Cellular Memory of HipA-Induced Growth Arrest: The Length of Cell Growth Arrest Becomes Shorter for Each Successive Induction
Abstract
:1. Introduction
2. Materials and Methods
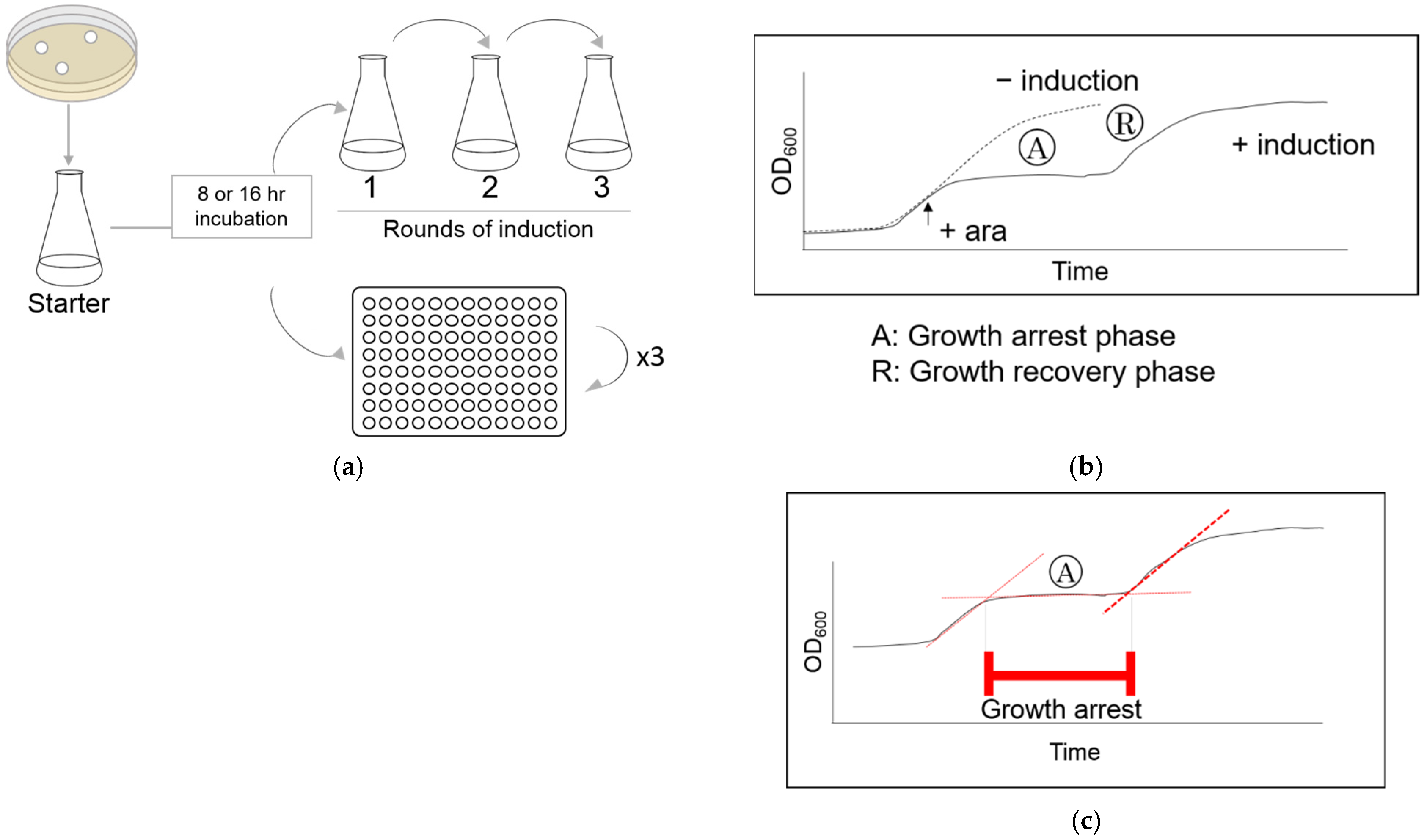
2.1. Bacterial Strain, Culture Conditions and Vectors
2.2. Measurement of Cell Growth
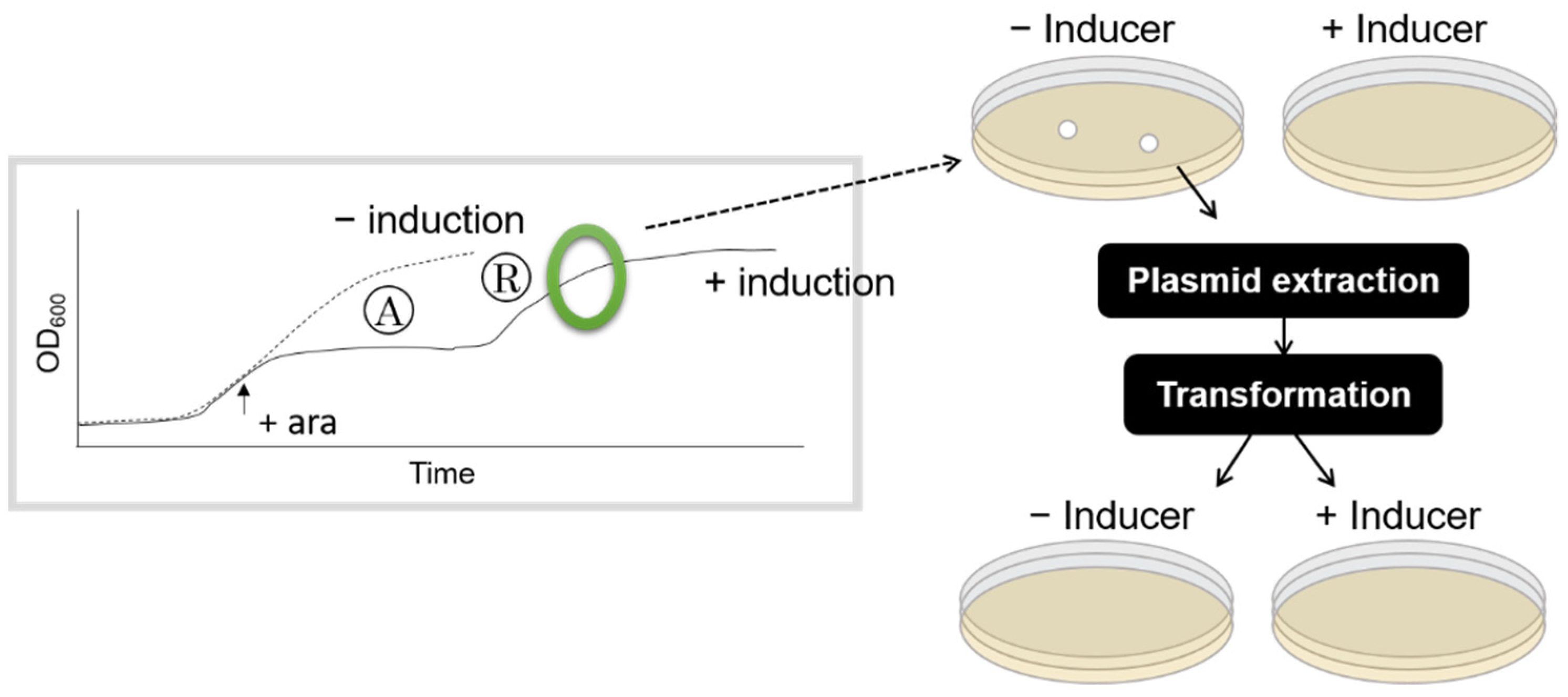
2.3. Induction of HipA during the Rapid Growth Recovery Phase
2.4. Computational and Statistical Analysis
2.5. Test of Genetic Mutation
3. Results
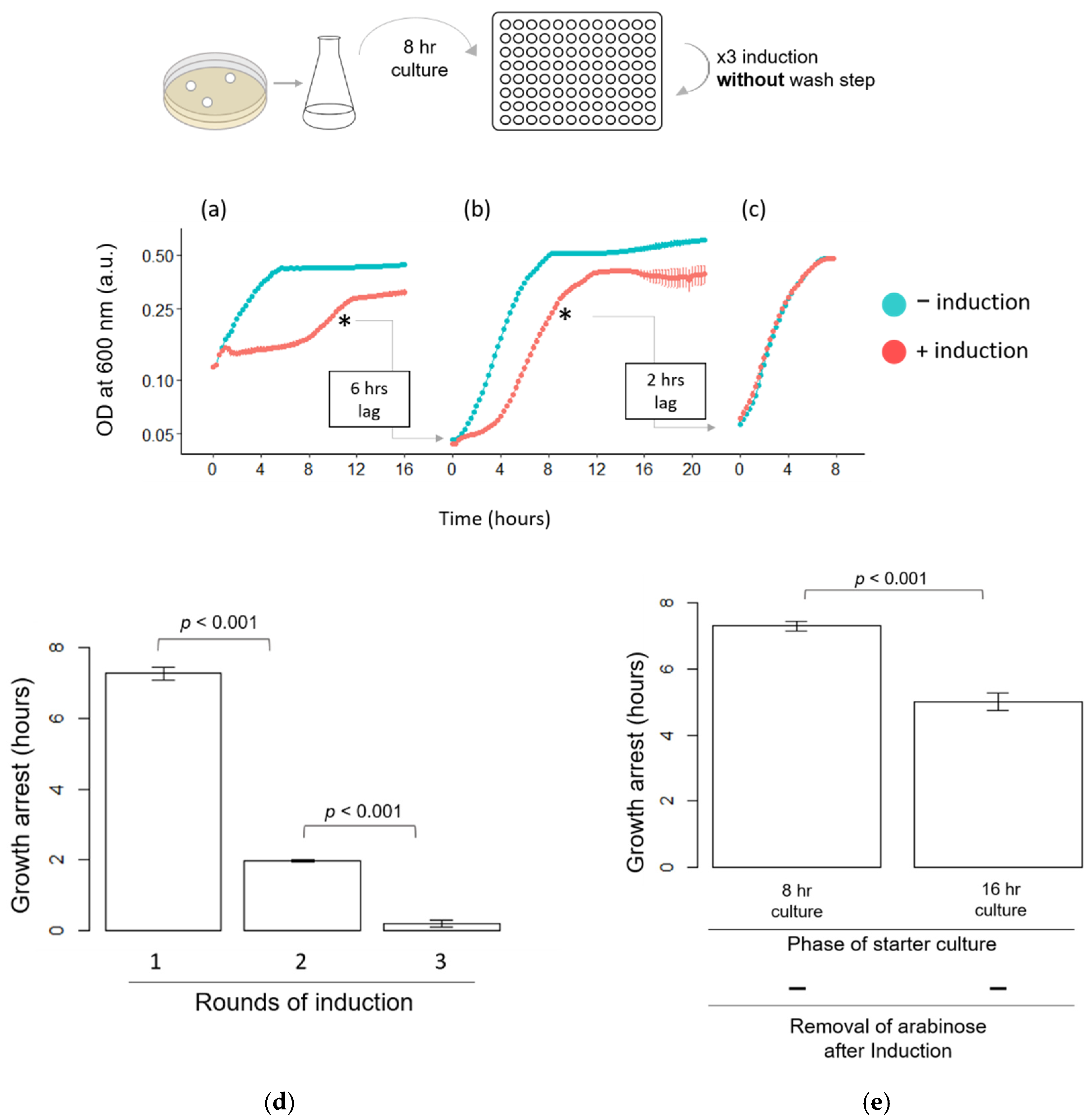
3.1. HipA-Induced Growth Arrest Phase Shortens in Each Round of Induction in Erlenmeyer Flasks
3.2. Shorter Growth Arrest Phase Is Not Due to an Increase in HipA-Resistant Mutants
3.3. Quantitative Analysis of HipA-Induced Growth Arrest in Microplates
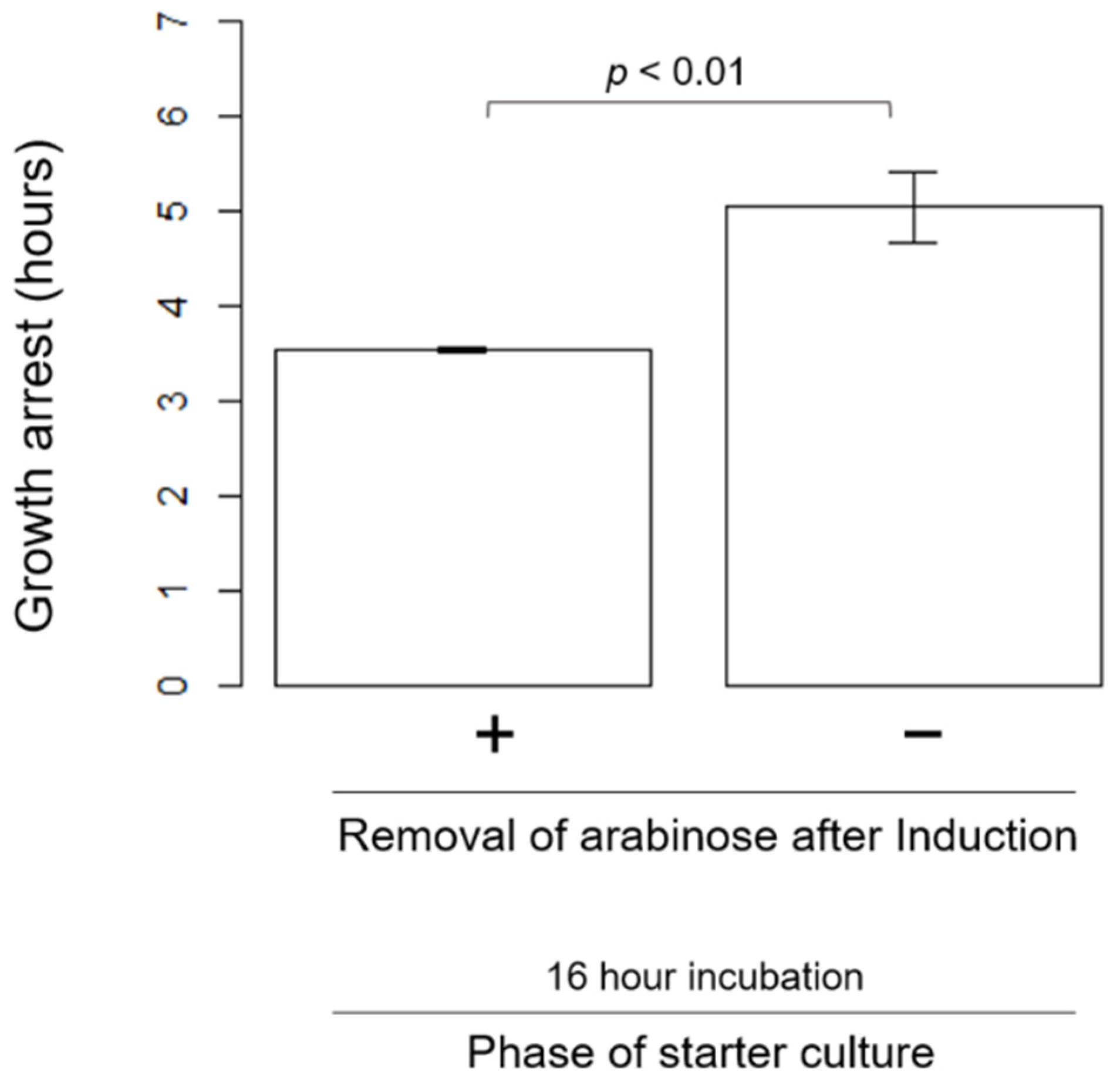
3.4. Other Factors Influencing the Length of HipA-Induced Growth Arrest Phase
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Shah, D.; Zhang, Z.; Khodursky, A.; Kaldalu, N.; Kurg, K.; Lewis, K. Persisters: A distinct physiological state of E. coli. BMC Microbiol. 2006, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Balaban, N.Q.; Merrin, J.; Chait, R.; Kowalik, L.; Leibler, S. Bacterial persistence as a phenotypic switch. Science 2004, 305, 1622–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigger, J.W. Treatment of staphylococcal infections with penicillin by intermittent sterilisation. Lancet 1944, 244, 497–500. [Google Scholar] [CrossRef]
