Relative Abundances of Species or Sequence Variants Can Be Misleading: Soil Fungal Communities as an Example
Abstract
1. Introduction
2. Materials and Methods
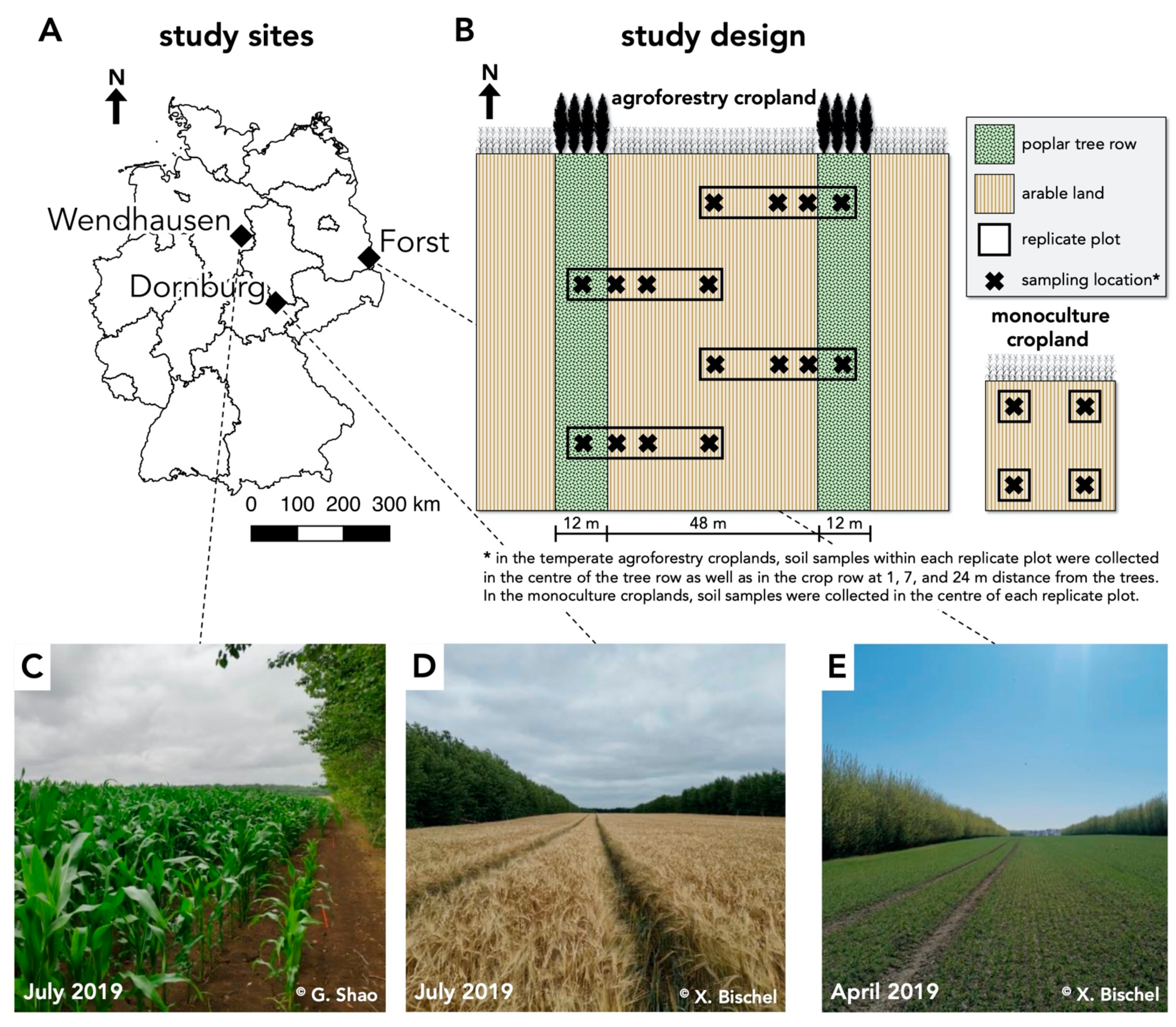
2.1. Study Sites and Study Design
2.2. Soil Collection, DNA Extraction, and Test for Polymerase Chain Reaction (PCR) Inhibitors
2.3. Library Preparation and Amplicon Sequencing
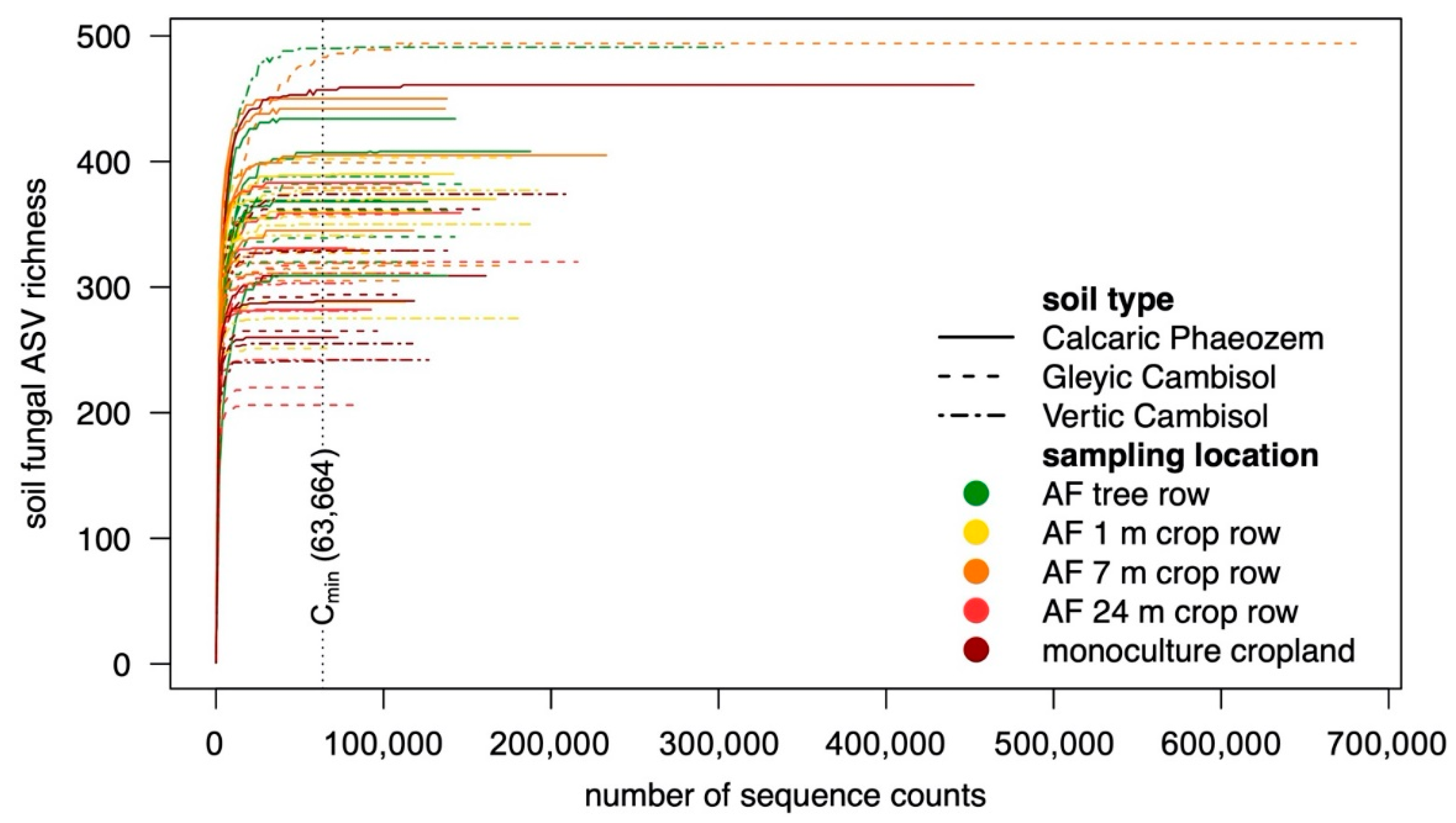
2.4. Processing of Amplicon Sequencing Data
2.5. Quantification of Soil Fungi
2.6. Statistical Analysis
3. Results
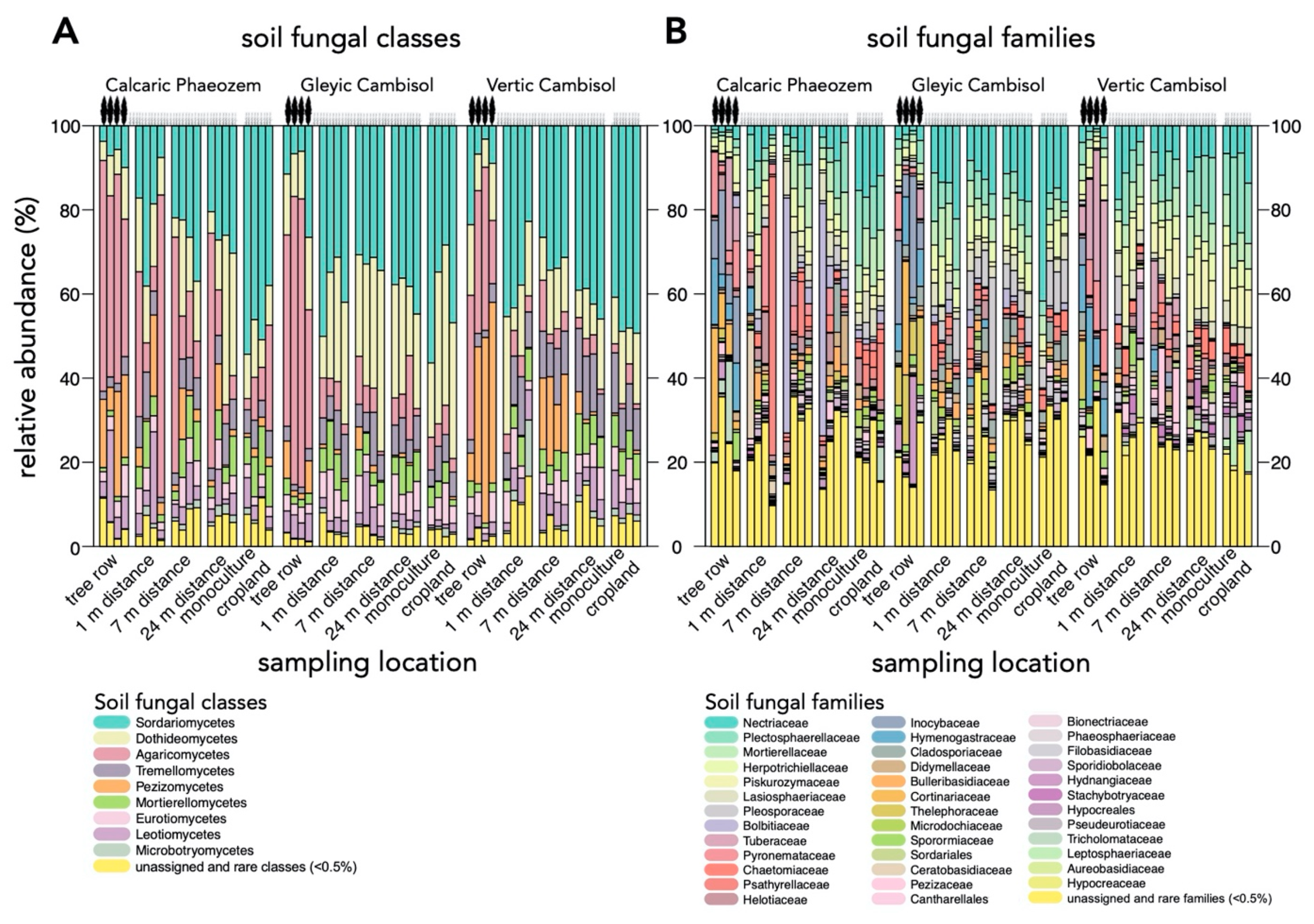
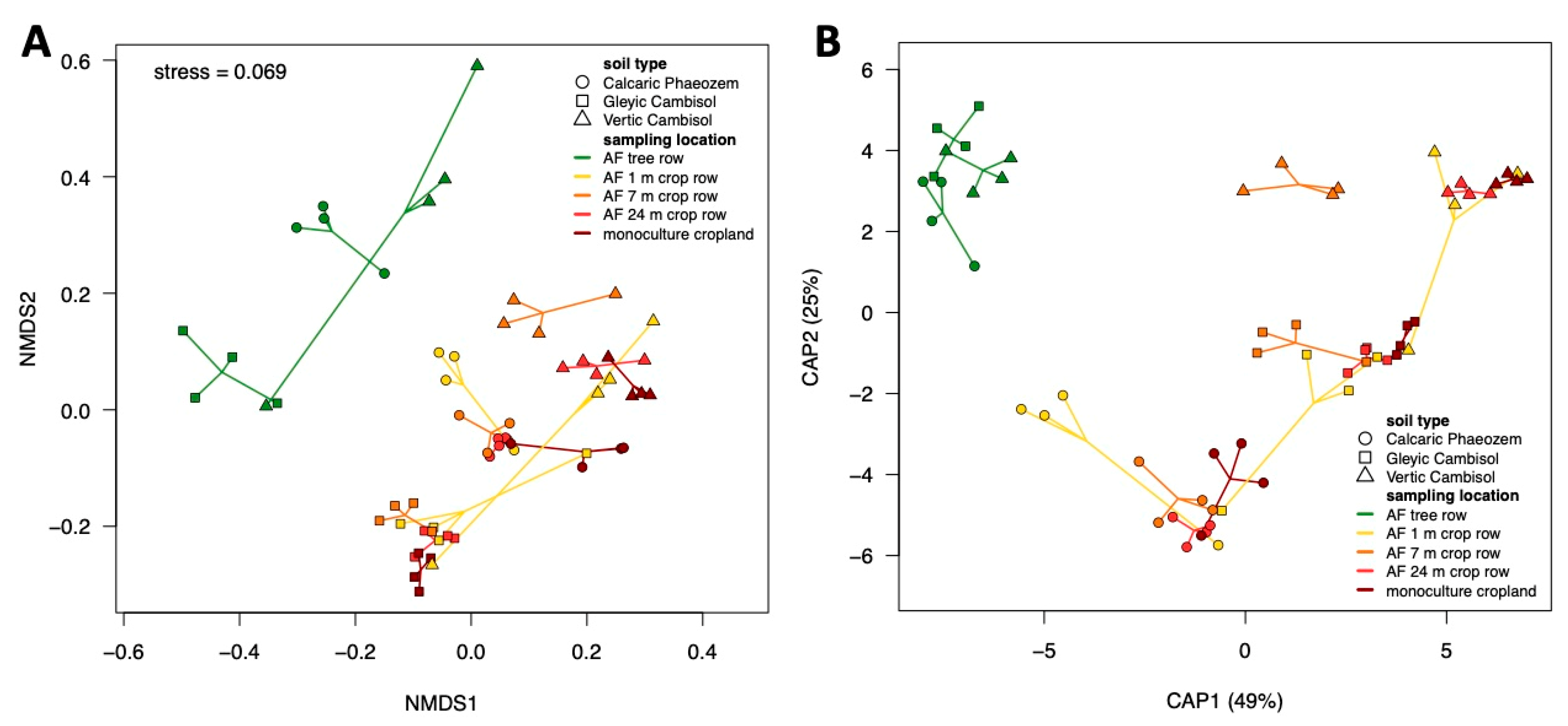
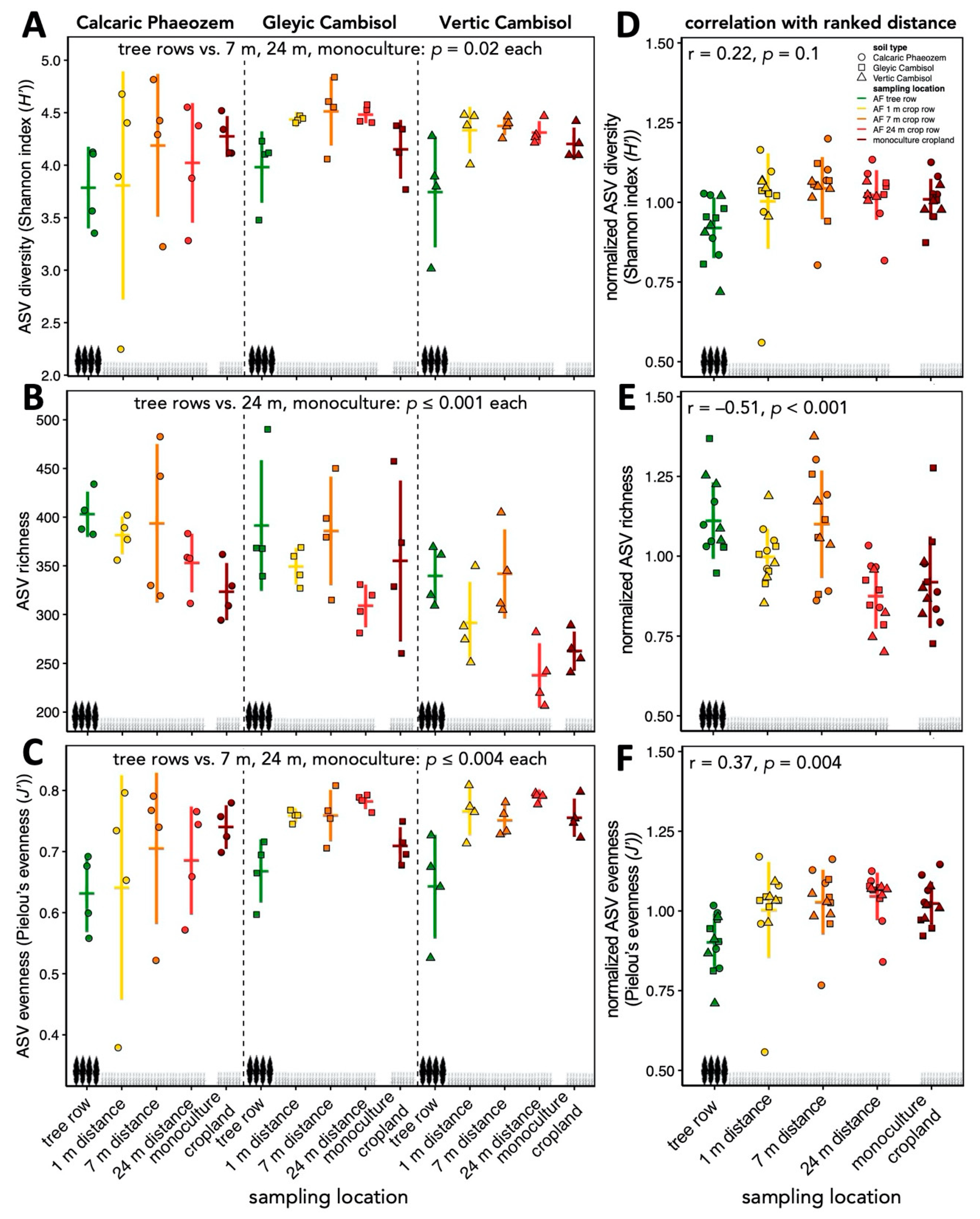
3.1. Community Composition and Diversity of Soil Fungi
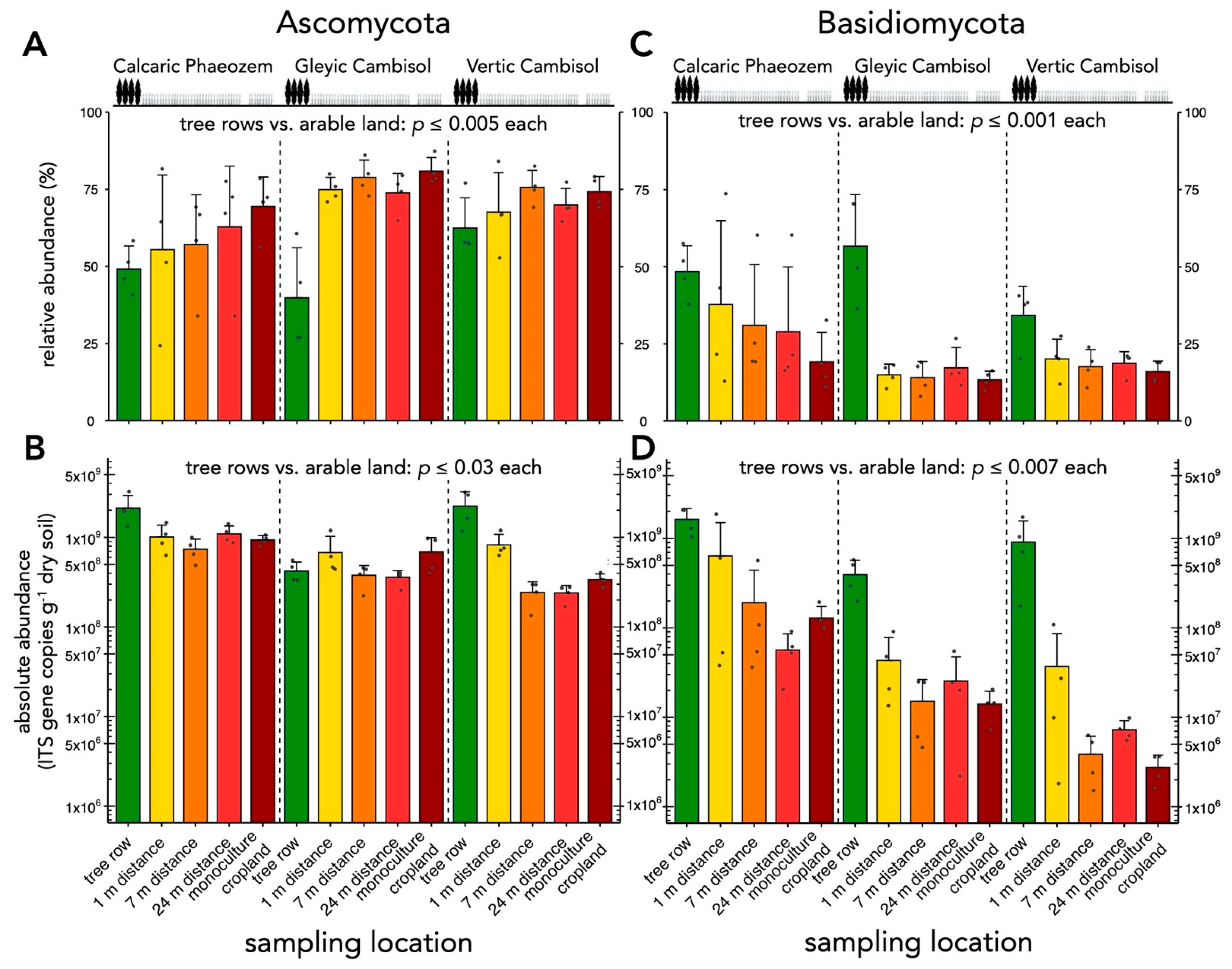
3.2. Comparison of Absolute and Relative Abundance of Selected Taxonomic Groups of Soil Fungi
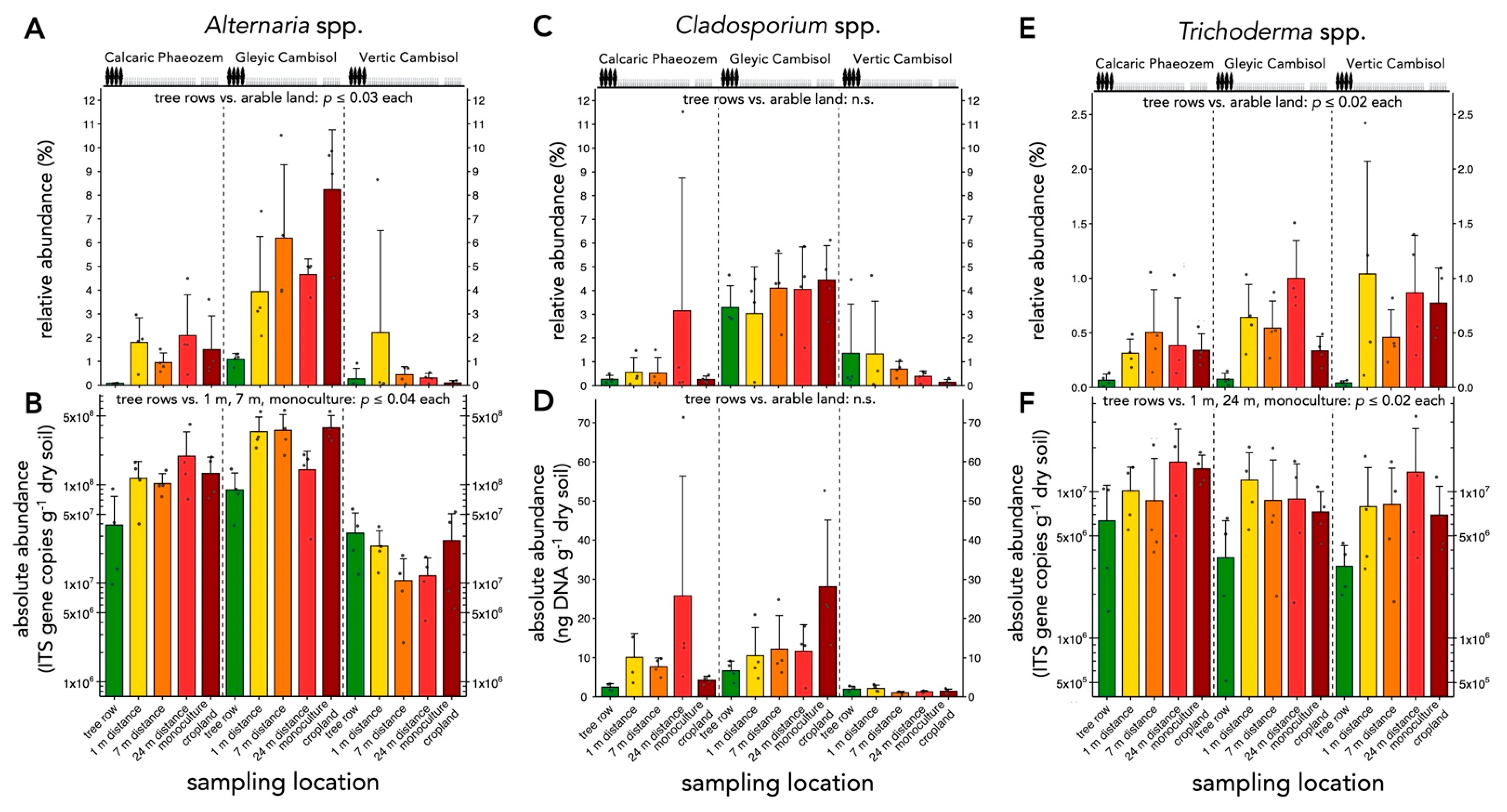
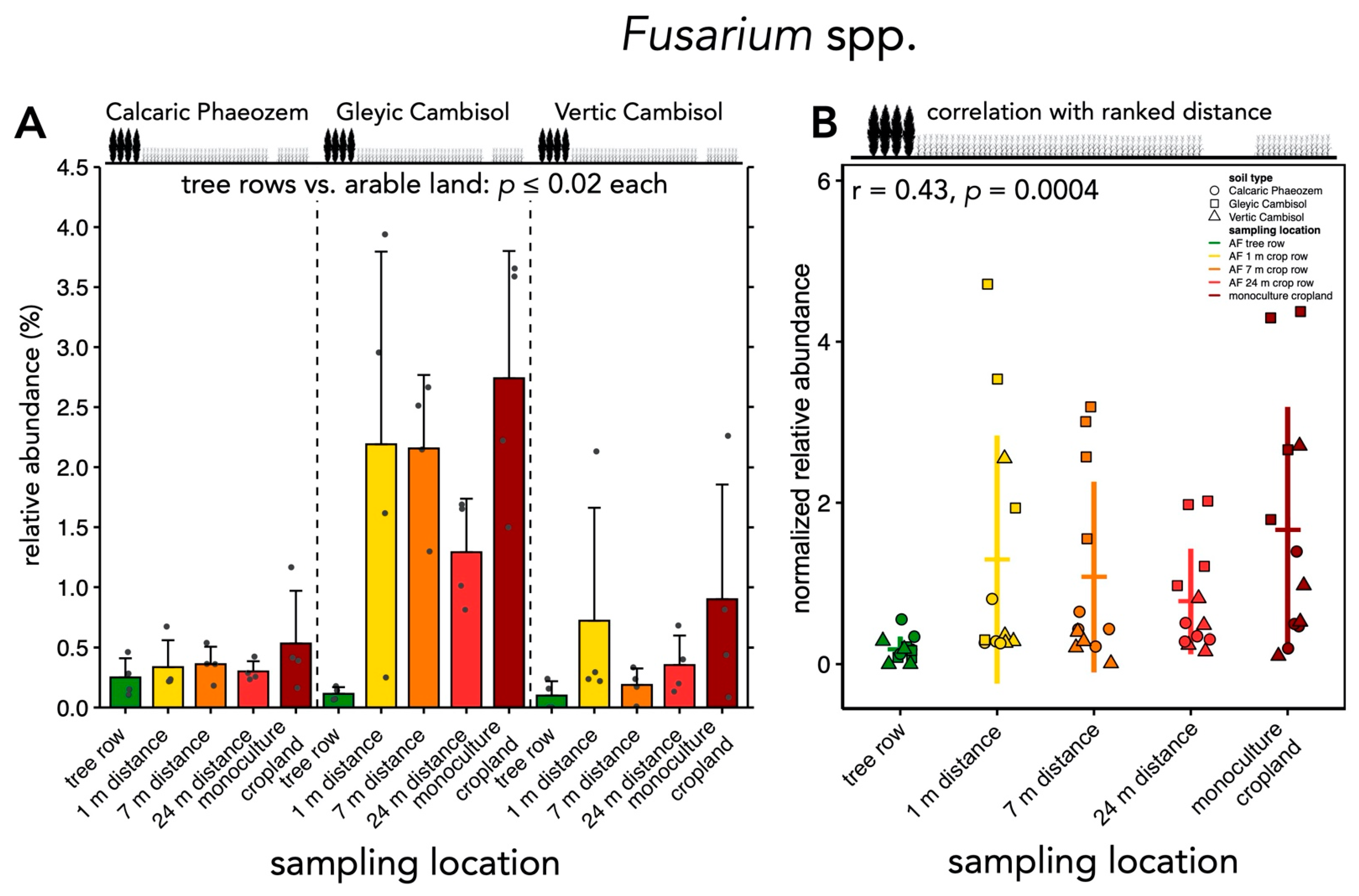
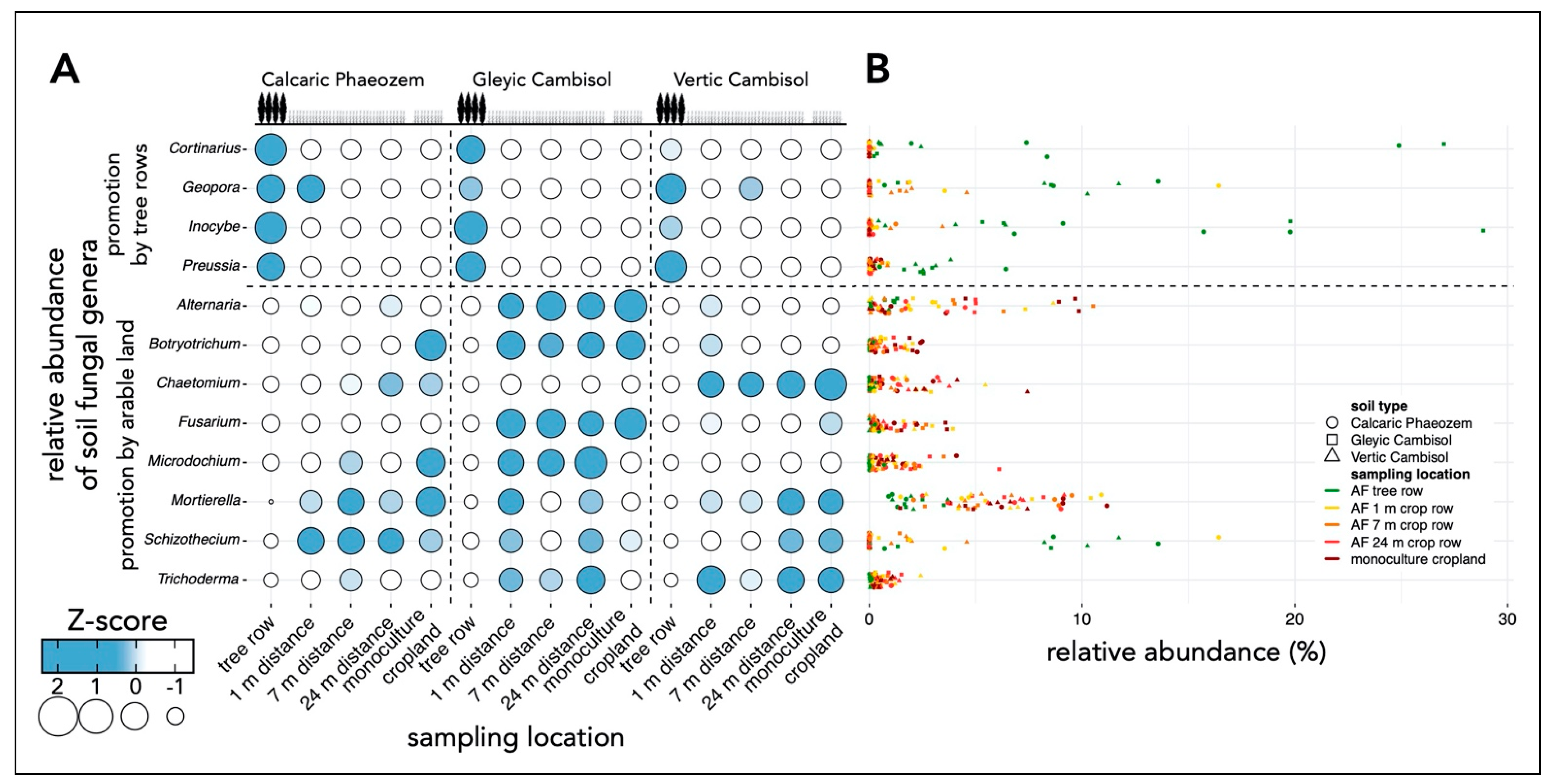
3.3. Relative Abundances of Selected Soil Fungal Genera under Agroforestry and Monoculture Cropland
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Notes
- Jose, S.; Gillespie, A.; Pallardy, S. Interspecific interactions in temperate agroforestry. Agrofor. Syst. 2004, 61-62, 237–255. [Google Scholar] [CrossRef]
