Comparison of Diplodia Tip Blight Pathogens in Spanish and North American Pine Ecosystems
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Collection and Isolation
2.2. Species Identification
2.3. PCR Amplification of SSR Loci and Data Analysis
2.4. DNA Sequencing and Phylogenetic Analysis
3. Results
3.1. Species Identification
3.2. PCR Amplification of SSR Loci and Data Analysis
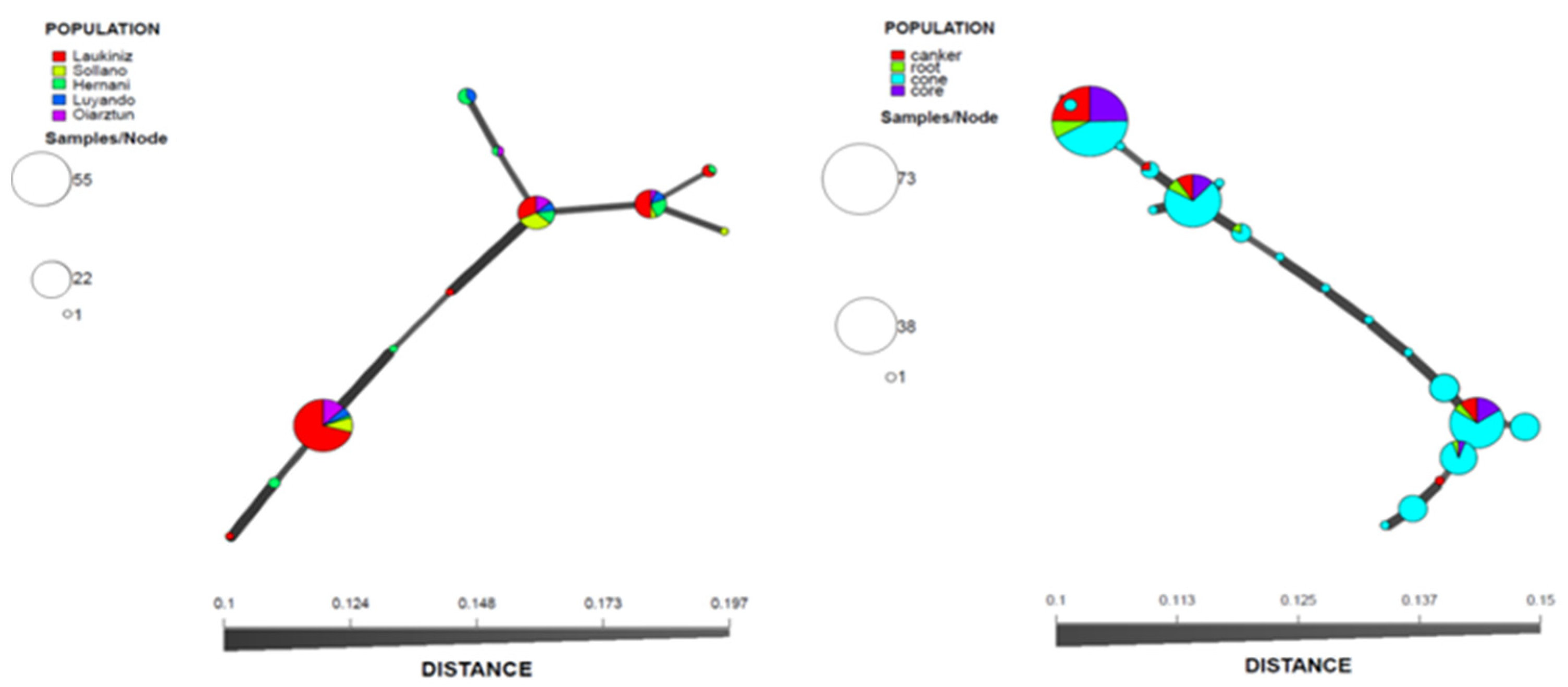
3.3. DNA Sequencing and Phylogenetic Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cavanilles, A. Lequeitio en 1857; Imprenta de J. Martín Alegría: Madrid, Spain, 1858; 151p. [Google Scholar]
- Secall, J. El “P. insignis”, Dougl., de la Escuela de Montes. Montes 1896, 458, 73–76. [Google Scholar]
- Michel, M. El Pino radiata (P. radiata D. Don) en la historia de la Comunidad Autónoma de Euskadi: Análisis de un proceso de forestalismo intensivo. Doctoral Thesis, Universidad Politécnica de Madrid, Madrid, Spain, 2004. [Google Scholar]
- Garayo, J.M. Las explotaciones forestales privadas de pino insignis en el País Vasco. Sustrai 1991, 22, 65–67. [Google Scholar]
- IKT. Inventario Forestal CAE 2005; Gobierno Vasco: Vitoria, Spain, 2005; Available online: http://www.nasdap.ejgv.euskadi.net (accessed on 5 August 2021).
