Abstract
Candida albicans is a commensal fungus of the vaginal mucosa and the principal etiological agent of vaginal candidiasis. Vaginal dysbiosis has been reported during vulvovaginal candidiasis (VVC), with a progressive decrease in Lactobacillus crispatus population and an increase in L. iners population. To date, the role of L. iners in VVC pathogenesis remains scarcely explored. Herein we investigated the in vitro effect of L. iners cell-free supernatant (CFS) on the ability of C. albicans to form biofilms. Biomass and metabolic activity were measured by crystal violet and XTT assays. Further, light microscopy was performed to determine the effect of L. iners CFS on biofilm cellular morphology. We found that L. iners CFS induced a significant increase in biofilm formation by C. albicans clinical isolates which were categorized as moderate or weak biofilm producers. This effect was associated with an enhancement of hyphal/pseudohyphal growth, and the expression levels of HWP1 and ECE1, which are typical hyphae-associated genes, were upregulated. Overall, these results suggest that L. iners contributes to the pathogenesis of VVC and highlight the complexity of the interaction between C. albicans and vaginal lactobacilli. Understanding these interactions could prove essential for the development of new strategies for treating VVC.
1. Introduction
Vulvovaginal candidiasis (VVC) is the second most common cause of lower reproductive tract infections in women of childbearing age. It is estimated that 75% of all women will suffer from VVC at least once in their lifetime [1], and nearly 10% of them are bound to experience recurrence. Recurrent VVC (RVVC), defined as four or more episodes of infection every year, markedly affects the quality of life, considering the severity of symptoms and the resulting psychological stress [2,3]. C. albicans is the etiological agent of almost all cases of VVC [1,4]. C. albicans is a commensal fungus of the vaginal mucosa; several host-related features and behavioral risk factors can affect the onset of VVC, including pregnancy, hyperglycemia, immunosuppression, antibiotic or glucocorticoid therapies, genetic predisposition, as well as vaginal microbiota composition. Oral contraceptive use, intrauterine devices, some hygiene habits, clothing, and sexual practices are all considered to be major behavioral risk factors associated with VVC [5].
C. albicans pathogenicity is supported by a plethora of virulence factors, including its ability to form biofilms, morphological transition between yeast and hyphal forms, expression of adhesins and invasins on its cell surface, secretion of enzymes such as secreted aspartyl proteases and phospholipases, and production of candidalysin, a cytolytic peptide toxin [6,7].
The ability of C. albicans to develop a complex biofilm on abiotic and biotic surfaces plays a key role in its pathogenicity [8]. Contrary to biofilms of other Candida spp., those formed by C. albicans display a more heterogeneous organization, formed by budding yeasts, a multilayer structure, and filamentous cells such as true hyphae and pseudohyphae, surrounded by extracellular matrix. The ability to form hyphae is critical for the development and maintenance of C. albicans biofilms. Indeed, hyphae are essential to supporting the structural integrity of biofilms and to provide a scaffold for the attachment of other yeast cells, pseudohyphae, as well as bacteria in polymicrobial biofilms [8,9,10].
Several studies have proposed that biofilm formation by C. albicans plays a crucial role in the development of vaginal candidiasis [11,12,13,14,15]. Wu et al. [16] supported the essential role of biofilm formation in the pathogenesis of vaginal candidiasis, demonstrating that histological damages of mucosal epithelial cells and local inflammation are associated with biofilm growth on the vaginal epithelium. In addition, it has been reported that C. albicans biofilm promotes the formation of persister cells, which are mainly responsible for the recalcitrance of vaginal candidiasis to antifungal drugs [14,17].
Lactobacillus spp. play a key role in maintaining vaginal health [18] and a decrease in their population, in particular of L. crispatus, is an important risk factor for the onset of vaginal infections [19,20]. However, recent studies have reported that not all Lactobacillus spp. are beneficial and protective in nature [21]. Tortelli et al. [22] showed that L. iners dominant communities were more permissive to vaginal colonization with potential pathogens such as C. albicans. Furthermore, Ceccarani et al. [20] demonstrated that VVC-positive women exhibited an increase in the relative abundance of L. iners, along with a decrease in that of L. crispatus.
L. iners is an unusual Lactobacillus species [23] with unique features. It shows a Gram-variable morphology, more complex nutritional requirements, produces few or no important protective factors, such as D-lactic acid and hydrogen peroxide; moreover, it is notable that it releases a cytolytic toxin [24]. L. iners dominance has been associated with adverse pregnancy outcomes [25,26] and infertility [27]. These characteristics have led to the hypothesis that L. iners can cause vaginal infections, including vaginal candidiasis [21,22,28]. Based on these assumptions, herein our main aim was to investigate the in vitro effect of L. iners cell-free supernatant (CFS) on the ability of C. albicans vaginal isolates to form biofilms. Further, we investigated the effects of L. iners CFS on biofilm cellular morphology, as well as on the expression of hyphae-associated genes.
2. Materials and Methods
2.1. Subjects
Fourteen nonpregnant, nondiabetic women aged between 18 and 48 years were enrolled at the Laboratory of Microbiology of the University Hospital “Santa Maria della Misericordia” (Perugia, Italy). Prior to enrollment, each of them completed a questionnaire indicating their health status and current symptoms of vaginal disease. All women signed an informed consent in accordance with the Declaration of Helsinki. This study was approved by the local ethical committee (Comitato Etico delle Aziende Sanitarie, Umbria, Italy) (CA 2020, 3802/19). All methods were performed in accordance with relevant guidelines and regulations. Each woman presented at least two of the following acute VVC signs and symptoms: vaginal discharge, itching, burning, and dyspareunia. Vaginal wet mount preparations were used to determine the number of polymorphonuclear cells per microscopic field, presence or absence of lactobacilli, and presence or absence of yeast and pseudohyphae/hyphae. The absence of other possible etiological agents responsible for vaginal infections was assessed by microbiological analysis.
2.2. Collection of C. albicans Isolates
All details pertaining to sample collection methods have been previously described [29,30]. Briefly, vaginal swabs from each patient were plated on CHROMagar Candida (VWR International P.B.I, Milan, Italy) and incubated at 37 °C for 48 h under aerobic conditions. Routine methods were used to identify yeast colonies, followed by confirmation with matrix-assisted laser/desorption ionization time-of-flight mass spectrometry (MALDI-TOF, bioMèrieux, Marcy-l’Étoile, France). All C. albicans strains were stored at −80 °C.
2.3. Microorganisms, Growth Media, and Growth Conditions
C. albicans ATCC 10231 and L. iners ATCC 55195 were purchased from the American Type Culture Collection (ATCC). C. albicans clinical isolates and reference strain were cultured on yeast extract peptone dextrose (YEPD) agar plates at 30 °C under aerobic conditions. L. iners ATCC 55195 was routinely grown in New York City (NYC) III broth at 37 °C for 24 h under anaerobic conditions. All microbial strains were freshly grown from frozen glycerol stocks (kept at −80 °C) before each experiment.
2.4. Growth Curve and L. iners CFS Preparation
An overnight NYC III broth culture of L. iners ATCC 55195 was 1:100 diluted in brain heart infusion (BHI) broth and incubated at 37 °C for 48 h under anaerobic conditions. At different timepoints (0, 2, 4, 6, 24, 28, 32, 48, and 52 h), 100 µL aliquots were withdrawn and diluted for CFU determination on NYC III agar plates, as previously described [31].
L. iners CFS was obtained at the end of exponential and decline growth phases. Briefly, after 24 h or 48 h of incubation, bacterial suspensions were centrifuged at 4000× g for 10 min, and the supernatant was aseptically decanted and sterilized using a syringe filter with a pore size of 0.45 µm. The pH of the supernatant was measured before storing it at −20 °C. Aliquots of L. iners CFS were immediately thawed before each experiment.
2.5. Biofilm Formation and Biomass Quantification
Biofilm formation was investigated as previously described by Gulati et al. [32]. Single colonies of C. albicans strains cultured on YEPD plates were inoculated into 5 mL of YEPD broth, followed by incubation at 30 °C for overnight, and cell densities were then assessed by measuring absorbance at OD600. The correlation between OD600 and CFU/mL was obtained by constructing a standard curve. Yeast cultures were then diluted with RPMI 1640 (with L-glutamine and 34.5 g/L MOPS, w/o sodium bicarbonate; pH 7) at 1 × 107 cells/mL, and 200 µL was transferred to each well of a 96-well microtiter plate (Corning Inc., New York, NY, USA). The plates were then incubated at 37 °C for 1.5 h under aerobic conditions. Subsequently, the medium was discarded, and the wells were washed twice with 200 µL PBS to remove nonadherent cells. The culture medium was replaced with 200 µL of fresh medium, and the plates were incubated again for 24, 48, and 72 h under the same conditions.
Biofilm biomass was quantified using the crystal violet (CV) staining method, as previously described [31,32]. After incubation, the broth was discarded, and the wells were air dried for 45 min. After washing twice with 200 µL PBS, biofilms were stained with 110 µL of 0.4% CV for 45 min, and then washed four times with 200 µL distilled water. The CV bound to the biofilm was solubilized by adding 200 µL of 95% ethanol, followed by incubation for 45 min. Absorbance was measured at OD590 using a 96-well microplate reader (Tecan, Männedorf, Switzerland). Biofilm formation capability was determined as reported by Stepanovic et al. [30]. Clinical isolates were categorized into weak, moderate, or strong biofilm producers based on the calculated OD values for each strain and negative controls.