- Van Noordwijk, M.; Lawson, G.; Hairiah, K.; Wilson, J. Root distribution of trees and crops: Competition and/or complementarity. In Tree-Crop Interactions: Agroforestry in a Changing Climate; CABI Publishing: Wallingford, UK, 2015; pp. 221–257. [Google Scholar]
- Quinkenstein, A.; Wöllecke, J.; Böhm, C.; Grünewald, H.; Freese, D.; Schneider, B.U.; Hüttl, R.F. Ecological benefits of the alley cropping agroforestry system in sensitive regions of Europe. Environ. Sci. Policy 2009, 12, 1112–1121. [Google Scholar] [CrossRef]
- Tsonkova, P.; Böhm, C.; Quinkenstein, A.; Freese, D. Ecological benefits provided by alley cropping systems for production of woody biomass in the temperate region: A review. Agrofor. Syst. 2012, 85, 133–152. [Google Scholar] [CrossRef]
- Smith, J.; Pearce, B.D.; Wolfe, M.S. Reconciling productivity with protection of the environment: Is temperate agroforestry the answer? Renew. Agric. Food Syst. 2013, 28, 80–92. [Google Scholar] [CrossRef]
- Allen, S.C.; Jose, S.; Nair, P.; Brecke, B.J.; Nkedi-Kizza, P.; Ramsey, C.L. Safety-net role of tree roots: Evidence from a pecan (Carya illinoensis K. Koch)–cotton (Gossypium hirsutum L.) alley cropping system in the southern United States. For. Ecol. Manag. 2004, 192, 395–407. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Lin, L.; Zepp, H. Agroforestry system reduces subsurface lateral flow and nitrate loss in Jiangxi Province, China. Agric. Ecosyst. Environ. 2011, 140, 441–453. [Google Scholar] [CrossRef]
