The Impact of Tick-Borne Diseases on the Bone
Abstract
1. Introduction
2. Anaplasmosis (Formerly Human Granulocytic Ehrlichiosis)
3. Ehrlichiosis
4. Babesiosis
5. Lyme Disease
6. Bourbon Virus Disease
7. Colorado Tick Fever Disease
8. Tick-Borne Encephalitis
9. Crimean–Congo Hemorrhagic Fever
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rochlin, I.; Toledo, A. Emerging tick-borne pathogens of public health importance: A mini-review. J. Med. Microbiol. 2020, 69, 781–791. [Google Scholar] [CrossRef]
- Rodino, K.G.; Theel, E.S.; Pritt, B.S. Tick-borne diseases in the United States. Clin. Chem. 2020, 66, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Bakken, J.S.; Dumler, J.S. Human granulocytic anaplasmosis. Infect. Dis. Clin. N. Am. 2015, 29, 341–355. [Google Scholar] [CrossRef]
- Fillâtre, P.; Revest, M.; Tattevin, P. Crimean-Congo hemorrhagic fever: An update. Med. Mal. Infect. 2019, 49, 574–585. [Google Scholar] [CrossRef]
- Krasteva, S.; Jara, M.; Frias-De-Diego, A.; Machado, G. Nairobi Sheep Disease Virus: A Historical and Epidemiological Perspective. Front. Vet. Sci. 2020, 7, 419. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lv, X.-L.; Zhang, X.; Han, S.Z.; Wang, Z.D.; Li, L.; Sun, H.T.; Ma, L.X.; Cheng, Z.L.; Shao, J.W.; et al. Identification of a new orthocnairovirus associated with human febrile illness in China. Nat. Med. 2021, 27, 434–439. [Google Scholar] [CrossRef]
- Krause, P.J. Human babesiosis. Int. J. Parasitol. 2019, 49, 165–174. [Google Scholar] [CrossRef]
- Saito, T.B.; Walker, D.H. Ehrlichioses: An Important One Health Opportunity. Vet. Sci. 2016, 3, 20. [Google Scholar] [CrossRef]
- Brault, A.C.; Savage, H.M.; Duggal, N.K.; Eisen, R.J.; Staples, J.E. Heartland Virus Epidemiology, Vector Association, and Disease Potential. Viruses 2018, 10, 498. [Google Scholar] [CrossRef] [PubMed]
- Bopp, N.E.; Kaiser, J.A.; Strother, A.E.; Barrett, A.D.T.; Beasley, D.W.C.; Benassi, V.; Milligan, G.N.; Preziosi, M.P.; Reece, L.M. Baseline mapping of severe fever with thrombocytopenia syndrome virology, epidemiology and vaccine research and development. NPJ Vaccines 2020, 5, 111. [Google Scholar] [CrossRef]
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.; Li, X.; Mead, P.S. Lyme borreliosis. Nat. Rev. Dis. Primers 2016, 2, 16090. [Google Scholar] [CrossRef] [PubMed]
- Madison-Antenucci, S.; Kramer, L.D.; Gebhardt, L.L.; Kauffman, E. Emerging Tick-Borne Diseases. Clin. Microbiol. Rev. 2020, 33, e00083-18. [Google Scholar] [CrossRef] [PubMed]
- Sahni, A.; Fang, R.; Sahni, S.K.; Walker, D.H. Pathogenesis of Rickettsial Diseases: Pathogenic and Immune Mechanisms of an Endotheliotropic Infection. Annu. Rev. Pathol. 2019, 14, 127–152. [Google Scholar] [CrossRef]
- Piotrowski, M.; Rymaszewska, A. Expansion of Tick-Borne Rickettsioses in the World. Microorganisms 2020, 8, 1906. [Google Scholar] [CrossRef] [PubMed]
- Kemenesi, G.; Bányai, K. Tick-Borne Flaviviruses, with a Focus on Powassan Virus. Clin. Microbiol. Rev. 2019, 32, e00106-17. [Google Scholar] [CrossRef]
- Bhatia, B.; Fledmann, H.; Marzi, A. Kyasanur Forest Disease and Alkhurma Hemorrhagic Fever Virus-Two Neglected Zoonotic Pathogens. Microorganisms 2020, 8, 1406. [Google Scholar] [CrossRef]
- Shah, S.Z.; Jabbar, B.; Ahmed, N.; Rehman, A.; Nasir, H.; Nadeem, S.; Jabbar, I.; Rahman, Z.U.; Azam, S. Epidemiology, Pathogenesis, and Control of a Tick-Borne Disease- Kyasanur Forest Disease: Current Status and Future Directions. Front. Cell. Infect. Microbiol. 2018, 8, 149. [Google Scholar] [CrossRef]
- Corrin, T.; Greig, J.; Harding, S.; Young, I.; Mascarenhas, M.; Waddell, L.A. Powassan virus, a scoping review of the global evidence. Zoonoses Public Health 2018, 65, 595–624. [Google Scholar] [CrossRef]
- Velay, A.; Paz, M.; Cesbron, M.; Gantner, P.; Solis, M.; Soulier, E.; Argemi, X.; Martinot, M.; Hansmann, Y.; Fafi-Kremer, S. Tick-borne encephalitis virus: Molecular determinants of neuropathogenesis of an emerging pathogen. Crit. Rev. Microbiol. 2019, 45, 472–493. [Google Scholar] [CrossRef]
- Talagrand-Reboul, E.; Boyer, P.H.; Bergström, S.; Vial, L.; Boulanger, N. Relapsing Fevers: Neglected Tick-Borne Diseases. Front. Cell. Infect. Microbiol. 2018, 8, 98. [Google Scholar] [CrossRef]
- Eisen, R.J.; Kugeler, K.J.; Eisen, L.; Beard, C.B.; Paddock, C.D. Tick-Borne Zoonoses in the United States: Persistent and Emerging Threats to Human Health. ILAR J. 2017, 58, 319–335. [Google Scholar] [CrossRef]