- Montoya, J.M.; Mesón, M. Selvicultura; Mundi Prensa: Madrid, Spain, 2004; Volume 1, 566p. [Google Scholar]
- Aragonés, A.; Barrena, I.; Espinel, S.; Herrán, A.; Ritter, E. Application of RAPD marker to analyse genetic relationship of P. radiata population. Ann. Sci. For. 1997, 54, 697–703. [Google Scholar] [CrossRef]
- Iturritxa, E. Evaluación del Estado de las Masas Forestales de P. radiata en las Provincias de Bizkaia y Araba; Informe técnico del Proyecto de Sanidad Forestal Neiker-Tecnalia; Vitoria, Spain, 2001; 190p. [Google Scholar]
- Espinel, S.; Aragonés, A.; Ritter, E. Performance of different provenances and of the local population of the monterrey pine (P. radiata D. Don) in northern Spain. Ann. Sci. For. 1995, 52, 515–519. [Google Scholar] [CrossRef]
- Cobos-Suarez, J.M.; Ruiz-Urrestarazu, M.M. Problemas fitosanitarios de la especie P. radiata D. Don en España, con especial referencia al País Vasco. Bol. San. Veg. Plagas 1990, 16, 37–53. [Google Scholar]
- Muñoz, C.; Pérez, V.; Cobos, P.; Hernández, R.; Sánchez, G. Sanidad Forestal: Guía en Imágenes de Plagas, Enfermedades y Otros Agentes Presentes en los Montes; Mundi-Prensa: Madrid, Spain, 2004; 575p. [Google Scholar]
- Fabre, B.; Piou, D.; Desprez-Loustau, M.-L.; Marcais, B. Can the emergence of pine Diplodia shoot blight in France be explained by changes in pathogen pressure linked to climate change? Glob. Chang. Biol. 2011, 17, 3218–3227. [Google Scholar] [CrossRef]
- Smith, D.R.; Stanosz, G.R.; Albers, J. Detection of the Diplodia shoot blight and canker pathogens from red and jack pine seeds using cultural methods. Can. J. Plant Pathol. 2015, 37, 61–66. [Google Scholar] [CrossRef]
- Boys, J.; Cherry, M.; Dayanandan, S. Microsatellite analysis reveals genetically distinct populations of red pine (P. resinosa Pinaceae). Am. J. Bot. 2005, 92, 833–841. [Google Scholar] [CrossRef]
- Rudolf, P.O. Pinus resinosa Ait. Volume 1. Conifers. In Silvics of North America; Burns, R.M., Honkala, B.H., Eds.; USDA Forest Service: Washington, DC, USA, 1990; pp. 442–455. [Google Scholar]
- Blodgett, J.T.; Kruger, E.L.; Stanosz, G.R. Sphaeropsis sapinea and water stress in a red pine plantation in central Wisconsin. Phytopathology 1997, 87, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Bronson, J.J.; Stanosz, G.R. Risk from Sirococcus conigenus to understory red pine seedlings in northern Wisconsin. For. Path. 2006, 36, 271–279. [Google Scholar] [CrossRef]
- Smith, R.D.; Stanosz, R.G. A species-specific PCR assay for detection of Diplodia pinea and D. scrobiculata in dead red and jack pines with collar rot symptoms. Plant Dis. 2006, 90, 307–313. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ostry, M.E.; Laflamme, G.; Katovich, S. Silvicultural approaches for management of eastern white pine to minimize impacts of damaging agents. Forest Pathol. 2010, 40, 332–346. [Google Scholar] [CrossRef]
- Oblinger, B.W.; Smith, D.R.; Stanosz, G.R. Red pine harvest debris as a potential source of inoculum of Diplodia shoot blight pathogens. For. Ecol. Manag. 2011, 262, 663–670. [Google Scholar] [CrossRef]
