Ten-Year Follow-Up of Patients Treated with Fecal Microbiota Transplantation for Recurrent Clostridioides difficile Infection from a Randomized Controlled Trial and Review of the Literature
Abstract
1. Introduction
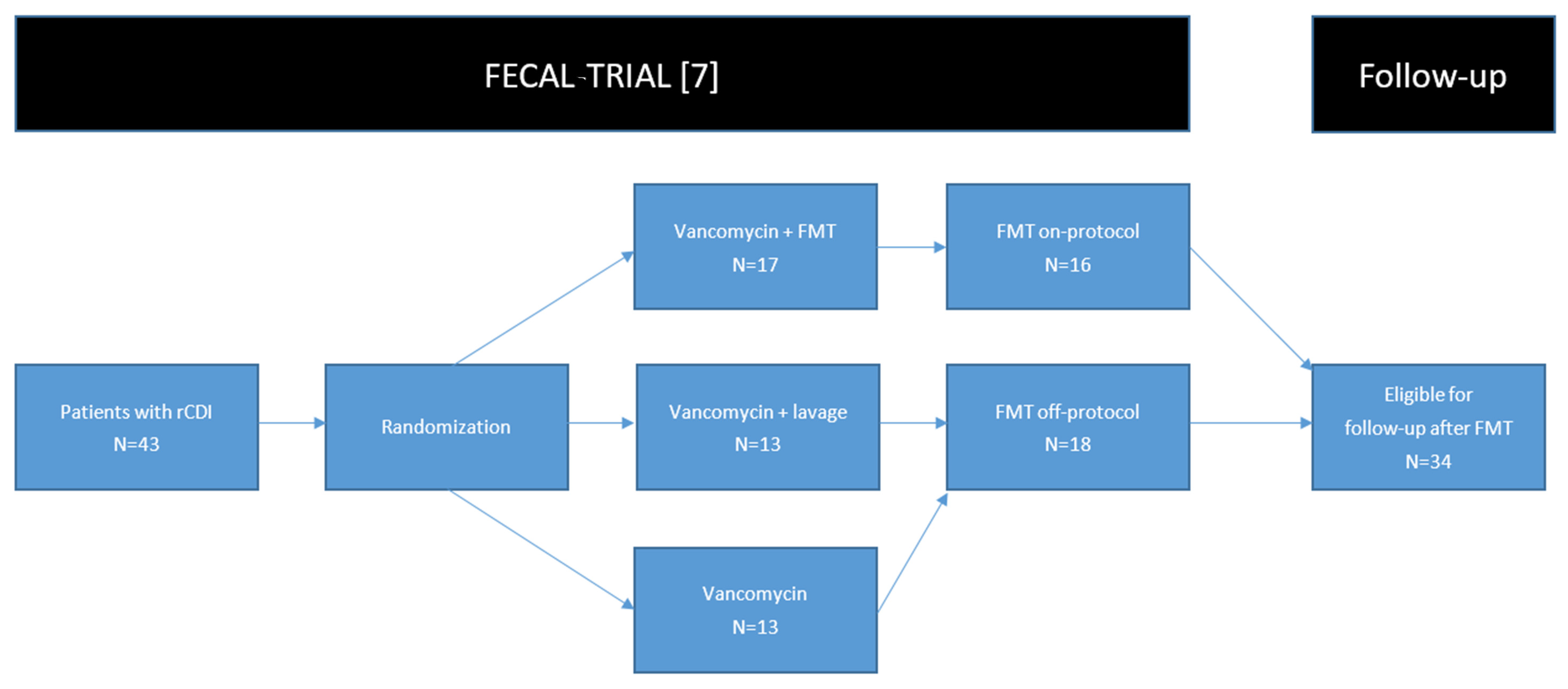
2. Patients and Methods
2.1. Patients
2.2. Collection of Follow-Up Data
2.3. Search of Literature
3. Results
3.1. Patients Treated with FMT
3.2. Patients Treated without FMT
3.3. Review of Literature
3.4. Outcome of rCDI
3.5. Infectious Complications
3.6. Weight Gain
3.7. Irritable Bowel Syndrome
3.8. Inflammatory Bowel Disease
3.9. Autoimmune Disorders
3.10. Malignancies
3.11. Other Disorders
4. Discussion
5. New Medical Conditions per Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deshpande, A.; Pasupuleti, V.; Thota, P.; Pant, C.; Rolston, D.D.K.; Hernandez, A.V.; Donskey, C.J.; Fraser, T.G. Risk Factors for Recurrent Clostridium difficile Infection: A Systematic Review and Meta-Analysis. Infect. Control Hosp. Epidemiol. 2015, 36, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Debast, S.B.; Bauer, M.P.; Kuijper, E.J. European Society of Clinical Microbiology and Infectious Diseases: Update of the treatment guidance document for Clostridium difficile infection. Clin. Microbiol. Infect. 2014, 20 (Suppl. 2), 1–26. [Google Scholar] [CrossRef]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, 987–994. [Google Scholar] [CrossRef]
- Ooijevaar, R.E.; van Beurden, Y.H.; Terveer, E.M.; Goorhuis, A.; Bauer, M.P.; Keller, J.J.; Mulder, C.J.J.; Kuijper, E.J. Update of treatment algorithms for Clostridium difficile infection. Clin Microbiol. Infect. 2018, 24, 452–462. [Google Scholar] [CrossRef]
- Terveer, E.M.; Vendrik, K.E.; Ooijevaar, R.E.; Lingen, E.V.; Boeije-Koppenol, E.; Nood, E.V.; Goorhuis, A.; Bauer, M.P.; van Beurden, Y.H.; Dijkgraaf, M.G.; et al. Faecal microbiota transplantation for Clostridioides difficile infection: Four years’ experience of the Netherlands Donor Feces Bank. United Eur. Gastroenterol. J. 2020, 8, 1236–1247. [Google Scholar] [CrossRef]
- Van Beurden, Y.H.; de Groot, P.F.; van Nood, E.; Nieuwdorp, M.; Keller, J.J.; Goorhuis, A. Complications, effectiveness, and long term follow-up of fecal microbiota transfer by nasoduodenal tube for treatment of recurrent Clostridium difficile infection. United Eur. Gastroenterol. J. 2017, 5, 868–879. [Google Scholar] [CrossRef]
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; de Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.; Tijssen, J.G.; et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef]