- Telford, S.R.; Goethert, H.K. Ecology of Francisella tularensis. Annu. Rev. Entomol. 2020, 65, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Yang, J.; Xie, Y.; Du, X.; Chen, H.; Zhou, H.; Chen, L. Bone function, dysfunction and its role in diseases including critical illness. Int. J. Biol. Sci. 2019, 15, 776–787. [Google Scholar] [CrossRef]
- Boskey, A.L. Bone composition: Relationship to bone fragility and antiosteoporotic drug effects. Bonekey Rep. 2013, 2, 447. [Google Scholar] [CrossRef]
- Feng, X. Chemical and Biochemical Basis of Cell-Bone Matrix Interaction in Health and Disease. Curr. Chem. Biol. 2009, 3, 189–196. [Google Scholar]
- Travlos, G.S. Normal structure, function, and histology of the bone marrow. Toxicol. Pathol. 2006, 34, 548–565. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M. Skeletal remodeling in health and disease. Nat. Med. 2007, 13, 791–801. [Google Scholar] [CrossRef]
- Rucci, N. Molecular biology of bone remodelling. Clin. Cases Miner. Bone Metab. 2008, 5, 49–56. [Google Scholar]
- Raggat, L.J.; Partridge, N.C. Cellular and Molecular Mechanisms of Bone Remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef]
- Oliveira, T.C.; Gomes, M.S.; Gomes, A.C. The crossroads between infection and bone loss. Microorganisms 2020, 8, 1765. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.L.; Romanò, D.; Logoluso, N.; Drago, L. Bone and joint infections in adults: A comprehensive classification proposal. Eur. J. Orthop. Surg. Traumatol. 2011, 1, 207. [Google Scholar] [CrossRef] [PubMed]
- Urish, K.L.; Cassat, J.E. Staphylococcus aureus osteomyelitis: Bone, bugs, and surgery. Infect. Immun. 2020, 88, e00932-19. [Google Scholar] [CrossRef]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving concepts in bone-immune interactions in health and disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef] [PubMed]
- Cain, C.J.; Rueda, R.; McLelland, B.; Collette, N.M.; Loots, G.G.; Manilay, J.O. Absence of sclerostin adversely affects B-cell survival. J. Bone Miner. Res. 2012, 27, 1451–1461. [Google Scholar] [CrossRef]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef]
- Bravenboer, N.; Oostlander, A.E.; van Bodegraven, A.A. Bone loss in patients with inflammatory bowel disease: Cause, detection and treatment. Curr. Opin. Gastroenterol. 2021, 37, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Ojogun, N.; Barnstein, B.; Huang, B.; Oskeritzian, C.A.; Homeister, J.W.; Miller, D.; Ryan, J.J.; Carlyon, J.A. Anaplasma phagocytophilum infects mast cells via alpha1,3-fucosylated but not sialylated glycans and inhibits IgE-mediated cytokine production and histamine release. Infect. Immun. 2011, 79, 2717–2726. [Google Scholar] [CrossRef] [PubMed]
- Granick, J.L.; Reneer, D.V.; Carlyon, J.A.; Borjesson, D.L. Anaplasma phagocytophilum infects cells of the megakaryocytic lineage through sialylated ligands but fails to alter platelet production. J. Med. Microbiol. 2018, 57, 416–423. [Google Scholar] [CrossRef][Green Version]
- Herron, M.J.; Ericson, M.E.; Kurtti, T.J.; Munderloh, U.G. The interactions of Anaplasma phagocytophilum, endothelial cells, and human neutrophils. Ann. N. Y. Acad. Sci. 2005, 1063, 374–382. [Google Scholar] [CrossRef]
- Almazán, C.; Fourniol, L.; Rouxel, C.; Alberdi, P.; Gandoin, C.; Lagrée, A.C.; Boulouis, H.J.; de la Fuente, J.; Bonnet, S.I. Experimental Ixodes ricinus-Sheep Cycle of Anaplasma phagocytophilum NV2Os Propagated in Tick Cell Cultures. Front. Vet. Sci. 2020, 7, 40. [Google Scholar] [CrossRef]
- Johns, J.L.; Discipulo, M.L.; Koehne, A.L.; Moorhead, K.A.; Nagamine, C.M. Influence of Genetic Background on Hematologic and Histopathologic Alterations during Acute Granulocytic Anaplasmosis in 129/SvEv and C57BL/6J Mice Lacking Type I and Type II Interferon Signaling. Comp. Med. 2017, 67, 127–137. [Google Scholar]
- Tate, C.M.; Mead, D.G.; Luttrell, M.P.; Howerth, E.W.; Dugan, V.G.; Munderloh, U.G.; Davidson, W.R. Experimental infection of white-tailed deer with Anaplasma phagocytophilum, etiologic agent of human granulocytic anaplasmosis. J. Clin. Microbiol. 2005, 43, 3595–3601. [Google Scholar] [CrossRef] [PubMed]
- Annen, K.; Friedman, K.; Eshoa, C.; Horowitz, M.; Gottschall, J.; Straus, T. Two cases of transfusion-transmitted Anaplasma phagocytophilum. Am. J. Clin. Pathol. 2012, 137, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.R.; Zimmerman, K.; Dascanio, J.J.; Pleasant, R.S.; Witonsky, S.G. Equine granulocytic anaplasmosis: A case report and review. J. Equine Vet. Sci. 2009, 29, 160–166. [Google Scholar] [CrossRef]
