Meet the Insidious Players: Review of Viral Infections in Head and Neck Cancer Etiology with an Update on Clinical Trials
Abstract
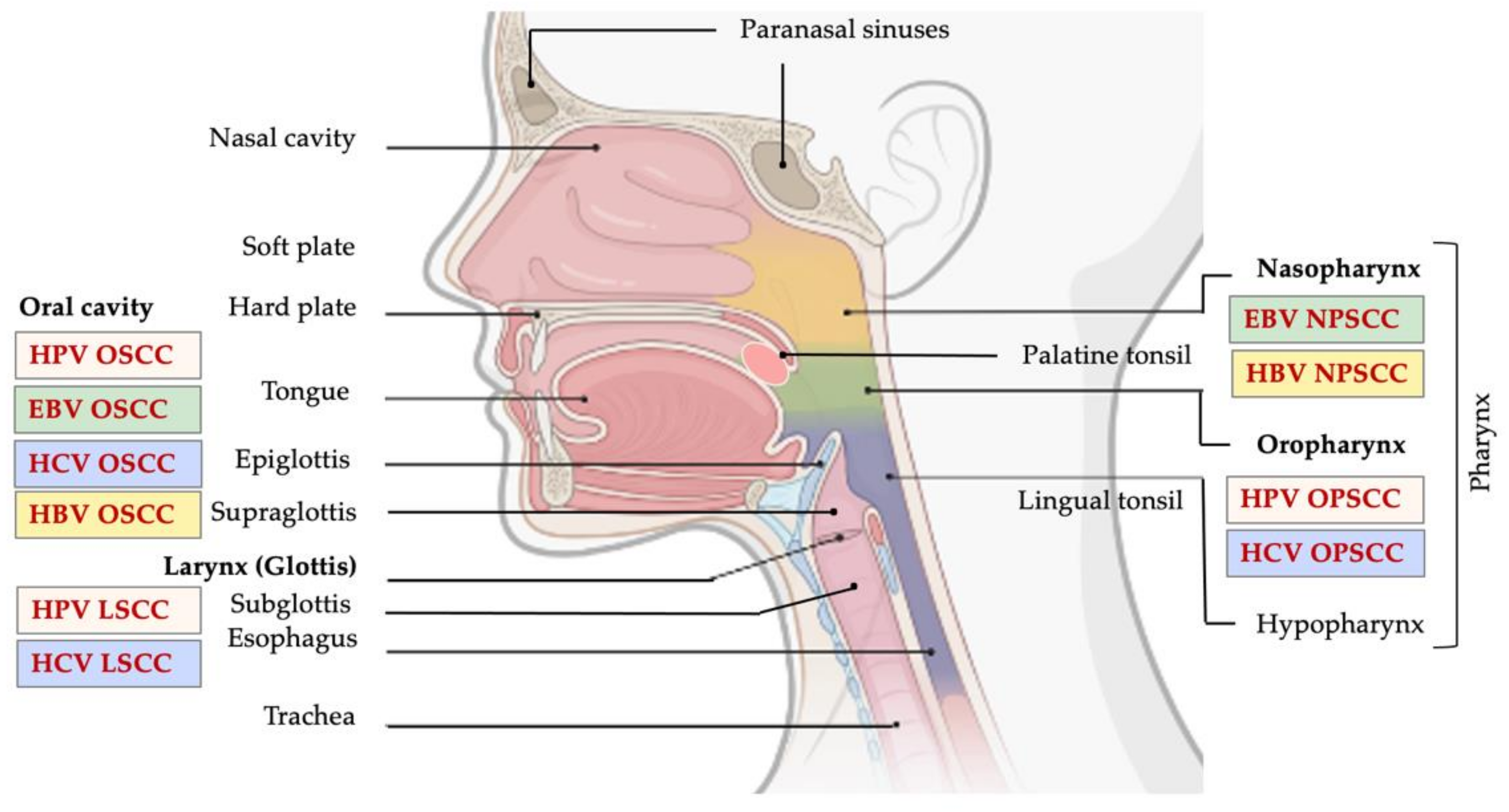
1. Introduction
2. Human Papillomavirus
2.1. HPV Genome and Mechanism of Infection
| Oncogenic Virus/Parameter | Human Papillomavirus (HPV) | Herpesvirus | Hepatitis Virus | |||
|---|---|---|---|---|---|---|
| HPV-Positive | HPV-Negative | Epstein-Barr Virus (EBV) | Hepatitis C | Hepatitis B | ||
| Virus-related | Nucleic acid | Circular double stranded DNA [16] | − | Linear double stranded DNA [36] | Single stranded RNA [37] | Double stranded circular DNA [38,39] |
| Genome | Approximately 8 kb in size [16] | − | Approximately 180 kb in size [36] | 9 600 bp in size [37,40] | The smallest genome with 3200 bp in size [38] | |
| Tropism | Kerationocytes and mucosal sufraces [16] | − | B-cells and epithelial cells [10,41] | Hepatocytes, lymphocytes, and salivary gland cells [42] | Hepatocytes and lymphocytes [43] | |
| Major viral oncoproteins | E6, E7 [15,16,24] | − | LMP1, LMP2A, EBNA1 [10,44] | NS3 or NS5A [40,42,45] | S, C, P and X [39,46] | |
| Virus transmission mode | Sexual contact, self-inoculation, vertical and horizontal transmissions [47] | − | Sexual contact, blood or saliva transmission [10] | Vertical transmission, horizontal transmission (sex or sharing of drug-injection needles) [39] | Sexual contact, self-inoculation, vertical and horizontal transmissions [47] | |
| Cancer-related | Anatomic site | Oral cavity, oropharynx, larynx [47,48] | All sites, but mostly oropharynx [36] | Nasopharynx [49] Oral cavity [50] | Oral cavity, oropharynx, larynx [45,51,52,53,54] | Oral cavity [54] Nasopharynx [43,55] |
| Histology | Nonkeratinized [56] | Keratinized [41] | Undifferentiated type NPC, squamous cell and non–keratinizing NPC [57] | Squamous cell [51,53] | Squamous cell or adenocarcinoma [58] | |
| Age | Under age of 50 [6] | Above age of 50 [6] | Above age of 50 [49,59] | Above age of 50 [42,54] | Above age of 50 [43] | |
| Gender | Mostly male [24] | Mostly male [24] | Mosty male [10] | Mostly male, 6.7-fold higher risk in male [42,54] | No significant difference [42,43] | |
| Incidence trend | Increasing | Decreasing | Increasing | Increasing | Increasing | |
2.2. HPV in HNSCC Development
2.3. Current Treatment Options and Update on Clinical Trials
| Clinical Trial/NCT Number/Year | Phase | Disease Stage | Patient Number (n) | Treatment Arms | Outcome |
|---|---|---|---|---|---|
| 2007–2015 [78] | N/A | OPSCC, stage I-IVb | n = 314 (n = 286 HPV-positive) | Arm 1: TORS4; Arm 2: TORS + RT6 (50–70 Gy); Arm 3: TORS + RT (30–70 Gy) + adjuvant CT (CDDP1/Carboplatin/Docetaxel/Cetuximab) | 5-year after surgery LR13 RFS17: 92% DMFS18: 90% OS8: 86% CSS3: 94% |
| ORATOR NCT01590355 2012–2019 [82] | Phase II | Early stage OPSCC | n = 68 (n = 60 p16-positive) | Arm 1: RT ± CT7 (70/63/56 Gy in 35 fxs for 7 weeks); Arm 2: TORS + neck dissection | Median follow-up (27 months): Arm 1: 25 months Arm 2: 29 months QoL11 (MDADI score) Arm 1: 86.9 Arm 2: 80.1 |
| ECOG-ACRIN 3311 NCT01898494 2013–2020 [77] | Phase II | HPV-positive, stage III-Iva OPSCC | n = 353 | Arm A: TORS; Arm B: TORS, low-dose IMRT (50 Gy); Arm C: TORS, standard-dose IMRT (60 Gy); Arm D: TORS, standard-dose IMRT (60–66 Gy) + CT (weekly CDDP 40 mg/m2) | 2-year PFS12: Arm A: 93.9% Arm B: 95.0% Arm C: 95.9% Arm D: 90.5% |
| ADEPT NCT01687413 2012–2020 [83] | Phase III | p16-positive OPSCC | n = 42 | Experimental group: postoperative IMRT (60 Gy in 30 fxs); Active comparator: RT (60 Gy in 30 fxs) + cisplatin (40 mg/m2 × 6 doses) | 1-year DFS: 100% vs. 90.9% 2-year LR control: 96.3% vs. 81.8% 2-year DM: 7.4% vs. 0% |
| ECOG-E1308 NCT01084083 2010–2015 [84] | Phase II | HPV-positive and/or p16-positive stage III-IV OPSCC | n = 80 | Group 1: CDDP (75 mg/m2) and paclitaxel (90 mg/m2), low dose IMRT (54 Gy in 27 fxs × 5 weeks), cetuximab (400 mg/m2 → 250 mg/m2); Group 2: CDDP (75 mg/m2) and paclitaxel (90 mg/m2), standard dose IMRT (69.3 Gy in 33 fxs × 6 week), cetuximab (400 mg/m2 → 250 mg/m2) | 2-year PFS and OS: 64% vs. 91% (IMRT 54 Gy); Primary CRR19: 73% |
| NCT01530997 2012–2020 [81] | Phase II | HPV-positive and/or p16-positive OPSCC, T0-T3, N0-N2c, M0 | n = 43; HPV+/p16+: 63.6% HPV−/p16+: 36.4% | De-intensification chemoradiation therapy; IMRT (54–60 Gy) + CDDP (30 mg/m2 × 6 doses + limited surgical evaluation | pCR20: 86% 2-year LC14: 100% |
| NCT02281955 2014–2020 [81,85] | Phase II | HPV-positive and/or p16-positive OPSCC, T0-T3, N0-N2c, M0 | n = 113; HPV+/p16+: 40.4 % HPV−/p16+: 10.5% HPV unknown/p16+: 49.1% | IMRT (60 Gy, 2Gy/fx) + CDDP (30–40 mg/m2 × 6 doses) or cetuximab (250 mg/m2) or Carboplatin (AUC 1.5 and paclitaxel 45 mg/m2) or Carboplatin AUC 3 + surgical evaluation | 2-year outcome: PFS: 88.4% LC: 96.4% RC15: 98.2% LRC16: 94.6% DMFS: 92.0% OS: 93.0% |
| RTOG 0129 NCT00047008 2003–2014 [67] | Phase III | Stage III-IV SCC of oral cavity, oropharynx, hypohparynx, larynx, T2, N2-3, M0 or T3-4) | n = 721; HPV+ (n = 206), HPV− (n = 117) | Arm 1: Standard fractionation RT (70 Gy in 35 fx, 2 Gy/fx) + CDDP (100 mg/m2); Arm 2: Accelerated fractionation RT (72 Gy in 42 fx) + CDDP (100 mg/m2) | 3-year outcome: OS (Arm 1 and Arm 2): 64.3% vs. 70.3%; OS (HPV-positive and HPV-negative group): 82.4% vs. 57.1%; PFS (HPV-positive and HPV-negative group): 73.7% vs. 43.4% |
| NCT01663259 2012–2020 (www.clinicaltrial.gov; accessed on 23 March 2021) | N/A | Stage III-IV (excluding N3 or T4), HPV-positive and/or p16-positive OPSCC | n = 42; HPV-positive (n = 42) | Cetuximab (400 mg/m2 → 250 mg/m2 concurrent with RT (70 Gy in 35 fx, 50–60 Gy) | 2-year outcome: RR21: 19% DFS: 81% OS: 95.2% FFLRP22: 87.9% |
