A Systematic Review (1990–2021) of Wild Animals Infected with Zoonotic Leishmania
Abstract
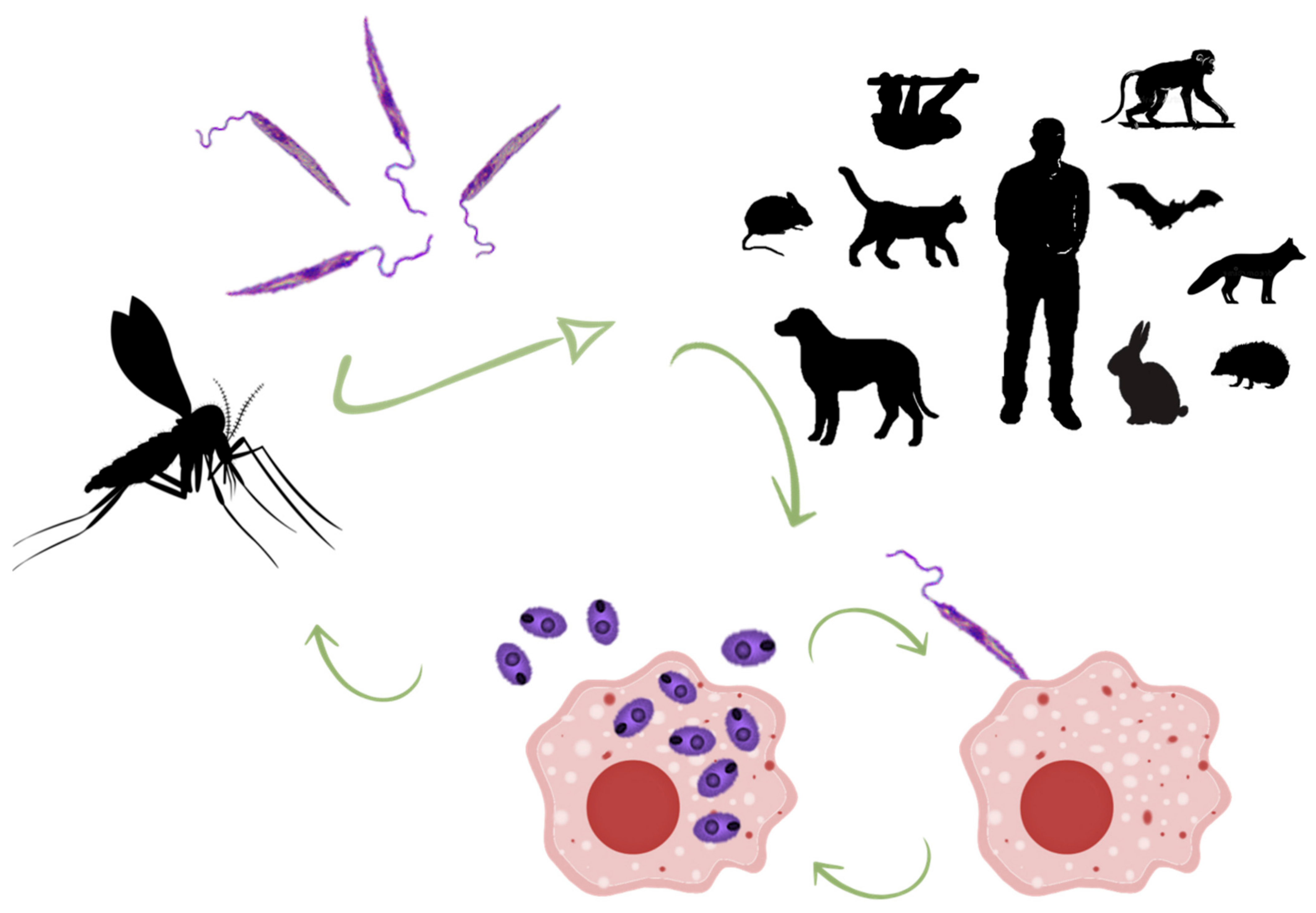
1. Introduction
2. Methods
2.1. Search Strategy and Databases
2.2. Exclusion Criteria
3. Results
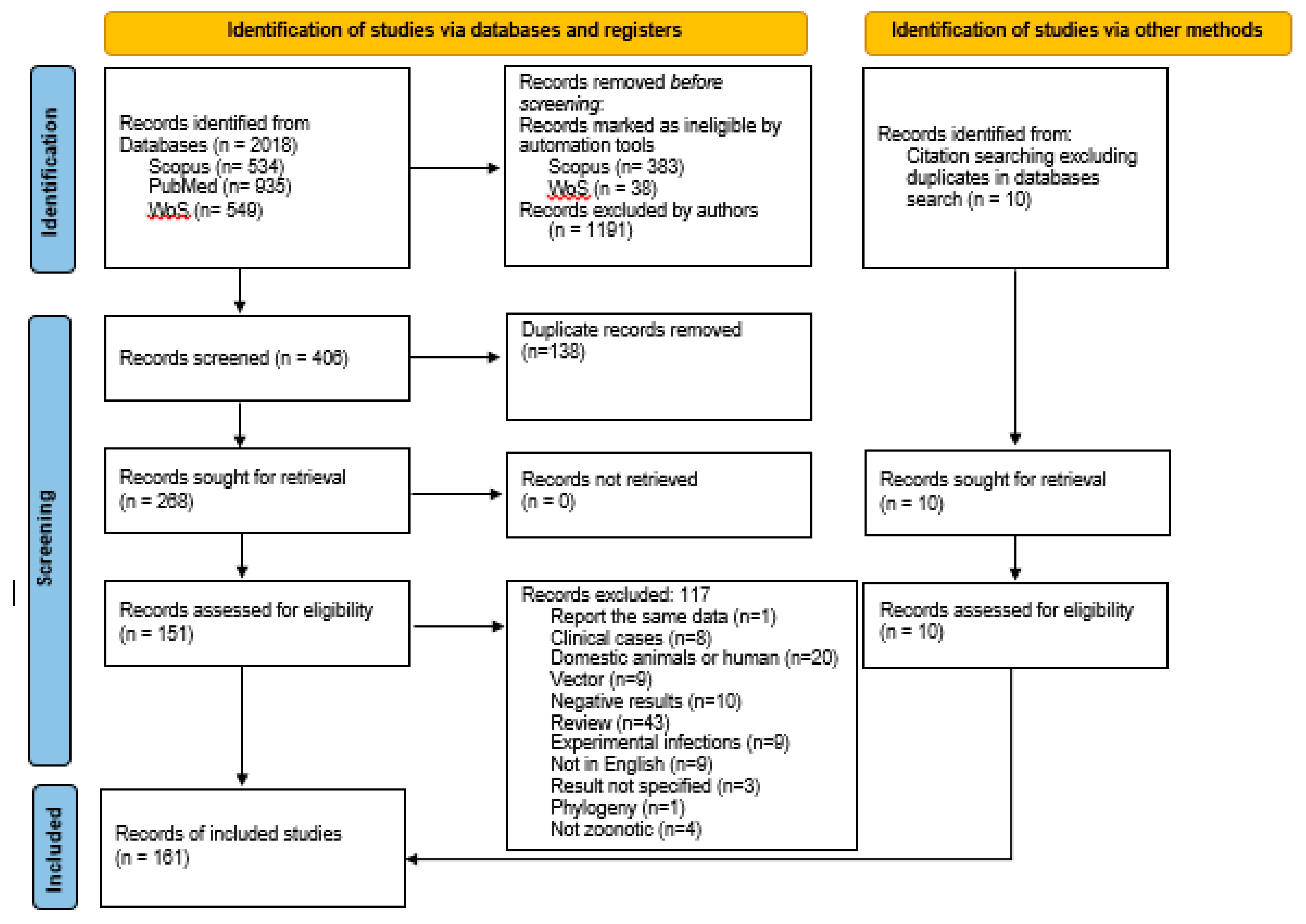
3.1. Result of the Search
3.2. Wild Animals Infected with Zoonotic Leishmania (Viannia) spp.
3.3. Wild Animals Infected with Leishmania amazonensis
3.4. Wild Animals Infected with Leishmania mexicana
3.5. Wild Animals Infected with Leishmania infantum (L. chagasi)
3.5.1. L. infantum in the Americas
3.5.2. L. infantum in Wild Animals from Europe, Asia and Africa
3.6. Wild Animals Infected with L. major
3.7. Wild Animals Infected with Leishmania tropica
3.8. Wild Animals Infected with Leishmania donovani
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Maxfield, L.; Crane, J.S. Leishmaniasis; StatPearls Pulblishing: Treasure Island, FL, USA, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK531456/ (accessed on 6 September 2020).
- Bern, C.; Maguire, J.H.; Alvar, J. Complexities of assessing the disease burden attributable to leishmaniasis. PLoS Negl. Trop. Dis. 2008, 2, e313. [Google Scholar] [CrossRef]
- World Health Organization. Leishmaniasis Situation and Trends; Global Health Observatory data; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Akhoundi, M.; Kuhls, K.; Canne, A.; Votýpka, J.; Marty, P.; Delaunay, P.; Sereno, D. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies. PLoS Negl. Trop. Dis. 2016, 10, e0004349. [Google Scholar] [CrossRef] [PubMed]
- Akhoundi, M.; Downing, T.; Votýpka, J.; KKuhls, K.; Lukeš, J.; Cannet, A.; Ravel, C.; Marty, P.; Delaunay, P.; Kasbari, M.; et al. Leishmania Infections: Molecular Targets and Diagnosis. Mol. Asp. Med. 2017, 57, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Schönian, G.; Mauricio, I.; Cupolillo, E. Is it time to revise the nomenclature of Leishmania? Trends Parasitol. 2010, 26, 466–469. [Google Scholar] [CrossRef]
- Pruzinova, K.; Sadlova, J.; Seblova, V.; Homola, M.; Votypka, J.; Volf, P. Comparison of bloodmeal digestion and the peritrophic matrix in four sand fly species differing in susceptibility to Leishmania donovani. PLoS ONE 2015, 10, e0128203. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Control of the Leishmaniases; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2010; pp. 1–186. [Google Scholar]
- Naucke, T.J.; Lorentz, S. First Report of Venereal and Vertical Transmission of Canine Leishmaniosis from Naturally Infected Dogs in Germany. Parasit. Vectors 2012, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Guedes, D.L.; van Henten, S.; Cnops, L.; Adriaensen, W.; Johan Griensven, J. Sexual Transmission of Visceral Leishmaniasis: A Neglected Story. Trends Parasitol. 2020, 36, 950–952. [Google Scholar] [CrossRef]
- Naucke, T.J.; Amelung, S.; Lorentz, S. First Report of Transmission of Canine Leishmaniosis through Bite Wounds from a Naturally Infected Dog in Germany. Parasit. Vectors 2016, 9, 1–4. [Google Scholar] [CrossRef]
- Coutinho, M.T.Z.; Linardi, P.M. Can Fleas from Dogs Infected with Canine Visceral Leishmaniasis Transfer the Infection to Other Mammals? Vet. Parasitol. 2007, 147, 320–325. [Google Scholar] [CrossRef]
- Lainson, R. Ecological Interactions in the Transmission of the Leishmaniases. Phil. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1988, 321, 389–404. [Google Scholar] [CrossRef]
- De Oliveira, F.M.; Costa, L.H.C.; de Barros, T.L.; Ito, P.K.R.K.; Colombo, F.A.; Carvalho, C.; Pedro, W.A.; Queiroz, L.H.; Nunes, C.M. First Detection of Leishmania Spp. DNA in Brazilian Bats Captured Strictly in Urban Areas. Acta Trop. 2015, 150, 176–181. [Google Scholar] [CrossRef]
- Hamad, I.; Forestier, C.L.; Peeters, M.; Delaporte, E.; Raoult, D.; Bittar, F. Wild Gorillas as a Potential Reservoir of Leishmania major. J. Infect. Dis. 2015, 211, 267–273. [Google Scholar] [CrossRef]
- Rescigno, M.; Urbano, M.; Valzasina, B.; Francolín, M.; Rotta, G.; Bonasio, R.; Granucci, F.; Kraehenbuhl, J.-P.; Ricciardi-Castagnoli, P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001, 2, 361–367. [Google Scholar] [CrossRef]
- Rodríguez, J.M. The origin of human milk bacteria: Is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv. Nutr. 2014, 5, 779–784. [Google Scholar] [CrossRef]
- World Health Organization. Leishmaniasis Facksheet. Available online: https://www.who.int/en/news-room/fact-sheets/detail/leishmaniasis (accessed on 8 January 2021).