- Uehlinger, F.D.; Clancey, N.P.; Lofstedt, J. Granulocytic anaplasmosis in a horse from Nova Scotia caused by infection with Anaplasma phagocytophilum. Can. Vet. J. 2011, 52, 537–540. [Google Scholar] [PubMed]
- Khatat, S.E.; Culang, D.; Gara-Boivin, C. Granulocytic anaplasmosis in 2 dogs from Quebec. Can. Vet. J. 2018, 59, 663–667. [Google Scholar]
- Yi, J.; Kim, K.-H.; Ko, M.K.; Lee, E.Y.; Choi, S.J.; Oh, M.D. Human Granulocytic Anaplasmosis as a Cause of Febrile Illness in Korea Since at Least 2006. Am. J. Trop. Med. Hyg. 2017, 96, 777–782. [Google Scholar] [CrossRef]
- Marko, D.; Perry, A.M.; Ponnampalam, A.; Nasr, M.R. Cytopenias and clonal expansion of gamma/delta T-cells in a patient with anaplasmosis: A potential diagnostic pitfall. J. Clin. Exp. Hematop. 2017, 56, 160–164. [Google Scholar] [CrossRef][Green Version]
- Stokes, W.; Lisboa, L.F.; Lindsay, L.R.; Fonseca, K. Case Report: Anaplasmosis in Canada: Locally Acquired Anaplasma phagocytophilum Infection in Alberta. Am. J. Trop. Med. Hyg. 2020, 103, 2478–2480. [Google Scholar] [CrossRef]
- Jereb, M.; Pecaver, B.; Tomazic, J.; Muzlovic, I.; Avsic-Zupanc, T.; Premru-Srsen, T.; Levicnik-Stezinar, S.; Karner, P.; Strle, F. Severe human granulocytic anaplasmosis transmitted by blood transfusion. Emerg. Infect. Dis. 2012, 18, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, C.M.; Kim, D.M.; Yun, N.R. Manifestation of anaplasmosis as cerebral infarction: A case report. BMC Infect. Dis. 2018, 18, 409. [Google Scholar] [CrossRef]
- Parkins, M.D.; Church, D.L.; Jiang, X.Y.; Gregson, D.B. Human granulocytic anaplasmosis: First reported case in Canada. Can. J. Infect. Dis. Med. Microbiol. 2009, 20, e100–e102. [Google Scholar] [CrossRef]
- Borjesson, D.; Macnamara, K.; Johns, J.; Winslow, G. Anaplasma phagocytophilum and Ehrlichia muris induce cytopenias and global defects in hematopoiesis. Clin. Microbiol. Infect. 2009, 15, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Bexfield, N.H.; Villiers, E.J.; Herrtage, M.E. Immune-mediated haemolytic anaemia and thrombocytopenia associated with Anaplasma phagocytophilum in a dog. J. Small Anim. Pract. 2005, 46, 543–548. [Google Scholar] [CrossRef]
- Klein, M.B.; Miller, J.S.; Nelson, C.M.; Goodman, J.L. Primary bone marrow progenitors of both granulocytic and monocytic lineages are susceptible to infection with the agent of human granulocytic ehrlichiosis. J. Infect. Dis. 1997, 176, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.B.; Hu, S.; Chao, C.C.; Goodman, J.L. The agent of human granulocytic ehrlichiosis induces the production of myelosuppressing chemokines without induction of proinflammatory cytokines. J. Infect. Dis. 2000, 182, 200–205. [Google Scholar] [CrossRef]
- Rikihisa, Y. Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum. Clin. Microbiol. Rev. 2011, 24, 469–489. [Google Scholar] [CrossRef]
- Cohen, S.B.; Yabsley, M.J.; Freye, J.D.; Dunlap, B.G.; Rowland, M.E.; Huang, J.; Dunn, J.R.; Jones, T.F.; Moncayo, A.C. Prevalence of Ehrlichia chaffeensis and Ehrlichia ewingii in ticks from Tennessee. Vector Borne Zoonotic Dis. 2010, 10, 435–440. [Google Scholar] [CrossRef]
- Pritt, B.S.; Allerdice, M.E.J.; Sloan, L.M.; Paddock, C.D.; Munderloh, U.G.; Rikihisa, Y.; Tajima, T.; Paskewitz, S.M.; Neitzel, D.F.; Hoang Johnson, D.K.; et al. Proposal to reclassify Ehrlichia muris as Ehrlichia muris subsp. muris subsp. nov. and description of Ehrlichia muris subsp. eauclairensis subsp. nov., a newly recognized tick-borne pathogen of humans. Int. J. Syst. Evol. Microbiol. 2017, 67, 2121–2126. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Bloch, K.C.; McBride, J.W. Human ehrlichiosis and anaplasmosis. Clin. Lab. Med. 2010, 30, 261–292. [Google Scholar] [CrossRef] [PubMed]
- Olano, J.P.; Masters, E.; Hogrefe, W.; Walker, D.H. Human monocytotropic ehrlichiosis, Missouri. Emerg. Infect. Dis. 2003, 9, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Dubie, T.; Mohammed, Y.; Terefe, G.; Muktar, Y.; Tesfaye, J. An insight review on canine ehrlichiosis with emphasis on its epidemiology and pathogenesity importance. Glob. J. Vet. Med. Res. 2014, 2, 59–67. [Google Scholar]
- Al-Badrani, B.A. Diagnostic study of ehrlichiosis in cattle of Mosul-Iraq. Bas. J. Vet. Res. 2013, 12, 87–97. [Google Scholar] [CrossRef]
- Saito, T.B.; Thirumalapura, N.R.; Shelite, T.R.; Rockx-Brouwer, D.; Popov, V.L.; Walker, D.H. An animal model of a newly emerging human ehrlichiosis. J. Infect. Dis. 2015, 211, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.B.; Pritt, B.S.; Sloan, L.M.; Paddock, C.D.; Musham, C.K.; Ramos, J.M.; Cetin, N.; Rosenbaum, E.R. First reported case of Ehrlichia ewingii involving human bone marrow. J. Clin. Microbiol. 2014, 52, 4102–4104. [Google Scholar] [CrossRef] [PubMed]