| CheckMate 141 NCT02105636 2014–2019 [86] | Phase III | Platinum-refractory, recurrent HNSCC | n = 361; Arm 1 (n = 240): p16-positive (n = 63) p16-negative (n = 50); Arm 2 (n = 121): p16+ (n = 29) p16− (n = 36) | Arm 1: Nivolumab (3 mg/kg, IV, every 2 weeks); Arm 2: Cetuximab/Methotrexate/Docetaxel (Cetuximab 400 mg/m2 → 250 mg/m2 or methotrexate 40–60 mg/m2 or docetaxel 30–40 mg/m2, weekly) | 18-month OS (Arm 1 and Arm 2): 7.49 vs. 5.06 months; 1-year OS: 36.0% vs. 16.6%; 6-month PFS: 19.7% vs. 9.9%; RR: 13.3% vs. 5.8%; Median OS in p16-positive patients: 9.1 vs. 4.4 months; Median OS in p16-negative patients: 7.5 vs. 5.8 months |
| KEYNOTE-012 NCT01848834 2013–2020 [87] | Phase Ib | PD-L1-positive, R/M5 HNSCC | n = 60; HPV-positive (n = 23), HPV-negative (n = 37) | Pembrolizumab (10 mg/kg, once every 2 weeks) | OR9 (central vs. investigator review): 18% vs. 21%; OS: 13 months |
| KEYNOTE-040 NCT02252042 2014–2020 [88] | Phase III | R/M HNSCC | n = 495;HPV-positive (n = 119), HPV-negative (n = 376) | Pembrolizumab group (200 mg, 3-week cycle); Active comparator group (Methotrexate 40–60 mg/m2 or docetaxel 75 mg/m2 or cetuximab 400 mg/m2 → 250 mg/m2) | 2-year outcome: OS (pembrolizumab vs. active comparator group): 8.4 vs. 6.9 months; PFS: 2.1 vs. 2.3 months ORR10: 14.6% vs. 10.1%; DOR 23: 18.4 vs. 5.0 months |
| KEYNOTE-048 NCT02358031 2015–2020 [89] | Phase III | R/M HNSCC | n = 882; HPV-positive (n = 190), HPV-negative (n = 692) | Pembrolizumab monotherapy (200 mg of 3-week cycle for 2 years); Pembrolizumab + CT (200 mg of 3-week cycle for 2 years + cisplatin 100 mg/m2 or carboplatin (AUC 5 + 5-FU 2 1000 mg/m2 up to 6 cycles); Cetuximab + CT (Control) (400 mg/m2 → 250 mg/m2 + cisplatin 100 mg/m2 or carboplatin (AUC 5 + 5-FU 1000 mg/m2 up to 6 cycles) | 47 months outcome: OS (Pembrolizumab + CT group vs. control group): 13.0 vs. 10.7 months; OS in PD-L1 CPS > 1 participants: 13.6 vs. 10.4 months; OS (Pembrolizumab monotherapy vs. control group): 11.5 vs. 10.7 months |
3. Epstein-Barr Virus
3.1. EBV Genome and Mechanism of Infection
3.2. EBV in HNSCC Development
3.3. Current Treatment Options and Update on Clinical Trials
| Clinical Trial/NCT Number/Year | Phase | Disease Stage | Patient Number (n) | Treatment Arms | Outcome |
|---|---|---|---|---|---|
| NCI-9742 NCT02339558 2015–2019 [119] | Phase II | Nonkeratinizing, R/M NPC, stage III-IVc | n = 45; Plasma EBV DNA detection (n = 44) | Nivolumab (3 mg/kg for 4 weeks) | ORR: 20.5% one-year outcome: OS: 59% PFS:19.3% |
| KEYNOTE-028 NCT02054806 2014–2020 [123] | Phase I | PD-L1-positive, R/M NPC | n = 27 | Pembrolizumab (10 mg/kg every 2-week cycle for 24 months) | ORR: 25.9%; one-year PFS: 33.4% |
| Adoptive T-cell transfer NCT02578641 2008–2011 [124] | Phase II | EBV-positive R/M NPC | n = 38 | Venesection → CT (gemcitabine 1000 mg/m2 and carboplatin AUC 2 every 4 weeks for 4 cycles) + EBV-CTLs 1 (1 × 108 cells/m2 on weeks 0, 2, 8, 16, 24, 32) | RR: 71.4%; Median OS: 29.9 months; two- and three-year OS: 62.9% vs. 37.1% |
| MVA-EBNA1/LMP2 (MVA-EL) NCT01256853 2006–2010 [126] | Phase I | EBV-positive NPC | n = 18 | MVA-EL vaccine (3 intradermal vaccinations at 3-week period, with doses of 5 × 107, 1 × 108, 2 × 108, 3.3 × 108, 5 × 108 plaque forming units (pfu) | T-cell response (one or both antigens): 15 patients |
| MVA-EL NCT01147991 2005–2010 [125] | Phase Ia | EBV-positive NPC | n = 16 | MVA-EL vaccine (3 intradermal vaccinations at 3-week period, doses of 5 × 107–5 × 108 pfu) | T-cell response (one or both antigens): 8 patients (7/14, EBNA1; 6/14 LMP2) |
4. Hepatitis C Virus
4.1. HCV Genome and Mechanism of Infection
4.2. HCV and HNSCC Development
5. Hepatitis B Virus
5.1. HBV Genome and Mechanism of Infection
5.2. HBV and HNSCC Development
5.3. Treatment Options for Hepatitis C and B Viruses
6. Merkel Cell Polyomavirus
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pezzuto, F.; Buonaguro, L.; Caponigro, F.; Ionna, F.; Starita, N.; Annunziata, C.; Buonaguro, F.M.; Tornesello, M.L. Update on Head and Neck Cancer: Current Knowledge on Epidemiology, Risk Factors, Molecular Features and Novel Therapies. Oncology 2015, 89, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Suresh, G.M.; Koppad, R.; Prakash, B.V.; Sabitha, K.S.; Dhara, P.S. Prognostic Indicators of Oral Squamous Cell Carcinoma. Ann. Maxillofac. Surg. 2019, 9, 364–370. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Primer 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The Molecular Landscape of Head and Neck Cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; D’Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-Associated Head and Neck Cancer: A Virus-Related Cancer Epidemic. Lancet Oncol. 2010, 11, 781–789. [Google Scholar] [CrossRef]
- Yang, J.J.; Yu, D.; Wen, W.; Shu, X.-O.; Saito, E.; Rahman, S.; Gupta, P.C.; He, J.; Tsugane, S.; Xiang, Y.-B.; et al. Tobacco Smoking and Mortality in Asia: A Pooled Meta-Analysis. JAMA Netw. Open 2019, 2, e191474. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, D.; Matsuo, K. Alcohol and Head and Neck Cancer. Cancer Metastasis Rev. 2017, 36, 425–434. [Google Scholar] [CrossRef]
- Dhull, A.K.; Atri, R.; Dhankhar, R.; Chauhan, A.K.; Kaushal, V. Major Risk Factors in Head and Neck Cancer: A Retrospective Analysis of 12-Year Experiences. World J. Oncol. 2018, 9, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Q.; Merhi, M.; Raza, A.; Inchakalody, V.P.; Abdelouahab, N.; Zar Gul, A.R.; Uddin, S.; Dermime, S. Role of Epstein-Barr Virus in the Pathogenesis of Head and Neck Cancers and Its Potential as an Immunotherapeutic Target. Front. Oncol. 2018, 8, 257. [Google Scholar] [CrossRef]
