Google Scholar] [CrossRef]
- Chen, C.; Cai, N.; Chen, J.; Wan, C. Clove Essential Oil as an Alternative Approach to Control Postharvest Blue Mold Caused by Penicillium Italicum in Citrus Fruit. Biomolecules 2019, 9, 197. [Google Scholar] [CrossRef]
- Rana, I.S.; Rana, A.S.; Rajak, R.C. Evaluation of Antifungal Activity in Essential Oil of the Syzygium Aromaticum (L.) by Extraction, Purification and Analysis of Its Main Component Eugenol. Braz. J. Microbiol. 2011, 42, 1269–1277. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Chen, H.; Fan, Y.; Shi, Z. Antifungal Activity of Eugenol against Botrytis Cinerea. Trop. Plant Pathol. 2010, 35, 137–143. [Google Scholar] [CrossRef]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Eugenol-Loaded Antimicrobial Nanoemulsion Preserves Fruit Juice against, Microbial Spoilage. Colloids Surf. B Biointerfaces 2014, 114, 392–397. [Google Scholar] [CrossRef]
- Hu, Q.; Gerhard, H.; Upadhyaya, I.; Venkitanarayanan, K.; Luo, Y. Antimicrobial Eugenol Nanoemulsion Prepared by Gum Arabic and Lecithin and Evaluation of Drying Technologies. Int. J. Biol. Macromol. 2016, 87, 130–140. [Google Scholar] [CrossRef]
- Vieira da Silva, S.A.; Clemente, A.; Rocha, J.; Direito, R.; Marques, H.C.; Sepodes, B.; Figueira, M.-E.; Ribeiro, M.H. Anti-Inflammatory Effect of Limonin from Cyclodextrin (Un)Processed Orange Juices in in Vivo Acute Inflammation and Chronic Rheumatoid Arthritis Models. J. Funct. Food. 2018, 49, 146–153. [Google Scholar] [CrossRef]
- Kuo, R.-Y.; Qian, K.; Morris-Natschke, S.L.; Lee, K.-H. Plant-Derived Triterpenoids and Analogues as Antitumor and Anti-HIV Agents. Nat. Prod. Rep. 2009, 26, 1321–1344. [Google Scholar] [CrossRef]
- Chidambara Murthy, K.N.; Jayaprakasha, G.K.; Kumar, V.; Rathore, K.S.; Patil, B.S. Citrus Limonin and Its Glucoside Inhibit Colon Adenocarcinoma Cell Proliferation through Apoptosis. J. Agric. Food Chem. 2011, 59, 2314–2323. [Google Scholar] [CrossRef]
- Mokbel, M.S.; Suganuma, T. Antioxidant and Antimicrobial Activities of the Methanol Extracts from Pummelo (Citrus Grandis Osbeck) Fruit Albedo Tissues. Eur. Food Res. Technol. 2006, 224, 39–47. [Google Scholar] [CrossRef]
- Okwu, D.E.; Awurum, A.N.; Okoronkwo, J.I. Phytochemical Composition and In Vitro Antifungal Activity Screening of Extracts from Citrus Plants against Fusarium Oxysporum of Okra Plant (Hibiscus Esculentus). Pest Technol. 2007, 145–148. [Google Scholar]
- Ayaz, M.; Ullah, F.; Sadiq, A.; Ullah, F.; Ovais, M.; Ahmed, J.; Devkota, H.P. Synergistic Interactions of Phytochemicals with Antimicrobial Agents: Potential Strategy to Counteract Drug Resistance. Chem. Biol. Interact. 2019, 308, 294–303. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Chen, Y.; Ji, L.; Yao, W. Synergistic Properties of Citral and Eugenol for the Inactivation of Foodborne Molds in Vitro and on Bread. LWT 2020, 122, 109063. [Google Scholar] [CrossRef]
- Zahi, M.R.; El Hattab, M.; Liang, H.; Yuan, Q. Enhancing the Antimicrobial Activity of D-Limonene Nanoemulsion with the Inclusion of ε-Polylysine. Food Chem. 2017, 221, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah, M.; Hashemi, M. Boosting Antifungal Effect of Essential Oils Using Combination Approach as an Efficient Strategy to Control Postharvest Spoilage and Preserving the Jujube Fruit Quality. Postharvest Biol. Technol. 2020, 164, 111159. [Google Scholar] [CrossRef]