- Qurollo, B.A.; Buch, J.; Chandrashekar, R.; Beall, M.J.; Breitschwerdt, E.B.; Yancey, C.B.; Caudill, A.H.; Comyn, A. Clinicopathological findings in 41 dogs (2008–2018) naturally infected with Ehrlichia ewingii. J. Vet. Intern. Med. 2019, 33, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.P.; Dumler, J.S.; Maley, W.R.; Klein, A.S.; Burdick, J.F.; Fred Poordad, F.; Thuluvath, P.J.; Markowitz, J.S. Human monocytic ehrlichiosis: An emerging pathogen in transplantation. Transplantation 2001, 71, 1678–1680. [Google Scholar] [CrossRef] [PubMed]
- MacNamara, K.C.; Racine, R.; Chatterjee, M.; Borjesson, D.; Winslow, G.M. Diminished hematopoietic activity associated with alterations in innate and adaptive immunity in a mouse model of human monocytic ehrlichiosis. Infect. Immun. 2009, 77, 4061–4069. [Google Scholar] [CrossRef]
- Smith, J.N.P.; Zhang, Y.; Li, J.J.; McCabe, A.; Jo, H.J.; Maloney, J.; MacNamara, K.C. Type I IFNs drive hematopoietic stem and progenitor cell collapse via impaired proliferation and increased RIPK1-dependent cell death during shock-like ehrlichial infection. PLoS Pathog. 2018, 14, e1007234. [Google Scholar] [CrossRef]
- Dumler, J.S.; Dawson, J.E.; Walker, D.H. Human ehrlichiosis: Hematopathology and immunohistologic detection of Ehrlichia chaffeensis. Hum Pathol. 1993, 24, 391–396. [Google Scholar] [CrossRef]
- Vannier, E.G.; Diuk-Wasser, M.A.; Ben, M.C.; Krause, P.J. Babesiosis. Infect. Dis. Clin. N. Am. 2015, 29, 357–370. [Google Scholar] [CrossRef]
- Joseph, J.T.; Roy, S.S.; Shams, N.; Visintainer, P.; Nadelman, R.B.; Hosur, S.; Nelson, J.; Wormser, G.P. Babesiosis in Lower Hudson Valley, New York, USA. Emerg. Infect. Dis. 2011, 17, 843–847. [Google Scholar] [CrossRef]
- Orinda, G.O.; Commins, M.A.; Waltisbuhl, D.J.; Goodger, B.V.; Wright, I.G. A study of autoantibodies to phosphatidyl-serine in Babesia bovis and Babesia bigemina infections in cattle. Vet. Immunol. Immunopathol. 1994, 40, 275–281. [Google Scholar] [CrossRef]
- Bhanot, P.; Parveen, N. Investigating disease severity in an animal model of concurrent babesiosis and Lyme disease. Int. J. Parasitol. 2019, 49, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.C.; Meyers, K.M.; Callan, M.B.; Bücheler, J.; Giger, U. Detection of platelet-bound and serum platelet-bindable antibodies for diagnosis of idiopathic thrombocytopenic purpura in dogs. J. Am. Vet. Med. Assoc. 1995, 206, 47–52. [Google Scholar]
- Huang, S.; Zhang, L.; Yao, L.; Li, J.; Chen, H.; Ni, Q.; Pan, C.; Jin, L. Human babesiosis in Southeast China: A case report. Int. J. Infect. Dis. 2018, 68, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Man, S.Q.; Qiao, K.; Cui, J.; Feng, M.; Fu, Y.F.; Cheng, X.J. A case of human infection with a novel Babesia species in China. Infect. Dis. Poverty 2016, 5, 28. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, R.; Jia, N.; Ning, N.; Zheng, Y.; Huo, Q.; Sun, Y.; Yuan, T.; Jiang, B.; Li, T.; et al. Human Case Infected with Babesia venatorum: A 5-Year Follow-Up Study. Open Forum Infect. Dis. 2020, 7, ofaa062. [Google Scholar] [CrossRef]
- Clark, I.A.; Jacobson, L.S. Do babesiosis and malaria share a common disease process? Ann. Trop. Med. Parasitol. 1998, 92, 483–488. [Google Scholar] [CrossRef]
- Orf, K.; Cunnington, A.J. Infection-related hemolysis and susceptibility to Gram-negative bacterial co-infection. Front Microbiol. 2015, 6, 666. [Google Scholar] [CrossRef] [PubMed]
- Scheepers, E.; Leisewitz, A.L.; Thompson, P.N.; Christopher, M.M. Serial haematology results in transfused and non-transfused dogs naturally infected with Babesia rossi. J. S. Afr. Vet Assoc. 2011, 82, 136–143. [Google Scholar] [CrossRef]
- Akel, T.; Mobarakai, N. Hematologic manifestations of babesiosis. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 6. [Google Scholar] [CrossRef]
- Bullard, J.M.; Ahsanuddin, A.N.; Perry, A.M.; Lindsay, L.R.; Iranpour, M.; Dibernardo, A.; Van Caeseele, P.G. The first case of locally acquired tick-borne Babesia microti infection in Canada. Can. J. Infect. Dis. Med. Microbiol. 2014, 25, e87–e89. [Google Scholar] [CrossRef] [PubMed]
- Hovius, K.E.; Rijpkema, S.G.; Westers, P.; van der Zeijst, B.A.; van Asten, F.J.; Houwers, D.J. A serological study of cohorts of young dogs, naturally exposed to Ixodes ricinus ticks, indicates seasonal reinfection by Borrelia burgdorferi sensu lato. Vet. Q. 1999, 21, 16–20. [Google Scholar] [CrossRef]