- Cardoso, L.; Schallig, H.; Persichetti, M.F.; Pennisi, M.G. New Epidemiological Aspects of Animal Leishmaniosis in Europe: The Role of Vertebrate Hosts Other than Dogs. Pathogens 2021, 10, 307. [Google Scholar] [CrossRef]
- Miró, G.; Müller, A.; Montoya, A.; Checa, R.; Marino, V.; Marino, E.; Fuster, F.; Escacena, C.; Descalzo, M.A.; Gálvez, R. Epidemiological Role of Dogs since the Human Leishmaniosis Outbreak in Madrid. Parasit. Vectors 2017, 10, 209. [Google Scholar] [CrossRef]
- Quaresma, P.F.; Rego, F.D.; Botelho, H.A.; da Silva, S.R.; Moura, A.J., Jr.; Teixeira Neto, R.G.; Madeira, F.M.; Carvalho, M.B.; Paglia, A.P.; Melo, M.N.; et al. Wild, synanthropic and domestic hosts of Leishmania in an endemic area of cutaneous leishmaniasis in Minas Gerais State, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 579–585. [Google Scholar] [CrossRef]
- World Health Organization. Control of the Leishmaniases: Report of a WHO Expert Committee; WHO Report Series; World Health Organization: Geneva, Switzerland, 1990; Volume 793. [Google Scholar]
- Ashford, R.W. Leishmaniasis Reservoirs and Their Significance in Control. Clin. Dermatol. 1996, 14, 523–532. [Google Scholar] [CrossRef]
- Roque, A.L.R.; Jansen, A.M. Wild and Synanthropic Reservoirs of Leishmania Species in the Americas. Int. J. Parasitol. Parasit. Wildl. 2014, 3, 251–262. [Google Scholar] [CrossRef]
- Souza, T.D.; Turchetti, A.P.; Fujiwara, R.T.; Paixão, T.A.; Santos, R.L. Visceral Leishmaniasis in Zoo and Wildlife. Vet. Parasitol. 2014, 200, 233–241. [Google Scholar] [CrossRef]
- Millán, J.; Ferroglio, E.; Solano-Gallego, L. Role of Wildlife in the Epidemiology of Leishmania infantum infection in Europe. Parasitol. Res. 2014, 113, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Quinnell, R.J.; Courtenay, O. Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology 2009, 136, 1915–1934. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.; Lozano, C.; Barker, D.C.; McCann, S.H.E.; Adler, G.H. Detection of Leishmania (Viannia) braziliensis Complex in Wild Mammals from Colombian Coffee Plantations by PCR and DNA Hybridization. Acta Trop. 1998, 69, 41–50. [Google Scholar] [CrossRef]
- Brandão-Filho, S.P.; Brito, M.E.; Carvalho, F.G.; Ishikaw, E.A.; Cupolillo, E.; Floeter-Winter, L.; Shaw, J.J. Wild and Synanthropic Hosts of Leishmania (Viannia) braziliensis in the Endemic Cutaneous Leishmaniasis Locality of Amaraji, Pernambuco State, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 291–296. [Google Scholar] [CrossRef]
- Tonelli, G.B.; Tanure, A.; Rego, F.D.; Carvalho, G.M.L.; Stumpp, R.; Ássimos, G.R.; Campos, A.M.; Lima, A.C.V.M.; Gontijo, C.M.F.; Paz, G.F.; et al. Leishmania (Viannia) braziliensis Infection in Wild Small Mammals in Ecotourism Area of Brazil. PLoS ONE 2017, 12, e0190315. [Google Scholar] [CrossRef]
- Lucas, J.; Lourenço, M.; Minuzzi-souza, T.T.C.; Silva, L.R.; Oliveira, A.M. High Frequency of Trypanosomatids in Gallery Forest Bats of a Neotropical Savanna. Acta Trop. 2018, 177, 200–206. [Google Scholar]
- De Lima, H.; De Guglielmo, Z.; Rodriguez, A.; Convit, J.; Rodriguez, N. Cotton rats (Sigmodon hispidus) and black rats (Rattus rattus) as possible reservoirs of Leishmania spp. in Lara State, Venezuela. Mem. Inst. Oswaldo Cruz 2002, 97, 169–174. [Google Scholar] [CrossRef]
- Trüeb, I.; Portela, R.D.; Franke, C.R.; Carneiro, I.O.; Ribeiro, G.J.; Soares, R.P.; Barrouin-Melo, S.M. Trypanosoma cruzi and Leishmania sp. Infection in Wildlife from Urban Rainforest Fragments in Northeast Brazil. J. Wildl. Dis. 2018, 54, 76–84. [Google Scholar] [CrossRef]
- Cardoso, R.M.; De Araújo, N.S.L.; Romero, G.A.S.; Souza, T.T.C.M.; Dietrich, A.G.; Mendes, J.D.; Reis, M.L.; Ferreira, J.B.C.; Hecht, M.M.; Gurgel-Gonçalves, R. Expanding the Knowledge about Leishmania Species in Wild Mammals and Dogs in the Brazilian Savannah. Parasit. Vectors 2015, 8, 171. [Google Scholar] [CrossRef]
- Cássia-Pires, R.; Boité, M.C.; D’Andrea, P.S.; Herrera, H.M.; Cupolillo, E.; Jansen, A.M.; Roque, A.L.R. Distinct Leishmania Species Infecting Wild Caviomorph Rodents (Rodentia: Hystricognathi) from Brazil. PLoS Negl. Trop. Dis. 2014, e3389. [Google Scholar] [CrossRef]
- Voltarelli, E.M.; Arraes, S.M.A.A.; Perles, T.F.; Lonardoni, M.V.C.; Teodoro, U.; Silveira, T.G.V. Serological survey for Leishmania sp. Infection in wild animals from the municipality of Maringá, Paraná state, Brazil. J. Venom. Anim. Toxins Incl. Trop. Dis. 2009, 15, 732–744. [Google Scholar] [CrossRef]
- Schallig, H.D.F.H.; da Silva, E.S.; van der Meide, W.F.; Schoone, G.J.; Contifjo, C.M.F. Didelphis marsupialis (Common Opossum): A Potential Reservoir Host for Zoonotic Leishmaniasis in the Metropolitan Region of Belo Horizonte (Minas Gerais, Brazil). Vector Borne Zoonotic Dis. 2007, 7, 387–393. [Google Scholar] [CrossRef]
- Lima, B.S.; Dantas-Torres, F.; de Carvalho, M.R.; Marinho-Junior, J.F.; de Almeida, E.L.; Brito, M.E.; Gomes, F.; Brandão-Filho, S.P. Small mammals as hosts of Leishmania spp. in a highly endemic area for zoonotic leishmaniasis in North-Eastern Brazil. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 592–597. [Google Scholar] [CrossRef]
- Aransay, A.M.; Scoulica, E.; Tselentis, Y. Detection and identification of Leishmania DNA within naturally infected sand flies by seminested PCR on minicircle kinetoplastic DNA. Appl. Environ. Microbiol. 2000, 66, 1933–1938. [Google Scholar] [CrossRef]
- Telleria, J.; Bosseno, M.F.; Tarifa, T.; Buitrago, R.; Martinez, E.; Torrez, M.; Le Pont, F.; Brenière, S.F. Putative reservoirs of Leishmania amazonensis in a Sub-Andean focus of Bolivia identified by kDNA-polymerase chain reaction. Mem. Inst. Oswaldo Cruz 1999, 94, 5–6. [Google Scholar] [CrossRef][Green Version]
- Shapiro, J.T.; da Costa Lima Junior, M.S.; Dorval, M.E.; de Oliveira, F.A.; Cepa Matos, M.F.; Bordignon, M.O. First record of Leishmania braziliensis presence detected in bats, Mato Grosso do Sul, southwest Brazil. Acta Trop. 2013, 128, 171–174. [Google Scholar] [CrossRef]
- Castro Ferreira, E.; Pereira, A.A.S.; Silveira, M.; Margonari, C.; Marcon, G.E.B.; França, A.O.; Souza, L.; Castro, L.S.; OscarBordignon, M.; Fischer, E.; et al. Leishmania (V.) braziliensis Infecting Bats from Pantanal Wetland, Brazil: First Records for Platyrrhinus lineatus and Artibeus planirostris. Acta Trop. 2017, 172, 217–222. [Google Scholar] [CrossRef]
- Castro, L.S.; Dorval, M.E.C.; Matheus, L.M.D.; Bednaski, A.V.; Facco, G.G.; Silveira, M.; Santos, C.F.; Gontijo, C.M.F.; Oliveira, A.P.G.; Ferreira, E.C. Leishmania Presence in Bats in Areas Endemic for Leishmaniasis in Central-West Brazil. Int. J. Parasitol. Parasit. Wildl. 2020, 11, 261–267. [Google Scholar] [CrossRef]
- Gómez-Hernández, C.; Bento, E.C.; Rezende-Oliveira, K.; Nascentes, G.A.N.; Barbosa, C.G.; Batista, L.R.; Tiburcio, M.G.S.; Pedrosa, A.L.; Lages-Silva, E.; Ramírez, J.D.; et al. Leishmania Infection in Bats from a Non-Endemic Region of Leishmaniasis in Brazil. Parasitology 2017, 144, 1980–1985. [Google Scholar] [CrossRef]
- Naiff, R.D.; Freitas, R.A.; Naiff, M.F.; Arias, J.R.; Barrett, T.V.; Momen, H.; Grimaldi Júnior, G. Epidemiological and Nosological aspects of Leishmania naiffi Lainson & Shaw, 1989. Mem Inst. Oswaldo Cruz 1991, 86, 317–321. [Google Scholar] [PubMed]
- Llanos-Cuentas, E.A.; Roncal, N.; Villaseca, P.; Paz, L.; Ogusuku, E.; Perez, J.E.; Cáceres, A.; Davies, C.R. Natural infections of Leishmania peruviana in animals in the Peruvian Andes. Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 15–20. [Google Scholar] [CrossRef]
- Quintal, A.P.; Ribeiro, E.S.; Rodrigues, F.P.; Rocha, F.S.; Floeter-Winter, L.M.; Nunes, C.M. Leishmania spp. in Didelphis albiventris and Micoureus paraguayanus (Didelphimorphia: Didelphidae) of Brazil. Vet. Parasitol. 2011, 176, 112–119. [Google Scholar] [CrossRef]
- Ocampo, C.B.; Ferro, M.C.; Cadena, H.; Gongora, R.; Pérez, M.; Valderrama-Ardila, C.H.; Quinnell, R.J.; Alexander, N. Environmental Factors Associated with American Cutaneous Leishmaniasis in a New Andean Focus in Colombia. Trop. Med. Int. Health 2012, 17, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- de Castro Ferreira, E.; Cruz, I.; Cañavate, C.; de Melo, L.A.; Pereira, A.A.S.; Madeira, F.A.M.; Nogueira Valério, S.A.; Cunha, H.M.; Paglia, A.P.; Ferreira Gontijo, C.M. Mixed Infection of Leishmania infantum and Leishmania braziliensis in Rodents from Endemic Urban Area of the New World. BMC Vet. Res. 2015, 11, 1–7. [Google Scholar] [CrossRef][Green Version]
- Silva, E.M.; Alves, L.C.; Guerra, N.R.; Farias, M.P.; Oliveira, E.L.; de Souza, R.C.; da Cunha, C.; Ramos, R.A.; Porto, W.J. Leishmania spp. in Didelphis spp. from Northeastern Brazil. J. Zoo Wildl. Med. 2016, 47, 942–944. [Google Scholar] [CrossRef] [PubMed]
- Paiz, L.; Donalisio, M.R.; Richini-Pereira, V.B.; Motoie, G.; Castagna, C.L.; Tolezano, J.E. Infection by Leishmania spp. in Free-Ranging Opossums (Didelphis albiventris) in an Environmentally Protected Area Inhabited by Humans in Southeastern Brazil. Vector Borne Zoonotic Dis. 2016, 16, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.A.S.; De Castro Ferreira, E.; Da Rocha Lima, A.C.V.M.; Tonelli, G.B.; Rêgo, F.D.; Paglia, A.D.; Andrade-Filho, J.D.; Paz, G.F.; Gontijo, C.M.F. Detection of Leishmania spp in Silvatic Mammals and Isolation of Leishmania (Viannia) braziliensis from Rattus rattus in an Endemic Area for Leishmaniasis in Minas Gerais State, Brazil. PLoS ONE 2017, 12, e0187704. [Google Scholar] [CrossRef]
- Donalisio, M.R.; Paiz, L.M.; da Silva, V.G.; Richini-Pereira, V.B.; von Zuben, A.P.B.; Castagna, C.L.; Motoie, G.; Hiramoto, R.M.; Tolezano, J.E. Visceral leishmaniasis in an environmentally protected area in southeastern Brazil: Epidemiological and laboratory cross-sectional investigation of phlebotomine fauna, wild hosts and canine cases. PLoS Negl. Trop. Dis. 2017, 11, e0005666. [Google Scholar] [CrossRef]
- Brandão, E.; Xavier, S.C.; Rocha, F.L.; Lima, C.F.; Candeias, Í.Z.; Lemos, F.G.; Azevedo, F.C.; Jansen, A.M.; Roque, A.L. Trypanosomatids in Small Mammals of an Agroecosystem in Central Brazil: Another Piece in the Puzzle of Parasite Transmission in an Anthropogenic Landscape. Pathogens 2019, 8, 190. [Google Scholar] [CrossRef]
- González, K.; Calzada, J.E.; Saldaña, A.; Rigg, C.A.; Alvarado, G.; Rodríguez-Herrera, B.; Kitron, U.D.; Adler, G.H.; Gottdenker, N.L.; Chaves, L.F.; et al. Survey of Wild Mammal Hosts of Cutaneous Leishmaniasis Parasites in Panamá and Costa Rica. Trop. Med. Health 2015, 43, 75–78. [Google Scholar] [CrossRef]
- Silveira, F.T.; Lainson, R.; Shaw, J.J.; Braga, R.R.; Ishikawa, E.E.; Souza, A.A. Cutaneous leishmaniasis in Amazonia: Isolation of Leishmania (Viannia) lainsoni from the rodent Agouti paca (Rodentia: Dasyproctidae), in the state of Para, Brazil. Rev. Inst. Med. Trop. Sao Paulo 1991, 33, 18–22. [Google Scholar] [CrossRef][Green Version]
- Oliveira, F.S.; Pirmez, C.; Pires, M.Q.; Brazil, R.P.; Pacheco, R.S. PCR-Based Diagnosis for Detection of Leishmania in Skin and Blood of Rodents from an Endemic Area of Cutaneous and Visceral Leishmaniasis in Brazil. Vet. Parasitol. 2005, 129, 219–227. [Google Scholar] [CrossRef]
- de Freitas, T.P.; D’Andrea, P.S.; de Paula, D.A.; Nakazato, L.; Dutra, V.; Bonvicino, C.R.; de Almeida, A.d.B.P.F.; Boa-Sorte, E.d.; Sousa, V.R.F. Natural infection of Leishmania (Viannia) braziliensis in Mus musculus captured in Mato Grosso, Brazil. Vector Borne Zoonotic Dis. 2012, 12, 81–83. [Google Scholar] [CrossRef]
- Brito, M.E.F.; Andrade, M.S.; Mendonça, M.G.; Silva, C.J.; Almeida, E.L.; Lima, B.S.; Félix, S.M.; Abath, F.G.C.; da Graça, G.C.; Porrozzi, R.; et al. Species Diversity of Leishmania (Viannia) Parasites Circulating in an Endemic Area for Cutaneous Leishmaniasis Located in the Atlantic Rainforest Region of Northeastern Brazil. Trop. Med. Int. Health 2009, 14, 1278–1786. [Google Scholar] [CrossRef]
- De Bruijn, M.H.L.; Barker, D.C. Diagnosis of New World leishmaniasis: Specific detection of species of the Leishmania braziliensis complex by amplification of kinetoplast DNA. Acta Trop. 1992, 52, 45–58. [Google Scholar] [CrossRef]