- Ostry, M.E.; Moore, M.J.; Kern, C.C.; Venette, R.; Palik, B. Multiple diseases impact survival of pine species planted in red pine stands harvested in spatially variable retention patterns. For. Ecol. Manag. 2012, 286, 66–72. [Google Scholar] [CrossRef]
- Haugen, L.M.; Ostry, M.E. Long-term impact of shoot blight disease on red pine saplings. Northern J. Appl. For. 2013, 30, 170–174. [Google Scholar] [CrossRef]
- Palmer, M.A.; McRoberts, R.E.; Nicholls, T.H. Sources of inoculum of Sphaeropsis Sapinea in forest tree nurseries. Phytopathology 1988, 78, 831–835. [Google Scholar] [CrossRef]
- Stanosz, G.R.; Smith, D.R.; Guthmiller, M.A.; Stanosz, J.C. Persistence of Sphaeropsis sapinea on or in asymptomatic shoots of red and jack pines. Mycologia 1997, 89, 525–530. [Google Scholar] [CrossRef]
- Stanosz, G.R.; Smith, D.R.; Leisso, R. Diplodia shoot blight and asymptomatic persistence of Diplodia pinea on or in stems of jack pine nursery seedlings. For. Pathol. 2007, 37, 145–154. [Google Scholar] [CrossRef]
- Adamson, K.; Laas, M.; Blumenstein, K.; Busskamp, J.; Langer, G.J.; Klavina, D.; Kaur, A.; Maaten, T.; Mullett, M.S.; Müller, M.M.; et al. Highly clonal structure and abundance of one haplotype characterise the Diplodia sapinea populations in Europe and Western Asia. J. Fungi 2021, 7, 634. [Google Scholar] [CrossRef] [PubMed]
- Stanosz, G.R.; Swart, W.J.; Smith, D.R. RAPD marker and isozyme characterization of Sphaeropsis sapinea isolates from diverse coniferous hosts and locations. Mycol. Res. 1999, 103, 1193–1202. [Google Scholar] [CrossRef]
- de Wet, J.; Wingfield, M.J.; Coutinho, T.A.; Wingfield, B.D. Characterization of Sphaeropsis sapinea isolates from South Africa, Mexico and Indonesia. Plant Dis. 2000, 84, 151–156. [Google Scholar] [CrossRef][Green Version]
- de Wet, J.; Wingfield, M.J.; Coutinho, T.A.; Wingfield, B.D. Characterisation of the ‘C’ morphotype of the pine pathogen Sphaeropsis sapinea. For. Ecol. Manag. 2002, 161, 181–188. [Google Scholar] [CrossRef]
- de Wet, J.; Burgess, T.; Slippers, B.; Preisig, O.; Wingfield, B.D.; Wingfield, M.J. Multiple gene genealogies and microsatellite markers reflect relationships between morphotypes of Sphaeropsis sapinea and distinguish a new species of Diplodia. Mycol. Res. 2003, 107, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Manzanos, T.; Aragonés, A.; Iturritxa, E. Diplodia scrobiculata, a latent pathogen of P. radiata reported in northern Spain. Phytopathol. Mediterr. 2017, 56, 274–277. [Google Scholar]
- Palmer, M.A.; Stewart, E.L.; Wingfield, M.J. Variation among isolates of Sphaeropsis sapinea in the north central USA. Phytopathology 1987, 77, 944–948. [Google Scholar] [CrossRef]
- Blodgett, J.T.; Stanosz, G.R. Sphaeropsis sapinea morphotypes differ in aggressiveness but both infect non wounded red or jack pines. Plant Dis. 1997, 81, 143–147. [Google Scholar] [CrossRef][Green Version]
- Peterson, G.W. Diplodia Blight of Pines, Forest Insect & Disease Leaflet 161, U.S. Agriculture Forest Service. Available online: www.na.fs.fed.us/spfo/pubs/fidls/diplodia/diplodiafidl.htm (accessed on 15 September 2021).