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Guidi, A.; Codecà, C.; Ferrari, D. Chemotherapy and Immunotherapy for Recurrent and Metastatic Head and Neck Cancer: A Systematic Review. Med. Oncol. 2018, 35, 37. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Huang, J.; Qiao, B.; Lam, A.K. Immune Checkpoint Pathways in Immunotherapy for Head and Neck Squamous Cell Carcinoma. Int. J. Oral Sci. 2020, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Grandis, J.R. Emerging Drugs for Head and Neck Cancer. Expert Opin. Emerg. Drugs 2015, 20, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Syrjänen, S. Oral Manifestations of Human Papillomavirus Infections. Eur. J. Oral Sci. 2018, 126 (Suppl. 1), 49–66. [Google Scholar] [CrossRef]
- Araldi, R.P.; Sant’Ana, T.A.; Módolo, D.G.; de Melo, T.C.; Spadacci-Morena, D.D.; de Cassia Stocco, R.; Cerutti, J.M.; de Souza, E.B. The Human Papillomavirus (HPV)-Related Cancer Biology: An Overview. Biomed. Pharmacother. 2018, 106, 1537–1556. [Google Scholar] [CrossRef]
- Brianti, P.; De Flammineis, E.; Mercuri, S.R. Review of HPV-Related Diseases and Cancers. New Microbiol. 2017, 40, 80–85. [Google Scholar]
- Tommasino, M. The Human Papillomavirus Family and Its Role in Carcinogenesis. Semin. Cancer Biol. 2014, 26, 13–21. [Google Scholar] [CrossRef]
- Broccolo, F.; Ciccarese, G.; Rossi, A.; Anselmi, L.; Drago, F.; Toniolo, A. Human Papillomavirus (HPV) and Epstein-Barr Virus (EBV) in Keratinizing versus Non- Keratinizing Squamous Cell Carcinoma of the Oropharynx. Infect. Agent. Cancer 2018, 13, 32. [Google Scholar] [CrossRef]
- Burd, E.M. Human Papillomavirus and Cervical Cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef]
- Sathish, N.; Wang, X.; Yuan, Y. Human Papillomavirus (HPV)-Associated Oral Cancers and Treatment Strategies. J. Dent. Res. 2014, 93, 29S–36S. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Dong, Y. Human Papillomavirus and Oral Squamous Cell Carcinoma: A Review of HPV-Positive Oral Squamous Cell Carcinoma and Possible Strategies for Future. Curr. Probl. Cancer 2017, 41, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Budu, V.A.; Decuseară, T.; Balica, N.C.; Mogoantă, C.A.; Rădulescu, L.M.; Chirilă, M.; Maniu, A.A.; Mistra, D.M.; Muşat, G.C.; Oprişcan, I.C.; et al. The Role of HPV Infection in Oropharyngeal Cancer. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2019, 60, 769–773. [Google Scholar]
- Blitzer, G.C.; Smith, M.A.; Harris, S.L.; Kimple, R.J. Review of the Clinical and Biologic Aspects of Human Papillomavirus-Positive Squamous Cell Carcinomas of the Head and Neck. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Williams, V.M.; Filippova, M.; Soto, U.; Duerksen-Hughes, P.J. HPV-DNA Integration and Carcinogenesis: Putative Roles for Inflammation and Oxidative Stress. Future Virol. 2011, 6, 45–57. [Google Scholar] [CrossRef]
- Parfenov, M.; Pedamallu, C.S.; Gehlenborg, N.; Freeman, S.S.; Danilova, L.; Bristow, C.A.; Lee, S.; Hadjipanayis, A.G.; Ivanova, E.V.; Wilkerson, M.D.; et al. Characterization of HPV and Host Genome Interactions in Primary Head and Neck Cancers. Proc. Natl. Acad. Sci. USA 2014, 111, 15544–15549. [Google Scholar] [CrossRef]
- Schiller, J.T.; Day, P.M.; Kines, R.C. Current Understanding of the Mechanism of HPV Infection. Gynecol. Oncol. 2010, 118, S12–S17. [Google Scholar] [CrossRef]
- Yim, E.-K.; Park, J.-S. The Role of HPV E6 and E7 Oncoproteins in HPV-Associated Cervical Carcinogenesis. Cancer Res. Treat. 2005, 37, 319–324. [Google Scholar] [CrossRef]
- Roman, A.; Munger, K. The Papillomavirus E7 Proteins. Virology 2013, 445, 138–168. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, L.; Hu, S.-Y.; Feng, R.-M.; Zhao, X.-L.; Zhang, Q.; Pan, Q.-J.; Zhang, X.; Qiao, Y.-L.; Zhao, F.-H. Risk Stratification of HPV 16 DNA Methylation Combined with E6 Oncoprotein in Cervical Cancer Screening: A 10-Year Prospective Cohort Study. Clin. Epigenetics 2020, 12, 62. [Google Scholar] [CrossRef]
- Balderas-Loaeza, A.; Anaya-Saavedra, G.; Ramirez-Amador, V.A.; Guido-Jimenez, M.C.; Kalantari, M.; Calleja-Macias, I.E.; Bernard, H.-U.; Garcia-Carranca, A. Human Papillomavirus-16 DNA Methylation Patterns Support a Causal Association of the Virus with Oral Squamous Cell Carcinomas. Int. J. Cancer 2007, 120, 2165–2169. [Google Scholar] [CrossRef]
- Ekanayake Weeramange, C.; Tang, K.D.; Vasani, S.; Langton-Lockton, J.; Kenny, L.; Punyadeera, C. DNA Methylation Changes in Human Papillomavirus-Driven Head and Neck Cancers. Cells 2020, 9, 61359. [Google Scholar] [CrossRef]
- Laaneväli, A.; Ustav, M.; Ustav, E.; Piirsoo, M. E2 Protein Is the Major Determinant of Specificity at the Human Papillomavirus Origin of Replication. PLoS ONE 2019, 14, e0224334. [Google Scholar] [CrossRef]
- Amaro-Filho, S.M.; Pereira Chaves, C.B.; Felix, S.P.; Basto, D.L.; de Almeida, L.M.; Moreira, M.A.M. HPV DNA Methylation at the Early Promoter and E1/E2 Integrity: A Comparison between HPV16, HPV18 and HPV45 in Cervical Cancer. Papillomavirus Res. 2018, 5, 172–179. [Google Scholar] [CrossRef]
- Khanal, S.; Shumway, B.S.; Zahin, M.; Redman, R.A.; Strickley, J.D.; Trainor, P.J.; Rai, S.N.; Ghim, S.-J.; Jenson, A.B.; Joh, J. Viral DNA Integration and Methylation of Human Papillomavirus Type 16 in High-Grade Oral Epithelial Dysplasia and Head and Neck Squamous Cell Carcinoma. Oncotarget 2018, 9, 30419–30433. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Graubard, B.I.; Broutian, T.; Pickard, R.K.L.; Tong, Z.-Y.; Xiao, W.; Kahle, L.; Gillison, M.L. Effect of Prophylactic Human Papillomavirus (HPV) Vaccination on Oral HPV Infections Among Young Adults in the United States. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 262–267. [Google Scholar] [CrossRef]
- Kato, N. Genome of Human Hepatitis C Virus (HCV): Gene Organization, Sequence Diversity, and Variation. Microb. Comp. Genom. 2000, 5, 129–151. [Google Scholar] [CrossRef]