- Marcelino, A.P.; Ferreira, E.C.; Avendanha, J.S.; Costa, C.F.; Chiarelli, D.; Almeida, G.; Moreira, E.C.; Leite, R.C.; dos Reis, J.K.P.; Gontijo, C.M.F. Molecular Detection of Leishmania braziliensis in Rattus norvegicus in an Area Endemic for Cutaneous Leishmaniasis in Brazil. Vet. Parasitol. 2011, 183, 54–58. [Google Scholar] [CrossRef]
- Vasconcelos, I.A.; Vasconcelos, A.W.; Fe Filho, N.M.; Queiroz, R.G.; Santana, E.W.; Bozza, M.; Sallenave, S.M.; Valim, C.; David, J.R.; Lopes, U.G. The identity of Leishmania isolated from sand flies and vertebrate hosts in a major focus of cutaneous leishmaniasis in Baturité, northeastern Brazil. Am. J. Trop Med. Hyg. 1994, 50, 158–164. [Google Scholar] [CrossRef]
- Martínez, M.F.; Kowalewski, M.M.; Giuliani, M.G.; Acardi, S.A.; Salomón, O.D. Molecular Identification of Leishmania in Free-Ranging Black and Gold Howler Monkeys (Alouatta caraya) in Northeastern Argentina. Acta Trop. 2020, 210, 105534. [Google Scholar] [CrossRef]
- Acardi, S.A.; Rago, M.V.; Liotta, D.J.; Fernandez-Duque, E.; Salomón, O.D. Leishmania (Viannia) DNA Detection by PCR-RFLP and Sequencing in Free-Ranging Owl Monkeys (Aotus azarai azarai) from Formosa, Argentina. Vet. Parasitol. 2013, 193, 256–259. [Google Scholar] [CrossRef]
- Savani, E.S.; de Almeida, M.F.; de Oliveira Camargo, M.C.; D’Auria, S.R.; Silva, M.M.; de Oliveira, M.L.; Sacramento, D. Detection of Leishmania (Leishmania) amazonensis and Leishmania (Leishmania) infantum chagasi in Brazilian bats. Vet. Parasitol. 2010, 168, 5–10. [Google Scholar] [CrossRef] [PubMed]
- De Lima, V.M.F.; Santiago, M.E.B.; Sanches, L.dC.; de Lima, B.D. Molecular diagnosis of Leishmania amazonensis ina captive spider monkey in Bauru, Sao Paulo, Brazil. J. Zoo Wildl. Med. 2012, 43, 943–945. [Google Scholar] [CrossRef]
- Kerr, S.F.; Emmons, L.H.; Melby, P.C.; Liu, C.; Perez, L.E.; Villegas, M.; Miranda, R. Leishmania amazonensis infections in Oryzomys acritus and Oryzomys nitidus from Bolivia. Am. J. Trop. Med. Hyg. 2006, 75, 1069–1073. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zamora-Ledesma, S.; Hernández-Camacho, N.; Villagrán-Herrera, M.E.; Sánchez-Moreno, M.; Concha-Valdez, F.G.; Jones, R.W.; Moreno-Pérez, M.A.; Camacho-Macías, B. Presence of Trypanosomatid Antibodies in Gray Foxes (Urocyon cinereoargenteus) and Domestic and Feral Dogs (Canis lupus familiaris). Vet. Parasitol. Reg. Stud. Rep. 2016, 5, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Berzunza-Cruz, M.; Rodríguez-Moreno, A.; Gutiérrez-Granados, G.; González-Salazar, C.; Stephens, C.R.; Hidalgo-Mihart, M.; Marina, C.F.; Rebollar-Téllez, E.A.; Bailón-Martínez, D.; Balcells, C.D.; et al. Leishmania (L.) mexicana Infected Bats in Mexico: Novel Potential Reservoirs. PLoS Negl. Trop. Dis. 2015, 9, e0003438. [Google Scholar] [CrossRef] [PubMed]
- Van Wynsberghe, N.R.; Canto-Lara, S.B.; Sosa-Bibiano, E.I.; Rivero-Cardenas, N.A.; Andrade-Narvaez, F.J. Comparison of small mammal prevalence of Leishmania (Leishmania) mexicana in five foci of cutaneous leishmaniasis in the State State of Campeche, Mexico. Rev. Inst. Med. Trop. Sao Paulo 2009, 51, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Van Wynsberghe, N.R.; Canto-Lara, S.B.; Mian-Centeno, A.G.; Itza-Ortiz, M.F.; Andrade-Narvaez, F.J. Retention of Leishmania (Leishmania) mexicana in naturally infected rodents from the State of Campeche, Mexico. Mem. Inst. Oswaldo Cruz 2000, 95, 595–600. [Google Scholar] [CrossRef][Green Version]
- Kerr, S.F.; McHugh, C.P.; Dronen, N.O., Jr. Leishmaniasis in Texas: Prevalence and seasonal transmission of Leishmania mexicana in Neotoma micropus. Am. J. Trop. Med. Hyg. 1995, 53, 73–77. [Google Scholar] [CrossRef]
- Raymond, R.W.; McHugh, C.P.; Witt, L.R.; Kerr, S.F. Temporal and Spatial Distribution of Leishmania mexicana Infections in a Population of Neotoma micropus. Mem. Inst. Oswaldo Cruz 2003, 98, 171–180. [Google Scholar] [CrossRef][Green Version]
- Canto-Lara, S.B.; Van Wynsberghe, N.R.; Vargas-González, A.; Ojeda-Farfán, F.F.; Andrade-Narváez, F.J. Use of Monoclonal Antibodies for the Identification of Leishmania Spp. Isolated from Humans and Wild Rodents in the State of Campeche, Mexico. Mem. Inst. Oswaldo Cruz 1999, 94, 305–309. [Google Scholar] [CrossRef]
- McHug, C.P.; Thies, M.L.; Melby, P.C.; Yantis, L.D.; Raymond, R.W.; Villegas, M.D.; Kerr, S.F. Short report: A disseminated infection of Leishmania mexicana in an eastern woodrat, Neotoma floridana, collected in Texas. Am. J. Trop. Med. Hyg. 2003, 69, 470–472. [Google Scholar] [CrossRef]
- Chable-Santos, J.B.; Van Wynsberghe, N.R.; Canto-Lara, S.B.; Andrade-Narvaez, F.J. Isolation of Leishmania (L.) mexicana from Wild Rodents and Their Possible Role in the Transmission of Localized Cutaneous Leishmaniasis in the State of Campeche, Mexico. Am. J. Trop. Med. Hyg. 1995, 53, 141–145. [Google Scholar] [CrossRef]
- Kipp, E.J.; Mariscal, J.; Armijos, R.X.; Weigel, M.; Waldrup, K. Genetic Evidence of Enzootic Leishmaniasis in a Stray Canine and Texas Mouse from Sites in West and Central Texas. Mem. Inst. Oswaldo Cruz 2016, 111, 652–654. [Google Scholar] [CrossRef]
- Muñoz-García, C.I.; Sánchez-Montes, S.; Villanueva-García, C.; Romero-Callejas, E.; Díaz-López, H.M.; Gordillo-Chávez, E.J.; Martínez-Carrasco, C.; Berriatua, E.; Rendón-Franco, E. The Role of Sloths and Anteaters as Leishmania Spp. Reservoirs: A Review and a Newly Described Natural Infection of Leishmania mexicana in the Northern Anteater. Parasitol. Res. 2019, 118, 1095–1101. [Google Scholar] [CrossRef]
- Rovirosa-Hernández, M.J.; Cortes-Ortíz, L.; García-Orduña, F.; Guzmán-Gómez, D.; López-Monteon, A.; Caba, M.; Ramos-Ligonio, A. Seroprevalence of Trypanosoma cruzi and Leishmania mexicana in free-ranging howler monkeys in southeastern Mexico. Am. J. Primatol. 2013, 75, 161–169. [Google Scholar] [CrossRef]
- Courtenay, O.; Quinnell, R.J.; Garcez, L.M.; Dye, C. Low Infectiousness of a Wildlife Host of Leishmania Infantum: The Crab-Eating Fox Is Not Important for Transmission. Parasitology 2002, 125, 407–414. [Google Scholar] [CrossRef]
- de Almeida Curi, N.H.; Miranda, I.; Talamoni, S.A. Serologic Evidence of Leishmania Infection in Free-Ranging Wild and Domestic Canids around a Brazilian National Park. Mem. Inst. Oswaldo Cruz 2006, 101, 99–101. [Google Scholar] [CrossRef]
- Gomes, R.B.; Mendonca, I.L.; Silva, V.C.; Ruas, J.; Silva, M.B.; Cruz, M.S.P.; Barral, A.; Costa, C.H.N. Antibodies against Lutzomya longipalpis saliva in the fox Cerdocyon thous and the sylvatic cycle of Leishmania chagasi. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 127–133. [Google Scholar] [CrossRef]
- Luppi, M.M.; Malta, M.C.C.; Silva, T.M.A.; Silva, F.L.; Motta, R.O.C.; Miranda, I.; Ecco, R.; Santos, R.L. Visceral Leishmaniasis in Captive Wild Canids in Brazil. Vet. Parasitol. 2008, 155, 146–151. [Google Scholar] [CrossRef]
- Jusi, M.M.G.; Starke-Buzetti, W.A.; de Sousa Oliveira, T.M.F.; da Silva Tenório, M.; de Oliveira de Sousa, L.; Zacarias Machad, R. Molecular and Serological Detection of Leishmania spp. in Captive Wild Animals from Ilha Solteira, SP, Brazil. Rev. Bras. de Parasitol. Veterinária 2011, 20, 219–222. [Google Scholar] [CrossRef]
- Richini-Pereira, V.B.; Marson, P.M.; Hayasaka, E.Y.; Victoria, C.; da Silva, R.C.; Langoni, H. Molecular Detection of Leishmania Spp. in Road-Killed Wild Mammals in the Central Western Area of the State of São Paulo, Brazil. J. Ven. Anim. Tox. Trop. Dis. 2014, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.C.; Melo, R.P.B.; Kim, P.C.P.; Guerra, N.R.; Alves, L.C.; Costa, D.F.; Alves, C.J.; Porto, W.J.N.; Mota, R.A. Molecular and Serological Investigation of Infectious Diseases in Captive and Free-Range Crab-Eating Fox (Cerdocyon thous-Linnaeus, 1776) from Northeastern Brazil. Acta Parasitol. 2018, 63, 184–189. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Curi, N.H.; Coelho, C.M.; Malta, M.C.C.; Magni, L.M.V.; Sábato, M.A.L.; Araújo, A.S.; Lobato, Z.I.P.; Santos, J.L.C.; Santos, H.A.; Ragozo, A.A.M.; et al. Pathogens of Wild Maned Wolves (Chrysocyon brachyurus) in Brazil. J. Wildl. Dis. 2012, 48, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Mol, J.P.S.; Soave, S.A.; Turchetti, A.P.; Pinheiro, G.R.G.; Pessanha, A.T.; Malta, M.C.C.; Tinoco, H.P.; Figueiredo, L.A.; Gontijo, N.F.; Paixão, T.A.; et al. Transmissibility of Leishmania infantum from Maned Wolves (Chrysocyon brachyurus) and Bush Dogs (Speothos venaticus) to Lutzomyia longipalpis. Vet. Parasitol. 2015, 212, 86–91. [Google Scholar] [CrossRef]
- Tolentino, N.; Pinheiro, G.R.G.; Ottino, J.; Rodrigues de Oliveira, A.; Coelho, C.M.; Tinoco, H.P.; Fujiwara, R.T.; Santos, R.L.; Ribeiro, V.M. Serological Evidence of Leishmania Infection by Employing ELISA and Rapid Tests in Captive Felids and Canids in Brazil. Vet. Parasitol. Reg. Stud. Rep. 2019, 17, 100308. [Google Scholar] [CrossRef]
- Lombardi, M.C.; Turchetti, A.P.; Tinoco, H.P.; Pessanha, A.T.; Soave, S.A.; Malta, M.C.C.; Paixão, T.A.; Santos, R.L. Diagnosis of Leishmania Infantum Infection by Polymerase Chain Reaction in Wild Mammals. Pesquisa Vet. Brasil. 2014, 34, 1243–1246. [Google Scholar] [CrossRef][Green Version]
- Paiz, L.M.; Fornazari, F.; Menozzi, B. Serological Evidence of Infection by Leishmania (Leishmania) infantum (Synonym: Leishmania (Leishmania) chagasi) in Free-Ranging Wild Mammals in a Nonendemic Region of the State of São Paulo, Brazil. Vector Borne Zoonotic Dis. 2015, 11, 667–673. [Google Scholar] [CrossRef]
- Dahroug, M.A.; Almeida, A.B.; Sousa, V.R.; Dutra, V.; Turbino, N.C.; Nakazato, L.; de Souza, R.L. Leishmania (Leishmania) chagasi in captive wild felids in Brazil. Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 73–74. [Google Scholar] [CrossRef]
- Dahroug, M.A.; Almeida, A.B.; Sousa, V.R.; Dutra, V.; Guimarães, L.D.; Soares, C.E.; Nakazato, L.; de Souza, R.L. The first case report of Leishmania (leishmania) chagasi in Panthera leo in Brazil. Asian Pac. J. Trop. Biomed. 2011, 3, 249–250. [Google Scholar] [CrossRef]
- de Rezende, M.B.; Herrera, H.M.; Carvalho, C.M.E.; Carvalho Anjos, E.A.; Ramos, C.A.N.; de Araújo, F.R.; Torres, J.M.; de Oliveira, C.E. Detection of Leishmania spp. in Bats from an Area of Brazil Endemic for Visceral Leishmaniasis. Trans. Emerg. Dis. 2017, 64, e36–e42. [Google Scholar] [CrossRef]
- Medkour, H.; Davoust, B.; Dulieu, F.; Maurizi, L.; Lamour, T.; Marié, J.L.; Mediannikov, O. Potential animal reservoirs (dogs and bats) of human visceral leishmaniasis due to Leishmania infantum in French Guiana. PLoS Negl. Trop. Dis. 2019, 13, e0007456. [Google Scholar] [CrossRef]
- De Lima, H.; Rodriguez, N.; Barrios, M.A.; Avila, A.; Canizales, I.; Gutierrez, S. Isolation and molecular identification of Leishmania chagasi from a bat (Carollia perspicillata) in northeastern Venezuela. Mem. Inst. Oswaldo Cruz 2008, 103, 412–414. [Google Scholar] [CrossRef]
- Pereira da Costa, A.; Costa, F.B.; Soares, H.S.; Ramirez, D.G.; de Carvalho, E.T.K.; Gennari, S.M.; Marcili, A. Trypanosoma cruzi and Leishmania infantum chagasi Infection in Wild Mammals from Maranhão State, Brazil. Vector Borne Zoonotic Dis. 2015, 15, 656–666. [Google Scholar] [CrossRef]
- de Araujo, V.A.; Boite, M.C.; Cupolillo, E.; Jansen, A.M.; Roque, A.L. Mixed infection in the anteater Tamandua tetradactyla (Mammalia: Pilosa) from Para State, Brazil: Trypanosoma cruzi, T. rangeli and Leishmania infantum. Parasitology 2013, 140, 455–460. [Google Scholar] [CrossRef]
- Humberg, R.M.; Oshiro, E.T.; Cruz, M.S.; Ribolla, P.E.; Alonso, D.P.; Ferreira, A.M.; Bonamigo, R.A.; Tasso, N., Jr.; de Oliveira, A.G. Leishmania chagasi in opossums (Didelphis albiventris) in an urban area endemic for visceral leishmaniasis, Campo Grande, Mato Grosso do Sul, Brazil. Am. J. Trop. Med. Hyg. 2012, 87, 470–472. [Google Scholar] [CrossRef][Green Version]
- Santiago, M.E.; Vasconcelos, R.O.; Fattori, K.R.; Munari, D.P.; Michelin, A.F.; Lima, V.M. An investigation of Leishmania spp. in Didelphis spp. from urban and peri-urban areas in Bauru (Sao Paulo, Brazil). Vet. Parasitol. 2007, 150, 283–290. [Google Scholar] [CrossRef]
- Carreira, J.C.; da Silva, A.V.; de Pita, P.D.; Brazil, R.P. Natural infection of Didelphis aurita (Mammalia: Marsupialia) with Leishmania infantum in Brazil. Parasit. Vectors 2012, 5, 111. [Google Scholar] [CrossRef]