- Storer, A.J.; Gordon, T.R.; Wood, D.L.; Bonello, P. Pitch canker disease of pines: Current and future impacts. J. For. 1997, 95, 21–26. [Google Scholar]
- Storer, A.J.; Gordon, T.R.; Clark, S.L. Association of the pitch canker fungus, Fusarium subglutinans f. sp. pini with Monterey pine seeds and seedlings in California. Plant Pathol. 2001, 47, 649–656. [Google Scholar] [CrossRef]
- Munck, I.A.; Stanosz, G.R. Longevity of inoculum production by Diplodia pinea on red pine cones. Phytopathology 2008, 98, S110. [Google Scholar]
- Palmer, M.A.; Nicholls, T.H. Shoot blight and collar rot of P. resinosa caused by Sphaeropsis sapinea in forest tree nurseries. Plant Dis. 1985, 69, 739–740. [Google Scholar] [CrossRef]
- Stanosz, G.R.; Smith, D.R.; Albers, J.S. Surveys for asymptomatic persistence of Sphaeropsis sapinea on or in stems of red pine seedlings from seven Great Lakes region nurseries. For. Pathol. 2005, 35, 233–244. [Google Scholar] [CrossRef]
- Vujanovic, V.; St-Arnaud, M.; Neumann, P.-J. Susceptibility of cones and seeds to fungal infection in a pine (Pinus spp.) collection. For. Pathol. 2000, 30, 305–320. [Google Scholar] [CrossRef]
- Flowers, J.; Nuckles, E.; Hartmann, J.; Vaillancourt, L. Latent infection of Austrian and Scots pine tissues by Sphaeropsis sapinea. Plant Dis. 2001, 85, 1107–1112. [Google Scholar] [CrossRef]
- Flowers, J.; Hartman, J.; Vaillancourt, L. Detection of latent Sphaeropsis sapinea infections in Austrian pine tissues using nested-polymerase chain reaction. Phytopathology 2003, 93, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.; Wingfield, M.J.; Coutinho, T.A. The role of latent Sphaeropsis sapinea infections in post-hail associated die-back of P. patula. For. Ecol. Manag. 2002, 164, 177–184. [Google Scholar] [CrossRef]
- Stanosz, G.R.; Blodgett, J.T.; Smith, D.R.; Kruger, E.L. Water stress and Sphaeropsis sapinea as a latent pathogen of red pine seedlings. New Phytol. 2001, 149, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Blodgett, J.T.; Bonello, P.; Stanosz, G.R. An effective medium for isolating Sphaeropsis sapinea from asymptomatic pines. For. Pathol. 2003, 33, 395–404. [Google Scholar] [CrossRef]
- Flowers, J.L.; Hartman, J.R.; Vaillancourt, L.J. Histology of Diplodia pinea in diseased and latently infected P. nigra shoots. For. Pathol. 2006, 36, 447–459. [Google Scholar] [CrossRef]
- Bihon, W.; Slippers, B.; Burgess, T.; Wingfield, M.J.; Wingfield, B.D. Sources of Diplodia pinea endophytic infections in P. patula and P. radiata seedlings in South Africa. For. Pathol. 2010, 41, 370–375. [Google Scholar] [CrossRef]
- Phillips, A.J.L.; Alves, A.; Abdollahzadeh JSlippers, B.; Wingfield, M.J.; Groenewald, J.Z.; Crous, P.W. The Botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 2013, 76, 51–167. [Google Scholar] [CrossRef]
- Grissino-Mayer, H.D. A manual and tutorial for the proper use of an increment borer. Tree-Ring Res. 2003, 59, 63–79. [Google Scholar]
- Munck, I.A.; Smith, D.R.; Sickley, T.; Stanosz, G.R. Site-related influences on cone-borne inoculum and asymptomatic persistence of Diplodia shoot blight fungi on or in mature red pines. For. Ecol. Manag. 2009, 257, 812–819. [Google Scholar] [CrossRef]