- Ianiro, G.; Maida, M.; Burisch, J.; Simonelli, C.; Hold, G.; Ventimiglia, M.; Gasbarrini, A.; Cammarota, G. Efficacy of different faecal microbiota transplantation protocols for Clostridium difficile infection: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2018, 6, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Ooijevaar, R.E.; Terveer, E.M.; Verspaget, H.W.; Kuijper, E.J.; Keller, J.J. Clinical Application and Potential of Fecal Microbiota Transplantation. Annu. Rev. Med. 2019, 70, 335–351. [Google Scholar] [CrossRef]
- Baxter, M.; Colville, A. Adverse events in faecal microbiota transplant: A review of the literature. J. Hosp. Infect. 2016, 92, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, M.; Wang, W.; Cao, X.; Piao, M.; Khan, S.; Yan, F.; Cao, H.; Wang, B. Systematic review: Adverse events of fecal microbiota transplantation. PLoS ONE 2016, 11, e0161174. [Google Scholar] [CrossRef] [PubMed]
- Terveer, E.M.; van Beurden, Y.H.; Goorhuis, A.; Seegers, J.; Bauer, M.P.; van Nood, E.; Dijkgraaf, M.G.W.; Mulder, C.J.J.; Vandenbroucke-Grauls, C.; Verspaget, H.W.; et al. How to: Establish and run a stool bank. Clin. Microbiol. Infect. 2017, 23, 924–930. [Google Scholar] [CrossRef] [PubMed]
- DeFilipp, Z.; Bloom, P.P.; Torres Soto, M.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.B.; Hohmann, E.L. Drug-Resistant E. coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Bunnik, E.M.; Aarts, N.; Chen, L.A. Physicians Must Discuss Potential Long-Term Risks of Fecal Microbiota Transplantation to Ensure Informed Consent. Am. J. Bioeth. 2017, 17, 61–63. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Greenwald, T.P.D.; Mcquillen, D.; Barto, A. Colonoscopy-assisted Fecal Microbiota Transplant for Outpatient Treatment of Recurrent or Refractory Clostridium Difficile Colitis; Long Term Follow-up of 58 Patients. J. Clin. Gastroenterol. Treat. 2016, 2. [Google Scholar] [CrossRef]
- Girotra, M.; Garg, S.; Anand, R.; Song, Y.; Dutta, S.K. Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection in the Elderly: Long-Term Outcomes and Microbiota Changes. Dig. Dis. Sci. 2016, 61, 3007–3015. [Google Scholar] [CrossRef]
- Jalanka, J.; Hillamaa, A.; Satokari, R.; Mattila, E.; Anttila, V.J.; Arkkila, P. The long-term effects of faecal microbiota transplantation for gastrointestinal symptoms and general health in patients with recurrent Clostridium difficile infection. Aliment Pharmacol. Ther. 2018, 47, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Chai, J.; Hammond, K.; Jeon, S.R.; Patel, Y.; Goldeh, C.; Kim, P. Long-term durability and safety of fecal microbiota transplantation for recurrent or refractory Clostridioides difficile infection with or without antibiotic exposure. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1731–1735. [Google Scholar] [CrossRef]