- Greenwood, D.; O’Grady, F. Trimodal response of Escherichia coli and Proteus mirabilis to penicillins. Nature 1970, 228, 457–458. [Google Scholar] [CrossRef]
- Pontes, M.H.; Groisman, E.A. Slow growth determines nonheritable antibiotic resistance in Salmonella enterica. Sci. Signal. 2019, 12, eaax3938. [Google Scholar] [CrossRef] [PubMed]
- Zalis, E.A.; Nuxoll, A.S.; Manuse, S.; Clair, G. Stochastic variation in expression of the tricarboxylic acid cycle produces persister cells. MBio 2019, 10, e01930-19. [Google Scholar] [CrossRef] [Green Version]
- Moyed, H.S.; Bertrand, K.P. hipA, a newly recognized gene of Escherichia coli k-12 that affects frequency of persistence after inhibition of murein synthesis. J. Bacteriol. 1983, 155, 768–775. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, Y.; Park, J.H.; Inouye, M. Toxin-antitoxin systems in bacteria and archaea. Annu. Rev. Genet. 2011, 45, 61–79. [Google Scholar] [CrossRef] [Green Version]
- Gerdes, K.; Christensen, S.K.; Lobner-Olesen, A. Prokaryotic toxin-antitoxin stress response loci. Nat. Rev. Microbiol. 