- Viettri, M.; Herrera, L.; Aguilar, C.M.; Morocoima, S.; Reyes, J.; Lares, M.; Lozano-Arias, D.; García-Alzate, R.; Chacón, T.; Feliciangeli, M.D.; et al. Molecular Diagnosis of Trypanosoma cruzi/Leishmania Spp. Coinfection in Domestic, Peridomestic and Wild Mammals of Venezuelan Co-Endemic Areas. Vet. Parasitol. Reg. Stud. Rep. 2018, 14, 123–130. [Google Scholar] [CrossRef]
- Corredor, A.; Gallego, J.F.; Tesh, R.B.; Pelaez, D.; Diaz, A.; Montilla, M.; Palau, M.T. Didelphis marsupialis, an apparent wild reservoir of Leishmania donovani chagasi in Colombia, South America. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 195. [Google Scholar] [CrossRef]
- Travi, B.L.; Jaramillo, C.; Montoya, J.; Segura, I.; Zea, A.; Goncalves, A.; Velez, I.D. Didelphis marsupialis, an important reservoir of Trypanosoma (Schizotrypanum) cruzi and Leishmania (Leishmania) chagasi in Colombia. Am. J. Trop. Med. Hyg. 1994, 50, 557–565. [Google Scholar] [CrossRef]
- Travi, B.L.; Osorio, Y.; Becerra, M.T.; Adler, G.H. Dynamics of Leishmania chagasi infection in small mammals of the undisturbed and degraded tropical dry forests of northern Colombia. Trans. R. Soc. Trop. Med. Hyg. 1998, 92, 275–278. [Google Scholar] [CrossRef]
- Zulueta, A.M.; Villarroel, E.; Rodriguez, N.; Feliciangeli, M.D.; Mazzarri, M.; Reyes, O.; Rodriguez, V.; Centeno, M.; Barrios, R.M.; Ulrich, M. Epidemiologic Aspects of American Visceral Leishmaniasis in an Endemic Focus in Eastern Venezuela. Am. J. Trop. Med. Hyg. 1999, 61, 945–950. [Google Scholar] [CrossRef][Green Version]
- Malta, M.C.; Tinoco, H.P.; Xavier, M.N.; Vieira, A.L.; Costa, E.A.; Santos, R.L. Naturally acquired visceral leishmaniasis in non-human primates in Brazil. Vet. Parasitol. 2010, 169, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Paiz, L.M.; Motoie, G.; Richini-Pereira, V.B.; Langoni, H.; Menozzi, B.D.; Tolezano, J.E.; Donalisio, M.R. Antibodies and molecular detection of Leishmania (Leishmania) infantum in samples of free-ranging marmosets (Primates: Callicitrichidae: Callithrix spp.) in an area of canine visceral leishmaniasis in southeastern Brazil. Vector Borne Zoonotic Dis. 2019, 19, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.S.; Sabioni, L.A.; Moraes de Seixas, M.M.; de Souza Filho, J.A.; Marcelino, A.P. Paloma Helena Fernandes Shimabukuro. Evidence of a Sylvatic Enzootic Cycle of Leishmania infantum in the State of Amapá, Brazil. Rev. Da Soc. Brasil. Med. Trop. 2020, 53, 13–15. [Google Scholar] [CrossRef]
- Rosypal, A.C.; Tripp, S.; Lewis, S.; Francis, J.; Stoskopf, M.K.; Larsen, R.S.; Lindsay, D. Survey of antibodies to Trypanosoma cruzi and Leishmania spp. in gray and red fox populations from North Carolina and Virginia. J. Parasitol. 2010, 96, 1230–1231. [Google Scholar] [CrossRef] [PubMed]
- Rosypal, A.C.; Alexander, A.; Byrd, D.; Weaver, M.; Stewart, R.; Gerhold, R.; Houston, A.; vanWhy, K.; Dubey, J.P. Survey of antibodies to Leishmania spp. in wild canids from Pennsylvania and Tennesee. J. Zoo Wildl. Med. 2013, 44, 1131–1133. [Google Scholar] [CrossRef]
- Millán, J.; Travaini, A.; Zanet, S.; López-Bao, J.V.; Trisciuoglio, A.; Ferroglio, E.; Rodríguez, A. Detection of Leishmania DNA in Wild Foxes and Associated Ticks in Patagonia, Argentina, 2000 Km South of Its Known Distribution Area. Parasit. Vectors 2016, 9, 241. [Google Scholar] [CrossRef]
- Reis, F.; Minuzzi-Souza Mariana, T.T.C.; Renata, N.; De Morais, I.O.B.; De Lima, T.M.; Hecht, M.; Nitz, N.; Gurgel-Gonçalves, R. Trypanosomatid Infections in Captive Wild Mammals and Potential Vectors at the Brasilia Zoo, Federal District, Brazil. Vet. Med. Sci. 2020, 6, 248–256. [Google Scholar] [CrossRef]
- Brandao, E.M.V.; Xavier, S.C.C.; Rocha, F.L.; Lima, C.F.M.; Roque, A.L.R. Wild and Domestic Canids and Their Interactions in the Transmission Cycles of Trypanosoma cruzi and Leishmania spp. in an Area of the Brazilian Cerrado. Pathogens 2020, 9, 818. [Google Scholar] [CrossRef]
- Porfirio, F.E.O.; Santos, F.M.; Carvalho de Macedo, G.; Barreto, W.T.G.; Campos, J.B.V.; Meyers, A.C.; André, M.R.; Perles, L.; Oliveira, C.E.; ChagasXavier, S.C.D.; et al. Maintenance of Trypanosoma cruzi, T. evansi and Leishmania spp. by Domestic Dogs and Wild Mammals in a Rural Settlement in Brazil-Bolivian Border. Int. J. Parasitol. Parasit. Wildl. 2018, 7, 398–404. [Google Scholar] [CrossRef]
- Lima, V.M.F.; Fattori, F.R.; Apargecida de Fátima Michelin, A.F.; Nogueira, F.S.; de Oliveira e Souza, L. Evidence of Leishmania spp. Antibodies and DNA in Bush Dogs (Speothos venaticus) in Brazil. J. Zoo Wildl. Med. 2009, 40, 91–94. [Google Scholar] [CrossRef]
- Johnson, R.N.; Young, D.G.; Butler, J.F.; Bogaert-Diaz, H. Possible Determination of the Vector and Reservoir of Leishmaniasis in the Dominican Republic. Am. J. Trop. Med. Hyg. 1992, 46, 282–287. [Google Scholar] [CrossRef]
- Medkour, H.; Davoust, B.; Levasseur, A.; Mediannikov, O. Molecular Evidence of Leishmania infantum and Leishmania guyanensis in Red Howler Monkey (Alouatta seniculus) from French Guiana. Vector Borne Zoonotic Dis. 2019, 12, 896–900. [Google Scholar] [CrossRef]
- Babuadze, G.; Alvar, J.; Argaw, D.; de Koning, H.P.; Iosava, M.; Kekelidze, M.; Tsertsvadze, N.; Tsereteli, D.; Chakhunashvili, G.; Mamatsashvili, T.; et al. Epidemiology of Visceral Leishmaniasis in Georgia. PLoS Negl. Trop. Dis. 2014, 8, e2725. [Google Scholar] [CrossRef]
- Mitková, B.; Hrazdilová, K.; Amico, G.D.; Duscher, G.G.; Suchentrunk, F.; Forejtek, P.; Gherman, M.; Matei, I.A.; Ionică, A.M.; Daskalaki, A.A.; et al. Eurasian Golden Jackal as Host of Canine Vector-Borne Protists. Parasit. Vectors 2017, 10, 183. [Google Scholar] [CrossRef]
- Mohebali, M.; Arzamani, K.; Zarei, Z.; Akhoundi, B.; Hajjaran, H.; Raeghi, S.; Heidari, Z.; Motavalli-Haghi, S.M.; Elikaee, S.; Mousazadeh-Mojarrad, A.; et al. Canine Visceral Leishmaniasis in Wild Canines (Fox, Jackal and Wolf) in Northeastern Iran Using Parasitological, Serological, and Molecular Methods. J. Arthopod Borne Dis. 2016, 10, 538–545. [Google Scholar]
- Talmi-Frank, D.; Kedem-Vaanunu, N.; King, R.; Bar-Gal, G.K.; Edery, N.; Jaffe, C.L.; Baneth, G. Leishmania tropica Infection in Golden Jackals and Red Foxes, Israel. Emerg. Infect. Dis. 2010, 16, 1973–1975. [Google Scholar] [CrossRef]
- Beck, A.; Beck, R.; Kusak, J.; Gudan, A.; Martinkovic, F.; Artukovic, B.; Hohšteter, M.; Huber, D.; Marinculic, A.; Grabarevic, Z. A Case of Visceral Leishmaniosis in a Gray Wolf (Canis lupus) from Croatia. J. Wildl. Dis. 2008, 44, 451–456. [Google Scholar] [CrossRef]
- Sobrino, R.; Ferroglio, E.; Oleaga, A.; Romano, A.; Millan, J.; Revilla, M.; Arnal, M.C.; Trisciuoglio, A.; Gortázar, C. Characterization of Widespread Canine Leishmaniasis among Wild Carnivores from Spain. Vet. Parasitol. 2008, 155, 198–203. [Google Scholar] [CrossRef]
- Oleaga, A.; Vicente, J.; Ferroglio, E.; Pegoraro de Macedo, M.R.; Casais, R.; Del Cerro, A.; Espí, A.; García, E.J.; Gortázar, C. Concomitance and Interactions of Pathogens in the Iberian Wolf (Canis lupus). Res. Vet. Sci. 2015, 101, 22–27. [Google Scholar] [CrossRef]
- Oleaga, A.; Zanet, S.; Espí, A.; Raquel, M.; De Macedo, P.; Gortázar, C.; Ferroglio, E. Leishmania in Wolves in Northern Spain: A Spreading Zoonosis Evidenced by Wildlife Sanitary Surveillance. Vet. Parasitol. 2018, 255, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Risueño, J.; Ortuño, M.; Pérez-Cutillas, P.; Goyena, E.; Maia, C.; Cortes, S.; Campino, L.; BernalL, J.; Muñoz, C.; ArcenillasI, L.; et al. Epidemiological and Genetic Studies Suggest a Common Leishmania infantum Transmission Cycle in Wildlife, Dogs and Humans Associated to Vector Abundance in Southeast Spain. Vet. Parasitol. 2018, 259, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Ortuño, M.; Latrofa, M.S.; Iborra, M.A.; Pérez, P.; Bernal, L.J.; Risueño, J.; Muñoz, C.; Bernal, A.; Sánchez-Lopez, P.F.; Segovia, M.; et al. Genetic Diversity and Phylogenetic Relationships between Leishmania infantum from Dogs, Humans and Wildlife in South - East Spain. Zoonoses Public Health 2019, 66, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Sastre, N.; Francino, O.; Ramírez, O.; Enseñat, C.; Sánchez, A.; Altet, L. Detection of Leishmania infantum in Captive Wolves from Southwestern Europe. Vet. Parasitol. 2008, 158, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Madrid, R.; Belinchón-Lorenzo, S.; Iniesta, V.; Fernández-Cotrina, J.; Parejo, J.C.; Serrano, F.J.; Monroy, I.; Baz, V.; Gómez-Luque, A.; Gómez-Nieto, L.C. First Detection of Leishmania infantum Kinetoplast DNA in Hair of Wild Mammals: Application of QPCR Method to Determine Potential Parasite Reservoirs. Acta Trop. 2013, 128, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Battisti, E.; Zanet, S.; Khalili, S.; Trisciuoglio, A.; Hertel, B.; Ferroglio, E. Molecular Survey on Vector-Borne Pathogens in Alpine Wild Carnivorans. Front. Vet. Sci. 2020, 7, 1–9. [Google Scholar] [CrossRef]
- Del Río, L.; Chitimia, L.; Cubas, A.; Victoriano, I.; De la Rúa, P.; Gerrikagoitia, X.; Barral, M.; Muñoz-García, C.I.; Goyena, E.; García-Martínez, D.; et al. Evidence for Widespread Leishmania infantum Infection among Wild Carnivores in L. infantum Periendemic Northern Spain. Prev. Vet. Med. 2014, 113, 430–435. [Google Scholar] [CrossRef]
- Millán, J.; Zanet, S.; Gomis, M.; Trisciuoglio, A.; Negre, N.; Ferroglio, E. An Investigation into Alternative Reservoirs of Canine Leishmaniasis on the Endemic Island of Mallorca (Spain). Trans. Emerg. Dis. 2011, 58, 352–357. [Google Scholar] [CrossRef]
- Gomes, J.; Rocha, H.; Carvalho, C.; Bandeira, V.; Fonseca, C.; Rosalino, L.M.; Cunha, M.V. Molecular Detection and Characterization of Leishmania infantum in Free-Ranging Egyptian Mongoose (Herpestes ichneumon). Int. J. Parasitol. Parasit. Wildl. 2020, 11, 158–162. [Google Scholar] [CrossRef]
- Tsakmakidis, G.; Pavlou, C.; Tamvakis, A.; Papadopoulos, T.; Christodoulou, V.; Angelopoulou, K.; Dovas, C.; Antoniou, M.; Anastasakis, C.; Diakou, A. Leishmania infection in lagomorphs and minks in Greece. Vet. Parasitol. Reg. Stud. Rep. 2019, 16, 100279. [Google Scholar] [CrossRef]
- Alcover, M.M.; Ribas, A.; Guillén, M.C.; Berenguer, D.; Tomás-Pérez, M.; Riera, C.; Fisa, R. Wild Mammals as Potential Silent Reservoirs of Leishmania infantum in a Mediterranean Area. Prev. Vet. Med. 2020, 175, 104874. [Google Scholar] [CrossRef]
- Calavera, M.A.; Latta, R.; Laricchiuta, P.; Passantino, G.; Abramo, F.; Mendoza-Roldan, J.A.; Otranto, D.; Zatelli, A. Clinical, haematological and biochemical findings in tigers infected by Leishmania infantum. BMC Vet. Res. 2020, 16, 214. [Google Scholar] [CrossRef]
- Mancianti, F.; Mignone, W.; Galastri, F. Serologic Survey for Leishmaniasis in Free-Living Red Foxes (Vulpes vulpes) in Italy. J. Wildl. Dis. 1994, 30, 454–456. [Google Scholar] [CrossRef][Green Version]
- Criado-Fornelio, A.; Gutierrez-Garcia, L.; Rodriguez-Caabeiro, F.; Reus-Garcia, E.; Roldan-Soriano, M.A.; Diaz-Sanchez, M.A. A Parasitological Survey of Wild Red Foxes (Vulpes vulpes) from the Province of Guadalajara, Spain. Vet. Parasitol. 2000, 92, 245–251. [Google Scholar] [CrossRef]
- Dipineto, L.; Manna, L.; Baiano, A.; Gala, M.; Fioretti, A.; Gravino, A.E.; Menna, L.F. Presence of Leishmania infantum in Red Foxes (Vulpes vulpes) in Southern Italy. J. Wildl. Dis. 2007, 43, 518–520. [Google Scholar] [CrossRef]
- Davoust, B.; Mary, C.; Marié, J.L. Detection of Leishmania in Red Foxes (Vulpes vulpes) from Southeastern France Using Real-Time Quantitative PCR. J. Wildl. Dis. 2014, 50, 130–132. [Google Scholar] [CrossRef]
- Karayiannis, S.; Ntais, P.; Messaritakis, I.; Tsirigotakis, N.; Dokianakis, E.; Antoniou, M. Detection of Leishmania infantum in Red Foxes (Vulpes vulpes) in Central Greece. Parasitology 2015, 142, 1574–1578. [Google Scholar] [CrossRef]
- Lledó, L.; Giménez-Pardo, C.; Saz, J.V.; Serrano, J.L. Wild Red Foxes (Vulpes vulpes) as Sentinels of Parasitic Diseases in the Province of Soria, Northern Spain. Vector Borne Zoonotic Dis. 2015, 15, 743–749. [Google Scholar] [CrossRef]