- Burgess, T.; Wingfield, M.J.; Wingfield, B.D. Simple sequence repeat (SSR) markers distinguish between morphotypes of Sphaeropsis sapinea. Appl. Environ. Microbiol. 2001, 67, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Bihon, W.; Burgess, T.; Slippers, B.; Wingfield, M.J.; Wingfield, B.D. Distribution of Diplodia pinea and its genotypic diversity within asymptomatic P. patula trees. Australas. Plant Pathol. 2011, 40, 540–548. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Kamvar, Z.N.; Brooks, J.C.; Grünwald, N.J. Novel R tools for analysis of genome-wide population genetic data with emphasis on clonality. Front. Genet. 2015, 208, 6. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, J.A.; Taylor, J.F. Genotypic diversity: Estimation and prediction in samples. Genetics 1988, 118, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Grünwald, N.J.; Goodwin, S.B.; Milgroom, M.G.; Fry, W.E. Analysis of genotypic diversity data for populations of microorganisms. Phytopathology 2003, 93, 738–746. [Google Scholar] [CrossRef]
- Agapow, P.M.; Burt, A. Indices of multilocus linkage disequilibrium. Mol. Ecol. Notes 2001, 1, 101–102. [Google Scholar] [CrossRef]
- Hedrick, P.W. A standardized genetic differentiation measure. Evolution 2005, 59, 1633–1638. [Google Scholar] [CrossRef]
- Archer, F.I.; Adams, P.E.; Schneiders, B.B. STRATAG: An R package for manipulating, summarizing and analysing population genetic data. Mol. Ecol. Resour. 2017, 17, 5–11. [Google Scholar] [CrossRef]
- Winter, D.J. MMOD: An R library for the calculation of population differentiation statistics. Mol. Ecol. Resour. 2012, 12, 1158–1160. [Google Scholar] [CrossRef]
- Jombart, T.; Devillard, S.; Balloux, F. Discriminant analysis of principal components: A new method for the analysis of genetically structured populations. BMC Genet. 2010, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Jombart, T. Adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Bruvo, R.; Michiels, N.K.; D’Souza, T.G.; Schulenburg, H. A simple method for the calculation of microsatellite genotype distances irrespective of ploidy level. Mol. Ecol. 2004, 13, 2101–2106. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Carbone, I.; Kohn, L. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Arnaud-Haond, S.; Duarte, C.M.; Alberto, F.; Serrão, E.A. Standardizing methods to address clonality in population studies. Mol. Ecol. 2007, 16, 5115–5139. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Batista, E.; Lopes, A.; Alves, A. Botryosphaeriaceae species on forest trees in Portugal: Diversity, distribution and pathogenicity. Eur. J. Plant Pathol. 2020, 158, 693–720. [Google Scholar] [CrossRef]
- Burgess, T.I.; Gordon, T.R.; Wingfield, M.J.; Wingfield, B.D. Geographic isolation of Diplodia scrobiculata and its association with native P. radiata. Mycol. Res. 2004, 108 Pt 12, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Morelet, M.; Chandelier, P. A case of variability in Sphaeropsis sapinea. Eur. J. Plant Pathol. 1993, 23, 317–320. [Google Scholar]