2005, 3, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Nariya, H.; Inouye, M. MazF, an mRNA interferase, mediates programmed cell death during multicellular myxococcus development. Cell 2008, 132, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Tan, Q.; Awano, N.; Inouye, M. YeeV is an Escherichia coli toxin that inhibits cell division by targeting the cytoskeleton proteins, FtsZ and MreB. Mol. Microbiol. 2011, 79, 109–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miki, T.; Ae Park, J.; Nagao, K.; Murayama, N.; Horiuchi, T. Control of segregation of chromosomal DNA by sex factor f in escherichia coli: Mutants of DNA gyrase subunit a suppress letD (ccdB) product growth inhibition. J. Mol. Biol. 1992, 225, 39–52. [Google Scholar] [CrossRef]
- Fraikin, N.; Goormaghtigh, F.; Van Melderen, L. Type II toxin-antitoxin systems: Evolution and revolutions. J. Bacteriol. 2020, 202, e00763-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhang, J.; Hoeflich, K.P.; Ikura, M.; Qing, G.; Inouye, M. MazF cleaves cellular mRNAs specifically at ACA to block protein synthesis in Escherichia coli. Mol. Cell 2003, 12, 913–923. [Google Scholar] [CrossRef]
- Masuda, H.; Inouye, M. Toxins of prokaryotic toxin-antitoxin systems with sequence-specific endoribonuclease activity. Toxins 2017, 9, 140. [Google Scholar] [CrossRef] [Green Version]
- Christensen, S.K.; Maenhaut-Michel, G.; Mine, N.; Gottesman, S.; Gerdes, K.; Van Melderen, L. Overproduction of the Lon protease triggers inhibition of translation in Escherichia coli: Involvement of the yefM-yoeB toxin-antitoxin system. Mol. Microbiol. 2004, 51, 1705–1717. [Google Scholar] [CrossRef]