2.6. Biofilm Metabolic Activity Assessment
Biofilm metabolic activity was assessed by performing the 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) reduction assay, as previously described [32]. Fresh XTT (Invitrogen, Waltham, MA, USA) was prepared in PBS at a final concentration of 0.5 mg/mL and stored at −80 °C. A phenazine methosulphate (PMS) solution was prepared at 0.32 mg/mL in sterile water. After biofilm incubation and cleaning, 200 µL of XTT–PMS solution (9:1 ratio of XTT and PMS) was added to each well, followed by incubation in the dark for 2 h at 37 °C under aerobic conditions. Absorbance was then measured at 492 nm using a 96-well microplate reader (Tecan, Männedorf, Switzerland).
2.7. Effect of L. iners CFS on C. albicans Biofilm Formation
The effect of L. iners CFS on C. albicans biofilm development was assessed according to the protocol described above. RPMI 1640 was mixed with L. iners CFS in a 1:1 ratio and added to Candida cultures both during the early adhesion and late maturation stages of biofilm development. In case of the control groups, biofilms were grown in RPMI 1640 mixed with fresh BHI broth. After 24 h of incubation, biofilm biomass and metabolic activity were determined, as previously described, and aliquots of CFS were used for pH determination.
2.8. Effect of L. iners CFS on C. albicans Biofilm Morphology
C. albicans clinical isolates and reference strain were incubated with L. iners CFS for 24 h, and after CV staining, they were microscopically analyzed with an inverted light microscope (Eurotek by Orma, Milan, Italy). Images were captured using a digital camera (200× and 400× magnifications).
2.9. Quantitative Analysis of Genes Associated with C. albicans Hyphal Formation
Total RNA was extracted from C. albicans ATCC 10231 and four clinical isolates, and the expression of hyphal-specific genes was determined. Briefly, total RNA was extracted from C. albicans biofilm cultures using TRIzol (Invitrogen, Waltham, MA, USA) and retrotranscribed into cDNA using the PrimeScript™ RT Reagent Kit with gDNA Eraser (Perfect Real Time) (Takara Bio Inc., Kusatsu, Japan). Quantitative real-time PCR (qRT-PCR) was performed using the QuantStudio 1 Real-Time PCR System (Applied Biosystems, Waltham, MA, USA). The analysis of ACT1, ECE1 (extent of cell elongation 1), and HWP1 (hyphal wall protein 1) expression levels was performed using SYBR Green Master Mix (Thermo Fisher Scientific, Waltham, MA, USA). Primer sequences were the same as those previously reported [29]. Real-time PCR was performed using the following cycling conditions: 3 min at 95 °C and 40 cycles of 10 s at 95 °C, 30 s of annealing at specific primer temperatures, and 45 s at 72 °C. Ct values of target genes were normalized on the ACT1 housekeeping gene to obtain ΔCt, while ΔΔCt was obtained as the difference between the average ΔCT of the treated sample and the untreated sample. Relative changes in gene expression from qRT-PCR experiments were analyzed using the 2−ΔΔCt method.
2.10. Statistical Analysis
All analyses were performed with Prism GraphPad 7. Values represent mean ± SEM of at least three independent experiments performed in triplicate. Data with normal distribution were analyzed using paired Student’s t-test or one-way analysis of variance and Dunnett’s multiple comparison test. For nonparametric variables, Wilcoxon matched-pairs signed-rank test or Kruskal–Wallis test and Dunn’s multiple comparison test were performed. p < 0.05 indicated statistical significance.
3. Results
3.1. Growth Curve of L. iners
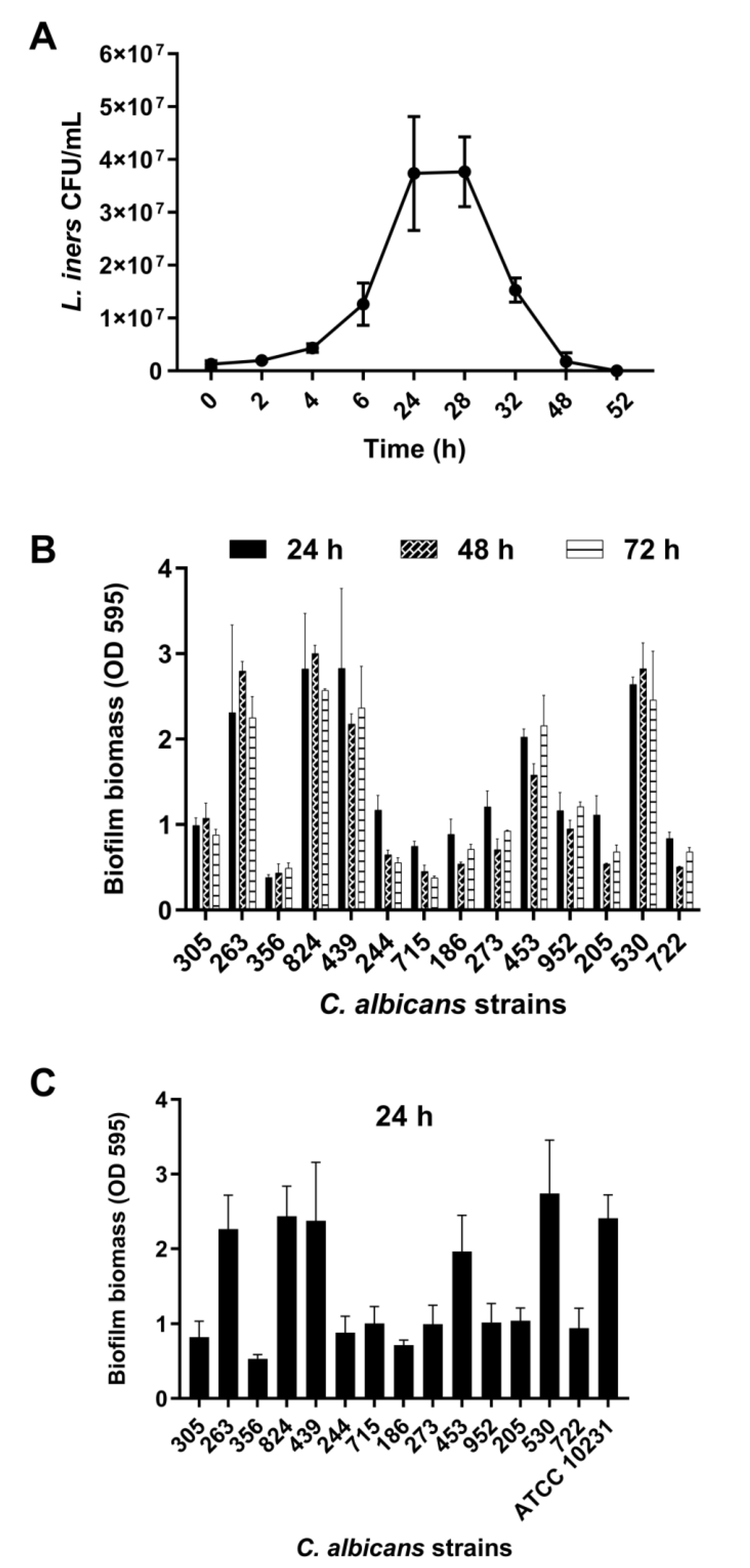
L. iners is an unusual member of the human vaginal microbiota and does not show growth on de Man–Rogosa–Sharpe medium, which is normally used to isolate vaginal lactobacilli. To obtain CFS, L. iners was grown in BHI broth. As evident from Figure 1A, L. iners showed the ability to adapt and grow in BHI media after approximately 3 h of incubation.
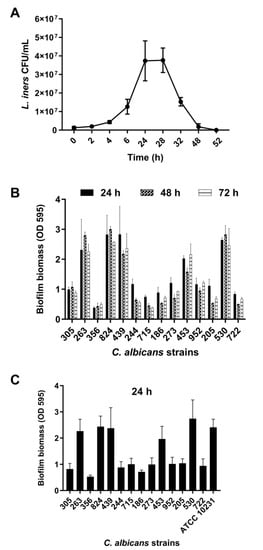
Figure 1.
(A) Growth curve of Lactobacillus iners. L. iners ATCC 55195 was incubated for 52 h in brain heart infusion (BHI) broth, and bacterial growth was determined at selected time points. Values represent mean ± SEM of colony-forming units (CFU)/mL from three independent experiments performed in duplicate. (B) Biofilm formation by Candida albicans clinical isolates in vitro. The isolates were grown for 24, 48, and 72 h. Biofilm biomass was evaluated using the crystal violet staining method, and values represent mean ± SEM of absorbance at OD595. Data are from at least three independent experiments with n = 6. (C) Biofilm formation by C. albicans clinical isolates in vitro in RPMI 1640 + BHI broth. Biofilms were grown for 24 h using 1:1 RPMI 1640 and BHI broth. Values represent mean ± SEM of absorbance at OD595 from at least three independent experiments with n = 6.