- Cleveland, C.A.; Swanepoel, L.; Brown, J.D.; Casalena, M.J.; Williams, L.; Yabsley, M.J. Surveillance for Borrelia spp. in Upland Game Birds in Pennsylvania, USA. Vet. Sci. 2020, 7, 82. [Google Scholar] [CrossRef]
- Tang, T.T.; Zhang, L.; Bansal, A.; Grynpas, M.; Moriarty, T.J. The Lyme Disease Pathogen Borrelia burgdorferi Infects Murine Bone and Induces Trabecular Bone Loss. Infect. Immun. 2017, 85, e00781-16. [Google Scholar] [CrossRef] [PubMed]
- Oksi, J.; Mertsola, J.; Reunanen, M.; Marjamäki, M.; Viljanen, M.K. Subacute multiple-site osteomyelitis caused by Borrelia burgdorferi. Clin. Infect. Dis. 1994, 19, 891–896. [Google Scholar] [CrossRef]
- Steere, A.C.; Schoen, R.T.; Taylor, E. The clinical evolution of Lyme arthritis. Ann. Intern. Med. 1987, 107, 725–731. [Google Scholar] [CrossRef]
- Schlesinger, P.A.; Duray, P.H.; Burke, B.A.; Steere, A.C.; Stillman, M.T. Maternal-fetal transmission of the Lyme disease spirochete, Borrelia burgdorferi. Ann. Intern. Med. 1985, 103, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Houtman, P.M.; Tazelaar, D.J. Joint and bone involvement in Dutch patients with Lyme borreliosis presenting with acrodermatitis chronica atrophicans. Neth. J. Med. 1999, 54, 5–9. [Google Scholar] [CrossRef]
- Hovmark, A.; Asbrink, E.; Olsson, I. Joint and bone involvement in Swedish patients with Ixodes ricinus-borne Borrelia infection. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 1986, 263, 275–284. [Google Scholar] [PubMed]
- Kvasnicka, H.M.; Thiele, J.; Ahmadi, T. Bone marrow manifestation of Lyme disease (Lyme borreliosis). Br. J. Haematol. 2003, 120, 723. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.P.; Rahn, D.W. Lyme disease and radiologic findings in Lyme arthritis. AJR Am. J. Roentgenol. 1992, 158, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.P.; Steere, A.C. Lyme arthritis: Radiologic findings. Radiology 1985, 154, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Munson, E.; Nardelli, D.T.; Du-Chateau, B.K.; Callister, S.M.; Schell, R.F. Hamster and murine models of severe destructive Lyme arthritis. Clin. Dev. Immunol. 2012, 2012, 504215. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Vanhoenacker, F.M.; Gielen, J. Unusual musculoskeletal manifestations of Lyme disease. JBR-BTR 2004, 87, 224–228. [Google Scholar]
- Steere, A.C. Musculoskeletal manifestations of Lyme disease. Am. J. Med. 1995, 98, 44S–48S. [Google Scholar] [CrossRef]
- Zlotnikov, N.; Javid, A.; Ahmed, M.; Eshghi, A.; Tang, T.T.; Arya, A.; Bansal, A.; Matar, F.; Parikh, M.; Ebady, R.; et al. Infection with the Lyme disease pathogen suppresses innate immunity in mice with diet-induced obesity. Cell. Microbiol. 2017, 19, e12689. [Google Scholar] [CrossRef] [PubMed]
- Isogai, E.; Isogai, H.; Kimura, K.; Hayashi, S.; Kubota, T.; Nishikawa, T.; Nakane, A.; Fujii, N. Cytokines in the serum and brain in mice infected with distinct species of Lyme disease Borrelia. Microb. Pathog. 1996, 21, 413–419. [Google Scholar] [CrossRef]
- Redlich, K.; Smolen, J.S. Inflammatory bone loss: Pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012, 11, 234–250. [Google Scholar] [CrossRef]
- Kosoy, O.I.; Lambert, A.J.; Hawkinson, D.J.; Pastula, D.M.; Goldsmith, C.S.; Hunt, D.C.; Staples, J.E. Novel thogotovirus associated with febrile illness and death, United States, 2014. Emerg. Infect. Dis. 2015, 21, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Bricker, T.L.; Shafiuddin, M.; Gounder, A.P.; Janowski, A.B.; Zhao, G.; Williams, G.D.; Jagger, B.W.; Diamond, M.S.; Bailey, T.; Kwon, J.H.; et al. Therapeutic efficacy of favipiravir against Bourbon virus in mice. PLoS Pathog. 2019, 15, e1007790. [Google Scholar] [CrossRef]
- Pace, E.J.; O’Reilly, M. Tickborne Diseases: Diagnosis and Management. Am. Fam. Physician 2020, 101, 530–540. [Google Scholar]
- Burgdorfer, W. Colorado tick fever. II. The behavior of Colorado tick fever virus in rodents. J. Infect. Dis. 1960, 107, 384–388. [Google Scholar] [CrossRef]
- Bowen, G.S.; Shriner, R.B.; Pokorny, K.S.; Kirk, L.J.; McLean, R.G. Experimental Colorado tick fever virus infection in Colorado mammals. Am. J. Trop. Med. Hyg. 1981, 30, 224–229. [Google Scholar] [CrossRef]
- Kadkhoda, K.; Semus, M.; Jelic, T.; Walkty, A. Case Report: A case of colorado tick fever acquired in Southwestern Saskatchewan. Am. J. Trop. Med. Hyg. 2018, 98, 891–893. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/coloradotickfever/faqs.html (accessed on 1 March 2021).