- Van Vuuren, S.F.; Viljoen, A.M. Interaction between the Non-Volatile and Volatile Fractions on the Antimicrobial Activity of Tarchonanthus Camphoratus. South Afr. J. Bot. 2009, 75, 505–509. [Google Scholar] [CrossRef]
- Li, W.; Chen, H.; He, Z.; Han, C.; Liu, S.; Li, Y. Influence of Surfactant and Oil Composition on the Stability and Antibacterial Activity of Eugenol Nanoemulsions. LWT-Food Sci. Technol. 2015, 62, 39–47. [Google Scholar] [CrossRef]
- Perumal, S.; Pillai, S.; Cai, L.W. Determination of Minimum Inhibitory Concentration of Euphorbia Hirta (L.) Extracts by Tetrazolium Microplate Assay. J. Nat. Prod. 2012, 5, 9. [Google Scholar]
- Chen, J.; Wu, L.; Lu, M.; Lu, S.; Li, Z.; Ding, W. Comparative Study on the Fungicidal Activity of Metallic MgO Nanoparticles and Macroscale MgO Against Soilborne Fungal Phytopathogens. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Campaniello, D.; Corbo, M.R.; Sinigaglia, M. Antifungal Activity of Eugenol against Penicillium, Aspergillus, and Fusarium Species. J. Food Prot. 2010, 73, 1124–1128. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, N.K.; Srivastava, A.; Kataria, A.; Dubey, S.; Sharma, S.; Kundu, B. Clove and Lemongrass Oil Based Non-Ionic Nanoemulsion for Suppressing the Growth of Plant Pathogenic Fusarium Oxysporum f.Sp. Lycopersici. Ind. Crops Prod. 2018, 123, 353–362. [Google Scholar] [CrossRef]
- Chen, C.; Wan, C.; Peng, X.; Chen, J. A Flavonone Pinocembroside Inhibits Penicillium Italicum Growth and Blue Mold Development in “Newhall” Navel Oranges by Targeting Membrane Damage Mechanism. Pestic. Biochem. Physiol. 2020, 165, 104505. [Google Scholar] [CrossRef]
- Kumar, A.; Pratap Singh, P.; Prakash, B. Unravelling the Antifungal and Anti-Aflatoxin B1 Mechanism of Chitosan Nanocomposite Incorporated with Foeniculum Vulgare Essential Oil. Carbohydr. Polym. 2020, 236, 116050. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F. An Overview on Chemical Aspects and Potential Health Benefits of Limonoids and Their Derivatives. Crit. Rev. Food Sci. Nutr. 2014, 54, 225–250. [Google Scholar] [CrossRef]
- Dandekar, D.V.; Jayaprakasha, G.K.; Patil, B.S. Hydrotropic Extraction of Bioactive Limonin from Sour Orange (Citrus Aurantium L.) Seeds. Food Chem. 2008, 109, 515–520. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, C.; Luo, T.; Wang, J.; Tang, Y.; Chen, Z.; Yu, L. Limonin: A Review of Its Pharmacology, Toxicity, and Pharmacokinetics. Molecules 2019, 24, 3679. [Google Scholar] [CrossRef]
- Roy, A.; Saraf, S. Limonoids: Overview of Significant Bioactive Triterpenes Distributed in Plants Kingdom. Biol. Pharm. Bull. 2006, 29, 191–201. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Sharifzadeh, A.; Shokri, H.; Khosravi, A.R.; Abbaszadeh, A. Antifungal Efficacy of Thymol, Carvacrol, Eugenol and Menthol as Alternative Agents to Control the Growth of Food-Relevant Fungi. J. Mycol. Médicale 2014, 24, e51–e56. [Google Scholar] [CrossRef]
- Zhao, R.; Song, R.; Sun, G.; Liu, S.; Li, B.; Cao, Y.; Li, Y. Cutoff Ostwald Ripening Stability of Eugenol-in-Water Emulsion by Co-Stabilization Method and Antibacterial Activity Evaluation. Food Hydrocoll. 2020, 107, 105925. [Google Scholar] [CrossRef]