The growth curve exhibits the exponential growth phase from 6 h to 24 h of incubation, followed by the stationary growth phase, lasting for 4 h, and ultimately the decline growth phase, beginning 28 h after incubation. CFS was recovered at the end of both the exponential (24 h CFS) and decline (48 h CFS) phases of growth. The average pH value was 6.0 (5.5–6.4) for both 24 and 48 h CFS.
3.2. Biofilm Formation by Vaginal Isolates of C. albicans
Fourteen clinical isolates of C. albicans obtained from women with VVC were analyzed for their ability to form biofilms under our experimental conditions. These isolates (1 × 107 CFU/mL) were incubated in flat-bottomed 96-well microtiter plates in RPMI 1640 at 37 °C for 24, 48, and 72 h, followed by biofilm biomass quantification. We found that all strains formed a mature biofilm within 24 h of incubation, with a clear heterogeneity between them (Figure 1B). Based on biofilm biomass quantification, the isolates were categorized into strong, moderate, or weak biofilm producers [33]. Eight isolates (305, 244, 715, 186, 273, 952, 205, 722) were moderate biofilm producers (0.60 < OD ≤ 1.21), five isolates (263, 824, 439, 453, 530) were strong biofilm producers (OD > 1.21), and one isolate (356) was a weak biofilm producer (OD ≤ 0.60). Biofilm formation was also determined in 50% RPMI 1640–50% BHI media after 24 h of incubation at 37 °C (Figure 1C). We observed that the presence of BHI did not influence the ability of C. albicans to form biofilms. Furthermore, in these experimental conditions, C. albicans ATCC 10231 showed the ability to produce a strong biofilm.
3.3. Effects of L. iners CFS on C. albicans Biofilm Formation
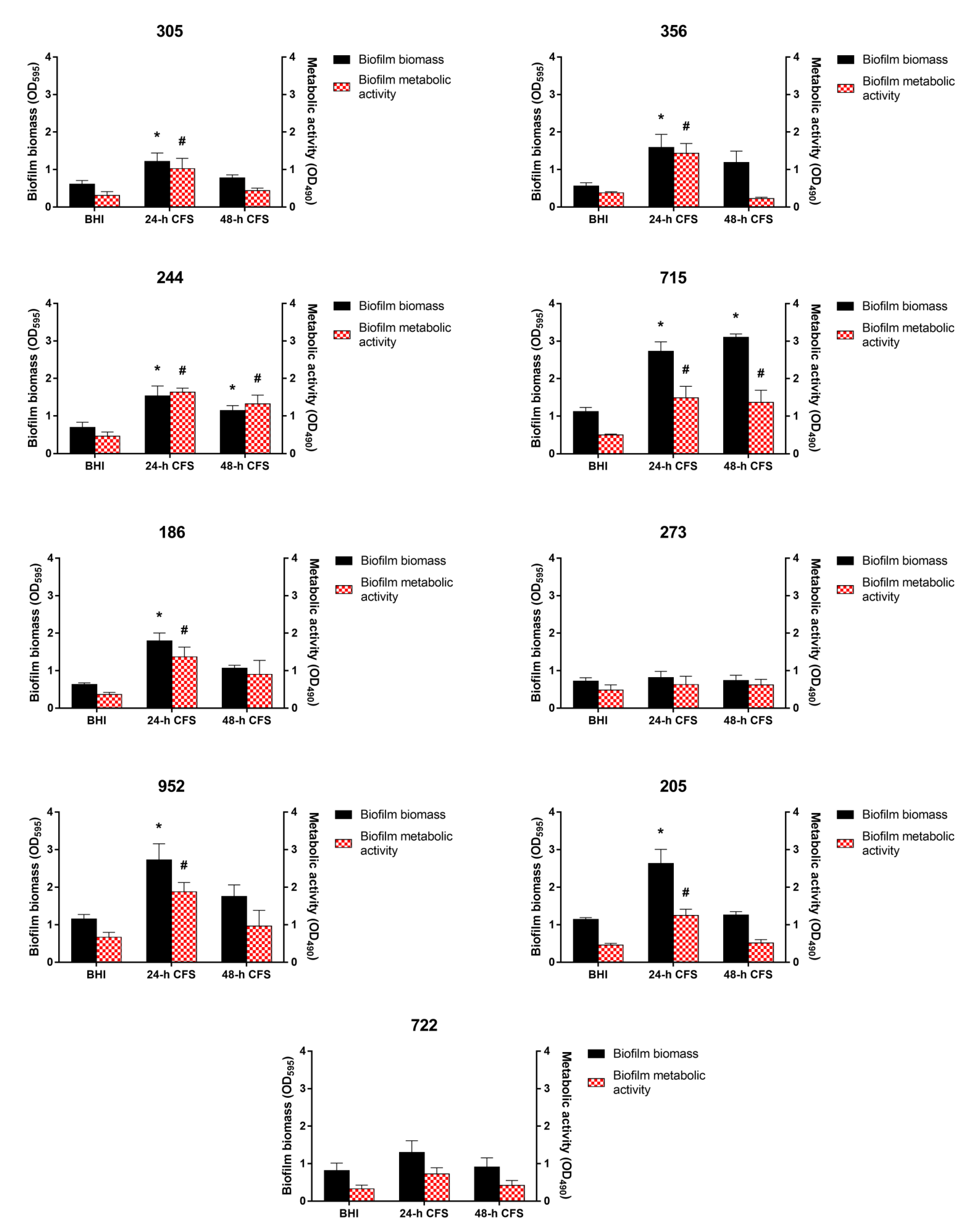
To evaluate the effects of L. iners CFS on C. albicans biofilm formation, 100 µL of 24 or 48 h CFS in BHI was mixed with 100 µL of each C. albicans isolate and C. albicans ATCC 10231 reference strain (1 × 107 CFU/mL) in RPMI 1640, followed by incubation for 24 h, as described earlier. Biofilm quantification was performed by biofilm biomass and metabolic activity determination. As is evident from Figure 2, seven of the nine moderate or weak biofilm producers displayed a significant increase in biofilm biomass and metabolic activity in the presence of L. iners 24 h CFS, whereas only two isolates (244 and 715) showed an increase in these parameters in the presence of L. iners 48 h CFS.
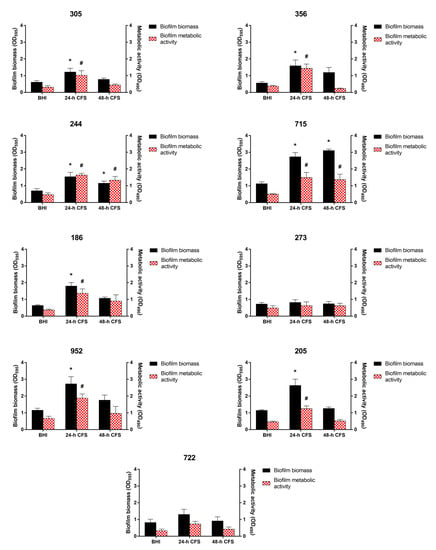
Figure 2.
Effect of Lactobacillus iners cell-free supernatant (CFS) on in vitro biofilm formation by Candida albicans clinical isolates characterized as moderate (305, 244, 715, 186, 273, 952, 205, 722) and weak (356) biofilm producers. Biofilms of C. albicans clinical isolates were grown for 24 h using RPMI 1640 + BHI broth or 24/48 h L. iners CFS in a 1:1 ratio. Biofilm biomass was evaluated using the crystal violet staining method (OD595, black bars, left y-axis), and metabolic activity was determined using the XTT reduction assay (OD490, red bars, right y-axis). Values represent mean ± SEM of at least three independent experiments performed in triplicate. Statistically significant differences were tested using one-way analysis of variance or Kruskal–Wallis test. * p < 0.05 biomass of biofilm grown with 24/48 h L. iners CFS vs. control biofilm biomass (BHI). # p < 0.05 metabolic activity of biofilm grown with 24/48 h L. iners CFS vs. control biofilm metabolic activity (BHI).