- Liang, T.J. Hepatitis B: The Virus and Disease. Hepatol. Baltim. Md 2009, 49, S13–S21. [Google Scholar] [CrossRef]
- Tsai, K.-N.; Kuo, C.-F.; Ou, J.-H.J. Mechanisms of Hepatitis B Virus Persistence. Trends Microbiol. 2018, 26, 33–42. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Wu, S.; Zhu, H. The Unexpected Structures of Hepatitis C Virus Envelope Proteins. Exp. Ther. Med. 2017, 14, 1859–1865. [Google Scholar] [CrossRef]
- Tsao, S.W.; Tsang, C.M.; Lo, K.W. Epstein-Barr Virus Infection and Nasopharyngeal Carcinoma. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef] [PubMed]
- Su, F.-H.; Chang, S.-N.; Chen, P.-C.; Sung, F.-C.; Huang, S.-F.; Chiou, H.-Y.; Su, C.-T.; Lin, C.-C.; Yeh, C.-C. Positive Association Between Hepatitis C Infection and Oral Cavity Cancer: A Nationwide Population-Based Cohort Study in Taiwan. PLoS ONE 2012, 7, e48109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weng, J.-J.; Wei, J.-Z.; Li, M.; Lu, J.-L.; Qin, Y.-D.; Jiang, H.; Qu, S.-H. Effects of Hepatitis B Virus Infection and Antiviral Therapy on the Clinical Prognosis of Nasopharyngeal Carcinoma. Cancer Med. 2020, 9, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Louis, C.U.; Straathof, K.; Bollard, C.M.; Ennamuri, S.; Gerken, C.; Lopez, T.T.; Huls, M.H.; Sheehan, A.; Wu, M.-F.; Liu, H.; et al. Adoptive Transfer of EBV-Specific T Cells Results in Sustained Clinical Responses in Patients With Locoregional Nasopharyngeal Carcinoma. J. Immunother. 2010, 33, 983–990. [Google Scholar] [CrossRef]
- Mahale, P.; Sturgis, E.M.; Tweardy, D.J.; Ariza-Heredia, E.J.; Torres, H.A. Association Between Hepatitis C Virus and Head and Neck Cancers. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef]
- Shih, C.; Yang, C.-C.; Choijilsuren, G.; Chang, C.-H.; Liou, A.-T. Hepatitis B Virus. Trends Microbiol. 2018, 26, 386–387. [Google Scholar] [CrossRef]
- Tumban, E. A Current Update on Human Papillomavirus-Associated Head and Neck Cancers. Viruses 2019, 11, 922. [Google Scholar] [CrossRef]
- D’souza, G.; Carey, T.E.; William, W.N.; Nguyen, M.L.; Ko, E.C.; Riddell, J.; Pai, S.I.; Gupta, V.; Walline, H.M.; Lee, J.J.; et al. Epidemiology of Head and Neck Squamous Cell Cancer among HIV-Infected Patients. J. Acquir. Immune Defic. Syndr. 1999 2014, 65, 603–610. [Google Scholar] [CrossRef]
- Tan, E.L.; Looi, L.M.; Sam, C.K. Evaluation of Plasma Epstein-Barr Virus DNA Load as a Prognostic Marker for Nasopharyngeal Carcinoma. Singap. Med. J. 2006, 47, 803–807. [Google Scholar]
- She, Y.; Nong, X.; Zhang, M.; Wang, M. Epstein-Barr Virus Infection and Oral Squamous Cell Carcinoma Risk: A Meta-Analysis. PLoS ONE 2017, 12, e0186860. [Google Scholar] [CrossRef]
- Borsetto, D.; Fussey, J.; Fabris, L.; Bandolin, L.; Gaudioso, P.; Phillips, V.; Polesel, J.; Boscolo-Rizzo, P. HCV Infection and the Risk of Head and Neck Cancer: A Meta-Analysis. Oral Oncol. 2020, 109, 104869. [Google Scholar] [CrossRef]
- Donà, S.; Borsetto, D.; Fussey, J.; Biscaro, V.; Vian, E.; Spinato, G.; Menegaldo, A.; Da Mosto, M.C.; Rigoli, R.; Polesel, J.; et al. Association between Hepatitis C and B Viruses and Head and Neck Squamous Cell Carcinoma. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2019, 121, 104209. [Google Scholar] [CrossRef]
- Nagao, Y.; Sata, M. High Incidence of Multiple Primary Carcinomas in HCV-Infected Patients with Oral Squamous Cell Carcinoma. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2009, 15, CR453–CR459. [Google Scholar]
- Nayyar, S.S.; Thiagarajan, S.; Malik, A.; D’Cruz, A.; Chaukar, D.; Patil, P.; Alahari, A.D.; Lashkar, S.G.; Prabhash, K. Head and Neck Squamous Cell Carcinoma in HIV, HBV and HCV Seropositive Patients—Prognosis and Its Predictors. J. Cancer Res. Ther. 2020, 16, 619–623. [Google Scholar] [CrossRef]
- Ye, Y.-F.; Xiang, Y.-Q.; Fang, F.; Gao, R.; Zhang, L.-F.; Xie, S.-H.; Liu, Z.; Du, J.-L.; Chen, S.-H.; Hong, M.-H.; et al. Hepatitis B Virus Infection and Risk of Nasopharyngeal Carcinoma in Southern China. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2015, 24, 1766–1773. [Google Scholar] [CrossRef]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide Burden of Cancer Attributable to HPV by Site, Country and HPV Type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- Zheng, X.-H.; Wang, R.-Z.; Li, X.-Z.; Zhou, T.; Zhang, J.-B.; Zhang, P.-F.; Lu, L.-X.; Jia, W.-H. Detection of Methylation Status of Epstein-Barr Virus DNA C Promoter in the Diagnosis of Nasopharyngeal Carcinoma. Cancer Sci. 2020, 111, 592–600. [Google Scholar] [CrossRef]
- Komori, M.F.; Kimura, T.; Kariya, S.; Onoda, T.; Takeda, S.; Mizukawa, N.; Iida, S.; Kimata, Y.; Nishizaki, K. Epidemiological Correlations between Head and Neck Cancer and Hepatitis B Core Antibody Positivity. Anticancer Res. 2020, 40, 2393–2403. [Google Scholar] [CrossRef]
- Prabhu, S.R.; Wilson, D.F. Evidence of Epstein-Barr Virus Association with Head and Neck Cancers: A Review. J. Can. Dent. Assoc. 2016, 82, g2. [Google Scholar]
- Lydiatt, W.; O’Sullivan, B.; Patel, S. Major Changes in Head and Neck Staging for 2018. Am. Soc. Clin. Oncol. Educ. Book Am. Soc. Clin. Oncol. Annu. Meet. 2018, 38, 505–514. [Google Scholar] [CrossRef]
- Knör, M.; Tziridis, K.; Agaimy, A.; Zenk, J.; Wendler, O. Human Papillomavirus (HPV) Prevalence in Nasal and Antrochoanal Polyps and Association with Clinical Data. PLoS ONE 2015, 10, e0141722. [Google Scholar] [CrossRef] [PubMed]