- Bergeron, M.; Lacombe, S.; Bradley, R.L.; Whalen, J.; Cogliastro, A.; Jutras, M.-F.; Arp, P. Reduced soil nutrient leaching following the establishment of tree-based intercropping systems in eastern Canada. Agrofor. Syst. 2011, 83, 321–330. [Google Scholar] [CrossRef]
- Pardon, P.; Reubens, B.; Reheul, D.; Mertens, J.; De Frenne, P.; Coussement, T.; Janssens, P.; Verheyen, K. Trees increase soil organic carbon and nutrient availability in temperate agroforestry systems. Agric. Ecosyst. Environ. 2017, 247, 98–111. [Google Scholar] [CrossRef]
- Varah, A.; Jones, H.; Smith, J.; Potts, S.G. Enhanced biodiversity and pollination in UK agroforestry systems. J. Sci. Food Agric. 2013, 93, 2073–2075. [Google Scholar] [CrossRef]
- Varah, A.; Jones, H.; Smith, J.; Potts, S.G. Temperate agroforestry systems provide greater pollination service than monoculture. Agric. Ecosyst. Environ. 2020, 301, 107031. [Google Scholar] [CrossRef]
- Pardon, P.; Reheul, D.; Mertens, J.; Reubens, B.; De Frenne, P.; De Smedt, P.; Proesmans, W.; Van Vooren, L.; Verheyen, K. Gradients in abundance and diversity of ground dwelling arthropods as a function of distance to tree rows in temperate arable agroforestry systems. Agric. Ecosyst. Environ. 2019, 270–271, 114–128. [Google Scholar] [CrossRef]