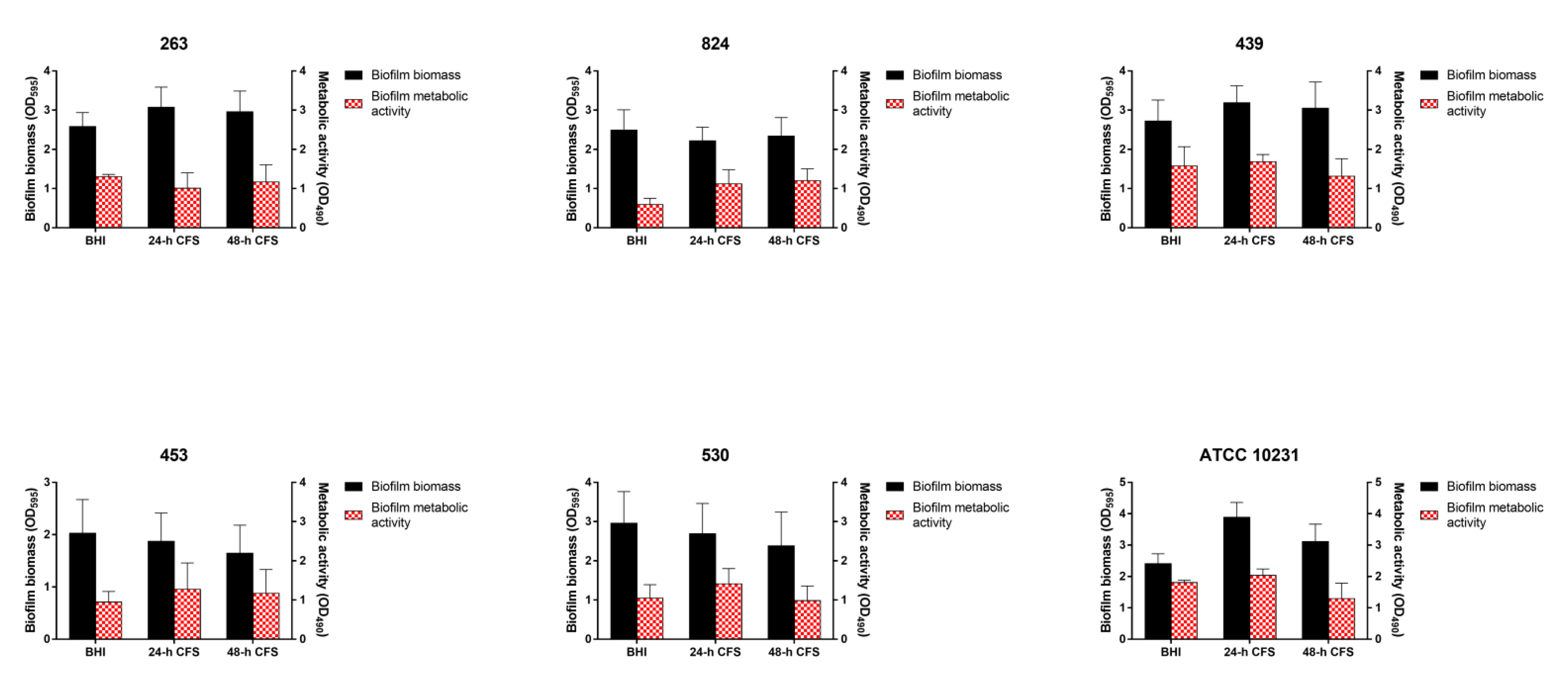
In contrast, L. iners 24 or 48 h CFS had no effects on the ability of strong biofilm producers to form biofilms (Figure 3).
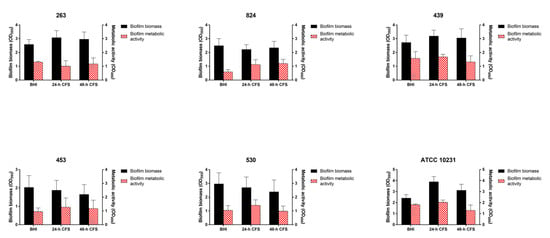
Figure 3.
Effect of Lactobacillus iners cell-free supernatant (CFS) on in vitro biofilm formation by Candida albicans clinical isolates characterized as strong biofilm producers and C. albicans ATCC 10231 reference strain. Biofilms of C. albicans clinical isolates and reference strain were grown for 24 h using RPMI 1640 + BHI broth or 24/48 h L. iners CFS in a 1:1 ratio. Biofilm biomass was evaluated using the crystal violet staining method (OD595, black bars, left y-axis), and metabolic activity was determined using the XTT reduction assay (OD490, red bars, right y-axis). Values represent mean ± SEM of at least three independent experiments performed in triplicate. Statistically significant differences were tested using one-way analysis of variance or Kruskal–Wallis test.
We also determined the pH of C. albicans biofilms developed in the absence or presence of L. iners 24 h CFS. C. albicans biofilm cultures showed a pH value of 6.4, indicating that pH was not significantly modulated and maintained near neutral.
3.4. Effects of L. iners CFS on C. albicans Biofilm Morphology
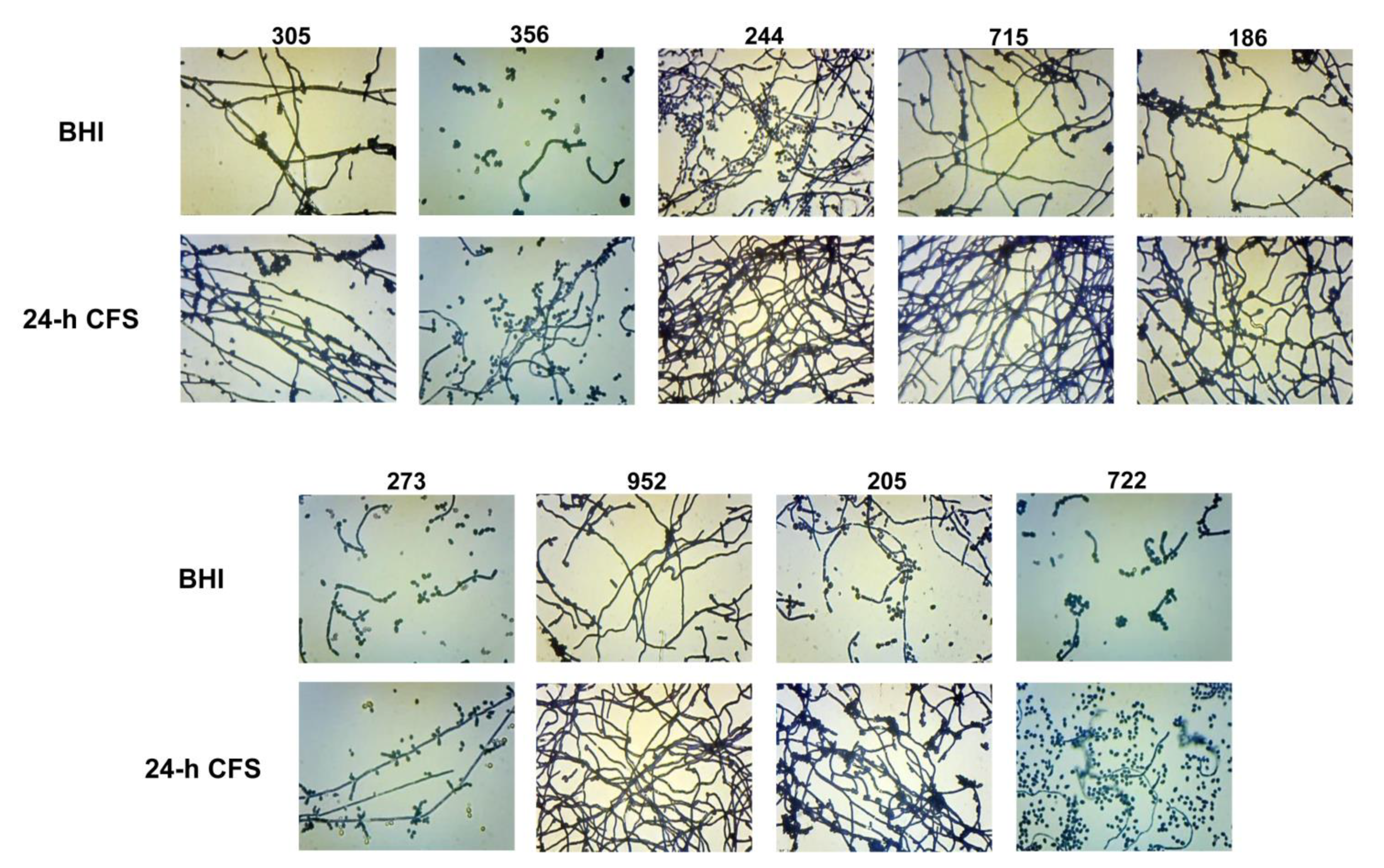
The yeast-to-hyphal transition is a key feature of biofilm formation by C. albicans. Herein we investigated the effects of L. iners 24 h CFS on C. albicans biofilm morphology. As shown in Figure 4, in comparison to respective controls, L. iners culture supernatants induced an increase in the growth of C. albicans filamentous forms by all moderate (305, 244, 715, 186, 273, 952, 205, 722) and weak (356) biofilm producers. In contrast, L. iners CFS did not affect hyphal growth in strong biofilm producers (clinical isolates or reference strain) (Supplementary Figure S1).