- Aizenman, E.; Engelberg-Kulka, H.; Glaser, G. An Escherichia coli chromosomal “addiction module” regulated by guanosine 3',5'-bispyrophosphate: A model for programmed bacterial cell death. Proc. Natl. Acad. Sci. USA 1996, 93, 6059–6063. [Google Scholar] [CrossRef] [Green Version]
- Germain, E.; Castro-Roa, D.; Zenkin, N.; Gerdes, K. Molecular mechanism of bacterial persistence by HipA. Mol. Cell 2013, 52, 248–254. [Google Scholar] [CrossRef]
- Kaspy, I.; Rotem, E.; Weiss, N.; Ronin, I.; Balaban, N.Q.; Glaser, G. HipA-mediated antibiotic persistence via phosphorylation of the glutamyl-tRNA-synthetase. Nat. Commun. 2013, 4, 3001. [Google Scholar] [CrossRef] [Green Version]
- Korch, S.B.; Henderson, T.A.; Hill, T.M. Characterization of the hipA7 allele of Escherichia coli and evidence that high persistence is governed by (p)ppGpp synthesis. Mol. Microbiol. 2003, 50, 1199–1213. [Google Scholar] [CrossRef]
- Semanjski, M.; Germain, E.; Bratl, K. The kinases HipA and HipA7 phosphorylate different substrate pools in Escherichia coli to promote multidrug tolerance. Sci. Signal. 2018, 11, eaat5750. [Google Scholar] [CrossRef]
- Korch, S.B.; Hill, T.M. Ectopic overexpression of wild-type and mutant hipA genes in Escherichia coli: Effects on macromolecular synthesis and persister formation. J. Bacteriol. 2006, 188, 3826–3836. [Google Scholar] [CrossRef] [Green Version]