- Oshiro, L.S.; Dondero, D.V.; Emmons, R.W.; Lennette, E.H. The development of Colorado tick fever virus within cells of the haemopoietic system. J. Gen. Virol. 1978, 39, 73–79. [Google Scholar] [CrossRef]
- Emmons, R.W.; Oshiro, L.S.; Johnson, H.N.; Lennette, E.H. Intra-erythrocytic location of Colorado tick fever virus. J. Gen. Virol. 1972, 17, 185–195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Philipp, C.S.; Callaway, C.; Chu, M.C.; Huang, G.H.; Monath, T.P.; Trent, D.; Evatt, B.L. Replication of Colorado tick fever virus within human hematopoietic progenitor cells. J. Virol. 1993, 67, 2389–2395. [Google Scholar] [CrossRef] [PubMed]
- AbuSamra, D.B.; Aleisa, F.A.; Al-Amoodi, A.S.; Jalal Ahmed, H.M.; Chin, C.J.; Abuelela, A.F.; Bergam, P.; Sougrat, R.; Merzaban, J.S. Not just a marker: CD34 on human hematopoietic stem/progenitor cells dominates vascular selectin binding along with CD44. Blood Adv. 2017, 1, 2799–2816. [Google Scholar] [CrossRef] [PubMed]
- Emmons, R.W. Colorado tick fever along the Pacific slope of North America. Jap. J. Med. Sci. Biol. 1967, 20, 166–170. [Google Scholar] [PubMed]
- Government of Canada, Diseases and Conditions. Available online: https://www.canada.ca/en/public-health/services/diseases/tick-borne-encephalitis/causes-tick-borne-encephalitis.html (accessed on 1 March 2021).
- Barp, N.; Trentini, A.; Di-Nuzzo, M.; Mondardini, V.; Francavilla, E.; Contini, C. Clinical and laboratory findings in tick-borne encephalitis virus infection. Parasite Epidemiol. Control. 2020, 10, e00160. [Google Scholar] [CrossRef] [PubMed]
- Bogovic, P.; Strle, F. Tick-borne encephalitis: A review of epidemiology, clinical characteristics, and management. World J. Clin. Cases 2015, 3, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Lotric-Furlan, S.; Strle, F. Thrombocytopenia--a common finding in the initial phase of tick-borne encephalitis. Infection 1995, 23, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Lotric-Furlan, S.; Strle, F. Thrombocytopenia, leukopenia and abnormal liver function tests in the initial phase of tick-borne encephalitis. Zentralbl. Bakteriol. 1995, 282, 275–278. [Google Scholar] [CrossRef]
- Bühler, T.; Boos, N.; Leuppi-Taegtmeyer, A.B.; Berger, C.T. Febrile illness and bicytopenia within hours after tick-borne encephalitis booster vaccination. NPJ Vaccines 2019, 4, 52. [Google Scholar] [CrossRef]
- Ruzek, J.S.D. Tick-borne encephalitis in domestic animals. Acta Virol. 2020, 64, 223–229. [Google Scholar]
- Wilhelmsson, P.; Jaenson, T.G.T.; Olsen, B.; Waldenström, J.; Lindgren, P.E. Migratory birds as disseminators of ticks and the tick-borne pathogens Borrelia bacteria and tick-borne encephalitis (TBE) virus: A seasonal study at Ottenby Bird Observatory in South-eastern Sweden. Parasites Vectors 2020, 13, 607. [Google Scholar] [CrossRef]