- Wanvestraut, R.H.; Jose, S.; Nair, P.R.; Brecke, B.J. Competition for water in a pecan (Carya illinoensis K. Koch)–cotton (Gossypium hirsutum L.) alley cropping system in the southern United States. Agrofor. Syst. 2004, 60, 167–179. [Google Scholar] [CrossRef]
- Reynolds, P.E.; Simpson, J.A.; Thevathasan, N.V.; Gordon, A.M. Effects of tree competition on corn and soybean photosynthesis, growth, and yield in a temperate tree-based agroforestry intercropping system in southern Ontario, Canada. Ecol. Eng. 2007, 29, 362–371. [Google Scholar] [CrossRef]
- Swieter, A.; Langhof, M.; Lamerre, J.; Greef, J.M. Long-term yields of oilseed rape and winter wheat in a short rotation alley cropping agroforestry system. Agrofor. Syst. 2019, 93, 1853–1864. [Google Scholar] [CrossRef]
- Pardon, P.; Reubens, B.; Mertens, J.; Verheyen, K.; De Frenne, P.; De Smet, G.; Van Waes, C.; Reheul, D. Effects of temperate agroforestry on yield and quality of different arable intercrops. Agric. Syst. 2018, 166, 135–151. [Google Scholar] [CrossRef]
- Jose, S.; Gold, M.A.; Garrett, H.E. The Future of Temperate Agroforestry in the United States. In Advances in Agroforestry; Springer International Publishing: Cham, Switzerland, 2012; pp. 217–245. [Google Scholar]