- Mamo, Y.; Woodworth, M.H.; Wang, T.; Dhere, T.; Kraft, C.S. Durability and Long-term Clinical Outcomes of Fecal Microbiota Transplant Treatment in Patients With Recurrent Clostridium difficile Infection. Clin. Infect. Dis. 2018, 66, 1705–1711. [Google Scholar] [CrossRef]
- Perler, B.K.; Chen, B.; Phelps, E.; Allegretti, J.R.; Fischer, M.; Ganapini, V.; Krajiceck, E.; Kumar, V.; Marcus, J.; Nativ, L.; et al. Long-Term Efficacy and Safety of Fecal Microbiota Transplantation for Treatment of Recurrent Clostridioides difficile Infection. J. Clin. Gastroenterol. 2020, 54, 701–706. [Google Scholar] [CrossRef]
- Wadhwa, A.; Al Nahhas, M.F.; Dierkhising, R.A.; Patel, R.; Kashyap, P.; Pardi, D.S.; Khanna, S.; Grover, M. High risk of post-infectious irritable bowel syndrome in patients with Clostridium difficile infection. Aliment Pharmacol. Ther. 2016, 44, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.A.; Stwalley, D.; Demont, C.; Dubberke, E.R. Clostridium difficile infection increases acute and chronic morbidity and mortality. Infect. Control Hosp. Epidemiol. 2019, 40, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Shorr, A.F.; Zilberberg, M.D.; Wang, L.; Baser, O.; Yu, H. Mortality and Costs in Clostridium difficile Infection Among the Elderly in the United States. Infect. Control Hosp. Epidemiol. 2016, 37, 1331–1336. [Google Scholar] [CrossRef]
- Keller, J.J.; Ooijevaar, R.E.; Hvas, C.L.; Terveer, E.M.; Lieberknecht, S.C.; Hogenauer, C.; Arkkila, P.; Sokol, H.; Gridnyev, O.; Megraud, F.; et al. A standardised model for stool banking for faecal microbiota transplantation: A consensus report from a multidisciplinary UEG working group. United Eur. Gastroenterol. J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Bittar, M.; Papa, E.; Kassam, Z.; Smith, M. Can you cause inflammatory bowel disease with fecal transplantation? A 31-patient case-series of fecal transplantation using stool from a donor who later developed Crohn’s disease. Gut Microbes 2017, 8, 205–207. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Guo, B.; Lufumpa, E.; Li, X.M.; Chen, L.H.; Meng, X.; Li, B.Z. Comparative of the Effectiveness and Safety of Biological Agents, Tofacitinib, and Fecal Microbiota Transplantation in Ulcerative Colitis: Systematic Review and Network Meta-Analysis. Immunol. Investig. 2020, 1–15. [Google Scholar] [CrossRef]
- Costello, S.P.; Soo, W.; Bryant, R.V.; Jairath, V.; Hart, A.L.; Andrews, J.M. Systematic review with meta-analysis: Faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Aliment Pharmacol. Ther. 2017, 46, 213–224. [Google Scholar] [CrossRef]
- Qazi, T.; Amaratunga, T.; Barnes, E.L.; Fischer, M.; Kassam, Z.; Allegretti, J.R. The risk of inflammatory bowel disease flares after fecal microbiota transplantation: Systematic review and meta-analysis. Gut Microbes 2017, 8, 574–588. [Google Scholar] [CrossRef]

| Inclusion | |