- Rotem, E.; Loinger, A.; Ronin, I.; Levin-Reisman, I.; Gabay, C.; Shoresh, N.; Biham, O.; Balaban, N.Q. Regulation of phenotypic variability by a threshold-based mechanism underlies bacterial persistence. Proc. Natl. Acad. Sci. USA 2010, 107, 12541–12546. [Google Scholar] [CrossRef] [Green Version]
- Nieto, C.; Cherny, I.; Khoo, S.K.; de Lacoba, M.G.; Chan, W.T.; Yeo, C.C.; Gazit, E.; Espinosa, M. The yefM-yoeB toxin-antitoxin systems of Escherichia coli and Streptococcus pneumoniae: Functional and structural correlation. J. Bacteriol. 2007, 189, 1266–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, H.; Tan, Q.; Awano, N.; Yamaguchi, Y.; Inouye, M. A novel membrane-bound toxin for cell division, CptA (YgfX), inhibits polymerization of cytoskeleton proteins, FtsZ and MreB, in Escherichia coli. FEMS Microbiol. Lett. 2012, 328, 174–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffer, S.M.; Westerhoff, H.V.; Hellingwerf, K.J.; Postma, P.W.; Tommassen, J. Autoamplification of a two-component regulatory system results in “learning” behavior. J. Bacteriol. 2001, 183, 4914–4917. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.A.; Berg, H.C. Temporal stimulation of chemotaxis in Escherichia coli. Proc. Natl. Acad. Sci. USA 1974, 71, 1388–1392. [Google Scholar] [CrossRef] [Green Version]