- Abbate, L.M.; Arfuso, F.; Napoli, E.; Gaglio, G.; Giannetto, S.; Latrofa, M.S.; Otranto, D.; Brianti, E. Leishmania infantum in Wild Animals in Endemic Areas of Southern Italy. Comp. Immunol. Microbiol. Infect. Dis. 2019, 67, 101374. [Google Scholar] [CrossRef]
- Medkour, H.; Laidoudi, Y.; Marié, J.L.; Fenollar, F.; Davoust, B.; Mediannikov, O. Molecular Investigation of Vector-Borne Pathogens in Red Foxes (Vulpes vulpes) from Southern France. J. Wildl. Dis. 2020, 56, 837–850. [Google Scholar] [CrossRef]
- Montoya, A.; Pérez De Quadros, L.; Mateo, M.; Hernández, L.; Gálvez, R.; Alcántara, G.; Checa, R.; Jiménez, M.Á.; Chicharro, C.; Israel Cruz, G.M. Leishmania infantum infection in Bennett’s wallabies (Macropus rufogriseus rufogriseus) in a spanish wildlife park. J. Zoo Wildl. Med. 2016, 47, 586–593. [Google Scholar] [CrossRef]
- Miró, G.; Troyano, A.; Montoya, A.; Fariñas, F.; Fermín, M.L.; Flores, L.; Rojo, C.; Checa, R.; Gálvez, R.; Marino, V.; et al. First report of L. infantum infection in the endangered orangutan (Pongo pygmaeus pygmaeus) in Madrid, Spain. Parasit. Vectors 2018, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Azami-Conesa, I.; Martínez-Díaz, R.A.; González, F.; Gómez-Muñoz, M.T. First Detection of Leishmania infantum in Common Urban Bats Pipistrellus pipistrellus in Europe. Res. Vet. Sci. 2020, 132, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Chemkhi, J.; Souguir, H.; BelHadjAli, I.; Driss, M.; Guizani, I.; Guerbouj, S. Natural Infection of Algerian Hedgehog, Atelerix algirus (Lereboullet 1842) with Leishmania Parasites in Tunisia. Acta Trop. 2015, 150, 42–51. [Google Scholar] [CrossRef]
- Souguir-Omrani, H.; Chemkhi, J.; Fathallah-Mili, A.; Saadi-BenAoun, Y.; BelHadjAli, I.; Guizani, I.; Guerbouj, S. Paraechinus aethiopicus (Ehrenberg 1832) and Atelerix algirus (Lereboullet 1842) Hedgehogs: Possible Reservoirs of Endemic Leishmaniases in Tunisia. Infect. Genet. Evol. 2018, 63, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Ruiz- Fons, F.; Ferroglio, E.; Gortázar, C. Leishmania infantum in Free-Ranging Hares, Spain, 2004–2010. Eurosurveillance 2013, 30, 80–81. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.V.; Moreno, I.; Domínguez, M.; de la Cruz, M.L.; Martín, A.B.; Rodríguez-Bertos, A.; López, R.; Navarro, A.; González, S.; Mazariegos, M.; et al. Application of a Specific Quantitative Real-Time PCR (QPCR) to Identify Leishmania infantum DNA in Spleen, Skin and Hair Samples of Wild Leporidae. Vet. Parasitol. 2017, 243, 92–99. [Google Scholar] [CrossRef]
- Ortega-García, M.V.; Salguero, F.J.; Rodríguez-Bertos, A.; Moreno, I.; García, N.; García-Seco, T.; Torre, G.L.; Domínguez, L.; Domínguez, M. A Pathological Study of Leishmania infantum Natural Infection in European Rabbits (Oryctolagus cuniculus) and Iberian Hares (Lepus granatensis). Trans. Emerg. Dis. 2019, 66, 2474–2781. [Google Scholar] [CrossRef]
- Ebani, V.V.; Poli, A.; Rocchigiani, G.; Bertelloni, F.; Nardoni, S.; Papini, R.A.; Mancianti, F. Serological Survey on Some Pathogens in Wild Brown Hares (Lepus europaeus) in Central Italy. Asian Pac. J. Trop. Med. 2016, 9, 465–469. [Google Scholar] [CrossRef]
- Rocchigiani, G.; Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Bascherini, A.; Leoni, A.; Mancianti, F.; Poli, A. Molecular Survey on the Occurrence of Arthropod-Borne Pathogens in Wild Brown Hares (Lepus europaeus) from Central Italy. Infect. Genet. Evol. 2018, 59, 142–147. [Google Scholar] [CrossRef]
- Chitimia, L.; Muñoz- García, C.I.; Sánchez-Velasco, D.; Lizana, V.; Del Río, L.; Murcia, L.; Fisa, R.; Rierad, C.; Giménez-Fonte, P.; Jiménez-Montalbán, P.; et al. Cryptic Leishmaniosis by Leishmania infantum, a Feature of Canines Only? A Study of Natural Infection in Wild Rabbits, Humans and Dogs in Southeastern Spain. Vet. Parasitol. 2011, 181, 12–16. [Google Scholar] [CrossRef]
- Díaz-Sáez, V.; Merino-Espinosa, G.; Morales-Yuste, M.; Corpas-López, V.; Pratlong, F.; Morillas-Márquez, F.; Martín-Sánchez, J. High Rates of Leishmania infantum and Trypanosoma nabiasi Infection in Wild Rabbits (Oryctolagus cuniculus) in Sympatric and Syntrophic Conditions in an Endemic Canine Leishmaniasis Area: Epidemiological Consequences. Vet. Parasitol. 2014, 202, 119–127. [Google Scholar] [CrossRef]
- Navea-Pérez, H.M.; Díaz- Sáez, V.; Corpas- López, V.; Merino- Espinosa, G.; Morillas- Márquez, F.; Martín- Sánchez, J. Leishmania infantum in Wild Rodents: Reservoirs or Just Irrelevant Incidental Hosts? Parasitol. Res. 2015, 114, 2363–2370. [Google Scholar] [CrossRef]
- Millán, J. Molecular Investigation of Vector-Borne Parasites in Wild Micromammals, Barcelona (Spain). Parasitol. Res. 2018, 117, 3015–3018. [Google Scholar] [CrossRef]
- Pourmohammadi, B.; Mohammadi-Azni, S.; Kalantari, M. Natural Infection of Nesokia indica with Leishmania major and Leishmania infantum Parasites in Damghan City, Northern Iran. Acta Trop. 2017, 170, 134–139. [Google Scholar] [CrossRef]
- Helhazar, M.; Leitão, J.; Duarte, A.; Tavares, L.; da Fonseca, I.P. Natural Infection of Synathropic Rodent Species Mus musculus and Rattus norvegicus by Leishmania infantum in Sesimbra and Sintra-Portugal. Parasit. Vectors 2013, 6, 1–6. [Google Scholar] [CrossRef]
- Echchakery, M.; Chicharro, C.; Boussaa, S.; Nieto, J.; Carrillo, E.; Sheila, O.; Moreno, J.; Boumezzough, A. Molecular Detection of Leishmania infantum and Leishmania tropica in Rodent Species from Endemic Cutaneous Leishmaniasis Areas in Morocco. Parasit. Vectors 2017, 10, 1–8. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; Al-Zahrani, M.A.; Al-Tuwaigri, A.S.; Al-Shammary, F.J.; Evans, D.A. Leishmania Infecting Man and Wild Animals in Saudi Arabia. 9. The Black Rat (Rattus rattus) a Probable Reservoir of Visceral Leishmaniasis in Gizan Province, South-West Saudi Arabia. Trans. R. Soc. Trop. Med. Hyg. 1992, 86, 513–514. Available online: https://academic.oup.com/trstmh/article/86/5/513/1922532 (accessed on 25 February 2021). [CrossRef]
- Zanet, S.; Sposimo, P.; Trisciuoglio, A.; Giannini, G.; Strumia, F.; Ferroglio, E. Epidemiology of Leishmania infantum, Toxoplasma gondii, and Neospora caninum in Rattus rattus in Absence of Domestic Reservoir and Definitive Hosts. Vet. Parasitol. 2014, 199, 247–249. [Google Scholar] [CrossRef]
- Papadogiannakis, E.; Spanakos, G.; Kontos, V.; Menounos, P.G.; Tegos, N.; Vakalis, N. Molecular Detection of Leishmania infantum in Wild Rodents (Rattus norvegicus) in Greece. Zoonoses Public Health 2010, 57, 2009–2011. [Google Scholar] [CrossRef] [PubMed]
- Pourmohammadi, B.; Motazedian, M.H.; Kalantari, M. Rodent Infection with Leishmania in a New Focus of Human Cutaneous Leishmaniasis, in Northern Iran. Ann. Trop. Med. Parasitol. 2008, 102, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Akhoundi, M.; Mohebali, M.; Asadi, M.; Mahmodi, M.R.; Amraei, K.; Mirzaei, A. Molecular Characterization of Leishmania spp. in Reservoir Hosts in Endemic Foci of Zoonotic Cutaneous Leishmaniasis in Iran. Folia Parasitol. 2013, 60, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Masoumeh, A.; Kourosh, A.; Mohsen, K.; Hossein, M.M.; Qasem, A.; Djaefar, M.F.M.; Esmaeil, N.M.; Tahereh, D. Laboratory Based Diagnosis of Leishmaniasis in Rodents as the Reservoir Hosts in Southern Iran, 2012. Asian Pac. J. Trop. Biomed. 2014, 4, S575–S580. [Google Scholar] [CrossRef]
- Nezamzadeh-Ezhiyeh, H.; Mirhendi, H.; Jafari, R.; Veysi, A.; Rassi, Y.; Oshaghi, M.A.; Arandian, M.H.; Abdoli, H.; Bahrami, S.; Ramazani, A.R.Z.; et al. An Eco-Epidemiological Study on Zoonotic Cutaneous Leishmaniasis in Central Iran. Iran. J. Public Health 2021, 50, 350–359. [Google Scholar] [CrossRef]
- Azizi, K.; Moemenbellah-Fard, M.D.; Fakoorziba, M.R.; Fekri, S. Gerbillus nanus (Rodentia: Muridae): A New Reservoir Host of Leishmania major. Ann. Trop. Med. Parasitol. 2011, 105, 431–437. [Google Scholar] [CrossRef][Green Version]
- Kassahun, A.; Sadlova, J.; Benda, P.; Kostalova, T.; Warburg, A.; Hailu, A.; Baneth, G.; Volf, P.; Votypka, J. Natural Infection of Bats with Leishmania in Ethiopia. Acta Trop. 2015, 150, 166–170. [Google Scholar] [CrossRef]
- Rouhani, S.; Mirzaei, A.; Spotin, A.; Parvizi, P. Novel Identification of Leishmania major in Hemiechinus auritus and Molecular Detection of This Parasite in Meriones libycus from an Important Foci of Zoonotic Cutaneous Leishmaniasis in Iran. J. Infect. Public Health 2014, 7, 210–212. [Google Scholar] [CrossRef]
- Pourmohammadi, B.; Mohammadi-Azni, S. Molecular Detection of Leishmania major in Hemiechinus auritus, A Potential Reservoir of Zoonotic Cutaneous Leishmaniasis in Damghan, Iran. J. Arthropod Borne Dis. 2019, 13, 334–343. [Google Scholar] [CrossRef]
- Tomás-Pérez, M.; Khaldi, M.; Riera, C.; Mozo-León, D.; Ribas, A.; Hide, M.; Barech, G.; Benyettoub, M.; Seghirib, K.; Doudou, S.; et al. First Report of Natural Infection in Hedgehogs with Leishmania major, a Possible Reservoir of Zoonotic Cutaneous Leishmaniasis in Algeria. Acta Trop. 2014, 135, 44–49. [Google Scholar] [CrossRef]
- Gicheru, M.M.; Jeneby, M.M.; Macharia, J.C.; Carlsson, H.E.; Suleman, M.A. Prevalence of Antibodies and Cell Mediated Immune Response against Leishmania major in Feral Nonhuman Primates from Kenya. Acta Trop. 2009, 109, 136–140. [Google Scholar] [CrossRef]
- Moemenbellah-Fard, M.D.; Kalantari, M.; Rassi, Y.; Javadian, E. The PCR- Based Detection of Leishmania major Infections in Meriones libycus (Rodentia: Muridae) from Southern Iran. Ann. Trop. Med. Parasitol. 2003, 97, 865–873. [Google Scholar] [CrossRef]
- Najafzadeh, N.; Sedaghat, M.M.; Sultan, S.S.; Spotin, A.; Zamani, A.; Taslimian, R.; Yaghoubinezhad, A.; Parvizi, P. The Existence of Only One Haplotype of Leishmania major in the Main and Potential Reservoir Hosts of Zoonotic Cutaneous Leishmaniasis Using Different Molecular Markers in a Focal Area in Iran. Revista Soc. Brasil. Med. Trop. 2014, 47, 599–606. [Google Scholar] [CrossRef]
- Faiman, R.; Abbasi, I.; Jaffe, C.; Motro, Y.; Nasereddin, A.; Schnur, L.F.; Torem, M.; Pratlong, F.; Dedet, J.P.; Warburg, A. A Newly Emerged Cutaneous Leishmaniasis Focus in Northern Israel and Two New Reservoir Hosts of Leishmania major. PLoS Negl. Trop. Dis. 2013, 7, e2058. [Google Scholar] [CrossRef]
- Kassahun, A.; Sadlova, J.; Dvorak, V.; Kostalova, T.; Rohousova, I.; Frynta, D.; Aghova, T.; Yasur-Landaud, D.; Lemma, W.; Hailu, A.; et al. Detection of Leishmania donovani and L. tropica in Ethiopian Wild Rodents. Acta Trop. 2015, 145, 39–44. [Google Scholar] [CrossRef]
- Massamba, N.N.; Mutinga, M.J.; Kamau, C.C. Characterisation of Leishmania Isolates from Laikipia District, Kenya. Acta Trop. 1998, 71, 293–303. [Google Scholar] [CrossRef]
- Doha, S.A.; Shehata, M.G.; Fahmy, A.R.; Samy, A.M. Natural and Experimental Evidence of Viscerotropic Infection Caused by Leishmania tropica from North Sinai, Egypt. J. Egypt. Soc. Parasitol. 2014, 44, 425–434. [Google Scholar] [CrossRef]
- Mukhtar, M.M.; Sharief, A.H.; El Saffi, S.H.; Harith, A.E.; Higazzi, T.B.; Adam, A.M.; Abdalla, H.S. Detection of Antibodies to Leishmania donovani in Animals in a Kala-Azar Endemic Region in Eastern Sudan: A Preliminary Report. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 33–36. [Google Scholar] [CrossRef]
- Tsokana, C.N.; Sokos, C.; Giannakopoulos, A.; Mamuris, Z.; Birtsas, P.; Papaspyropoulos, K.; Valiakos, G.; Spyrou, V.; Lefkaditis, M.; Chatzopoulos, D.C.; et al. First Evidence of Leishmania Infection in European Brown Hare (Lepus europaeus) in Greece: GIS Analysis and Phylogenetic Position within the Leishmania spp. Parasitol. Res. 2016, 115, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Morsy, T.A.; Al-Dakhil, M.A.; El-Bahrawy, A.F. Natural Leishmania infection in sand cats captured in Riyadh district, Saudi Arabia. J. Egypt. Soc. Parasitol. 1999, 29, 69–74. [Google Scholar] [PubMed]
- Morsy, T.A.; el Shazly, A.M.; el Kady, G.A.; Sabry, A.H.; Handousa, A.A.; Ragheb, D.A.; Kotb, M.M. Natural Leishmania infections in two stray dogs and two Gerbillus pyramidum in Dakahlia Governorate, Egypt. J. Egypt Soc. Parasitol. 1994, 24, 383–394. [Google Scholar] [PubMed]
| Section | Subgenus | Species | Clinical Disease in humans | Geographic Area |
|---|---|---|---|---|
| Euleishmania | Leishmania | L. aethiopica | CL, DCL | Ethiopia, Kenya |