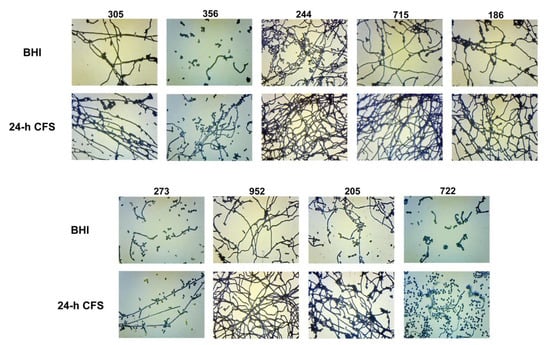
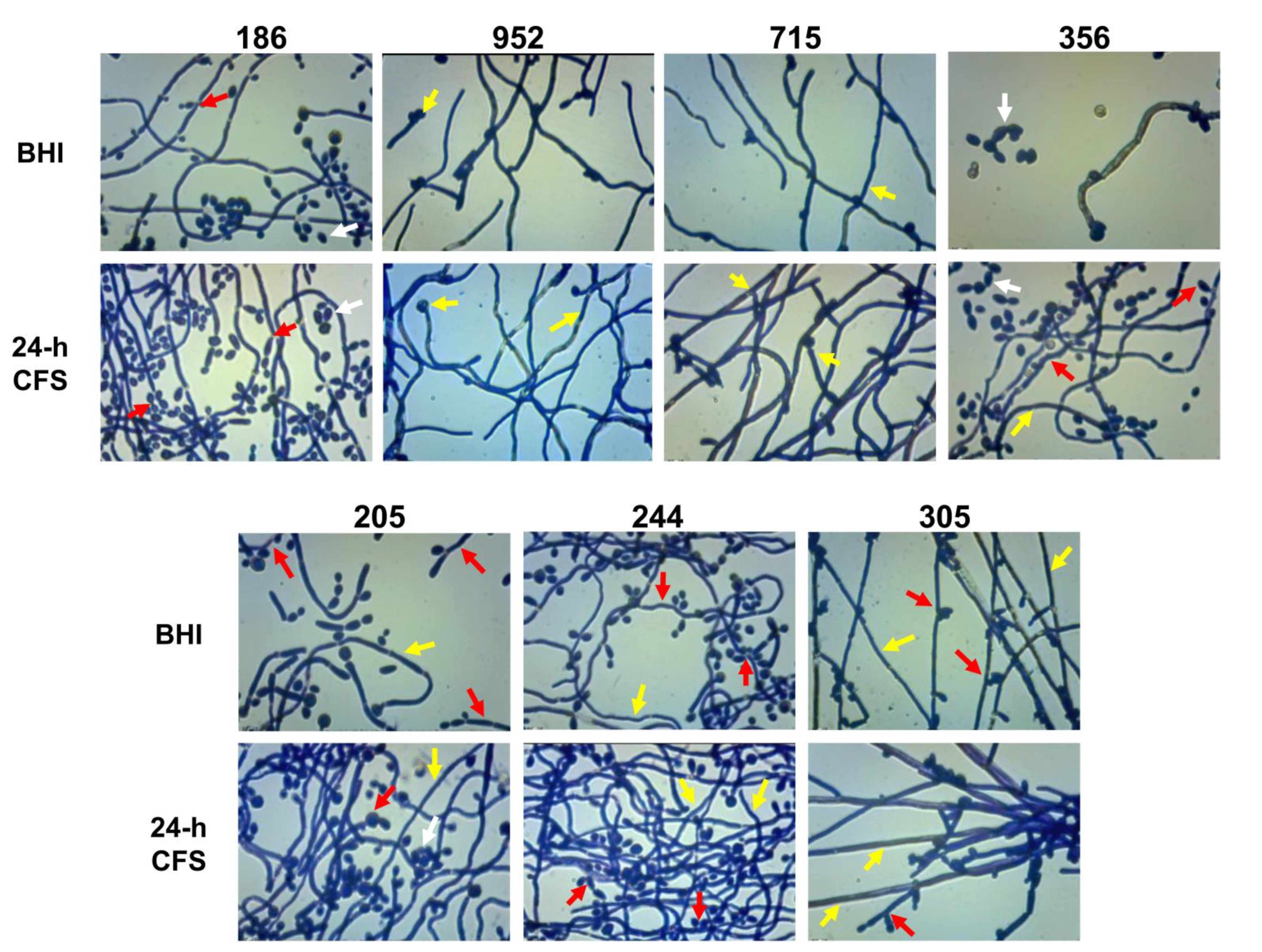
Figure 4.
Effect of Lactobacillus iners cell-free supernatant (CFS) on biofilm morphology of Candida albicans clinical isolates characterized as moderate (305, 244, 715, 186, 273, 952, 205, 722) and weak (356) biofilm producers. Biofilms of C. albicans clinical isolates were grown for 24 h using RPMI 1640 + BHI broth or 24 h L. iners CFS in a 1:1 ratio and stained with crystal violet. Images of biofilms were acquired with an inverted light microscope at 200×. Representative microscopic images from three different experiments are shown.
The biofilm morphology of all the moderate/weak isolates (n = 7) which showed an increase in both biofilm formation as well as filamentous growth was further microscopically analyzed at magnification 400×. Relative to respective controls (BHI), in the presence of L. iners 24 h CFS, we observed that apart from the clinical isolate 186 that formed a biofilm predominantly composed of clusters of yeast cells and pseudohyphae, and the strains 952 and 715, which formed true hyphae, all the remaining strains tested produced biofilms that were composed of both the filamentous forms (Figure 5). In addition, for all moderate/weak biofilm producers, we investigated the effects of pH on pseudohyphal/hyphal growth induced by L. iners 24 h CFS. We observed C. albicans biofilm formation at pH 4.9. All tested strains failed to produce hyphae, and thus, resultant biofilms were composed of only clusters of yeast cells (data not shown).
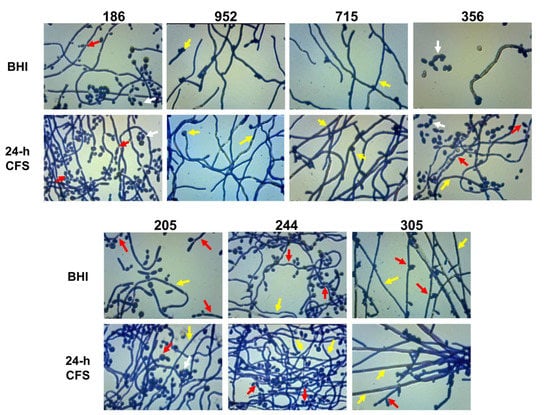
Figure 5.
Lactobacillus iners cell-free supernatant (CFS) promotes filamentous growth by Candida albicans. The effect of L. iners CFS on hyphal/pseudohyphal growth of C. albicans characterized as moderate/weak biofilm producers was microscopically determined after crystal violet staining. Optical microscopy images (magnification 400×) of C. albicans isolates showed that the hyphae/pseudohyphae mass was significantly higher in the presence of L. iners CFS than in controls cultured in BHI. Red arrows show branched C. albicans pseudohyphae, which appear ellipsoidal in shape, pointing to constrictions at septal sites, bearing single or multiple lateral blastoconidia. Yellow arrows show well-structured biofilms mainly composed of hyphae characterized as unconstricted filaments with parallel-sided walls and true septa. Clusters of unicellular oval or spherical yeasts (white arrows), some of them replicating as budding daughter cells, are also shown.
3.5. L. iners Culture Supernatants Upregulated the Expression of C. albicans Hyphal-Specific Genes
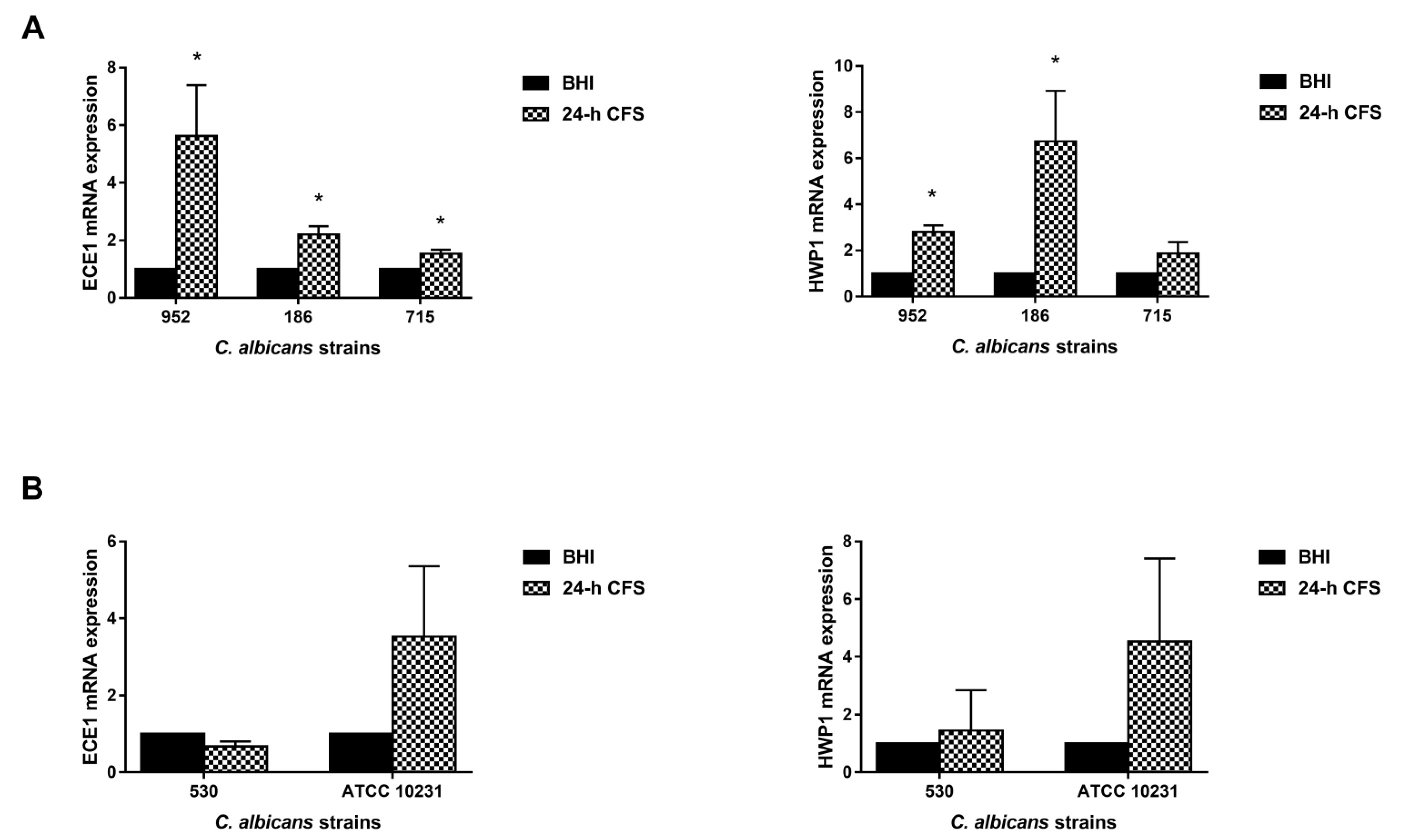
Our previous studies have shown that two key hyphae-associated genes, ECE1 and HWP1 [34,35,36], were overexpressed during human vaginal candidiasis, and that their upregulation was associated with NLRP3 inflammasome activation, a crucial player in the immunopathogenesis of VVC [29,37]. Based on these results, here we analyzed their expression level to gain insights into the mechanism by which L. iners 24 h CFS can modulate the pseudohyphal/hyphal growth in C. albicans.