- Lambert, G.; Kussell, E. Memory and fitness optimization of bacteria under fluctuating environments. PLoS Genet. 2014, 10, e1004556. [Google Scholar] [CrossRef] [Green Version]
- Wolf, D.M.; Fontaine-Bodin, L.; Bischofs, I.; Price, G.; Keasling, J.; Arkin, A.P. Memory in microbes: Quantifying history-dependent behavior in a bacterium. PLoS ONE 2008, 3, e1700. [Google Scholar] [CrossRef] [Green Version]
- Jõers, A.; Tenson, T. Growth resumption from stationary phase reveals memory in Escherichia coli cultures. Sci. Rep. 2016, 6, 24055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyaue, S.; Suzuki, E.; Komiyama, Y.; Kondo, Y.; Morikawa, M.; Maeda, S. Bacterial memory of persisters: Bacterial persister cells can retain their phenotype for days or weeks after withdrawal from colony-biofilm culture. Front. Microbiol. 2018, 9, 1396. [Google Scholar] [CrossRef] [Green Version]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli k-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzman, L.M.; Belin, D.; Carson, M.J.; Beckwith, J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose pBAD promoter. J. Bacteriol. 1995, 177, 4121–4130. [Google Scholar] [CrossRef] [Green Version]
- Sambrook, J.; Fritsch, E.R.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://cran.R-project.Org/web/packages/ggplot2/index.html (accessed on 30 January 2021).