- Mansfield, K.L.; Johnson, N.; Phipps, L.P.; Stephenson, J.R.; Fooks, A.R.; Solomon, T. Tick-borne encephalitis virus—A review of an emerging zoonosis. J. Gen. Virol. 2009, 90, 1781–1794. [Google Scholar] [CrossRef]
- Růžek, D.; Dobler, G.; Mantke, O.D. Tick-borne encephalitis: Pathogenesis and clinical implications. Travel Med. Infect. Dis. 2010, 8, 223–232. [Google Scholar] [CrossRef]
- Whitehouse, C.A. Crimean-Congo hemorrhagic fever. Antivir. Res. 2004, 64, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Ergonul, O. Crimean-Congo hemorrhagic fever virus: New outbreaks, new discoveries. Curr. Opin. Virol. 2012, 2, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, D.; Barut, H.; Duygu, F.; Çevik, B.; Kurt, S.; Sümbül, O. Characteristics of headache and its relationship with disease severity in patients with Crimean-Congo hemorrhagic fever. Agri 2018, 30, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Peyrefitte, C.; Marianneau, P.; Tordo, N.; Bouloy, M. Crimean-Congo haemorrhagic fever. Rev. Sci. Tech. 2015, 34, 391–401. [Google Scholar] [CrossRef]
- Bente, D.A.; Alimonti, J.B.; Shieh, W.J.; Camus, G.; Ströher, U.; Zaki, S.; Jones, S.M. Pathogenesis and immune response of Crimean-Congo hemorrhagic fever virus in a STAT-1 knockout mouse model. J. Virol. 2010, 84, 11089–11100. [Google Scholar] [CrossRef] [PubMed]
- Cross, R.W.; Prasad, A.N.; Borisevich, V.; Geisbert, J.B.; Agans, K.N.; Deer, D.J.; Fenton, K.A.; Geisbert, T.W. Crimean-Congo hemorrhagic fever virus strains Hoti and Afghanistan cause viremia and mild clinical disease in cynomolgus monkeys. PLoS Negl. Trop. Dis. 2020, 14, e0008637. [Google Scholar] [CrossRef]
- Swanepoel, R.; Gill, D.E.; Shepherd, A.J.; Leman, P.A.; Mynhardt, J.H.; Harvey, S. The clinical pathology of Crimean-Congo hemorrhagic fever. Rev. Infect. Dis. 1989, 11, S794–S800. [Google Scholar] [CrossRef]
- Cagatay, A.; Kapmaz, M.; Karadeniz, A.; Basaran, S.; Yenerel, M.; Yavuz, S.; Midilli, K.; Ozsut, H.; Eraksoy, H.; Calangu, S. Haemophagocytosis in a patient with Crimean Congo haemorrhagic fever. J. Med. Microbiol. 2007, 56, 1126–1128. [Google Scholar] [CrossRef][Green Version]
- Fisgin, N.T.; Fisgin, T.; Tanyel, E.; Doganci, L.; Tulek, N.; Guler, N.; Duru, F. Crimean-Congo hemorrhagic fever: Five patients with hemophagocytic syndrome. Am. J. Hematol. 2008, 83, 73–76. [Google Scholar] [CrossRef]
- Morimoto, A.; Nakazawa, Y.; Ishii, E. Hemophagocytic lymphohistiocytosis: Pathogenesis, diagnosis, and management. Pediatr. Int. 2016, 58, 817–825. [Google Scholar] [CrossRef]
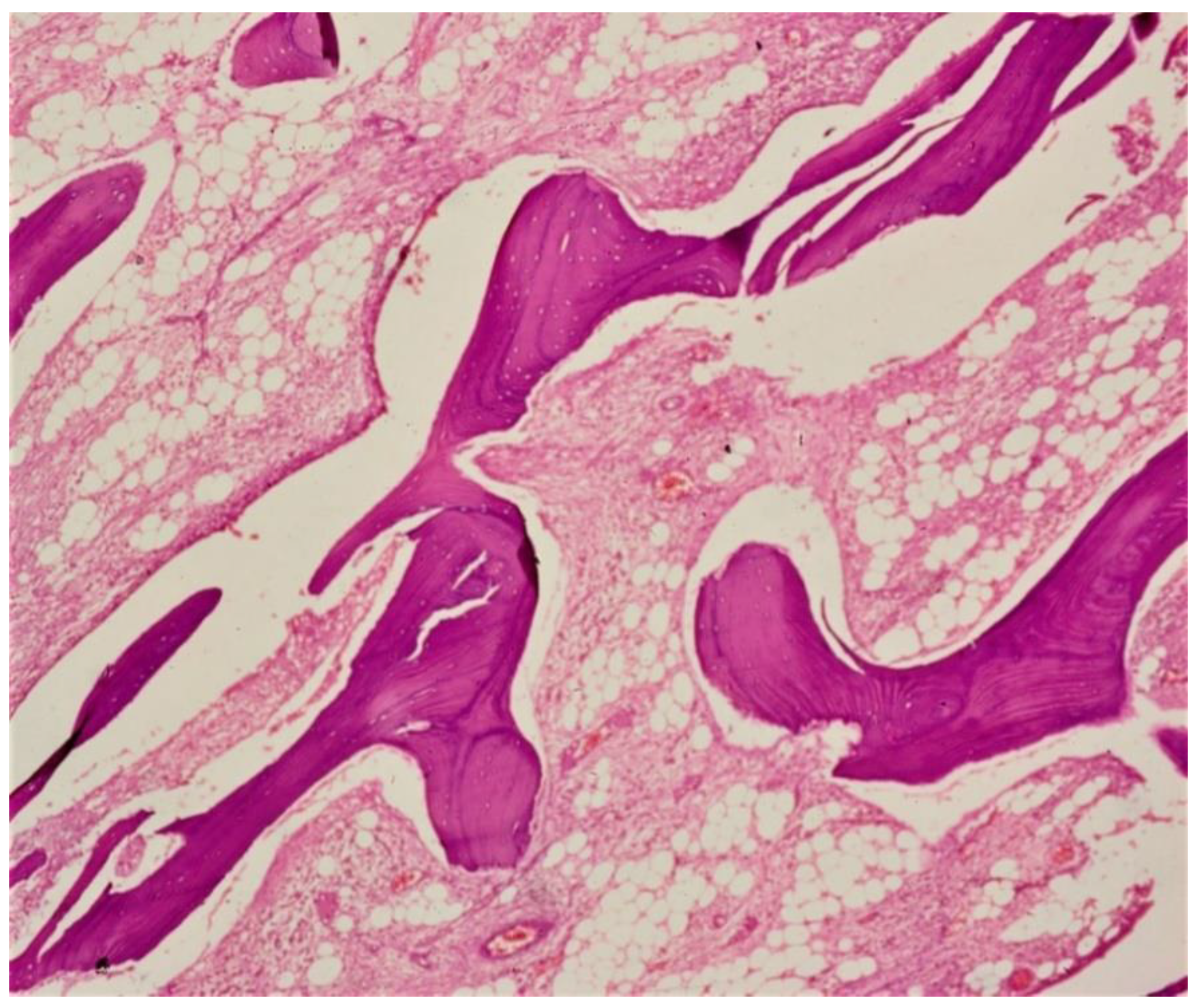
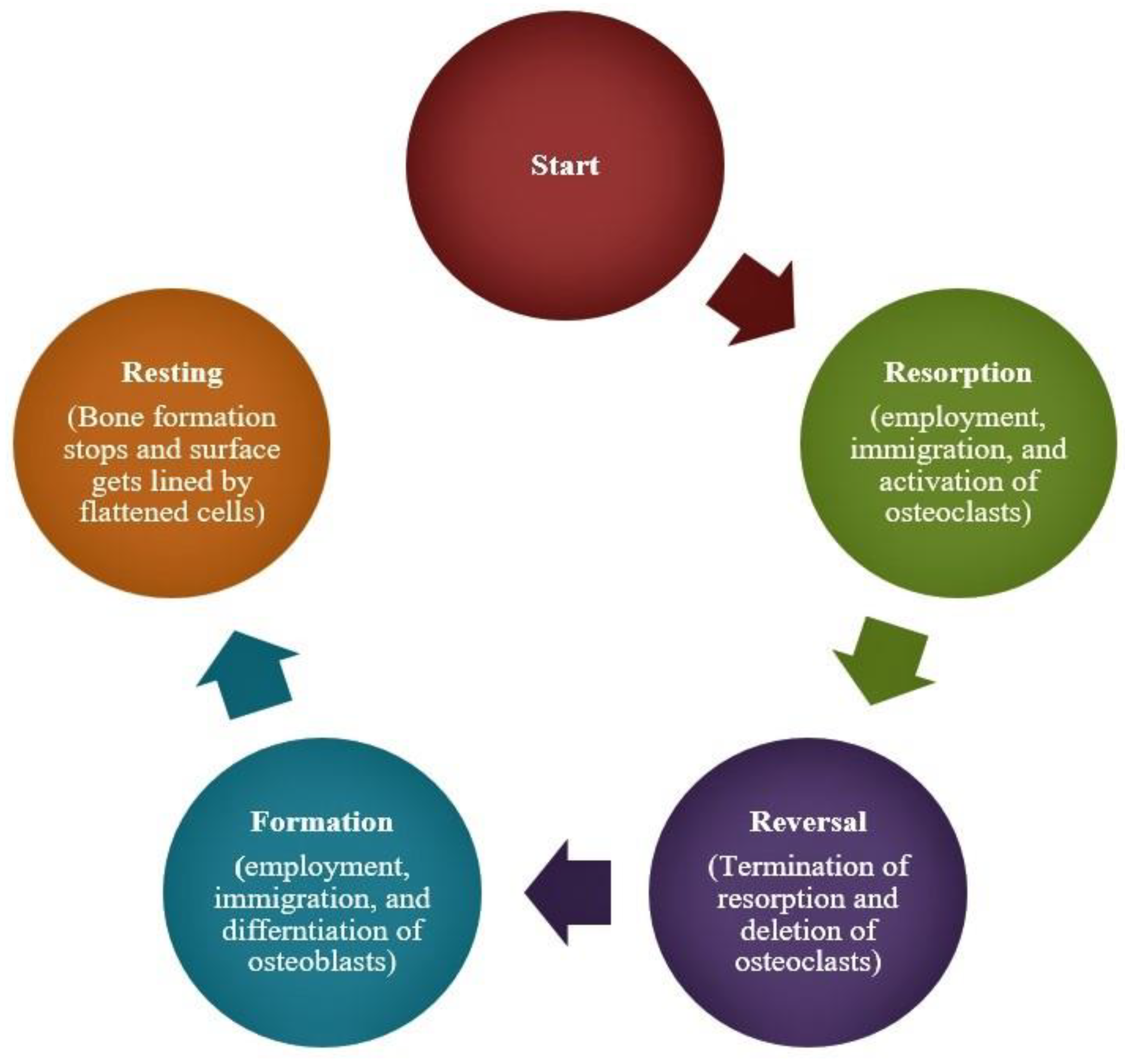