- Thakur, J.S.; Chaitanya, A.; Minhas, R.S.; Azad, R.K.; Sharma, D.R.; Mohindroo, N.K. Killian’s Polyp Mimicking Malignant Tumor. Ann. Maxillofac. Surg. 2015, 5, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Oton-Gonzalez, L.; Rotondo, J.C.; Cerritelli, L.; Malagutti, N.; Lanzillotti, C.; Bononi, I.; Ciorba, A.; Bianchini, C.; Mazziotta, C.; De Mattei, M.; et al. Association between Oncogenic Human Papillomavirus Type 16 and Killian Polyp. Infect. Agent. Cancer 2021, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.D.; Burtness, B.; Le, Q.T.; Ferris, R.L. The Changing Therapeutic Landscape of Head and Neck Cancer. Nat. Rev. Clin. Oncol. 2019, 16, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.D.; Burtness, B.; Ferris, R.L. Immunotherapy for Head and Neck Cancer: Recent Advances and Future Directions. Oral Oncol. 2019, 99, 104460. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, S.K.; Bauman, J.E. Current Concepts in Chemotherapy for Head and Neck Cancer. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 145–154. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Göttgens, E.L.; Ostheimer, C.; Span, P.N.; Bussink, J.; Hammond, E.M. HPV, Hypoxia and Radiation Response in Head and Neck Cancer. Br. J. Radiol. 2018, 92, 20180047. [Google Scholar] [CrossRef]
- Alterio, D.; Marvaso, G.; Ferrari, A.; Volpe, S.; Orecchia, R.; Jereczek-Fossa, B.A. Modern Radiotherapy for Head and Neck Cancer. Semin. Oncol. 2019, 46, 233–245. [Google Scholar] [CrossRef]
- Schwartz, D.L.; Hayes, D.N. The Evolving Role of Radiotherapy for Head and Neck Cancer. Hematol. Oncol. Clin. N. Am. 2020, 34, 91–108. [Google Scholar] [CrossRef]
- Ionna, F.; Guida, A.; Califano, L.; Motta, G.; Salzano, G.; Pavone, E.; Aversa, C.; Longo, F.; Villano, S.; Ponzo, L.M.; et al. Transoral Robotic Surgery in Head and Neck District: A Retrospective Study on 67 Patients Treated in a Single Center. Infect. Agent. Cancer 2020, 15, 40. [Google Scholar] [CrossRef]
- Mydlarz, W.K.; Chan, J.Y.K.; Richmon, J.D. The Role of Surgery for HPV-Associated Head and Neck Cancer. Oral Oncol. 2015, 51, 305–313. [Google Scholar] [CrossRef]
- Fundakowski, C.E.; Lango, M. Considerations in Surgical versus Non-Surgical Management of HPV Positive Oropharyngeal Cancer. Cancers Head Neck 2016, 1, 6. [Google Scholar] [CrossRef][Green Version]
- Golusiński, W. Functional Organ Preservation Surgery in Head and Neck Cancer: Transoral Robotic Surgery and Beyond. Front. Oncol. 2019, 9, 293. [Google Scholar] [CrossRef]
- Colevas, A.D.; Yom, S.S.; Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Brizel, D.M.; Burtness, B.; Busse, P.M.; Caudell, J.J.; et al. NCCN Guidelines Insights: Head and Neck Cancers, Version 1.2018. J. Natl. Compr. Canc. Netw. 2018, 16, 479–490. [Google Scholar] [CrossRef]
- Chinn, S.B.; Myers, J.N. Oral Cavity Carcinoma: Current Management, Controversies, and Future Directions. J. Clin. Oncol. 2015, 33, 3269–3276. [Google Scholar] [CrossRef]
- Ferris, R.L.; Flamand, Y.; Weinstein, G.S.; Li, S.; Quon, H.; Mehra, R.; Garcia, J.J.; Chung, C.H.; Gillison, M.L.; Duvvuri, U.; et al. Transoral Robotic Surgical Resection Followed by Randomization to Low- or Standard-Dose IMRT in Resectable P16+ Locally Advanced Oropharynx Cancer: A Trial of the ECOG-ACRIN Cancer Research Group (E3311). J. Clin. Oncol. 2020, 38, 6500. [Google Scholar] [CrossRef]
- Moore, E.J.; Van Abel, K.M.; Price, D.L.; Lohse, C.M.; Olsen, K.D.; Jackson, R.S.; Martin, E.J. Transoral Robotic Surgery for Oropharyngeal Carcinoma: Surgical Margins and Oncologic Outcomes. Head Neck 2018, 40, 747–755. [Google Scholar] [CrossRef]
- Zenga, J.; Suko, J.; Kallogjeri, D.; Pipkorn, P.; Nussenbaum, B.; Jackson, R.S. Postoperative Hemorrhage and Hospital Revisit after Transoral Robotic Surgery: Postoperative Hemorrhage After TORS. Laryngoscope 2017, 127, 2287–2292. [Google Scholar] [CrossRef]
- Owadally, W.; Hurt, C.; Timmins, H.; Parsons, E.; Townsend, S.; Patterson, J.; Hutcheson, K.; Powell, N.; Beasley, M.; Palaniappan, N.; et al. PATHOS: A Phase II/III Trial of Risk-Stratified, Reduced Intensity Adjuvant Treatment in Patients Undergoing Transoral Surgery for Human Papillomavirus (HPV) Positive Oropharyngeal Cancer. BMC Cancer 2015, 15, 602. [Google Scholar] [CrossRef]
- Chera, B.S.; Amdur, R.J.; Tepper, J.; Qaqish, B.; Green, R.; Aumer, S.L.; Hayes, N.; Weiss, J.; Grilley-Olson, J.; Zanation, A.; et al. Phase 2 Trial of De-Intensified Chemoradiation Therapy for Favorable-Risk Human Papillomavirus–Associated Oropharyngeal Squamous Cell Carcinoma. Int. J. Radiat. Oncol. 2015, 93, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.C.; Theurer, J.; Prisman, E.; Read, N.; Berthelet, E.; Tran, E.; Fung, K.; de Almeida, J.R.; Bayley, A.; Goldstein, D.P.; et al. Radiotherapy versus Transoral Robotic Surgery and Neck Dissection for Oropharyngeal Squamous Cell Carcinoma (ORATOR): An Open-Label, Phase 2, Randomised Trial. Lancet Oncol. 2019, 20, 1349–1359. [Google Scholar] [CrossRef]
- Psyrri, A.; Rampias, T.; Vermorken, J.B. The Current and Future Impact of Human Papillomavirus on Treatment of Squamous Cell Carcinoma of the Head and Neck. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 2101–2115. [Google Scholar] [CrossRef]
- Marur, S.; Li, S.; Cmelak, A.J.; Gillison, M.L.; Zhao, W.J.; Ferris, R.L.; Westra, W.H.; Gilbert, J.; Bauman, J.E.; Wagner, L.I.; et al. E1308: Phase II Trial of Induction Chemotherapy Followed by Reduced-Dose Radiation and Weekly Cetuximab in Patients With HPV-Associated Resectable Squamous Cell Carcinoma of the Oropharynx—ECOG-ACRIN Cancer Research Group. J. Clin. Oncol. 2017, 35, 490–497. [Google Scholar] [CrossRef]