| L. amazonensis | CL, DCL, MCL | Bolivia, Brazil and Venezuela | ||
| L. donovani | VL, PKDL | Central Africa, South Asia, Middle East, India and China | ||
| L. infantum | VL, CL | North Africa, South Europe, Middle East, Central Asia and North, Central and South America | ||
| L. major | CL | Central and North Africa, Middle East and Central Asia | ||
| L. mexicana | CL, DCL | USA, Mexico, Ecuador, Peru and Venezuela | ||
| L. tropica | CL, VL | Central and North Africa, Middle East, Central Asia and India | ||
| L. venezuelensis | CL | Northern South America | ||
| Viannia | L. braziliensis | CL, MCL | Brazil, Bolivia, Peru, Guatemala and Venezuela | |
| L. guyanensis | CL, MCL | Bolivia, Brazil, French Guyana and Suriname | ||
| L. lainsoni | CL | Brazil, Bolivia and Peru | ||
| L. lindenbergi | CL | Brazil | ||
| L. naiffi | CL | Brazil, French Guyana | ||
| L. panamensis | CL, MCL | Brazil, Panama, Venezuela and Colombia | ||
| L. peruviana | CL, MCL | Peru, Bolivia | ||
| L. shawi | CL | Brazil | ||
| Paraleishmania | L. colombiensis | CL, VL | Colombia |
| Host | Prevalence | Organs/Tissue Analysed | Methods for Detection | Leishmania (Viannia) species | Country | References |
|---|---|---|---|---|---|---|
| Order Carnivora | ||||||
| Cerdocyon thous (crab-eating fox) | 20–100% | blood, serum | PCR (kDNA), DAT | LVsp | Brazil | [34,37] |
| Conepatus chinga rex (Molina’s hog-nose skunk) | 50% | Skin + liver + spleen | Inoculation to hamster, isoenzyme analysis, hybridisation, PCR (kDNA) | Lb | Bolivia | [41] |
| Lycalopex (Pseudalopex) vetulus (hoary fox) | 100% | serum | DAT | LVsp | Brazil | [37] |
| Nasua nasua (ring-tailed coati) | 50% | serum | DAT | LVsp | Brazil | [37] |
| Procyon cancrivorous (crab-eating raccoon) | 50% | serum | DAT | LVsp | Brazil | [37] |
| Order Cingulata | ||||||
| Dasypus novemcinctus (armadillo) | 15.6% | blood, LN, liver, skin, spleen | Culture, zymodeme analysis | Lb | Brazil | [46] |
| Dasypus sp. | 100% | blood | PCR (kDNA) | LVsp | Brazil | [34] |
| Order Chiroptera | ||||||
| Artibeus planirostris (frugivorous) | 4.3% | skin | PCR (kDNA), PCR (HSP70) + RFLP, PCR (G6DP) + sequencing | Lb | Brazil | [43] |
| Cynomops planirostris (insectivorous) | 11.1% | liver, skin | PCR (kDNA), nPCR (SSU) + sequencing | Lb | Brazil | [44] |
| Desmodus rotundus (hematophagous) | 3.2% | blood | PCR (kDNA), PCR (Cyt B) + sequencing | Lb | Brazil | [43] |
| Eumops perotis (insectivorous) | 5.6% | blood | PCR (kDNA), PCR (Cyt B) + sequencing | Lb | Brazil | [45] |
| Glossophaga soricina (insectivorous) | 0.9–40% | blood, liver, spleen | PCR (ITS1) + RFLP, PCR (kDNA, PCR (Cyt B) + sequencing | Lb | Brazil | [42,44,45] |
| Lasiurus cinereus (insectivorous) | 20% | liver, skin | PCR (kDNA), nPCR (SSU) + sequencing | Lb, LVsp | Brazil | [44] |
| Molossus molossus (insectivorous) | 44–25% | blood | PCR (ITS1) + RFLP, PCR (kDNA), PCR (Cyt b) + sequencing | Lb | Brazil | [42,45] |
| Platyrrhinus lineatus (frugivorous) | 13.3% | skin | PCR (kDNA), PCR (HSP70) + RFLP, PCR (G6DP) + sequencing | Lb | Brazil | [43] |
| Several species: Artibeus lituratus, Carollia perspicillata, Diphylla ecaudata and Glossophaga soricina) | 19.8% | oral swab | PCR (SSU) + sequencing | LVsp | Brazil | [32] |
| Order Didelphimorphia | ||||||
| Didelphis albiventris (white-eared opossum) | 1.6–50% | blood, BM, liver, serum, skin (tail/ear), spleen | culture, imprints, isoenzymes, PCR (kDNA), qPCR (kDNA) PCR (ITS1), PCR (HSP70), PCR (HSP70) + RFLP, PCR (ITS) + RFLP, nPCR (SSU) + sequencing, IFAT, DAT | Lb, LVsp, Lg, Lpe | Peru, Brazil | [21,30,37,38,39,47,50,51,52,53,54] |
| Didelphis marsupialis (common opossum) | 20–33.3% | ear | PCR (kDNA), hybridisation, xenodiagnoses vector | Lb, LVsp | Colombia | [29,49] |
| Didelphis sp. | 90% | blood | PCR (kDNA), culture | LVsp | Brazil | [34] |
| Marmosa sp. | 16.7–25% | skin, spleen | PCR (kDNA), smears, culture | LVsp | Brazil | [30,39] |
| Gracilinanus agilis (agile gracile opossum) | 1.4–75% | blood, BM, liver, skin (tail/ear), spleen | PCR (kDNA), PCR (HSP70), PCR (HSP70) + RFLP, PCR (ITS) + RFLP | Lb, LVsp, Lg | Brazil | [21,55] |
| Marmosops incanus (grey slender opossum) | 50% | ear skin | PCR (HSP70) + RFLP | Brazil | [21] | |
| Micoureus demerarae (woolly mouse opossum) | 66.7% | ear | PCR (kDNA), hybridisation, xenodiagnoses vector | Lb | Colombia | [29] |
| Monodelphis domestica | 25% | skin, spleen | PCR (kDNA) | LVsp | Brazil | [39] |
| Micoureus pagaruayanus (woolly-mouse opossum) | 4.2–11.6% | skin | PCR (kDNA), qPCR (kDNA), nPCR (SSU), nPCR (G6DP) | Lb, LVsp | Brazil | [48] |
| Micoureus sp. | 100% | blood | PCR (kDNA) | LVsp or Lsp. | Brazil | [34] |
| Order Lagomorpha | ||||||
| Sylvilagus brasiliensis (tapeti) | 100% (n = 1) | ear | PCR (kDNA), hybridisation, xenodiagnoses vector | Lb | Colombia | [29] |
| Order Pilosa | ||||||
| Choloepus hoffmani (two-toed sloth) | 75% | blood | Culture, PCR (kDNA), PCR (HSP70) | Lpa | Panama | [56] |
| Order Primates | ||||||
| Alouatta caraya (black howler) | 8.3% | ear tissue | PCR (ITS) + RFLP + sequencing | Lb, LVsp | Argentina | [64] |
| Aotus azarai (Azara’s night monkey) | 44.4% | blood, spleen | PCR (miniexon) + RFLP + sequencing | Lb | Argentina | [65] |
| Callithrix sp. | 100% | blood | PCR (kDNA) | LVsp | Brazil | [34] |
| Cebus apella (tufted capuchin) | 100% | serum | DAT | LVsp | Brazil | [37] |
| Order Rodentia | ||||||
| Agouti paca (paca) | 100% | skin | culture, isoenzymes, inoculation hamster | Ll | Brazil | [57] |
| Akodon arviculoides | 4% | spleen | smears, PCR (kDNA) | LVsp | Brazil | [30] |
| Akodon cursor | 9.7% | liver, skin (tail), spleen | culture (liver and skin), PCR (kDNA) | Lb | Brazil | [31] |
| Akodon sp. | 2.6% | blood, skin | culture + isoenzymes, PCR | LVsp | Peru | [47] |
| Cerradomys (sin. Oryzomys) subflavus | 7.8–50% | BM, liver, skin (tail/ear), spleen | culture (skin), PCR (kDNA), PCR (kDNA) + hybridisation, nPCR (SSU) + sequencing, PCR (HSP70) + RFLP | Lb, LVsp | Brazil | [31,39,50,53,57] |
| Calomys expulsus | 3.3% | liver | PCR (kDNA) | LVsp | Brazil | [55] |
| Dasyprocta azarae (Agouti) | 75% | serum | DAT | Brazil | [37] | |
| Holochilus scieurus | 7.1–15% | skin, spleen | imprints, PCR (kDNA) | LVsp | Brazil | [30,39] |
| Melanomys caliginosus | 21.4% | ear | PCR (kDNA) + hybridisation | Lb | Colombia | [29] |
| Microryzomys minutus | 50% | ear | PCR (kDNA) + hybridisation | Lb | Colombia | [29] |
| Mus musculus | 55–100% | blood, BM, liver, skin (tail/ear), spleen | PCR (kDNA) + RFLP, nPCR (SSU) + sequencing | Lb, LVsp | Brazil | [34,50,59] |
| Necromys (sin. Bolomys) lasiurus | 4.9–100% | BM, liver, skin (tail/ear), spleen | culture, imprints, PCR (kDNA), PCR (kDNA) + RFLP, serodeme, isoenzyme, PCR (ITS) + RFLP, PCR (D7 24Sα rRNA = trypanosomatids) & PCR (ITS) + sequencing, nPCR (SSU) + sequencing | Lb | Brazil | [30,35,50,59,60] |
| Nectomys squamipes | 7.2–28.1% | skin, spleen | culture, smears, serodeme, isoenzyme, PCR (ITS) + RFLP, PCR (kDNA), serology rK39 Ag, inoculation to hamster, zymodeme | Lb, LVsp | Brazil | [30,39,60] |
| Oecomys trinitatus | 100% (n = 1) | ear skin | PCR (kDNA) | LV sp | Colombia | [49] |
| Oligoryzomis nigripes | 26.8% | liver | PCR (kDNA) | LV sp | Brazil | [55] |
| Oxymyicterus dasytrichus | 33.3% | liver | culture, PCR (kDNA), PCR (HSP70) + RFLP | Lb | Brazil | [31] |
| Phyllotis andinum | 1.2% | blood, skin | culture + isoenzymes, PCR | LVsp, Lpe | Peru | [47] |
| Proechymis sp. | 100% (n = 1) | liver, skin | PCR (kDNA) | LVsp | Brazil | [61] |
| Rhipidomys macrurus | 29.6% | ear skin | PCR (D7 24Sα rRNA trypanosomatids) and PCR ITS + sequencing | Lb | Brazil | [35] |
| Rattus norvegicus (brown rat) | 26.9–66.6% | blood, BM, liver, skin (tail/ear), spleen, | nPCR (SSU) + sequencing | Lb | Brazil | [50,62] |
| Rattus rattus (black rat) | 2.5–50% | blood, BM, liver, skin (tail/ear), spleen | culture, hybridisation, smears, serodeme, isoenzyme, PCR (kDNA), PCR (kDNA) + RFLP, PCR (ITS) + RFLP, nPCR (SSU)+ sequencing, PCR (HSP70) + RFLP, serology rK39 Ag | Lb, LVsp | Brazil, Colombia, Venezuela | [29,30,33,39,50,53,57,60,63] |
| Sigmodon hispidus (hispid cotton rat) | 0.3%-100% (n = 1) | blood, ear skin | culture, PCR (kDNA), PCR (kDNA) + RFLP or hybridisation | Lb, LVsp | Venezuela Colombia | [33,49] |
| Trichomys apereoides | 6.3–15.6% | liver, skin (tail/ear) | PCR (HSP70) + RFLP | Lb, Lg | Brazil | [21] |
| Trichomys fosteri | 2.5% | spleen | PCR (kDNA), PCR (HSP70) | Ln | Brazil | [36] |
| Trichomys inermis | 3% | spleen | PCR (kDNA), PCR HSP70 | Ls | Brazil | [36] |
| Trichomys laurentis | 2–3.9% | spleen | PCR (kDNA), PCR (HSP70) | Lb, Ls, Ln, Lg | Brazil | [36] |
| Trichomys sp. | 100% (n = 1) | blood | PCR (kDNA) | LVsp | Brazil | [34] |
| Zygodontomys bruneus | 100% (n = 1) | ear skin | PCR (kDNA) | LVsp | Colombia | [49] |
| Host | Prevalence | Organs/Tissue Analysed | Methods for Detection | Country | Reference |
|---|---|---|---|---|---|
| Order Carnivora | |||||
| Conepatus chinga rex (Molina’s hog-nose skunk) | 50% | liver, skin and spleen | Inoculation to hamster, Isoenzyme typing, PCR (kDNA) PCR (trypanosomatids) + hybridisation | Bolivia | [41] |
| Order Chiroptera | |||||
| Artibeus lituratus (nectarivorous) | 1.6% | liver, skin and spleen | nPCR (SSU), qPCR (kDNA), PCR (ITS1) + RFLP | Brazil | [14,66] |
| Artibeus planirostris (nectarivorous) | n.s. | skin | qPCR (kDNA), PCR (ITS1) + RFLP | Brazil | [14] |
| Desmodus rotundus (haematofagous) | n.s. | liver, spleen | qPCR (kDNA), PCR (ITS1) + RFLP | Brazil | [14] |
| Eumops glaucinus (insectivorous) | 8.3% | liver, skin and spleen | nPCR (SSU), qPCR (kDNA), PCR (ITS1) + RFLP | Brazil | [14,66] |
| Eumops auripendulus (insectivorous) | 25% | liver, spleen | nPCR (SSU) | Brazil | [66] |
| Eumops perotis (insectivorous) | 5.6% | blood | PCR (kDNA), PCR (Cyt b) + sequencing | Brazil | [45] |
| Glossophaga soricina (insectivorous) | 2.8–4.2% | blood, liver and spleen | nPCR (SSU), PCR (kDNA), PCR (Cyt B) + sequencing | Brazil | [45,66] |
| Molossus molossus (insectivorous) | 1–1.6% | blood, liver and spleen | nPCR (SSU), PCR (kDNA), PCR (Cyt B) + sequencing | Brazil | [45,66] |
| Molossus rufus (insectivorous) | 1% | liver, skin, spleen | nPCR (SSU), qPCR (kDNA), PCR (ITS1) + RFLP | Brazil | [14,66] |
| Myotis nigricans (insectivorous) | 2.9% | liver, spleen | nPCR (SSU), qPCR (kDNA), PCR (ITS1) + RFLP | Brazil | [14,66] |
| Nyctinomops laticaudatus (insectivorous) | 10% | liver, spleen | nPCR (SSU) | Brazil | [66] |
| Phyllostomus hastatus (omnivorous) | 2.9% | blood | PCR (kDNA), PCR (Cyt b) + sequencing | Brazil | [45] |
| Platyrrhinus lineatus (omnivorous) | 18.2% | blood, spleen | qPCR (kDNA), PCR (ITS1) + RFLP, PCR (kDNA), PCR (Cyt b) + sequencing | Brazil | [14,45] |
| Sturnira lilium (nectarivorous) | 25% | liver, spleen | nPCR (SSU) | Brazil | [66] |
| Order Didelphimorphia | |||||
| Marmosa (Micoureus) paraguayanus (woolly-mouse opossum) | 1.1% | skin | PCR (kDNA), qPCR (kDNA), nPCR (SSU), PCR (G6PD), sequencing | Brazil | [48] |
| Order Primata | |||||
| Alouatta caraya (black howler monkey) | 2.8% | ear tissue | PCR (ITS) + RFLP + sequencing | Argentina | [64] |
| Ateles paniscus (spider monkey) | 100% | blood | PCR (kDNA), PCR (ITS) + RFLP | Brazil | [67] |
| Order Rodentia | |||||
| Akodon spp. | 7.1% | blood | PCR (kDNA) + hybridisation | Bolivia | [41] |
| Necromys (sin. Bolomys) lasiurus | 20% | ear skin | PCR-D7 24Sα rRNA (trypanosomatids) and PCR (ITS) + sequencing | Brazil | [35] |
| Oligoryzomys spp. (rice rat) | 25% | blood | PCR (kDNA) + hybridisation | Bolivia | [41] |
| Hylaeamys (Oryzomys) acritus | 33.3% | tail skin | PCR (kDNA) + sequencing | Bolivia | [68] |
| Oryzomys nitidus | 13.3% | tail skin | PCR (kDNA) + sequencing | Bolivia | [68] |
| Host | Prevalence | Organs/Tissue Analysed | Methods for Detection | Country | References |
|---|---|---|---|---|---|
| Order Carnivora | |||||
| Conepatus chinga rex (Molina’s hog-nosed skunk) | 50% | Liver + skin + spleen (macerate) | Inoculation to hamster, isoenzyme analysis, hybridisation | Bolivia | [41] |
| Urocyon cinereoargenteus (fox) | 100% | serum | ELISA | Mexico | [69] |