| Number of patients: | 34 |
| Number of cured patients: | 30 (88%) |
| Average age in years at inclusion (SD): | 71.7 (11.6) |
| Charlson comorbidity index (SD): | 2.53 (2.1) |
| Karnofsky performance status (SD): | 51 (18) |
| Mean number of FMTs given: | 1.26 |
| Follow-up | |
| Number of patients still alive: | 7 (21%) |
| Mean duration of follow-up in years (range): | 4.5 (0.1–11) |
| Mean duration of follow-up in years (range) of patients still alive: | 10.5 (9.5–11) |
| New episodes of CDI: | 4 (12%) |
| Course of non-CDI antibiotics: | 29 (85%) |
| Long-term complications of FMT: | 0 |
| Long-term complications of CDI: | 1 (3%) |
| Lost to follow-up *: | 3 (9%) |
| Onset of new autoimmune or gastrointestinal (GI) disorders: | 0 |
| Cause of Death | Number of Patients | Time Passed Since FMT (Months) | Relatable to FMT/CDI |
|---|---|---|---|
| Renal failure | 3 | 2, 22, 25 | No |
| Pneumonia | 3 | 35, 48, NA | Unlikely |
| Stroke | 2 | 8, 14 | No |
| Urosepsis/sepsis | 2 | 3, 41 | Unlikely |
| Dementia and natural aging | 3 | 44, 57, NA | No |
| Myocardial infarction | 3 | 36, 84, NA | No |
| Cholecystitis | 1 | 47 | No |
| Cirrhosis | 1 | 24 | No |
| Myelodysplastic syndrome | 1 | 22 | No |
| Peritonitis carcinomatosa | 1 | 65 | No |
| Malignancy | 1 | 10 | No |
| CDI and peritonitis | 1 | 13 | Yes |
| Unclear | 2 | NA | NA |
| Total | 24 |
| Author | Study Design | Patients Treated with FMT | Deaths | Patients Included for Follow-Up | Mean Age | Mean Follow-Up | Primary Cure * | Patients with New Episodes of CDI | Patients Receiving Antibiotics Post-FMT ** | Safety: SAEs Possibly Attributable to FMT |
|---|---|---|---|---|---|---|---|---|---|---|
| Van Beurden et al. [6]. | Retrospective cohort | 43 | 8 (19%) | 39 | 73 | 21 months | 82% | 7 (18%) | NA | Not reported |
| Perler et al. [20]. | Retrospective cohort | 528 | 52 (10%) | 207 | 58 | 34 months | 89% | 51 (25%) | 100 (48%) | Not reported |
| Lee et al. [18]. | Retrospective cohort | 94 | 37 (39%) | 23 | NA | 6 years | NA | 0 | 12 (52%) | Not reported |
| Girotra et al. [16]. | Prospective observational cohort | 29 | 0 | 29 | 80 | 12 months | 100% | 0 | NA | Not reported |
| Mamo et al. [19]. | Retrospective cohort | 232 | 26 (11%) | 137 | 66 | 22 months | NA | 24 (18%) | 61 (45%) | Not reported |
| Greenwald et al. [15]. | Retrospective cohort | 79 | 0 | 58 | 69 | 18 months | NA | 11 (19%) | NA | Not reported |
| Jalanka et al. [17]. | Retrospective cohort | NA | 0 | 55 | 57 | 3.4 years | NA | 2 (4%) | 26 (47%) | Not reported |
| Author | Onset of New Medical Condition * Patients (%) | Deterioration of Medical Condition ** Patients (%) | Amelioration of Medical Condition ** Patients (%) |
|---|---|---|---|
| Perler et al. [20] | 105 (51%) | 11 (5%) | 15 (7%) |