- Blodgett, J.T.; Stanosz, G.R. Differences in aggressiveness of Sphaeropsis sapinea RAPD marker group isolates on several conifers. Plant Dis. 1999, 83, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Munck, I.A.; Stanosz, G. Quantification of Conidia of Diplodia spp. Extracted from Red and Jack Pine Cones. Plant Dis. 2009, 93, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Munck, I.A.; Stanosz, G.R. Longevity of inoculum production by Diplodia pinea on red pine cones. For. Pathol. 2010, 40, 58–63. [Google Scholar] [CrossRef]
- Hanso, M.; Drenkhan, R. Diplodia pinea is a new pathogen on Austrian pine (P. nigra) in Estonia. Plant Pathol. 2009, 58, 797. [Google Scholar] [CrossRef]
- Oliva, J.; Boberg, J.; Stenlid, J. First report of Sphaeropsis sapinea on Scots pine (P. sylvestris) and Austrian pine (P. nigra) in Sweden. New Dis. Rep. 2013, 27, 23. [Google Scholar] [CrossRef]
- Müller, M.; Hantula, J.; Wingfield, M.; Drenkhan, R. Diplodia sapinea found on Scots pine in Finland. For. Pathol. 2018, 49, e12483. [Google Scholar] [CrossRef]
- Adamson, K.; Klavina, D.; Drenkhan, R.; Gaitnieks, T.; Hanso, M. Diplodia sapinea is colonizing the native scots pine (P. sylvestris) in the northern baltics. Eur. J. Plant Pathol. 2015, 143, 343–350. [Google Scholar] [CrossRef]
- Swart, W.J.; Wingfield, M.J. Biology and control of Sphaeropsis sapinea on P. species in South Africa. Plant Dis. 1991, 75, 761–766. [Google Scholar] [CrossRef]
- Santini, A.; Pepori, A.; Ghelardini, L.; Capretti, P. Persistence of some pine pathogens in coarse woody debris in a forest. For. Ecol. Manag. 2008, 256, 502–506. [Google Scholar] [CrossRef]
- Manzanos, T.; Stanosz GSmith DMuenchow, J.; Schratz, P.; Brenning, A.; Aragones, A.; Iturritxa, E. Mating type ratios and pathogenicity in Diplodia shoot blight fungi populations: Comparative analysis. Eur. J. For. Pathol. 2018, 49, e12475. [Google Scholar] [CrossRef]
- Taherdoost, H. Sampling Methods in Research Methodology, How to Choose a Sampling Technique for Research. Int. J. Acad. Res. Manag. 2016, 5, 18–27. [Google Scholar] [CrossRef]
- Brodde, L.; Adamson, K.; Julio Camarero, J.; Castaño, C.; Drenkhan, R.; Lehtijärvi, A.; Luchi, N.; Migliorini, D.; Sánchez-Miranda, Á.; Stenlid, J.; et al. Diplodia tip blight on its way to the North: Drivers of disease emergence in Northern Europe. Front. Plant Sci. 2019, 9, 1818. [Google Scholar] [CrossRef]
- Burgess, T.I.; Wingfield, M.J.; Wingfield, B.D. Global distribution of Diplodia pinea genotypes revealed using simple sequence repeat (SSR) markers. Australas. Plant Pathol. 2004, 33, 513–519. [Google Scholar] [CrossRef]
- Doğmuş-Lehtijärvi, T.; Aday, G.; Lehtijärvi, A.; Oskay, F.; Kaya, Ö.D. Occurrence and Genetic Similarity of Diplodia pinea on Shoots and Cones in Seed Orchards of P. spp. in North-Western Turkey. Plant Prot. Sci. 2014, 50, 217–220. [Google Scholar] [CrossRef]
- Monat, J.P. The benefits of global scaling in multi-criteria decision analysis. Judgm. Decis. Mak. 2009, 4, 492–508. [Google Scholar]
- Ramos, P.; Petisco, E.; Martín, J.M.; Rodríguez, E. Downscaled climate change projections over Spain: Application to water resources. Int. J. Water Resour. Dev. 2012, 29, 201–218. [Google Scholar] [CrossRef]