| Bacterial | Viral | Parasitic |
|---|---|---|
| Anaplasmosis (Anaplasma phagocytophilum) [3] | Nairoviral diseases: Crimean-Congo Hemorrhagic Fever (CCHFV) [4]; Nairobi Sheep Disease (NSDV) [5]; Songling Virus Disease (SGLV) [6] | Babesiosis (Babesia microti, B. divergens, B. duncani, B. venatorum) [7] |
| Ehrlichiosis (Ehrlichia chaffeensis, E. ewingii, E. muris) [8] | Phenuiviral diseases: Heartland Virus Disease (HRTV) [9]; Severe Fever with Thrombocytopenia Syndrome (SFTSV) [10] | |
| Lyme Disease (Borreliella afzelii, B. burgdorferi sensu stricto, B. garinii, B. mayonii) [11] | Orthomyxoviral diseases: Bourbon virus disease (BRBV) [12] | |
| Rickettsioses, including Flinders Island (R. honei), Israeli (R. conoriisubsp. israelensis), Mediterranean (R. conoriisubsp. conorii), Japanese (R. japonica) and Rocky Mountain (R. rickettsii) Spotted Fevers; Indian (R. conoriisubsp. indica), Queensland (R. australis) and Siberian Tick Typhus (R. sibiricasubsp. sibirica); Far Eastern (R. heilongjiangensis) and Lymphangitis-Associated (R. sibirica subsp. mongolitimonae) Rickettsioses; African Tick Bite (R. rickettsii) and Astrakhan (R. conorii subsp. caspia) Fevers; SENLAT (R. raoultii) [13,14] | Flaviviral diseases [15]: Alkhurma Hemorrhagic Fever (AHFV) [16]; Kyasanur Forest Disease (KFDV) [17]; Omsk Hemorrhagic Fever (OHFV) [15]; Powassan Disease (POWV) [15,18]; Tick-borne Encephalitis (TBEV) [19] | |
| Tick-borne Relapsing Fever (Borrelia crocidurae, B. duttoni, B. hermsii, B. hispanica, B. miyamotoi, B. parkeri, B. persica, B. turicatae) [20] | Reoviral diseases: Colorado Tick Fever Disease (CTFV); Eyach Virus Disease (EYAV) [21] | |
| Tularemia (Francisella tularensis) [22] |
| Tick-Borne Disease | Impact on Bone | |
|---|---|---|
| Disrupted Bone Marrow Function | Bone Loss | |
| Anaplasmosis | √ | - |
| Ehrlichiosis | √ | - |
| Babesiosis | √ | - |
| Lyme disease | - | √ |
| Bourbon virus disease | √ | - |
| Colorado tick fever disease | √ | - |
| Tick-borne encephalitis | √ | - |
| Crimean-Congo Hemorrhagic Fever | √ | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, I.; Moriarty, T.J. The Impact of Tick-Borne Diseases on the Bone. Microorganisms 2021, 9, 663. https://doi.org/10.3390/microorganisms9030663
Farooq I, Moriarty TJ. The Impact of Tick-Borne Diseases on the Bone. Microorganisms. 2021; 9(3):663. https://doi.org/10.3390/microorganisms9030663
Chicago/Turabian StyleFarooq, Imran, and Tara J. Moriarty. 2021. "The Impact of Tick-Borne Diseases on the Bone" Microorganisms 9, no. 3: 663. https://doi.org/10.3390/microorganisms9030663
APA StyleFarooq, I., & Moriarty, T. J. (2021). The Impact of Tick-Borne Diseases on the Bone. Microorganisms, 9(3), 663. https://doi.org/10.3390/microorganisms9030663