- Beuschel, R.; Piepho, H.-P.; Joergensen, R.G.; Wachendorf, C. Similar spatial patterns of soil quality indicators in three poplar-based silvo-arable alley cropping systems in Germany. Biol. Fertil. Soils 2019, 55, 1–14. [Google Scholar] [CrossRef]
- Beule, L.; Corre, M.D.; Schmidt, M.; Göbel, L.; Veldkamp, E.; Karlovsky, P. Conversion of monoculture cropland and open grassland to agroforestry alters the abundance of soil bacteria, fungi and soil-N-cycling genes. PLoS ONE 2019, 14, e0218779. [Google Scholar] [CrossRef] [PubMed]
- Beule, L.; Lehtsaar, E.; Corre, M.D.; Schmidt, M.; Veldkamp, E.; Karlovsky, P. Poplar Rows in Temperate Agroforestry Croplands Promote Bacteria, Fungi, and Denitrification Genes in Soils. Front. Microbiol. 2020, 10, 3108. [Google Scholar] [CrossRef]
- Lacombe, S.; Bradley, R.L.; Hamel, C.; Beaulieu, C. Do tree-based intercropping systems increase the diversity and stability of soil microbial communities? Agric. Ecosyst. Environ. 2009, 131, 25–31. [Google Scholar] [CrossRef]
- Bainard, L.D.; Koch, A.M.; Gordon, A.M.; Klironomos, J.N. Temporal and compositional differences of arbuscular mycorrhizal fungal communities in conventional monocropping and tree-based intercropping systems. Soil Biol. Biochem. 2012, 45, 172–180. [Google Scholar] [CrossRef]