- Yang, C.Y.; Bialecka-Fornal, M.; Weatherwax, C.; Larkin, J.W.; Prindle, A.; Liu, J.; Garcia-Ojalvo, J.; Süel, G.M. Encoding membrane-potential-based memory within a microbial community. Cell Syst. 2020, 10, 417–423.e3. [Google Scholar] [CrossRef] [PubMed]
- Blauwkamp, T.A.; Ninfa, A.J. Antagonism of pii signalling by the amtb protein of Escherichia coli. Mol. Microbiol. 2003, 48, 1017–1028. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, J.L.; Uknalis, J.; Oscar, T.P.; Schwarz, J.G.; Vimini, B.; Parveen, S. The effect of previous life cycle phase on the growth kinetics, morphology, and antibiotic resistance of Salmonella typhimurium dt104 in brain heart infusion and ground chicken extract. Front. Microbiol. 2019, 10, 1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riber, L.; Koch, B.M.; Kruse, L.R.; Germain, E.; Løbner-Olesen, A. Hipa-mediated phosphorylation of SeqA does not affect replication initiation in Escherichia coli. Front. Microbiol. 2018, 9, 2637. [Google Scholar] [CrossRef] [Green Version]
- Correia, F.F.; D’Onofrio, A.; Rejtar, T.; Li, L.; Karger, B.L.; Makarova, K.; Koonin, E.V.; Lewis, K. Kinase activity of overexpressed HipA is required for growth arrest and multidrug tolerance in Escherichia coli. J. Bacteriol. 2006, 188, 8360–8367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumacher, M.A.; Min, J.; Link, T.M.; Guan, Z.; Xu, W.; Ahn, Y.H.; Soderblom, E.J.; Kurie, J.M.; Evdokimov, A.; Moseley, M.A.; et al. Role of unusual p loop ejection and autophosphorylation in HipA-mediated persistence and multidrug tolerance. Cell Rep. 2012, 2, 518–525. [Google Scholar] [CrossRef] [Green Version]


| Samples | Cells Exhibiting Arabinose-Inducible Toxicity (n = 50) | Plasmids Conferring HipA Toxicity (n = 50) |
|---|---|---|
| Initial | 100% | 100% |
| After the first round of induction and regrowth | 100% | 100% |
| After the second round of induction and regrowth | 100% | 100% |
| After the third round of induction and regrowth | 100% | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Hatimi, S.; Tupa, P.R.; Masuda, H. Cellular Memory of HipA-Induced Growth Arrest: The Length of Cell Growth Arrest Becomes Shorter for Each Successive Induction. Microorganisms 2021, 9, 2594. https://doi.org/10.3390/microorganisms9122594
Lin C-Y, Hatimi S, Tupa PR, Masuda H. Cellular Memory of HipA-Induced Growth Arrest: The Length of Cell Growth Arrest Becomes Shorter for Each Successive Induction. Microorganisms. 2021; 9(12):2594. https://doi.org/10.3390/microorganisms9122594
Chicago/Turabian StyleLin, Chun-Yi, Sanya Hatimi, Peter Robert Tupa, and Hisako Masuda. 2021. "Cellular Memory of HipA-Induced Growth Arrest: The Length of Cell Growth Arrest Becomes Shorter for Each Successive Induction" Microorganisms 9, no. 12: 2594. https://doi.org/10.3390/microorganisms9122594
APA StyleLin, C.-Y., Hatimi, S., Tupa, P. R., & Masuda, H. (2021). Cellular Memory of HipA-Induced Growth Arrest: The Length of Cell Growth Arrest Becomes Shorter for Each Successive Induction. Microorganisms, 9(12), 2594. https://doi.org/10.3390/microorganisms9122594