- Chera, B.S.; Kumar, S.; Shen, C.; Amdur, R.; Dagan, R.; Green, R.; Goldman, E.; Weiss, J.; Grilley-Olson, J.; Patel, S.; et al. Plasma Circulating Tumor HPV DNA for the Surveillance of Cancer Recurrence in HPV-Associated Oropharyngeal Cancer. J. Clin. Oncol. 2020, 38, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and Clinical Activity of Pembrolizumab for Treatment of Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-012): An Open-Label, Multicentre, Phase 1b Trial. Lancet Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Soulières, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.-J.; Soria, A.; Machiels, J.-P.; Mach, N.; Mehra, R.; et al. Pembrolizumab versus Methotrexate, Docetaxel, or Cetuximab for Recurrent or Metastatic Head-and-Neck Squamous Cell Carcinoma (KEYNOTE-040): A Randomised, Open-Label, Phase 3 Study. Lancet Lond. Engl. 2019, 393, 156–167. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab Alone or with Chemotherapy versus Cetuximab with Chemotherapy for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-048): A Randomised, Open-Label, Phase 3 Study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Huang, S.H.; Perez-Ordonez, B.; Massey, C.; Siu, L.L.; Weinreb, I.; Hope, A.; Kim, J.; Bayley, A.J.; Cummings, B.; et al. Outcomes of HPV-Related Oropharyngeal Cancer Patients Treated by Radiotherapy Alone Using Altered Fractionation. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2012, 103, 49–56. [Google Scholar] [CrossRef]
- Ma, D.J.; Price, K.A.; Moore, E.J.; Patel, S.H.; Hinni, M.L.; Garcia, J.J.; Graner, D.E.; Foster, N.R.; Ginos, B.; Neben-Wittich, M.; et al. Phase II Evaluation of Aggressive Dose De-Escalation for Adjuvant Chemoradiotherapy in Human Papillomavirus-Associated Oropharynx Squamous Cell Carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Sim, F.; Leidner, R.; Bell, R.B. Immunotherapy for Head and Neck Cancer. Hematol. Oncol. Clin. N. Am. 2019, 33, 301–321. [Google Scholar] [CrossRef] [PubMed]
- Taberna, M.; Oliva, M.; Mesía, R. Cetuximab-Containing Combinations in Locally Advanced and Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2019, 9, 383. [Google Scholar] [CrossRef]
- Kabolizadeh, P.; Kubicek, G.J.; Heron, D.E.; Ferris, R.L.; Gibson, M.K. The Role of Cetuximab in the Management of Head and Neck Cancers. Expert Opin. Biol. Ther. 2012, 12, 517–528. [Google Scholar] [CrossRef]
- Sano, D.; Fujisawa, T.; Tokuhisa, M.; Shimizu, M.; Sakagami, T.; Hatano, T.; Nishimura, G.; Ichikawa, Y.; Iwai, H.; Oridate, N. Real-World Treatment Outcomes of the EXTREME Regimen as First-Line Therapy for Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck: A Multi-Center Retrospective Cohort Study in Japan. Anticancer Res. 2019, 39, 6819–6827. [Google Scholar] [CrossRef]
- Kozakiewicz, P.; Grzybowska-Szatkowska, L. Application of Molecular Targeted Therapies in the Treatment of Head and Neck Squamous Cell Carcinoma (Review). Oncol. Lett. 2018. [Google Scholar] [CrossRef]
- Gavrielatou, N.; Doumas, S.; Economopoulou, P.; Foukas, P.G.; Psyrri, A. Biomarkers for Immunotherapy Response in Head and Neck Cancer. Cancer Treat. Rev. 2020, 84, 101977. [Google Scholar] [CrossRef]
- Kao, H.-F.; Lou, P.-J. Immune Checkpoint Inhibitors for Head and Neck Squamous Cell Carcinoma: Current Landscape and Future Directions. Head Neck 2019, 41 (Suppl. 1), 4–18. [Google Scholar] [CrossRef]
- Ferris, R.L. Immunology and Immunotherapy of Head and Neck Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3293–3304. [Google Scholar] [CrossRef]
- Berman, T.A.; Schiller, J.T. Human Papillomavirus in Cervical Cancer and Oropharyngeal Cancer: One Cause, Two Diseases: HPV in Cervical and Oropharyngeal Ca. Cancer 2017, 123, 2219–2229. [Google Scholar] [CrossRef]
- Timbang, M.R.; Sim, M.W.; Bewley, A.F.; Farwell, D.G.; Mantravadi, A.; Moore, M.G. HPV-Related Oropharyngeal Cancer: A Review on Burden of the Disease and Opportunities for Prevention and Early Detection. Hum. Vaccines Immunother. 2019, 15, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Herrero, R.; Quint, W.; Hildesheim, A.; Gonzalez, P.; Struijk, L.; Katki, H.A.; Porras, C.; Schiffman, M.; Rodriguez, A.C.; Solomon, D.; et al. Reduced Prevalence of Oral Human Papillomavirus (HPV) 4 Years after Bivalent HPV Vaccination in a Randomized Clinical Trial in Costa Rica. PLoS ONE 2013, 8, e68329. [Google Scholar] [CrossRef] [PubMed]
- Hirth, J.M.; Chang, M.; Resto, V.A.; Guo, F.; Berenson, A.B. Prevalence of Oral Human Papillomavirus by Vaccination Status among Young Adults (18–30 Years Old). Vaccine 2017, 35, 3446–3451. [Google Scholar] [CrossRef]
- Bakkalci, D.; Jia, Y.; Winter, J.R.; Lewis, J.E.; Taylor, G.S.; Stagg, H.R. Risk Factors for Epstein Barr Virus-Associated Cancers: A Systematic Review, Critical Appraisal, and Mapping of the Epidemiological Evidence. J. Glob. Health 2020, 10, 010405. [Google Scholar] [CrossRef]
- Correia, S.; Palser, A.; Elgueta Karstegl, C.; Middeldorp, J.M.; Ramayanti, O.; Cohen, J.I.; Hildesheim, A.; Fellner, M.D.; Wiels, J.; White, R.E.; et al. Natural Variation of Epstein-Barr Virus Genes, Proteins, and Primary MicroRNA. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Ogembo, J.G.; Kannan, L.; Ghiran, I.; Nicholson-Weller, A.; Finberg, R.W.; Tsokos, G.C.; Fingeroth, J.D. Human Complement Receptor Type 1/CD35 Is an Epstein-Barr Virus Receptor. Cell Rep. 2013, 3, 371–385. [Google Scholar] [CrossRef]
- Odumade, O.A.; Hogquist, K.A.; Balfour, H.H. Progress and Problems in Understanding and Managing Primary Epstein-Barr Virus Infections. Clin. Microbiol. Rev. 2011, 24, 193–209. [Google Scholar] [CrossRef]
- Wang, L.W.; Jiang, S.; Gewurz, B.E. Epstein-Barr Virus LMP1-Mediated Oncogenicity. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Yin, H.; Qu, J.; Peng, Q.; Gan, R. Molecular Mechanisms of EBV-Driven Cell Cycle Progression and Oncogenesis. Med. Microbiol. Immunol. (Berl.) 2019, 208, 573–583. [Google Scholar] [CrossRef]
- Mainou, B.A.; Everly, D.N.; Raab-Traub, N. Unique Signaling Properties of CTAR1 in LMP1-Mediated Transformation. J. Virol. 2007, 81, 9680–9692. [Google Scholar] [CrossRef]