- Battie-Laclau, P.; Taschen, E.; Plassard, C.; Dezette, D.; Abadie, J.; Arnal, D.; Benezech, P.; Duthoit, M.; Pablo, A.-L.; Jourdan, C.; et al. Role of trees and herbaceous vegetation beneath trees in maintaining arbuscular mycorrhizal communities in temperate alley cropping systems. Plant Soil 2019, 453, 153–171. [Google Scholar] [CrossRef]
- Davis, E.F. The Toxic Principle of Juglans Nigra as Identified with Synthetic Juglone and Its Toxic Effects on Tomato and Alfalfa Plants. Am. J. Bot. 1928, 15, 620. [Google Scholar]
- Dighton, J. Fungi in Ecosystem Processes; CRC Press: London, UK, 2018; Volume 31. [Google Scholar]
- Brandfass, C.; Karlovsky, P. Upscaled CTAB-Based DNA Extraction and Real-Time PCR Assays for Fusarium culmorum and F. graminearum DNA in Plant Material with Reduced Sampling Error. Int. J. Mol. Sci. 2008, 9, 2306–2321. [Google Scholar] [CrossRef] [PubMed]
- Guerra, V.; Beule, L.; Lehtsaar, E.; Liao, H.-L.; Karlovsky, P. Improved Protocol for DNA Extraction from Subsoils Using Phosphate Lysis Buffer. Microorg. 2020, 8, 532. [Google Scholar] [CrossRef]
- Ihrmark, K.; Bödeker, I.T.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandström-Durling, M.; Clemmensen, K.E.; et al. New primers to amplify the fungal ITS2 region-evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M., Gelfand, D., Shinsky, J., White, T., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Beule, L.; Karlovsky, P. Tree rows in temperate agroforestry croplands alter the composition of soil bacterial communities. PLoS ONE 2021, 16, e0246919. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Abarenkov, K.; Zirk, A.; Piirmann, T.; Pöhönen, R.; Ivanov, F.; Nilsson, H.R.; Kõljalg, U. UNITE QIIME Release for Eukaryotes 2. Verison 04.02.2020 2020, UNITE Community.
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2′s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Beule, L.; Karlovsky, P. Improved normalization of species count data in ecology by scaling with ranked subsampling (SRS): Application to microbial communities. PeerJ 2020, 8, e9593. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Team RC: Vienna, Austria, 2017. [Google Scholar]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Simpson, G.; Solymos, P.; Stevens, M.; Wagner, H. The Vegan Package v. 2.5–6; 2019
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Mul-tiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar]
- Anderson, M.J.; Willis, T.J. Canonical Analysis of Principal Coordinates: A Useful Method of Constrained Or-dination for Ecology. Ecology 2003, 84, 511–525. [Google Scholar] [CrossRef]
- Kindt, R.; Kindt, M. The BiodiviersityR Package v. 2.11–3; 2019
- Hervé, M. Package “RVAideMemoire”v. 0.9–75; 2020.