- Bachi, P.R.; Peterson, J.L. Emhancement of Sphaeropsis sapinea stem invasion of pines by water deficits. Plant Dis. 1985, 69, 798–799. [Google Scholar]

| Origin | Host Species | Sample Type | Sample ID | Origin | Host Species | Sample Type | Sample ID |
|---|---|---|---|---|---|---|---|
| Bajo Deba, Spain | Pinus pinaster | Canker | BCI2 | P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM198 |
| Plentzia-Munguia, Spain | Pinus radiata | Root | BCI5 | P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM199 |
| Alto Deba, Spain | Pinus radiata | Cone | BC14 | P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM200 |
| Estribaciones del Gorbea, Spain | Pinus radiata | Cone | BC15 | P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM201 |
| Encartaciones, Spain | Pinus radiata | Cone | BC17 | P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM202 |
| Encartaciones, Spain | Pinus radiata | Cone | BC18 | P2 (Sollano), Spain | Pinus radiata | Cone | BC22 |
| Encartaciones, Spain | Pinus radiata | Cone | BC19 | P2 (Sollano), Spain | Pinus radiata | Cone | BC23 |
| Alto Deba, Spain | Pinus radiata | Cone | BC39 | P2 (Sollano), Spain | Pinus radiata | Cone | BC24 |
| Alto Deba, Spain | Pinus radiata | Cone | BC40 | P2 (Sollano), Spain | Pinus radiata | Cone | BC25 |
| Goierri, Spain | Pinus nigra | Cone | BC41 | P2 (Sollano), Spain | Pinus radiata | Cone | BC26 |
| Goierri, Spain | Pinus nigra | Cone | BC42 | P2 (Sollano), Spain | Pinus radiata | Cone | BC27 |
| Montaña Alavesa, Spain | Pinus nigra | Cone | BC44 | P2 (Sollano), Spain | Pinus radiata | Cone | BC29 |
| Llanada Alavesa, Spain | Pinus radiata | Cone | BC45 | P2 (Sollano), Spain | Pinus radiata | Cone | BC30 |
| Llanada Alavesa, Spain | Pinus radiata | Cone | BC46 | P2 (Sollano), Spain | Pinus radiata | Cone | BC31 |
| Llanada Alavesa, Spain | Pinus radiata | Cone | BC47 | P2 (Sollano), Spain | Pinus radiata | Cone | BC33 |
| Gernika Bermeo, Spain | Pinus radiata | Cone | BC48 | P2 (Sollano), Spain | Pinus radiata | Cone | BC34 |
| Gernika Bermeo, Spain | Pinus radiata | Cone | BC49 | P2 (Sollano), Spain | Pinus radiata | Cone | BC35 |
| Gernika Bermeo, Spain | Pinus radiata | Cone | BC50 | P2 (Sollano), Spain | Pinus radiata | Cone | BC36 |
| Gernika Bermeo, Spain | Pinus radiata | Cone | BC51 | P2 (Sollano), Spain | Pinus radiata | Cone | BC38 |
| Gernika Bermeo, Spain | Pinus radiata | Cone | BC52 | P3 (Hernani), Spain | Pinus radiata | Cone | BC60 |
| Duranguesado, Spain | Pinus radiata | Cone | BC53 | P3 (Hernani), Spain | Pinus radiata | Cone | BC61 |
| Markina-Ondarroa, Spain | Pinus radiata | Cone | BC54 | P3 (Hernani), Spain | Pinus radiata | Cone | BC62 |
| Donostia San Sebastian, Spain | Pinus radiata | Cone | BC55 | P3 (Hernani), Spain | Pinus radiata | Cone | BC63 |
| Valles Alaveses, Spain | Pinus attenuata | Cone | BC56 | P3 (Hernani), Spain | Pinus radiata | Cone | BC64 |
| Gernika Bermeo, Spain | Pinus radiata | Cone | BC57 | P3 (Hernani), Spain | Pinus radiata | Cone | BC65 |
| Goierri, Spain | Pinus radiata | Cone | BC76 | P3 (Hernani), Spain | Pinus radiata | Cone | BC66 |
| Goierri, Spain | Pinus radiata | Cone | BC77 | P3 (Hernani), Spain | Pinus radiata | Cone | BC67 |