| Order Chiroptera | |||||
| Pteronotus personatus | 25% | heart, liver, skin and spleen | PCR (kDNA), PCR (SSU) | Mexico | [70] |
| Artibeus jamaicensis | 5.8% | heart, liver, skin and spleen | PCR (kDNA), PCR (SSU) | Mexico | [70] |
| Artibeus lituratus | 7.3% | heart, liver, skin and spleen | PCR (kDNA), PCR (SSU) | Mexico | [70] |
| Carollia sowelli | 4.4% | heart, liver, skin and spleen | PCR (kDNA), PCR (SSU) | Mexico | [70] |
| Choeroniscus godmani | 23.1% | heart, liver, skin and spleen | PCR (kDNA), PCR (SSU) | Mexico | [70] |
| Desmodus rotundus | 7.1% | heart, liver, skin and spleen | PCR (kDNA), PCR (SSU) | Mexico | [70] |
| Dermanura phaeotis | 8.1% | heart, liver, skin and spleen | PCR (kDNA), PCR (SSU) | Mexico | [70] |
| Glossophaga commissarissi | 75% | heart, liver, skin and spleen | PCR (kDNA), PCR (SSU) | Mexico | [70] |
| Glossophaga soricina | 26.9% | heart, liver, skin and spleen | PCR (kDNA), PCR (SSU) | Mexico | [70] |
| Leptonycteris curasoae | 50% | heart, liver, skin and spleen | PCR (kDNA), PCR (SSU) | Mexico | [70] |
| Phyllostomus discolor | 100% (n = 1) | heart, liver, skin and spleen | PCR (kDNA), PCR (SSU) | Mexico | [70] |
| Stumira lilium | 11.1% | heart, liver, skin and spleen | PCR (kDNA), PCR (SSU) | Mexico | [70] |
| Stumira ludovici | 4% | heart, liver, skin and spleen | PCR (kDNA), PCR (SSU) | Mexico | [70] |
| Order Didelphimorphia | |||||
| Marmosa mexicana (Mexican mouse opossum) | 66.7% | base of the tail | PCR (kDNA) | Mexico | [71] |
| Order Pilosa | |||||
| Tamandua mexicana (northern tamandua) | 6.3% | spleen | PCR (ALAT), PCR (ITS1) + sequencing | Mexico | [79] |
| Order Primates | |||||
| Alouatta palliate (mantled howler monkey) | 5% | serum | ELISA, IFAT and WB | Mexico | [80] |
| Alouatta pigra (Guatemalan black howler) | 37.5% | serum | ELISA, IFAT and WB | Mexico | [80] |
| Order Rodentia | |||||
| Heteromys gaumeri | 46.3% | base of the tail | PCR (kDNA) | Mexico | [71] |
| Heteromys desmarestianus | 100% | base of the tail | PCR (kDNA) | Mexico | [71] |
| Neotoma micropus (woodrats) | 7.3–50% | skin, ear tissue | Culture, PCR (kDNA), Culture of lesions + PCR + isoenzyme analysis of cultures | USA | [73,74] |
| Neotoma floridana (eastern woodrat) | 100% | ear, foot | Smears, PCR | USA | [76] |
| Handleyomys (Oryzomys) melanotis | 65–100% | skin (base-tail, lesions), liver and spleen | Culture, Mab, imprints and PCR (kDNA) | Mexico | [71,72,75,77] |
| Ototylomis phyllotis | 75.5–100% | skin (base-tail), liver | Culture, Mab, imprints and PCR (kDNA) | Mexico | [71,72,75] |
| Peromyscus attwateri | 100% (n = 1) | skin (neck) | PCR (ITS1) + sequencing | USA | [78] |
| Peromyscus yucatanicus | 28.6–100% | skin (base-tail), heart and kidney, liver, spleen | Culture, PCR (kDNA) and imprints | Mexico | [71,72] |
| Rattus rattus (black rat) | 2.9–19% | blood | Culture, PCR (kDNA) + RFLP/hybridisation | Venezuela, Brazil | [33,58] |
| Reithrodontomys gracilis | 66.6% | skin (base-tail) | Culture, PCR (kDNA) | Mexico | [71] |
| Sigmodon hispidus (cotton rat) | 58.8–100% | liver, skin (base-tail, lesion) and spleen | Imprints, culture, Mab and PCR (kDNA) | Mexico | [71,72,75,77] |
| Trichomys apereoides | 27.8% | blood | PCR (kDNA) + hybridisation | Brazil | [58] |
| Host | Prevalence | Organs/Tissue Analysed | Method of Detection | Country | Reference |
|---|---|---|---|---|---|
| Order Carnivora | |||||
| Cerdocyon thous (crab-eating fox) | 4–100% | BM, heart, liver, lung, mesenteric LN, serum skin and spleen | Smears, ELISA, culture, PCR, inoculation to hamster, IFAT, xenodiagnosis vector, PCR (kDNA) + sequencing, PCR (kDNA) + sequencing * | Brazil | [81,82,83,84,85,86,87,89] |
| Chrysocyon brachyurus (maned wolf) | 10–75% | BM, serum and skin | ELISA, IFAT, PCR (kDNA), PCR (kDNA) + sequencing, IC rk39, xenodiagnoses in vector | Brazil | [82,84,85,88,89,90] |
| Eira barbara (tayra) | n.s. | serum | DAT (n = 3) | Brazil | [92] |
| Galictis cuja (lesser grison) | n.s. | serum | DAT (n = 3) | Brazil | [92] |
| Leopardus pardalis (ocelot) | 75% | serum | ELISA, | Brazil | [90] |
| Lycalopex (Pseudalopex) vetulus (hoary fox) | 33.3% | BM, serum | IFAT, ELISA, PCR (kDNA) | Brazil | [84] |
| Nasua nasua (coati) | n.s. | serum | DAT (n = 2) | Brazil | [92] |
| Speothos venaticus (bush dogs) | 33.3–100% | blood, LN, serum, skin, spleen and other tissues (liver, kidney, lung and large intestine) | PCR (kDNA), PCR (kDNA) + sequencing, histopathology, IHC, ELISA, IFAT, IC rk39, xenodiagnoses in vector | Brazil | [84,85,89,90,91] |
| Panthera onca (jaguar) | 20–50% | blood, LN and serum | PCR (kDNA) + RFLP, ELISA, IC | Brazil | [90,93] |
| Panthera tigris altaica (Siberian tiger) | 50% | serum | ELISA, IC | Brazil | [90] |
| Panthera leo (lion) | 50–100% | blood, serum | PCR (kDNA) + RFLP, ELISA | Brazil | [90,94] |
| Puma concolor (cougar) | 71.4% | blood, LN | PCR (kDNA) + RFLP | Brazil | [93] |
| Procyon cancrivorus (crab-eating racoon) | 33.3% | kidney | PCR (kDNA) + sequencing * | Brazil | [86] |
| Order Chiroptera | |||||
| Artibeus planirostris (frugivorous) | 7.4–16.7% | blood | PCR (kDNA), PCR (Cyt B) + sequencing | Brazil | [45,95] |
| Artibeus lituratus (frugivorous) | 40.9% | blood | qPCR (kDNA) | Brazil | [95] |
| Desmodus rotundus (hematophagous) | 50% | liver, skin | qPCR (kDNA), PCR (ITS1) + RFLP, PCR (kDNA), nPCR (SSU) + sequencing | Brazil | [14,44] |
| Carollia perspicillata (frugivorous) | 3–27.3% | blood, spleen | Culture, qPCR (kDNA), qPCR (SSU), PCR (kDNA), PCR (ITS2) + sequencing | Venezuela, Brazil and French Guiana | [95,96,97] |
| Eumops perotis (insectivorous) | 11.1% | blood | PCR (kDNA), PCR (Cyt B) + sequencing | Brazil | [45] |
| Eptesicus furinalis (frugivorous) | 100% (n = 1) | blood | PCR (kDNA), PCR (Cyt B) + sequencing | Brazil | [45] |
| Glossophaga soricina (nectarivorous) | 0.7–100% | blood, liver and spleen | nPCR (SSU), PCR (kDNA), PCR (Cyt B) + sequencing, qPCR (kDNA), PCR (kDNA) and nPCR (SSU) + sequencing | Brazil | [44,45,66,95] |
| Myotis nigricans (insectivorous) | 33.3% | liver | PCR (kDNA) + nPCR (SSU) + sequencing | Brazil | [44] |
| Molossus molossus (insectivorous) | 0.5%–100% | blood, liver and spleen | nPCR (SSU), qPCR (kDNA), PCR (ITS1) + RFLP, PCR (kDNA), PCR (Cyt B) + sequencing, PCR (kDNA) + nPCR (SSU) + sequencing | Brazil | [14,44,45,66] |
| Molossus pretiosus (insectivorous) | 21.1% | liver, skin | PCR (kDNA) + nPCR (SSU) + sequencing | Brazil | [44] |
| Molossus rufus (insectivorous) | 20–100% | liver, spleen | qPCR (kDNA), PCR (ITS1) + RFLP, nPCR (SSU) + sequencing | Brazil | [14,44] |
| Molossidae spp. (insectivorous) | 40% | liver, skin | PCR (kDNA)+ nPCR (SSU) + sequencing | Brazil | [44] |
| Nyctinomops laticaudatus (insectivorous) | 40% | liver, skin | PCR (kDNA) and nPCR (SSU) + sequencing | Brazil | [44] |
| Nyctinomops macrotis (insectivorous) | 60% | liver, skin | PCR (kDNA) + nPCR (SSU) + sequencing | Brazil | [44] |
| Platyrrhynus lineatus (frugivorous) | 15.4% | blood | qPCR (kDNA) | Brazil | [95] |
| Phyllostomus hastatus (omnivorous) | 5.9% | blood | PCR (kDNA), PCR (Cyt B) + sequencing | Brazil | [45] |
| Phyllostomus discolor (omnivorous) | 100% (n = 1) | blood | qPCR (kDNA) | Brazil | [95] |
| Pteronotus parnellii (insectivorous) | 100% (n = 1) | blood | PCR (SSU), PCR (GAPDP) | Brazil | [98] |
| Bats (n.s.) | 0.1% | oral swab | PCR (SSU) + sequencing * | Brazil | [32] |
| Order Cingulata | |||||
| Dasypus septemcinctus (seven-banded armadillo) | 100% (n = 1) | liver | PCR (kDNA) + sequencing * | Brazil | [86] |
| Order Didelphimorphia | |||||
| Didelphis albiventris (white-eared opossum) | 6.3–22.2% | blood, BM, liver, lung, kidney, skin and spleen | Culture, PCR (ITS1) + RFLP, PCR (kDNA), PCR (kDNA) + sequencing, nPCR (SSU) + sequencing, PCR (kDNA), PCR (ITS1) | Brazil | [39,50,54,86,100] |
| Didelphis aurita (big-eared opossum) | 6.3% | LN, serum and spleen | Spleen smears, PCR (kDNA) + hybridisation IC rk39 | Brazil | [102] |
| Didelphis marsupialis (common opossum) | 7.1–40.5% | blood, BM, liver, serum, skin and spleen | smears, Culture, inoculation to hamster + isoenzyme, Mab, PCR (kDNA) + hybridisation, IFAT, DAT, PCR+RFLP, nPCR (SSU), PCR (ITS1) | Brazil, Colombia and Venezuela | [38,103,104,105,106,107] |
| Didelphis sp. D. albiventris D. aurita | 91.6% | blood, BM | PCR (kDNA) ELISA, FML-ELISA, smears, culture | Brazil | [101] |
| Order Lagomorpha | |||||
| Lepus europaeus (European hare) | n.s. | serum | DAT (n = 1) | Brazil | [92] |
| Order Pilosa | |||||
| Myrmecophaga tridactyla (giant anteater) | 33.3% | heart, kidney, lung and mesenteric LN | PCR (kDNA) + sequencing * | Brazil | [86] |
| Tamandua tetradactyla (lesser anteater) | 50–100% | BM, liver, lung and mesenteric LN | PCR (kDNA), PCR (ITS1) + sequencing | Brazil | [86,99] |
| Order Primates | |||||
| Alouatta caraya (black howler) | 3.7% | ear tissue | PCR (ITS) + RFLP + sequencing | Brazil, Argentina | [64] |
| Alouatta guariba (brown howler monkey) | 12.5 | blood | PCR (kDNA) | Brazil | [108] |
| Alouatta seniculus (red howler monkey) | 22.2% | blood | PCR (kDNA), PCR (ITS2), PCR (SSU), IC | French Guiana | [119] |
| Aotus nigriceps (black-headed night monkey) | 100% (n = 1) | blood | qPCR (kDNA) | Brazil | [108] |
| Callicebus nigrifons (black-fronted titi) | 33.3% | blood, liver, lung, intestine and spleen | qPCR (kDNA), IHC, | Brazil | [108] |
| Callithrix jacchus (white-tufted-ear marmoset) | 100% (n = 1) | serum | DAT | Brazil | [92] |
| Callithrix penicillata, C. jacchus | 26.9% | blood, skin | DAT, PCR + sequencing | Brazil | [109] |
| Cebus xanthosternos (golden-bellied capuchin) | 60% | blood | qPCR (kDNA) | Brazil | [108] |
| Leontopithecus chrysomelas (golden-headed lion tamarin) | 20% | blood | qPCR (kDNA) | Brazil | [108] |
| Pithecia irrorata (bald-faced saki) | 50% | blood | qPCR (kDNA) | Brazil | [108] |
| Saguinus imperator (emperor tamarin) | 100% | blood | qPCR (kDNA) | Brazil | [108] |
| Order Rodentia | |||||
| Cavia aperea (Brazilian guinea pig) | 25% | heart | PCR (kDNA) + sequencing | Brazil | [86] |
| Cerradomys (Oryzomys) subflavus | 25% | BM, liver and spleen | nPCR (SSU) + sequencing | Brazil | [50] |
| Coendu (Sphiggurus) villosus (prehensile tailed porcupine) | n.s. | serum | DAT (n = 2) | Brazil | [92] |
| Coendou (Sphiggurus) spinosus (Paraguayan hairy dwarf porcupine) | 20% | heart, kidney, liver and spleen | PCR (kDNA) + sequencing | Brazil | [86] |
| Clyomis laticeps | 5.2% | spleen | PCR (kDNA) + PCR (HSP70) | Brazil | [36] |
| Dasyprocta azarae | 16.7% | spleen | PCR (kDNA) + PCR (HSP70) | Brazil | [36] |
| Dasyprocta sp. | n.s. | blood, skin | PCR (kDNA) + PCR (ITS), PCR (HSP70) + sequencing | Brazil | [110] |
| Holochilus scieurus | 10% | skin, spleen | PCR (kDNA) | Brazil | [39] |
| Hydrochoerus hydrochaeris (capybara) | 50% | lung | PCR (kDNA) + sequencing | Brazil | [86] |
| Mus musculus (house mice) | 20% | BM, liver, tail–ear skin and spleen | nPCR (SSU) + sequencing | Brazil | [50] |
| Nectomys squamipes | 7% | skin, spleen | PCR (kDNA) | Brazil | [39] |
| Proechymis canicollis | 8.8% | skin, spleen | PCR + hybridisation | Colombia | [106] |
| Proechymis cuvieri | n.s. | blood, skin | PCR (kDNA) + PCR (ITS), PCR (HSP70) + sequencing | Brazil | [110] |
| Rhipidomys mastacalis | 28.5% | liver | PCR (HSP70) + RFLP | Brazil | [21] |
| Rattus norvegicus (brown rat) | 16.7% | liver, tail–ear skin, | nPCR (SSU) + sequencing | Brazil | [50] |
| Rattus rattus (black rat) | 0.1–100% | blood, BM, liver, skin and spleen | PCR (kDNA), PCR (kDNA) + hybridisation, PCR (HSP70) + RFLP, PCR (kDNA), nPCR (SSU) + sequencing, PCR (HSP70) + RFLP | Venezuela Brazil | [21,39,50,53,58,107] |
| Trichomys apereoides | 6.3–11.1% | skin, ear skin | PCR (kDNA) + hybridisation PCR (HSP70) + RFLP | Brazil | [21,58] |
| Trichomys laurentis | 1% | spleen | PCR (kDNA) | Brazil | [36] |
| Wild animals infected with Leishmania spp. in the Americas | |||||
| Host | Prevalence | Organs/tissue Analysed | Method of Detection | Country | Reference |
| Order Carnivora | |||||
| Canis latrans (coyote) | 1.6% | serum | IC rAgK39 | USA | [112] |