- Fotheringham, J.A.; Coalson, N.E.; Raab-Traub, N. Epstein-Barr Virus Latent Membrane Protein-2A Induces ITAM/Syk- and Akt-Dependent Epithelial Migration through Av-Integrin Membrane Translocation. J. Virol. 2012, 86, 10308–10320. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Longnecker, R. Epstein-Barr Virus Latent Membrane Protein 2A Mediates Transformation through Constitutive Activation of the Ras/PI3-K/Akt Pathway. J. Virol. 2007, 81, 9299–9306. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.B.; Manet, E.; Gruffat, H.; Busson, P.; Blondel, M.; Fahraeus, R. EBNA1: Oncogenic Activity, Immune Evasion and Biochemical Functions Provide Targets for Novel Therapeutic Strategies against Epstein-Barr Virus- Associated Cancers. Cancers 2018, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Lung, M.L.; Cheung, A.K.L.; Ko, J.M.Y.; Lung, H.L.; Cheng, Y.; Dai, W. The Interplay of Host Genetic Factors and Epstein-Barr Virus in the Development of Nasopharyngeal Carcinoma. Chin. J. Cancer 2014, 33, 556–568. [Google Scholar] [CrossRef]
- Liu, S.-L.; Sun, X.-S.; Li, X.-Y.; Tang, L.-Q.; Chen, Q.-Y.; Lin, H.-X.; Liang, Y.-J.; Yan, J.-J.; Lin, C.; Guo, S.-S.; et al. The Diagnostic and Prognostic Values of Plasma Epstein-Barr Virus DNA for Residual Cervical Lymphadenopathy in Nasopharyngeal Carcinoma Patients: A Retrospective Study. Cancer Commun. Lond. Engl. 2019, 39, 14. [Google Scholar] [CrossRef]
- Ngan, H.-L.; Wang, L.; Lo, K.-W.; Lui, V.W.Y. Genomic Landscapes of EBV-Associated Nasopharyngeal Carcinoma vs. HPV-Associated Head and Neck Cancer. Cancers 2018, 10, 210. [Google Scholar] [CrossRef]
- Ma, B.B.Y.; Hui, E.P.; Chan, A.T.C. Investigational Drugs for Nasopharyngeal Carcinoma. Expert Opin. Investig. Drugs 2017, 26, 677–685. [Google Scholar] [CrossRef]
- Chen, B.J.; Chapuy, B.; Ouyang, J.; Sun, H.H.; Roemer, M.G.M.; Xu, M.L.; Yu, H.; Fletcher, C.D.M.; Freeman, G.J.; Shipp, M.A.; et al. PD-L1 Expression Is Characteristic of a Subset of Aggressive B-Cell Lymphomas and Virus-Associated Malignancies. Clin. Cancer Res. 2013, 19, 3462–3473. [Google Scholar] [CrossRef]
- Ma, B.B.Y.; Lim, W.-T.; Goh, B.-C.; Hui, E.P.; Lo, K.-W.; Pettinger, A.; Foster, N.R.; Riess, J.W.; Agulnik, M.; Chang, A.Y.C.; et al. Antitumor Activity of Nivolumab in Recurrent and Metastatic Nasopharyngeal Carcinoma: An International, Multicenter Study of the Mayo Clinic Phase 2 Consortium (NCI-9742). J. Clin. Oncol. 2018, 36, 1412–1418. [Google Scholar] [CrossRef]
- Lee, A.W.M.; Ma, B.B.Y.; Ng, W.T.; Chan, A.T.C. Management of Nasopharyngeal Carcinoma: Current Practice and Future Perspective. J. Clin. Oncol. 2015, 33, 3356–3364. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Hong, S.; Yang, Y.; Yu, G.; Jia, J.; Peng, P.; Wu, X.; Lin, Q.; Xi, X.; et al. Gemcitabine plus Cisplatin versus Fluorouracil plus Cisplatin in Recurrent or Metastatic Nasopharyngeal Carcinoma: A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet 2016, 388, 1883–1892. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, J.; Hong, S.; Zhan, J.; Chen, N.; Qin, T.; Tang, Y.; Zhang, Y.; Kang, S.; Zhou, T.; et al. EBV-Driven LMP1 and IFN-γ up-Regulate PD-L1 in Nasopharyngeal Carcinoma: Implications for Oncotargeted Therapy. Oncotarget 2014, 5, 12189–12202. [Google Scholar] [CrossRef]
- Hsu, C.; Lee, S.-H.; Ejadi, S.; Even, C.; Cohen, R.B.; Le Tourneau, C.; Mehnert, J.M.; Algazi, A.; van Brummelen, E.M.J.; Saraf, S.; et al. Safety and Antitumor Activity of Pembrolizumab in Patients With Programmed Death-Ligand 1-Positive Nasopharyngeal Carcinoma: Results of the KEYNOTE-028 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 4050–4056. [Google Scholar] [CrossRef]
- Chia, W.-K.; Teo, M.; Wang, W.-W.; Lee, B.; Ang, S.-F.; Tai, W.-M.; Chee, C.-L.; Ng, J.; Kan, R.; Lim, W.-T.; et al. Adoptive T-Cell Transfer and Chemotherapy in the First-Line Treatment of Metastatic and/or Locally Recurrent Nasopharyngeal Carcinoma. Mol. Ther. 2014, 22, 132–139. [Google Scholar] [CrossRef]
- Taylor, G.S.; Steven, N.M. Therapeutic Vaccination Strategies to Treat Nasopharyngeal Carcinoma. Chin. Clin. Oncol. 2016, 5, 23. [Google Scholar] [CrossRef]
- Hui, E.P.; Taylor, G.S.; Jia, H.; Ma, B.B.Y.; Chan, S.L.; Ho, R.; Wong, W.-L.; Wilson, S.; Johnson, B.F.; Edwards, C.; et al. Phase I Trial of Recombinant Modified Vaccinia Ankara Encoding Epstein–Barr Viral Tumor Antigens in Nasopharyngeal Carcinoma Patients. Cancer Res. 2013, 73, 1676–1688. [Google Scholar] [CrossRef]
- Lin, C.-L.; Lo, W.-F.; Lee, T.-H.; Ren, Y.; Hwang, S.-L.; Cheng, Y.-F.; Chen, C.-L.; Chang, Y.-S.; Lee, S.P.; Rickinson, A.B.; et al. Immunization with Epstein-Barr Virus (EBV) Peptide-Pulsed Dendritic Cells Induces Functional CD8+ T-Cell Immunity and May Lead to Tumor Regression in Patients with EBV-Positive Nasopharyngeal Carcinoma. Cancer Res. 2002, 62, 6952–6958. [Google Scholar]
- Taylor, G.S.; Jia, H.; Harrington, K.; Lee, L.W.; Turner, J.; Ladell, K.; Price, D.A.; Tanday, M.; Matthews, J.; Roberts, C.; et al. A Recombinant Modified Vaccinia Ankara Vaccine Encoding Epstein–Barr Virus (EBV) Target Antigens: A Phase I Trial in UK Patients with EBV-Positive Cancer. Clin. Cancer Res. 2014, 20, 5009–5022. [Google Scholar] [CrossRef]
- Hunt, J.; Hagan, J.; Nobles, J.; Wold, C.; Fazekas-May, M.; Gilbert, J.; Friedlander, P.L. Outcome Analysis of Patients with Squamous Cell Carcinoma of the Head and Neck and Hepatitis C Virus. Laryngoscope 2005, 115, 1882–1886. [Google Scholar] [CrossRef]
- Nobles, J.; Wold, C.; Fazekas-May, M.; Gilbert, J.; Friedlander, P.L. Prevalence and Epidemiology of Hepatitis C Virus in Patients with Squamous Cell Carcinoma of the Head and Neck. Laryngoscope 2004, 114, 2119–2122. [Google Scholar] [CrossRef]