- Hao, W.; Zhong, G.; Hu, M.; Luo, J.; Weng, Q.; Rizwan-ul-Haq, M. Control of Citrus Postharvest Green and Blue Mold and Sour Rot by Tea Saponin Combined with Imazalil and Prochloraz. Postharvest Biol. Tech. 2010, 56, 39–43. [Google Scholar] [CrossRef]
- Tao, N.; OuYang, Q.; Jia, L. Citral Inhibits Mycelial Growth of Penicillium Italicum by a Membrane Damage Mechanism. Food Control 2014, 41, 116–121. [Google Scholar] [CrossRef]
- Carmo, E.S.; de Oliveira Lima, E.; de Souza, E.L.; de Sousa, F.B. Effect of Cinnamomum Zeylanicum Blume Essential Oil on the Rowth and Morphogenesis of Some Potentially Pathogenic Aspergillus Species. Braz. J. Microbiol. 2008, 39, 91–97. [Google Scholar] [CrossRef]
- Chen, C.; Qi, W.; Peng, X.; Chen, J.; Wan, C. Inhibitory Effect of 7-Demethoxytylophorine on Penicillium Italicum and Its Possible Mechanism. Microorganisms 2019, 7, 36. [Google Scholar] [CrossRef]
- Yan, F.; Li, C.; Ye, X.; Lian, Y.; Wu, Y.; Wang, X. Antifungal Activity of Lipopeptides from Bacillus Amyloliquefaciens MG3 against Colletotrichum Gloeosporioides in Loquat Fruits. Biol. Control 2020, 146, 104281. [Google Scholar] [CrossRef]
- Daum, G.; Lees, N.D.; Bard, M.; Dickson, R. Biochemistry, Cell Biology and Molecular Biology of Lipids of Saccharomyces Cerevisiae. Yeast 1998, 14, 1471–1510. [Google Scholar] [CrossRef]
- Das, S.; Singh, V.K.; Dwivedy, A.K.; Chaudhari, A.K.; Deepika; Dubey, N.K. Eugenol Loaded Chitosan Nanoemulsion for Food Protection and Inhibition of Aflatoxin B1 Synthesizing Genes Based on Molecular Docking. Carbohydr. Polym. 2021, 255, 117339. [Google Scholar] [CrossRef]



| Groups | Concentrations a | Mycelial Growth in PDB (3rd Day) | Mycelial Growth on PDA (6th Day) | MIC a | MFC a |
|---|---|---|---|---|---|
| EG | 1280 | − | − | 320 | 320 |
| 640 | − | − | |||
| 320 | − | − | |||
| 160 | + | ||||
| 80 | + | ||||
| EGL | 1280 | − | − | 160 | 320 |
| 640 | − | − | |||
| 320 | − | − | |||
| 160 | − | + | |||
| 80 | + | ||||
| PDB + Stain | 0 | + | |||
| PDB | 0 | − |
| Groups | Infected Oranges (%) | Infected Cuts (%) | ||
|---|---|---|---|---|
| 3 d | 5 d | 3 d | 5 d | |
| CK | 75.0 ± 12.5 a | 100.0 ± 0.0 a | 59.7 ± 6.4 a | 84.7 ± 6.4 a |
| EG | 37.5 ± 12.5 b | 58.3 ± 7.2 b | 29.2 ± 11.0 b | 38.9 ± 8.7 b |
| EGL | 20.8 ± 7.2 b | 29.2 ± 7.2 c | 12.5 ± 4.2 c | 19.4 ± 6.4 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhao, R.; Li, Y.; Zhou, Z. Limonin Enhances the Antifungal Activity of Eugenol Nanoemulsion against Penicillium Italicum In Vitro and In Vivo Tests. Microorganisms 2021, 9, 969. https://doi.org/10.3390/microorganisms9050969
Li Y, Zhao R, Li Y, Zhou Z. Limonin Enhances the Antifungal Activity of Eugenol Nanoemulsion against Penicillium Italicum In Vitro and In Vivo Tests. Microorganisms. 2021; 9(5):969. https://doi.org/10.3390/microorganisms9050969
Chicago/Turabian StyleLi, Yi, Runan Zhao, Yan Li, and Zhiqin Zhou. 2021. "Limonin Enhances the Antifungal Activity of Eugenol Nanoemulsion against Penicillium Italicum In Vitro and In Vivo Tests" Microorganisms 9, no. 5: 969. https://doi.org/10.3390/microorganisms9050969
APA StyleLi, Y., Zhao, R., Li, Y., & Zhou, Z. (2021). Limonin Enhances the Antifungal Activity of Eugenol Nanoemulsion against Penicillium Italicum In Vitro and In Vivo Tests. Microorganisms, 9(5), 969. https://doi.org/10.3390/microorganisms9050969