| Cerdocyon thous (crab-eating fox) | 15.3–100% | blood, serum and skin | qPCR (kDNA), IFAT, IC | Brazil | [114,115] |
| Chrysocyon brachyurus (maned wolf) | 42.9% | blood | qPCR (kDNA) | Brazil | [114] |
| Lontra longicaudis (neotropical otter) | 50% | blood | qPCR (kDNA) | Brazil | [114] |
| Lycalopex (Pseudalopex) griseus (South American grey fox) | 37.5% | blood | PCR (kDNA) + sequencing | Argentina | [113] |
| Lycalopex (Pseudalopex) vetulus (hoary fox) | 7.1–50% | blood, serum | qPCR (kDNA), IFAT | Brazil | [114,115] |
| Nasua nasua (coati) | 50% | blood | qPCR (kDNA), IFAT | Brazil | [114,116] |
| Puma concolor (cougar) | 100% (n = 1) | blood | qPCR (kDNA) | Brazil | [114] |
| Spheotos venaticus (bush dog) | 33.3–100% | Blood, serum, liver and LN | ELISA, PCR (kDNA) | Brazil | [91,117] |
| Vulpes fulvus (American red fox) | 9.1% | serum | IC rK39 | USA | [112] |
| Urocyon cinereoargenteus (gray fox) | 2% | serum | IC rK39 | USA | [111] |
| Order Chiroptera | |||||
| Molossus molossus (insectivorous) | 7.4% | liver | PCR (kDNA)+ nPCR (SSU) + sequencing | Brazil | [44] |
| Molossus pretiosus (insectivorous) | 5.2% | liver | PCR (kDNA)+ nPCR (SSU) + sequencing | Brazil | [44] |
| Nyctinomops macrotis (insectivorous) | 6.7% | liver | PCR (kDNA)+ nPCR (SSU) + sequencing | Brazil | [44] |
| Order Pilosa | |||||
| Myrmecophaga tridactyla (giant anteater) | 36.4% | blood | qPCR (kDNA) | Brazil | [114] |
| Tamandua tetradactyla (lesser anteater) | 33.3% | blood | qPCR (kDNA) | Brazil | [114] |
| Order Primates | |||||
| Alouatta guariba (brown howler monkey) | 37.5% | blood | PCR (kDNA) | Brazil | [91] |
| Aotus nigriceps (black-headed night monkey) | 20% | blood | qPCR (kDNA) | Brazil | [114] |
| Chiropotes satanas (black-bearded saki) | 50% | blood | qPCR (kDNA) | Brazil | [114] |
| Lagothrix cana (gray-woolly monkey) | 33.3% | blood | qPCR (kDNA) | Brazil | [114] |
| Leontopithecus chrysomelas (golden-headed lion tamarin) | 16.7% | blood | qPCR (kDNA) | Brazil | [114] |
| Order Rodentia | |||||
| Rattus rattus (black rat) | 9.1% | serum | IFAT | Dominican Republic | [118] |
| Sciurus granatensis (red-tailed squirrel) | 100% (n = 1) | blood | nPCR (SSU) | Venezuela | [103] |
| Host | Prevalence | Organs/Tissue Analysed | Methods for Detection | Country | References |
|---|---|---|---|---|---|
| Order Carnivora | |||||
| Canis aureus (golden jackal) | 3–11.6% | blood, BM, liver, LN, serum, spleen | qPCR (ITS1), PCR (kDNA), IC rk39, smear, culture, PCR (α-tubulin and GAPDH) | Georgia, Israel, Iran and Romania | [120,121,122,123] |
| Canis lupus (grey wolf) | 6–100% | blood, hair, liver, LN, skin, serum, spleen | PCR (cysteine protease B), qPCR (kDNA), PCR (kDNA) + RFLP, PCR (ITS2) + RFLP, PCR (kDNA) + sequencing, ELISA | Croatia, Italy, Spain | [124,125,126,127,128,129,130,131,132] |
| Felis silvestris (wildcat) | 25–100% | liver, LN, skin, spleen | qPCR (kDNA), PCR (ITS2) + sequencing, PCR (kDNA) + sequencing, qPCR (kDNA) + RFLP + sequencing | Spain | [127,128,133] |
| Genetta genetta (common genet) | 10–100% | blood, liver, skin and spleen | PCR (kDNA) + RFLP, qPCR (kDNA), PCR (ITS2) + sequencing, PCR (kDNA) + sequencing, qPCR (kDNA) + RFLP + sequencing, PCR (kDNA & ITS2) + RFLP | Spain | [125,127,128,129,133,134] |
| Herpestes ichneumon (Egyptian mongoose) | 4.7–28.6% | blood, spleen, | PCR (kDNA) + RFLP, PCR (kDNA) + sequencing, PCR (ITS1) | Spain, Portugal | [125,135] |
| Lutra lutra (Eurasian otter) | 70% | spleen | PCR (kDNA) + sequencing | Spain | [127] |
| Lynx pardinus (Iberian lynx) | 25% | blood, spleen, | PCR (kDNA) + RFLP | Spain | [125] |
| Martes foina (beech marten) | 29–100% | liver, LN, hair, skin and spleen | qPCR (kDNA), qPCR (ITS2) + sequencing, PCR (kDNA) + sequencing, qPCR (kDNA) + sequencing, PCR (kDNA & ITS2) + RFLP | Spain | [127,128,129,131,133,137] |
| Martes martes (European pine marten) | 30–62% | blood, liver, spleen | PCR (kDNA) + RFLP, qPCR (kDNA), qPCR (ITS2) + sequencing, PCR (kDNA) + sequencing | Spain | [127,133,134] |
| Meles meles (European badger) | 26–53% | liver, spleen | qPCR (kDNA), PCR (ITS2) + sequencing, PCR (kDNA) + sequencing | Spain, Italy | [132,133] |
| Mustela lutreola (European Mink) | 2.1–50% | liver, spleen, serum | qPCR (kDNA), PCR (ITS2) + sequencing, PCR (ITS1), ELISA | Greece, Spain | [133,136] |
| Mustela putorius (European polecat) | 25% | liver, spleen | qPCR (kDNA), PCR (ITS2) + sequencing | Spain | [133] |
| Mustela vison (American mink) | 100% (n = 1) | liver, spleen | qPCR (kDNA) | Spain | [137] |
| Panthera tigris (Tiger) | 25% | serum, LN and swab (oral, conjunctival and nasal) | IFAT, qPCR | Italy | [138] |
| Sciurus vulgaris (red squirrel) | 20% | liver, skin, pleen | qPCR (kDNA) | Spain | [137] |
| Ursus arctos (brown bear) | 100% (n = 1) | liver, skin, spleen | PCR (kDNA), PCR (ITS2) + RFLP | Spain | [129] |
| Vulpes vulpes (red fox) | 2.6–74.6% | blood, BM, hair, liver, LN, skin, spleen, serum | PCR (Repeat Region), PCR (kDNA), PCR (kDNA) + RFLP, qPCR (kDNA), qPCR (ITS2) + sequencing, qPCR (ITS1) + RFLP, PCR (α-tubulin and GAPDH) + sequencing, PCR (kDNA) + RFLP, PCR (kDNA) + sequencing, PCR (ITS2) + RFLP, PCR (ITS1) + sequencing, ELISA, IFAT, WB, IC rk39, smear, culture | France, Georgia and Greece, Iran, Italy and Spain | [120,122,125,127,128,129,131,132,139,140,141,142,143,144,145,146] |
| Order Chiroptera | |||||
| Pipistrellus pipistrellus (common urban bat) | 59.2% | blood clot, hair, spleen | PCR (Repeat region) + sequencing | Spain | [149] |
| Order Diprotodontia | |||||
| Macropus rufogriseus (Bennett’s wallaby) | 33.3% | blood, BM, liver, lung, LN, kidney, skin, spleen | PCR (ITS1 and ITS2) + sequencing, IC rk39 | Spain | [147] |
| Order Eulipotyphla | |||||
| Atelerix algirus (Algerian hedgehog) | 100% | blood, eye swab, heart, kidney, liver, LN, skin, spleen | PCR (kDNA), PCR (ITS1), PCR (mini-exon), PCR (Repeat region), PCR (SSU), smear | Tunisia | [150,151] |
| Erinaceus europaeus (European hedgehog) | 34.4–100% | hair, serum, skin, spleen | qPCR (kDNA), ELISA | Spain | [131,137] |
| Order Lagomorpha | |||||
| Lepus europaeus (European hare) | 0.9–43.6% | blood, spleen, serum | PCR (kDNA) + RFLP, PCR (ITS1), PCR (ITS1) + sequencing, ELISA, IFAT | Greece, Italy and Spain | [136,152,155,156] |
| Lepus granatensis (Iberian hare) | 10.1–100% | hair, skin, spleen, serum | PCR (kDNA) + RFLP, qPCR (kDNA), nPCR (SSU), IFAT, DFA | Spain | [152,153,154] |
| Oryctolagus cuniculus (European rabbit) | 0.6–59% | blood, BM, hair, heart, liver, LN, skin, spleen, serum | qPCR (kDNA), PCR (ITS1) + RFLP, ELISA, nPCR (SSU), qPCR (kDNA) + RFLP + sequencing, PCR (kDNA) + RFLP, PCR (ITS2) + RFLP, PCR (ITS1), smears, culture, IFAT, DFA, ELISA, IC rk39 | Greece, Italy and Spain | [128,129,136,145,153,154,157,158] |
| Order Primates | |||||
| Pongo pygmaeus (north west Bornean orangutan) | 100% | BM, serum | Microscopy, IFAT, nPCR (ITS1) | Spain | [148] |
| Order Rodentia | |||||
| Apodemus sylvaticus (wood mouse) | 20–50% | blood, BM, liver, skin, spleen | PCR (ITS1) + sequencing, PCR-ELISA (kDNA), qPCR (kDNA) + RFLP + sequencing, PCR (ITS2) + RFLP, smear, culture | Spain | [128,129,159] |
| Crocidura russula (white-toothed shrew) | 13.3% | blood and/or spleen | qPCR (kDNA) | Spain | [160] |
| Mus musculus (house mouse) | 22–50% | blood, BM, liver, skin, spleen | qPCR (kDNA) + sequencing, PCR (ITS1) + sequencing, PCR-ELISA (kDNA), nPCR (SSU and ITS1) + sequencing, smear | Morocco, Portugal and Spain | [162,163] |
| Mus spretus (Algerian mouse) | 4.3–42.9% | blood, liver, skin, spleen and serum | qPCR (kDNA), ELISA | Spain | [137,160] |
| Nesokia indica (short-tailed bandicoot rat) | 39% | liver, skin, spleen, | nPCR (kDNA), smear | Iran | [162] |
| Rattus norvegicus (brown rat) | 5.9–100% | hair, liver, skin, spleen | nPCR (SSU), nPCR (ITS1) + sequencing, qPCR (kDNA), PCR (kDNA), PCR (kDNA) + RFLP, PCR (ITS2) + RFLP, smear | Greece, Morocco, Portugal and Spain | [129,131,162,163,166] |
| Rattus rattus (black rat) | 7.5–33.3% | blood, BM, liver, skin, spleen | PCR (kDNA) + sequencing, PCR (ITS1) + sequencing, PCR-ELISA (kDNA), nPCR (SSU), nPCR (ITS1) + sequencing, smear, culture, inoculation to hamster, isoenzymes | Italy, Morocco, Saudi Arabia and Spain | [159,163,164,165] |
| Host | Prevalence | Organs/Tissue Analysed | Methods for Detection | Country | References |
|---|---|---|---|---|---|
| Order Chiroptera | |||||
| Nycteris hispida | 100% (n = 1) | spleen | qPCR (kDNA and ITS1) + sequencing | Ethiopia | [172] |
| Order Eulipotyphla | |||||
| Atelerix algirus (Algerian hedgehog) | 36.8–100% | blood, eye swab, heart, kidney, liver, LN, skin, spleen | qPCR (kDNA), PCR (kDNA), PCR (ITS1) + RFLP, nPCR (kDNA), PCR (ITS1) + sequencing + RFLP, PCR (mini-exon) + sequencing + RFLP, nPCR (Repeat region) + sequencing + RFLP, PCR (SSU) + sequencing, smear, ELISA, WB | Algeria, Tunisia | [150,151,175] |
| Hemiechinus auritus (long-eared hedgehogs) | 33.3–53.3% | liver, skin, spleen | nPCR (ITS1) + sequencing, nPCR (kDNA), semi-nPCR (kDNA), smear | Iran | [173,174] |
| Paraechinus aethiopicus (desert hedgehog) | 40–100% | blood, eye swab, kidney, liver, LN, skin, spleen, serum | qPCR (kDNA), PCR (ITS1) + RFLP, PCR (kDNA and SSU) + sequencing, nPCR (Repeat region) + RFLP + sequencing, ELISA, WB | Algeria, Tunisia | [151,175] |
| Order Primates | |||||
| Cercopithecus mitis (syke’s monkeys) | 67.2% | serum | ELISA, WB | Kenya | [176] |
| Chlorocebus aethiops (vervet monkeys) | 60.6% | serum | ELISA, WB, lymphocyte proliferation assay | Kenya | [176] |
| Gorilla gorilla (gorilla) | 13.2% | faeces | qPCR (SSU), qPCR (SSU) + sequencing, PCR (ITS) + sequencing, PCR (Cytb) + sequencing | Cameroon | [15] |
| Papio cynocephalus anubis (olive baboons) | 77.2% | serum | ELISA, WB | Kenya | [176] |
| Order Rodentia | |||||
| Gerbillus nanus | 11.8% | liver, skin, spleen | PCR (kDNA), smear | Iran | [171] |
| Meriones hurrianae | 7.7% | liver, skin, spleen | PCR (kDNA), smear | Iran | [171] |
| Meriones libycus | 5.7–100% | liver, skin, spleen | PCR (kDNA), nPCR (ITS1), PCR (ITS1) + RFLP + sequencing, semi-nPCR (kDNA), PCR (Cytb) + sequencing, nPCR (ITS1) + sequencing, nPCR (ITS2) + RFLP, smear, inoculation to hamster, inoculation to BALB/c mice | Iran | [167,168,169,170,173,177,178] |
| Meriones persicus | 33% | skin | PCR (ITS1) + RFLP + sequencing, smear | Iran | [168] |
| Meriones tristrami | 58.3% | skin | PCR (ITS1) + RFLP | Israel | [179] |
| Microtus guentheri | 16.5% | skin | PCR (ITS1) + RFLP | Israel | [179] |
| Microtus socialis | 50% | liver, skin, spleen | smear | Iran | [161] |
| Mus musculus (house mouse) | 2.3–33% | liver, skin, spleen | PCR (ITS1) + RFLP, smear | Israel, Morocco and Iran | [161,179] |
| Nesokia indica | 8–63.4% | liver, skin, spleen | PCR (ITS1) + RFLP + sequencing, PCR (kDNA), nPCR (kDNA), smear | Iran | [161,167,168] |
| Rhombomys opimus (great gerbil) | 13.4–35% | skin | PCR (ITS1) + RFLP + sequencing, semi-nPCR (kDNA), PCR (Cytb) + sequencing, smear, IHC, inoculation to hamster, inoculation to BALB/c mice | Iran | [168,169] |
| Tatera indica | 3.7–50% | liver, skin, spleen | PCR (kDNA), PCR (ITS1) + RFLP + sequencing, semi-nPCR (kDNA) + sequencing, PCR (Cytb), smear | Iran | [168,169,171] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azami-Conesa, I.; Gómez-Muñoz, M.T.; Martínez-Díaz, R.A. A Systematic Review (1990–2021) of Wild Animals Infected with Zoonotic Leishmania. Microorganisms 2021, 9, 1101. https://doi.org/10.3390/microorganisms9051101
Azami-Conesa I, Gómez-Muñoz MT, Martínez-Díaz RA. A Systematic Review (1990–2021) of Wild Animals Infected with Zoonotic Leishmania. Microorganisms. 2021; 9(5):1101. https://doi.org/10.3390/microorganisms9051101
Chicago/Turabian StyleAzami-Conesa, Iris, María Teresa Gómez-Muñoz, and Rafael Alberto Martínez-Díaz. 2021. "A Systematic Review (1990–2021) of Wild Animals Infected with Zoonotic Leishmania" Microorganisms 9, no. 5: 1101. https://doi.org/10.3390/microorganisms9051101
APA StyleAzami-Conesa, I., Gómez-Muñoz, M. T., & Martínez-Díaz, R. A. (2021). A Systematic Review (1990–2021) of Wild Animals Infected with Zoonotic Leishmania. Microorganisms, 9(5), 1101. https://doi.org/10.3390/microorganisms9051101