Three moderate biofilm producers were chosen: the isolate 186 forming only pseudohyphae and the 952 and 715 forming only true hyphae. Transcriptional levels were quantified by qRT-PCR. The expression level of each gene was normalized with that of a housekeeping gene (ACT1) for 24 h CFS-treated as well as -untreated C. albicans biofilms; data are presented as relative expression fold change. In comparison with the control, L. iners 24 h CFS significantly upregulated the expression levels of HWP1 and ECE1 in all three and two of the three moderate biofilm producers, respectively (Figure 6).
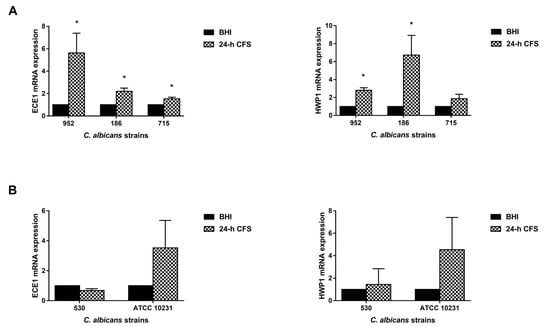
Figure 6.
Effect of Lactobacillus iners 24 h cell-free supernatant (CFS) on the expression of hyphal-specific genes of Candida albicans clinical isolates and C. albicans ATCC 10231 reference strain. Total RNA was extracted from biofilms grown with or without 24 h L. iners CFS, retrotranscribed into cDNA, and real-time PCR was performed to assess expression of ACT1, ECE1, and HWP1. (A) Relative gene expression levels of ECE1 and HWP1 of three C. albicans clinical isolates characterized as moderate biofilm producers and (B) one characterized as strong biofilm producer with the ATCC 10231 reference strain. Data were analyzed using the 2−ΔΔCt method, and values represent mean ± SEM of three (panel A) and two (panel B) independent experiments performed in triplicate. Statistically significant differences were tested with paired Student’s t-test or Wilcoxon matched-pairs signed-rank test. * p < 0.05 relative gene expression of 24 h L. iners CFS-treated biofilms vs. control biofilms.
Transcriptional levels were also analyzed in two strong biofilm producers (one clinical isolate, 530, and the reference strain ATCC 10231), but L. iners CFS did not significantly modulate the expression levels of HWP1 or ECE1 in them.
4. Discussion
C. albicans is a common commensal fungus of the vaginal mucosa and also a pathogen that is responsible for almost 90% of all VVC cases [38]. It was recently reported that VVC-positive women present a depletion in the populations of health-associated Lactobacillus spp., such as L. crispatus. A decrease in the population of L. crispatus is associated with an increase in that of L. iners [13,20], an unusual Lactobacillus species that does not seem to have a protective effect against VVC [23,28]. Deciphering the interaction between C. albicans and L. iners is crucial for increasing our knowledge of C. albicans pathogenicity and for developing new diagnostic tools and effective therapeutic approaches.
The ability of C. albicans to produce biofilms is considered to be one of the most important determinants of this common gynecological disease [38] and a critical factor responsible for conferring resistance to antimycotic drugs, such as fluconazole and amphotericin B [11,39,40,41], and consequently for RVVC development. To the best of our knowledge, we investigated, for the first time, the in vitro effects of L. iners CFS on the biofilm formation ability of C. albicans vaginal isolates. We found that L. iners CFS enhanced the biofilm-formation ability of several C. albicans isolates. According to previous studies [13,14,41], vaginal isolates display an ability to form mature, heterogeneous biofilms. We observed that L. iners CFS induced a significant increase in both biofilm biomass and metabolic activity of 77.7% of C. albicans clinical isolates that were moderate or weak biofilm producers, transforming them from moderate or weak biofilm producers to strong biofilm producers and consequently increasing their virulence. Indeed, clinical isolates capable of forming robust, well-structured biofilms are more pathogenic than weak biofilm producers [41,42]. These data suggest that L. iners-dominated vaginal microbiome can not only contribute to the onset of VVC, but also affect the management of such infections, increasing the probability of recurrence. McKloud et al. [13] suggested that women experiencing RVVC for >6 months show an imbalance of the vaginal microbiota, characterized by an abundance of L. iners and a reduction in health-associated Lactobacillus spp. Besides, McKloud et al. [13] demonstrated that L. iners was unable to modulate biofilm formation by C. albicans. One reason for this apparent discrepancy with our data could be differences in experimental conditions. We studied the effects of L. iners CFS on the biofilm formation ability of C. albicans clinical isolates, whereas McKloud et al. used reference strains of L. iners and C. albicans (DSMZ 13,335 and 5314, respectively). Furthermore, McKloud et al. used C. albicans and L. iners cocultures, instead of L. iners CFS.
The ability to produce filamentous forms is critical for the development and maintenance of C. albicans biofilms [8,10,43,44]. We found that L. iners CFS enhanced the growth of the filamentous forms only in those strains that became strong biofilm producers from moderate or weak biofilm producers. Our findings are consistent with those of previous studies, indicating a direct relationship between the ability of C. albicans to form biofilms and pseudohyphal/hyphal growth [45,46,47]. Therefore, it is possible that L. iners-dominated vaginal microbiome favors the formation of robust, stable C. albicans biofilms on the vaginal mucosa as well as on implanted devices, such as intrauterine devices [48], which is one of the most used methods to prevent fertilization [49]. It is well-established that pH plays a key role in the yeast-to-hyphal morphogenetic transition [35,50]; neutral pH provides a permissive environment for hyphal formation [51], while low pH prevents hyphal formation, thus promoting the growth of fungus as yeast cells [52]. Our data showed that the pH of C. albicans biofilms was near neutral, and that the presence of L. iners CFS, differently to other lactobacilli [53,54,55], did not reduce the pH value. This could be because L. iners produces less lactic acid than L. crispatus, and lactic acid is the main acidifier in the vaginal environment [56,57]. Considering that C. albicans is capable of alkalinizing the external environment [51], it is possible that in vaginal econiches where C. albicans coexists with L. iners, the pH is less acidic or even near neutral.
To elucidate the potential mechanism underlying the ability of L. iners to induce the growth of filamentous forms of C. albicans, we also assessed the expression levels of HWP1 and ECE1, which encode proteins essential for hyphal formation and play a key role in, at least, two phases of VVC pathogenesis: adhesion and tissue damage. In particular, HWP1 is crucial for C. albicans adhesion; it promotes its binding to epithelial cells, enabling colonization [58,59] and biofilm formation [12,60,61,62]. ECE1 is highly expressed by hyphae during the invasion of epithelial cells. It encodes candidalysin, a toxin recently identified by Moyes et al. [7], which directly damages host epithelial membranes, triggering a danger-response signaling pathway with consequent activation of the epithelial immune response [63]. Our results showed that L. iners CFS upregulated the expression of both HWP1 and ECE1. These results are in line with those of our previous studies, which reported an overexpression of these genes in vaginal swabs of symptomatic women with VVC [29,37]. The limitations of this study are the small number of C. albicans clinical isolates tested and the use of L. iners CFS instead of live bacteria, which could be more representative of all the interactions occurring in vivo. A further limitation is that of not having tested supernatants from clinical isolates of L. iners. Their use would be helpful in confirming our data.