- Seiter, S.; Ingham, E.R.; William, R.D. Dynamics of soil fungal and bacterial biomass in a temperate climate alley cropping system. Appl. Soil Ecol. 1999, 12, 139–147. [Google Scholar] [CrossRef]
- Frey, S.; Elliott, E.; Paustian, K. Bacterial and fungal abundance and biomass in conventional and no-tillage agroecosystems along two climatic gradients. Soil Biol. Biochem. 1999, 31, 573–585. [Google Scholar] [CrossRef]
- Lundell, T.K.; Mäkelä, M.R.; de Vries, R.P.; Hildén, K.S. Chapter Eleven—Genomics, Lifestyles and Future Pro-spects of Wood-Decay and Litter-Decomposing Basidiomycota. In Advances in Botanical Research; Martin, F.M., Ed.; Fungi; Academic Press: London, UK, 2014; Volume 70, pp. 329–370. [Google Scholar]
- Oelbermann, M.; Voroney, R.P.; Gordon, A. Carbon sequestration in tropical and temperate agroforestry systems: A review with examples from Costa Rica and southern Canada. Agric. Ecosyst. Environ. 2004, 104, 359–377. [Google Scholar] [CrossRef]
- Lou, J.; Yang, L.; Wang, H.; Wu, L.; Xu, J. Assessing soil bacterial community and dynamics by integrated high-throughput absolute abundance quantification. PeerJ 2018, 6, e4514. [Google Scholar] [CrossRef]
- Jian, C.; Luukkonen, P.; Yki-Järvinen, H.; Salonen, A.; Korpela, K. Quantitative PCR provides a simple and accessible method for quantitative microbiota profiling. PLoS ONE 2020, 15, e0227285. [Google Scholar] [CrossRef]
- Barlow, J.T.; Bogatyrev, S.R.; Ismagilov, R.F. A quantitative sequencing framework for absolute abundance measurements of mucosal and lumenal microbial communities. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Props, R.; Kerckhof, F.-M.; Rubbens, P.; De Vrieze, J.; Sanabria, E.H.; Waegeman, W.; Monsieurs, P.; Hammes, F.; Boon, N. Absolute quantification of microbial taxon abundances. ISME J. 2017, 11, 584–587. [Google Scholar] [CrossRef]
- Vandeputte, D.; Kathagen, G.; D’Hoe, K.; Vieira-Silva, S.M.; Valles-Colomer, M.; Sabino, J.; Wang, J.; Tito, R.Y.; De Commer, L.; Darzi, Y.; et al. Quantitative microbiome profiling links gut community variation to microbial load. Nat. Cell Biol. 2017, 551, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Boinot, S.; Poulmarc’H, J.; Mézière, D.; Lauri, P.E.; Sarthou, J.-P. Distribution of overwintering invertebrates in temperate agroforestry systems: Implications for biodiversity conservation and biological control of crop pests. Agric. Ecosyst. Environ. 2019, 285, 106630. [Google Scholar] [CrossRef]
- Hiddink, G.A.; Termorshuizen, A.J.; van Bruggen, A.H.C. Mixed Cropping and Suppression of Soilborne Dis-eases. In Genetic Engineering, Biofertilisation, Soil Quality and Organic Farming; Lichtfouse, E., Ed.; Sustainable Agriculture Reviews; Springer: Dordrecht, The Netherlands, 2010; pp. 119–146. ISBN 978-90-481-8741-6. [Google Scholar]
- Beule, L.; Lehtsaar, E.; Rathgeb, A.; Karlovsky, P. Crop Diseases and Mycotoxin Accumulation in Temperate Agroforestry Systems. Sustain. J. Rec. 2019, 11, 2925. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The genomics of opportunistic success. Nat. Rev. Genet. 2011, 9, 749–759. [Google Scholar] [CrossRef]
- Pérez, L.M.; Roco, A. In vitro biocontrol activity of Trichoderma harzianum on Alternaria alternata in the presence of growth regulators. Electron. J. Biotechnol. 2001, 4. [Google Scholar] [CrossRef]
- Sempere, F.; Santamarina, M.P. In vitro biocontrol analysis of Alternaria alternata (Fr.) Keissler under different environmental conditions. Mycopathol. 2007, 163, 183–190. [Google Scholar] [CrossRef]
- Matarese, F.; Sarrocco, S.; Gruber, S.; Seidl-Seiboth, V.; Vannacci, G. Biocontrol of Fusarium head blight: Interactions between Trichoderma and mycotoxigenic Fusarium. Microbiol. 2012, 158, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, R.; Yu, J.; Saravanakumar, K.; Chen, J. Antagonistic and Biocontrol Potential of Trichoderma asperellum ZJSX5003 Against the Maize Stalk Rot Pathogen Fusarium graminearum. Indian J. Microbiol. 2016, 56, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Li, Y.; Yu, C.; Wang, Q.-Q.; Wang, M.; Sun, J.; Gao, J.-X.; Chen, J. Effect of Trichoderma harzianum on maize rhizosphere microbiome and biocontrol of Fusarium Stalk rot. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Z.A.; Akhtar, M.S.; Futai, K. (Eds.) Mycorrhizae: Sustainable Agriculture and Forestry; Springer: Dordrecht, The Netherlands, 2008; ISBN 978-1-4020-8769-1. [Google Scholar]
- Khasa, P.; Chakravarty, P.; Robertson, A.; Thomas, B.R.; Dancik, B. The mycorrhizal status of selected poplar clones introduced in Alberta. Biomass-Bioenergy 2002, 22, 99–104. [Google Scholar] [CrossRef]
- Perrin, R. Interactions between mycorrhizae and diseases caused by soil-borne fungi. Soil Use Manag. 1990, 6, 189–194. [Google Scholar] [CrossRef]
- Marschner, H.; Dell, B. Nutrient uptake in mycorrhizal symbiosis. Plant Soil 1994, 159, 89–102. [Google Scholar] [CrossRef]
- Lindahl, B.D.; Tunlid, A. Ectomycorrhizal fungi—Potential organic matter decomposers, yet not saprotrophs. New Phytol. 2015, 205, 1443–1447. [Google Scholar] [CrossRef]
- Shah, F.; Nicolás, C.; Bentzer, J.; Ellström, M.; Smits, M.M.; Rineau, F.; Canbäck, B.; Floudas, D.; Carleer, R.; Lackner, G.; et al. Ectomycorrhizal fungi decompose soil organic matter using oxidative mechanisms adapted from saprotrophic ancestors. New Phytol. 2016, 209, 1705–1719. [Google Scholar] [CrossRef]
- Long, D.; Liu, J.; Han, Q.; Wang, X.; Huang, J. Ectomycorrhizal fungal communities associated with Populus simonii and Pinus tabuliformis in the hilly-gully region of the Loess Plateau, China. Sci. Rep. 2016, 6, 24336. [Google Scholar] [CrossRef] [PubMed]
- Buée, M.; Reich, M.; Murat, C.; Morin, E.; Nilsson, R.H.; Uroz, S.; Martin, F. 454 Pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. New Phytol. 2009, 184, 449–456. [Google Scholar] [CrossRef]
- Lindahl, B.D.; De Boer, W.; Finlay, R.D. Disruption of root carbon transport into forest humus stimulates fungal opportunists at the expense of mycorrhizal fungi. ISME J. 2010, 4, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Bödeker, I.T.M.; Clemmensen, K.E.; De Boer, W.; Martin, F.; Olson, Å.; Lindahl, B.D. Ectomycorrhizal Cortinarius species participate in enzymatic oxidation of humus in northern forest ecosystems. New Phytol. 2014, 203, 245–256. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beule, L.; Arndt, M.; Karlovsky, P. Relative Abundances of Species or Sequence Variants Can Be Misleading: Soil Fungal Communities as an Example. Microorganisms 2021, 9, 589. https://doi.org/10.3390/microorganisms9030589
Beule L, Arndt M, Karlovsky P. Relative Abundances of Species or Sequence Variants Can Be Misleading: Soil Fungal Communities as an Example. Microorganisms. 2021; 9(3):589. https://doi.org/10.3390/microorganisms9030589
Chicago/Turabian StyleBeule, Lukas, Markus Arndt, and Petr Karlovsky. 2021. "Relative Abundances of Species or Sequence Variants Can Be Misleading: Soil Fungal Communities as an Example" Microorganisms 9, no. 3: 589. https://doi.org/10.3390/microorganisms9030589
APA StyleBeule, L., Arndt, M., & Karlovsky, P. (2021). Relative Abundances of Species or Sequence Variants Can Be Misleading: Soil Fungal Communities as an Example. Microorganisms, 9(3), 589. https://doi.org/10.3390/microorganisms9030589