| Infectious disorders: n = 84 | |||
| AD: n = 3 | Rheumatoid arthritis: n = 1 | IBS: n = 10 | |
| Malignancies: n = 8 | IBD: n = 4 | ||
| IBD/IBS: n = 12 | IBD: n = 10 | Alopecia areata: n = 1 | |
| Other: n = 33 | |||
| Lee et al. [18] | 8 (35%) | Not available | 7 (30%) |
| Infectious disorders: n = 0 | IBD: n = 4 | ||
| AD: n = 1 | Diabetes mellitus: n = 1 | ||
| Malignancies: n = 1 | Parkinson’s disease: n = 2 | ||
| IBD/IBS: n = 2 | |||
| Other: n = 4 | |||
| Mamo et al. [19] | 43 (31%) | Not available | 12 (7%) |
| Rheumatoid arthritis: n = 1 | |||
| Infectious disorders: n = 1 | IBS: n = 3 | ||
| Autoimmune disorders: n = 0 | IBD: n = 2 | ||
| Malignancies: n = 1 | Diverticulosis: n = 2 | ||
| IBD/IBS: n = 0 | Diabetes mellitus: n = 1 | ||
| Other: n = 40 | CVID: n = 1 | ||
| Jalanka et al. [17] | 16 (29%) | 4 (7%) | 8 (11%) |
| Infectious disorders: n = 0 | |||
| AD: n = 1 | Diabetes mellitus: n = 2 | IBD: n = 5 | |
| Malignancies: n = 0 | |||
| IBD/IBS: n = 5 | AD: n = 2 | AD: n = 3 | |
| Other: n = 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ooijevaar, R.E.; van Nood, E.; Goorhuis, A.; Terveer, E.M.; van Prehn, J.; Verspaget, H.W.; van Beurden, Y.H.; Dijkgraaf, M.G.W.; Keller, J.J. Ten-Year Follow-Up of Patients Treated with Fecal Microbiota Transplantation for Recurrent Clostridioides difficile Infection from a Randomized Controlled Trial and Review of the Literature. Microorganisms 2021, 9, 548. https://doi.org/10.3390/microorganisms9030548
Ooijevaar RE, van Nood E, Goorhuis A, Terveer EM, van Prehn J, Verspaget HW, van Beurden YH, Dijkgraaf MGW, Keller JJ. Ten-Year Follow-Up of Patients Treated with Fecal Microbiota Transplantation for Recurrent Clostridioides difficile Infection from a Randomized Controlled Trial and Review of the Literature. Microorganisms. 2021; 9(3):548. https://doi.org/10.3390/microorganisms9030548
Chicago/Turabian StyleOoijevaar, R. E., E. van Nood, A. Goorhuis, E. M. Terveer, J. van Prehn, H. W. Verspaget, Y. H. van Beurden, M. G. W. Dijkgraaf, and J. J. Keller. 2021. "Ten-Year Follow-Up of Patients Treated with Fecal Microbiota Transplantation for Recurrent Clostridioides difficile Infection from a Randomized Controlled Trial and Review of the Literature" Microorganisms 9, no. 3: 548. https://doi.org/10.3390/microorganisms9030548
APA StyleOoijevaar, R. E., van Nood, E., Goorhuis, A., Terveer, E. M., van Prehn, J., Verspaget, H. W., van Beurden, Y. H., Dijkgraaf, M. G. W., & Keller, J. J. (2021). Ten-Year Follow-Up of Patients Treated with Fecal Microbiota Transplantation for Recurrent Clostridioides difficile Infection from a Randomized Controlled Trial and Review of the Literature. Microorganisms, 9(3), 548. https://doi.org/10.3390/microorganisms9030548