Overall, our findings demonstrate that L. iners promotes C. albicans virulence by enhancing pseudohyphal/hyphal growth and biofilm formation by moderate and weak biofilm producers, implying that the presence of L. iners in the vaginal environment, unlike other Lactobacillus spp., might not be a good indicator of vaginal health. Further in vitro and in vivo studies are warranted on this intriguing topic.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microorganisms9122577/s1. Figure S1. Effect of Lactobacillus iners cell-free supernatant (CFS) on biofilm morphology of Candida albicans clinical isolates characterized as strong biofilm producers.
Author Contributions
Conceptualization, C.M. and S.S.; methodology, S.S., S.V., M.G., E.L.; validation, S.S. and C.M.; formal analysis, S.S., S.V., M.G., E.N., S.R., R.G. and S.P.; investigation, S.S. and S.V.; resources, C.M.; writing—original draft preparation, S.S., C.M. and R.G.; writing—review and editing, R.G. and C.M.; supervision, C.M.; funding acquisition, C.M. All authors approved the submitted version and agree to be personally accountable for the author’s own contributions and for ensuring that questions related to the accuracy or integrity of any part of the work, even the ones in which the author was not personally involved, are appropriately investigated, resolved, and documented in the literature. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by University of Perugia (Departmental funds for basic research-2019-Monari).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by local ethical committee (Comitato Etico delle Aziende Sanitarie, Umbria, Italy) protocol code CA 2020, 3802/19 (16 September 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sobel, J.D. Vulvovaginal candidosis. Lancet 2007, 369, 1961–1971. [Google Scholar] [CrossRef]
- Aballea, S.; Guelfucci, F.; Wagner, J.; Khemiri, A.; Dietz, J.P.; Sobel, J.; Toumi, M. Subjective health status and health-related quality of life among women with Recurrent Vulvovaginal Candidosis (RVVC) in Europe and the USA. Health Qual. Life Outcomes 2013, 11, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrstrom, S.M.; Kornfeld, D.; Thuresson, J.; Rylander, E. Signs of chronic stress in women with recurrent candida vulvovaginitis. Am. J. Obstet. Gynecol. 2005, 193, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.A.; Sobel, J.D. Vulvovaginal Candidiasis Caused by Non-albicans Candida Species: New Insights. Curr. Infect. Dis. Rep. 2010, 12, 465–470. [Google Scholar] [CrossRef]
- Goncalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 2016, 42, 905–927. [Google Scholar] [CrossRef] [Green Version]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Moyes, D.L.; Wilson, D.; Richardson, J.P.; Mogavero, S.; Tang, S.X.; Wernecke, J.; Hofs, S.; Gratacap, R.L.; Robbins, J.; Runglall, M.; et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 2016, 532, 64–68. [Google Scholar] [CrossRef] [Green Version]
- Ponde, N.O.; Lortal, L.; Ramage, G.; Naglik, J.R.; Richardson, J.P. Candida albicans biofilms and polymicrobial interactions. Crit. Rev. Microbiol. 2021, 47, 91–111. [Google Scholar] [CrossRef]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef] [Green Version]
- Harriott, M.M.; Noverr, M.C. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: Effects on antimicrobial resistance. Antimicrob. Agents Chemother. 2009, 53, 3914–3922. [Google Scholar] [CrossRef] [Green Version]
- Harriott, M.M.; Lilly, E.A.; Rodriguez, T.E.; Fidel, P.L.; Noverr, M.C. Candida albicans forms biofilms on the vaginal mucosa. Microbiology 2010, 156, 3635–3644. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Cerdeira, C.; Martinez-Herrera, E.; Carnero-Gregorio, M.; Lopez-Barcenas, A.; Fabbrocini, G.; Fida, M.; El-Samahy, M.; Gonzalez-Cespon, J.L. Pathogenesis and Clinical Relevance of Candida Biofilms in Vulvovaginal Candidiasis. Front. Microbiol. 2020, 11, 544480. [Google Scholar] [CrossRef]
- McKloud, E.; Delaney, C.; Sherry, L.; Kean, R.; Williams, S.; Metcalfe, R.; Thomas, R.; Richardson, R.; Gerasimidis, K.; Nile, C.J.; et al. Recurrent Vulvovaginal Candidiasis: A Dynamic Interkingdom Biofilm Disease of Candida and Lactobacillus. mSystems 2021, 6, e0062221. [Google Scholar] [CrossRef]
- Sherry, L.; Kean, R.; McKloud, E.; O’Donnell, L.E.; Metcalfe, R.; Jones, B.L.; Ramage, G. Biofilms Formed by Isolates from Recurrent Vulvovaginal Candidiasis Patients Are Heterogeneous and Insensitive to Fluconazole. Antimicrob. Agents Chemother. 2017, 61, e01065-17. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Cerdeira, C.; Gregorio, M.C.; Molares-Vila, A.; Lopez-Barcenas, A.; Fabbrocini, G.; Bardhi, B.; Sinani, A.; Sanchez-Blanco, E.; Arenas-Guzman, R.; Hernandez-Castro, R. Biofilms and vulvovaginal candidiasis. Colloids Surf. B Biointerfaces 2019, 174, 110–125. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, S.; Li, H.; Shen, L.; Dong, C.; Sun, Y.; Chen, H.; Xu, B.; Zhuang, W.; Deighton, M.; et al. Biofilm Formation of Candida albicans Facilitates Fungal Infiltration and Persister Cell Formation in Vaginal Candidiasis. Front. Microbiol. 2020, 11, 1117. [Google Scholar] [CrossRef]
- Silva, S.; Rodrigues, C.F.; Araujo, D.; Rodrigues, M.E.; Henriques, M. Candida Species Biofilms’ Antifungal Resistance. J. Fungi 2017, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Petrova, M.I.; Lievens, E.; Malik, S.; Imholz, N.; Lebeer, S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front. Physiol. 2015, 6, 81. [Google Scholar] [CrossRef] [Green Version]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4680–4687. [Google Scholar] [CrossRef] [Green Version]
- Ceccarani, C.; Foschi, C.; Parolin, C.; D’Antuono, A.; Gaspari, V.; Consolandi, C.; Laghi, L.; Camboni, T.; Vitali, B.; Severgnini, M.; et al. Diversity of vaginal microbiome and metabolome during genital infections. Sci. Rep. 2019, 9, 14095. [Google Scholar] [CrossRef] [Green Version]
- Kalia, N.; Singh, J.; Kaur, M. Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: A critical review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 5. [Google Scholar] [CrossRef]
- Tortelli, B.A.; Lewis, W.G.; Allsworth, J.E.; Member-Meneh, N.; Foster, L.R.; Reno, H.E.; Peipert, J.F.; Fay, J.C.; Lewis, A.L. Associations between the vaginal microbiome and Candida colonization in women of reproductive age. Am. J. Obstet. Gynecol. 2020, 222, 471.e1–471.e9. [Google Scholar] [CrossRef]
- Vaneechoutte, M. Lactobacillus iners, the unusual suspect. Res. Microbiol. 2017, 168, 826–836. [Google Scholar] [CrossRef]
- Rampersaud, R.; Planet, P.J.; Randis, T.M.; Kulkarni, R.; Aguilar, J.L.; Lehrer, R.I.; Ratner, A.J. Inerolysin, a cholesterol-dependent cytolysin produced by Lactobacillus iners. J. Bacteriol. 2011, 193, 1034–1041. [Google Scholar] [CrossRef] [Green Version]
- Bayar, E.; Bennett, P.R.; Chan, D.; Sykes, L.; MacIntyre, D.A. The pregnancy microbiome and preterm birth. Semin. Immunopathol. 2020, 42, 487–499. [Google Scholar] [CrossRef]
- Zheng, N.; Guo, R.; Yao, Y.; Jin, M.; Cheng, Y.; Ling, Z. Lactobacillus iners Is Associated with Vaginal Dysbiosis in Healthy Pregnant Women: A Preliminary Study. Biomed. Res. Int. 2019, 2019, 6079734. [Google Scholar] [CrossRef]
- Campisciano, G.; Iebba, V.; Zito, G.; Luppi, S.; Martinelli, M.; Fischer, L.; De Seta, F.; Basile, G.; Ricci, G.; Comar, M. Lactobacillus iners and gasseri, Prevotella bivia and HPV Belong to the Microbiological Signature Negatively Affecting Human Reproduction. Microorganisms 2020, 9, 39. [Google Scholar] [CrossRef]
- Petrova, M.I.; Reid, G.; Vaneechoutte, M.; Lebeer, S. Lactobacillus iners: Friend or Foe? Trends Microbiol. 2017, 25, 182–191. [Google Scholar] [CrossRef]
- Roselletti, E.; Monari, C.; Sabbatini, S.; Perito, S.; Vecchiarelli, A.; Sobel, J.D.; Cassone, A. A Role for Yeast/Pseudohyphal Cells of Candida albicans in the Correlated Expression of NLRP3 Inflammasome Inducers in Women with Acute Vulvovaginal Candidiasis. Front. Microbiol. 2019, 10, 2669. [Google Scholar] [CrossRef]