- Rangel, J.d.B.; Thuler, L.C.S.; Pinto, J.F.d.C. Prevalence of Hepatitis C Virus Infection and Its Impact on the Prognosis of Head and Neck Cancer Patients. Oral Oncol. 2018, 87, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.R.; Lodi, G.; Chandler, K.; Kumar, N. Development of Squamous Cell Carcinoma in Hepatitis C Virus-Associated Lichen Planus. Oral Oncol. 1997, 33, 58–59. [Google Scholar] [CrossRef]
- Carrozzo, M.; Carbone, M.; Gandolfo, S.; Valente, G.; Colombatto, P.; Ghisetti, V. An Atypical Verrucous Carcinoma of the Tongue Arising in a Patient with Oral Lichen Planus Associated with Hepatitis C Virus Infection. Oral Oncol. 1997, 33, 220–225. [Google Scholar] [CrossRef]
- Carrozzo, M. Oral Diseases Associated with Hepatitis C Virus Infection. Part 1. Sialadenitis and Salivary Glands Lymphoma. Oral Dis. 2008, 14, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.; Mankotia, D.S.; Irshad, K. An Insight into the Diagnosis and Pathogenesis of Hepatitis C Virus Infection. World J. Gastroenterol. 2013, 19, 7896–7909. [Google Scholar] [CrossRef] [PubMed]
- Geng, M.; Xin, X.; Bi, L.-Q.; Zhou, L.-T.; Liu, X.-H. Molecular Mechanism of Hepatitis B Virus X Protein Function in Hepatocarcinogenesis. World J. Gastroenterol. 2015, 21, 10732–10738. [Google Scholar] [CrossRef] [PubMed]
- Bonilla Guerrero, R.; Roberts, L.R. The Role of Hepatitis B Virus Integrations in the Pathogenesis of Human Hepatocellular Carcinoma. J. Hepatol. 2005, 42, 760–777. [Google Scholar] [CrossRef] [PubMed]
- Takata, Y.; Takahashi, T.; Fukuda, J. Prevalence of Hepatitis Virus Infection in Association with Oral Diseases Requiring Surgery. Oral Dis. 2002, 8, 95–99. [Google Scholar] [CrossRef]
- Lv, J.-W.; Chen, Y.-P.; Huang, X.-D.; Zhou, G.-Q.; Chen, L.; Li, W.-F.; Tang, L.-L.; Mao, Y.-P.; Guo, Y.; Xu, R.-H.; et al. Hepatitis B Virus Screening and Reactivation and Management of Patients with Nasopharyngeal Carcinoma: A Large-Scale, Big-Data Intelligence Platform-Based Analysis from an Endemic Area: HBV Screening and Management of NPC. Cancer 2017, 123, 3540–3549. [Google Scholar] [CrossRef]
- Yeo, W.; Hui, E.P.; Chan, A.T.C.; Ho, W.M.; Lam, K.C.; Chan, P.K.S.; Mok, T.S.K.; Lee, J.J.; Mo, F.K.F.; Johnson, P.J. Prevention of Hepatitis B Virus Reactivation in Patients With Nasopharyngeal Carcinoma With Lamivudine. Am. J. Clin. Oncol. 2005, 28, 379–384. [Google Scholar] [CrossRef]
- Xu, X.; Lai, Y.; Hua, Z.-C. Apoptosis and Apoptotic Body: Disease Message and Therapeutic Target Potentials. Biosci. Rep. 2019, 39, BSR20180992. [Google Scholar] [CrossRef]
- Mulder, F.J.; Klufah, F.; Janssen, F.M.E.; Farshadpour, F.; Willems, S.M.; de Bree, R.; Zur Hausen, A.; van den Hout, M.F.C.M.; Kremer, B.; Speel, E.-J.M. Presence of Human Papillomavirus and Epstein-Barr Virus, but Absence of Merkel Cell Polyomavirus, in Head and Neck Cancer of Non-Smokers and Non-Drinkers. Front. Oncol. 2020, 10, 560434. [Google Scholar] [CrossRef]
- Mohebbi, E.; Noormohamadi, Z.; Sadeghi-Rad, H.; Sadeghi, F.; Yahyapour, Y.; Vaziri, F.; Rahimi, A.; Rahimi Jamnani, F.; Mehrabi, S.; Siadat, S.D.; et al. Low Viral Load of Merkel Cell Polyomavirus in Iranian Patients with Head and Neck Squamous Cell Carcinoma: Is It Clinically Important? J. Med. Virol. 2018, 90, 344–350. [Google Scholar] [CrossRef]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal Integration of a Polyomavirus in Human Merkel Cell Carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef]
- Muñoz, J.P.; Blanco, R.; Osorio, J.C.; Oliva, C.; Diaz, M.J.; Carrillo-Beltrán, D.; Aguayo, R.; Castillo, A.; Tapia, J.C.; Calaf, G.M.; et al. Merkel Cell Polyomavirus Detected in Head and Neck Carcinomas from Chile. Infect. Agent. Cancer 2020, 15, 4. [Google Scholar] [CrossRef]
- Saini, A.T.; Miles, B.A. Merkel Cell Carcinoma of the Head and Neck: Pathogenesis, Current and Emerging Treatment Options. OncoTargets Ther. 2015, 8, 2157–2167. [Google Scholar] [CrossRef][Green Version]
- Saláková, M.; Košlabová, E.; Vojtěchová, Z.; Tachezy, R.; Šroller, V. Detection of Human Polyomaviruses MCPyV, HPyV6, and HPyV7 in Malignant and Non-Malignant Tonsillar Tissues. J. Med. Virol. 2016, 88, 695–702. [Google Scholar] [CrossRef]
- Windon, M.; Fakhry, C.; Rooper, L.; Ha, P.; Schoppy, D.; Miles, B.; Koch, W.; Vosler, P.; Eisele, D.; D’Souza, G. The Role of Age and Merkel Cell Polyomavirus in Oral Cavity Cancers. Otolaryngol. Head Neck Surg. 2020, 163, 1194–1197. [Google Scholar] [CrossRef]
- Herberhold, S.; Hellmich, M.; Panning, M.; Bartok, E.; Silling, S.; Akgül, B.; Wieland, U. Human Polyomavirus and Human Papillomavirus Prevalence and Viral Load in Non-Malignant Tonsillar Tissue and Tonsillar Carcinoma. Med. Microbiol. Immunol. 2017, 206, 93–103. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmutović, L.; Bilajac, E.; Hromić-Jahjefendić, A. Meet the Insidious Players: Review of Viral Infections in Head and Neck Cancer Etiology with an Update on Clinical Trials. Microorganisms 2021, 9, 1001. https://doi.org/10.3390/microorganisms9051001
Mahmutović L, Bilajac E, Hromić-Jahjefendić A. Meet the Insidious Players: Review of Viral Infections in Head and Neck Cancer Etiology with an Update on Clinical Trials. Microorganisms. 2021; 9(5):1001. https://doi.org/10.3390/microorganisms9051001
Chicago/Turabian StyleMahmutović, Lejla, Esma Bilajac, and Altijana Hromić-Jahjefendić. 2021. "Meet the Insidious Players: Review of Viral Infections in Head and Neck Cancer Etiology with an Update on Clinical Trials" Microorganisms 9, no. 5: 1001. https://doi.org/10.3390/microorganisms9051001
APA StyleMahmutović, L., Bilajac, E., & Hromić-Jahjefendić, A. (2021). Meet the Insidious Players: Review of Viral Infections in Head and Neck Cancer Etiology with an Update on Clinical Trials. Microorganisms, 9(5), 1001. https://doi.org/10.3390/microorganisms9051001