- Roselletti, E.; Perito, S.; Sabbatini, S.; Monari, C.; Vecchiarelli, A. Vaginal Epithelial Cells Discriminate Between Yeast and Hyphae of Candida albicans in Women Who Are Colonized or Have Vaginal Candidiasis. J. Infect. Dis. 2019, 220, 1645–1654. [Google Scholar] [CrossRef]
- Sabbatini, S.; Monari, C.; Ballet, N.; Decherf, A.C.; Bozza, S.; Camilloni, B.; Perito, S.; Vecchiarelli, A. Anti-Biofilm Properties of Saccharomyces cerevisiae CNCM I-3856 and Lacticaseibacillus rhamnosus ATCC 53103 Probiotics against G. vaginalis. Microorganisms 2020, 8, 1294. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Lohse, M.B.; Ennis, C.L.; Gonzalez, R.E.; Perry, A.M.; Bapat, P.; Arevalo, A.V.; Rodriguez, D.L.; Nobile, C.J. In Vitro Culturing and Screening of Candida albicans Biofilms. Curr. Protoc. Microbiol. 2018, 50, e60. [Google Scholar] [CrossRef] [PubMed]
- Stepanovic, S.; Vukovic, D.; Hola, V.; Di Bonaventura, G.; Djukic, S.; Cirkovic, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Tsuchimori, N.; Sharkey, L.L.; Fonzi, W.A.; French, S.W.; Edwards, J.E., Jr.; Filler, S.G. Reduced virulence of HWP1-deficient mutants of Candida albicans and their interactions with host cells. Infect. Immun. 2000, 68, 1997–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudbery, P.E. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 2011, 9, 737–748. [Google Scholar] [CrossRef]
- Finkel, J.S.; Mitchell, A.P. Genetic control of Candida albicans biofilm development. Nat. Rev. Microbiol. 2011, 9, 109–118. [Google Scholar] [CrossRef]
- Roselletti, E.; Perito, S.; Gabrielli, E.; Mencacci, A.; Pericolini, E.; Sabbatini, S.; Cassone, A.; Vecchiarelli, A. NLRP3 inflammasome is a key player in human vulvovaginal disease caused by Candida albicans. Sci. Rep. 2017, 7, 17877. [Google Scholar] [CrossRef] [Green Version]
- Gaziano, R.; Sabbatini, S.; Roselletti, E.; Perito, S.; Monari, C. Saccharomyces cerevisiae-Based Probiotics as Novel Antimicrobial Agents to Prevent and Treat Vaginal Infections. Front. Microbiol. 2020, 11, 718. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Wang, H.; Zhu, L. Quercetin Assists Fluconazole to Inhibit Biofilm Formations of Fluconazole-Resistant Candida Albicans in In Vitro and In Vivo Antifungal Managements of Vulvovaginal Candidiasis. Cell. Physiol. Biochem. 2016, 40, 727–742. [Google Scholar] [CrossRef]
- Muzny, C.A.; Schwebke, J.R. Biofilms: An Underappreciated Mechanism of Treatment Failure and Recurrence in Vaginal Infections. Clin. Infect. Dis. 2015, 61, 601–606. [Google Scholar] [CrossRef] [Green Version]
- Sherry, L.; Rajendran, R.; Lappin, D.F.; Borghi, E.; Perdoni, F.; Falleni, M.; Tosi, D.; Smith, K.; Williams, C.; Jones, B.; et al. Biofilms formed by Candida albicans bloodstream isolates display phenotypic and transcriptional heterogeneity that are associated with resistance and pathogenicity. BMC Microbiol. 2014, 14, 182. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, R.; May, A.; Sherry, L.; Kean, R.; Williams, C.; Jones, B.L.; Burgess, K.V.; Heringa, J.; Abeln, S.; Brandt, B.W.; et al. Integrating Candida albicans metabolism with biofilm heterogeneity by transcriptome mapping. Sci. Rep. 2016, 6, 35436. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Ribot, J.L. Candida albicans biofilms: More than filamentation. Curr. Biol. 2005, 15, R453–R455. [Google Scholar] [CrossRef] [Green Version]
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, M.; Uppuluri, P.; Zhao, X.R.; Carlisle, P.L.; Vipulanandan, G.; Villar, C.C.; Lopez-Ribot, J.L.; Kadosh, D. Expression of UME6, a key regulator of Candida albicans hyphal development, enhances biofilm formation via Hgc1- and Sun41-dependent mechanisms. Eukaryot Cell 2013, 12, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Ramage, G.; VandeWalle, K.; Lopez-Ribot, J.L.; Wickes, B.L. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol. Lett. 2002, 214, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, M.; Thompson, D.S.; Lazzell, A.; Carlisle, P.L.; Pierce, C.; Monteagudo, C.; Lopez-Ribot, J.L.; Kadosh, D. UME6, a novel filament-specific regulator of Candida albicans hyphal extension and virulence. Mol. Biol. Cell 2008, 19, 1354–1365. [Google Scholar] [CrossRef] [Green Version]
- Auler, M.E.; Morreira, D.; Rodrigues, F.F.; Abr Ao, M.S.; Margarido, P.F.; Matsumoto, F.E.; Silva, E.G.; Silva, B.C.; Schneider, R.P.; Paula, C.R. Biofilm formation on intrauterine devices in patients with recurrent vulvovaginal candidiasis. Med. Mycol. 2010, 48, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Pal, Z.; Urban, E.; Dosa, E.; Pal, A.; Nagy, E. Biofilm formation on intrauterine devices in relation to duration of use. J. Med. Microbiol. 2005, 54, 1199–1203. [Google Scholar] [CrossRef]
- Hall, R.A.; Cottier, F.; Muhlschlegel, F.A. Molecular networks in the fungal pathogen Candida albicans. Adv. Appl. Microbiol. 2009, 67, 191–212. [Google Scholar] [CrossRef]
- Vylkova, S.; Carman, A.J.; Danhof, H.A.; Collette, J.R.; Zhou, H.; Lorenz, M.C. The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. mBio 2011, 2, e00055-11. [Google Scholar] [CrossRef] [Green Version]
- Davis, D. Adaptation to environmental pH in Candida albicans and its relation to pathogenesis. Curr. Genet. 2003, 44, 1–7. [Google Scholar] [CrossRef]
- Jiang, Q.; Stamatova, I.; Kari, K.; Meurman, J.H. Inhibitory activity in vitro of probiotic lactobacilli against oral Candida under different fermentation conditions. Benef. Microbes 2015, 6, 361–368. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Yang, E.; Yan, L.; Li, T.; Zhuang, H. Antimicrobial Compounds Produced by Vaginal Lactobacillus crispatus Are Able to Strongly Inhibit Candida albicans Growth, Hyphal Formation and Regulate Virulence-related Gene Expressions. Front. Microbiol. 2017, 8, 564. [Google Scholar] [CrossRef] [Green Version]
- Zangl, I.; Pap, I.J.; Aspock, C.; Schuller, C. The role of Lactobacillus species in the control of Candida via biotrophic interactions. Microb. Cell 2019, 7, 1–14. [Google Scholar] [CrossRef]
- Witkin, S.S.; Mendes-Soares, H.; Linhares, I.M.; Jayaram, A.; Ledger, W.J.; Forney, L.J. Influence of vaginal bacteria and D- and L-lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: Implications for protection against upper genital tract infections. mBio 2013, 4, e00460-13. [Google Scholar] [CrossRef] [Green Version]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef]
- Orsi, C.F.; Sabia, C.; Ardizzoni, A.; Colombari, B.; Neglia, R.G.; Peppoloni, S.; Morace, G.; Blasi, E. Inhibitory effects of different lactobacilli on Candida albicans hyphal formation and biofilm development. J. Biol. Regul. Homeost. Agents 2014, 28, 743–752. [Google Scholar]
- Samot, J.; Rouabhia, M. Effect of Dermaseptin S4 on C. albicans Growth and EAP1 and HWP1 Gene Expression. Probiotics Antimicrob. Proteins 2021, 13, 287–298. [Google Scholar] [CrossRef]
- Finkel, J.S.; Xu, W.; Huang, D.; Hill, E.M.; Desai, J.V.; Woolford, C.A.; Nett, J.E.; Taff, H.; Norice, C.T.; Andes, D.R.; et al. Portrait of Candida albicans adherence regulators. PLoS Pathog. 2012, 8, e1002525. [Google Scholar] [CrossRef] [Green Version]
- Holland, L.M.; Schroder, M.S.; Turner, S.A.; Taff, H.; Andes, D.; Grozer, Z.; Gacser, A.; Ames, L.; Haynes, K.; Higgins, D.G.; et al. Comparative phenotypic analysis of the major fungal pathogens Candida parapsilosis and Candida albicans. PLoS Pathog. 2014, 10, e1004365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, C.; Yu, J.; Lu, Y. Hyphal development in Candida albicans from different cell states. Curr. Genet. 2018, 64, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Rogiers, O.; Frising, U.C.; Kucharikova, S.; Jabra-Rizk, M.A.; van Loo, G.; Van Dijck, P.; Wullaert, A. Candidalysin Crucially Contributes to Nlrp3 Inflammasome Activation by Candida albicans Hyphae. mBio 2019, 10, e02221-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).