| Goierri, Spain | Pinus radiata | Cone | BC78 | P3 (Hernani), Spain | Pinus radiata | Cone | BC68 |
| Tolosa, Spain | Pinus radiata | Cone | BC79 | P3 (Hernani), Spain | Pinus radiata | Cone | BC69 |
| Tolosa, Spain | Pinus radiata | Cone | BC80 | P3 (Hernani), Spain | Pinus radiata | Cone | BC70 |
| Tolosa, Spain | Pinus radiata | Cone | BC81 | P3 (Hernani), Spain | Pinus radiata | Cone | BC71 |
| Tolosa, Spain | Pinus nigra | Cone | BC82 | P3 (Hernani), Spain | Pinus radiata | Cone | BC72 |
| Tolosa, Spain | Pinus nigra | Cone | BC83 | P3 (Hernani), Spain | Pinus radiata | Cone | BC73 |
| Tolosa, Spain | Pinus nigra | Cone | BC84 | P3 (Hernani), Spain | Pinus radiata | Cone | BC74 |
| Tolosa, Spain | Pinus radiata | Cone | BC86 | P3 (Hernani), Spain | Pinus radiata | Cone | BC75 |
| Donostia San Sebastian, Spain | Pinus radiata | Cone | BC87 | P4 (Luyando), Spain | Pinus radiata | Root | BCM157 |
| Donostia San Sebastian, Spain | Pinus radiata | Cone | BC89 | P4 (Luyando), Spain | Pinus radiata | Root | BCM175 |
| Donostia San Sebastian, Spain | Pinus radiata | Cone | BC90 | P4 (Luyando), Spain | Pinus radiata | Root | BCM176 |
| Donostia San Sebastian, Spain | Pinus radiata | Cone | BC91 | P4 (Luyando), Spain | Pinus radiata | Root | BCM177 |
| Donostia San Sebastian, Spain | Pinus radiata | Cone | BC92 | P4 (Luyando), Spain | Pinus radiata | Root | BCM178 |
| Donostia San Sebastian, Spain | Pinus radiata | Cone | BC93 | P4 (Luyando), Spain | Pinus radiata | Core | BCM179 |
| Urola Costa, Spain | Pinus radiata | Cone | BC94 | P4 (Luyando), Spain | Pinus radiata | Root | BCM181 |
| Urola Costa, Spain | Pinus radiata | Cone | BC95 | P4 (Luyando), Spain | Pinus radiata | Root | BCM183 |
| Urola Costa, Spain | Pinus radiata | Cone | BC96 | P4 (Luyando), Spain | Pinus radiata | Core | BCM187 |
| Urola Costa, Spain | Pinus radiata | Cone | BC97 | P5 (Oiarztun), Spain | Pinus radiata | Core | BCM158 |
| Bajo Deba, Spain | Pinus radiata | Cone | BC98 | P5 (Oiarztun), Spain | Pinus radiata | Core | BCM159 |
| Bajo Deba, Spain | Pinus radiata | Cone | BC99 | P5 (Oiarztun), Spain | Pinus radiata | Core | BCM160 |
| Encartaciones, Spain | Pinus radiata | Cone | BC100 | P5 (Oiarztun), Spain | Pinus radiata | Core | BCM162 |
| Encartaciones, Spain | Pinus radiata | Cone | BC101 | P5 (Oiarztun), Spain | Pinus radiata | Core | BCM163 |
| Encartaciones, Spain | Pinus nigra | Cone | BC104 | P5 (Oiarztun), Spain | Pinus radiata | Core | BCM164 |
| Encartaciones, Spain | Pinus radiata | Cone | BC105 | P5 (Oiarztun), Spain | Pinus radiata | Core | BCM173 |
| Encartaciones, Spain | Pinus radiata | Cone | BC106 | P5 (Oiarztun), Spain | Pinus radiata | Core | BCM180 |
| Encartaciones, Spain | Pinus radiata | Cone | BC108 | P5 (Oiarztun), Spain | Pinus radiata | Root | BCM209 |
| Valles Alaveses, Spain | Pinus pinaster | Cone | BC109 | P5 (Oiarztun), Spain | Pinus radiata | Root | BCM211 |
| Estribaciones del Gorbea, Spain | Pinus radiata | Cone | BC112 | P5 (Oiarztun), Spain | Pinus radiata | Root | BCM212 |
| Arratia Nervión, Spain | Pinus nigra | Cone | BC113 | P5 (Oiarztun), Spain | Pinus radiata | Root | BCM213 |
| Arratia Nervión, Spain | Pinus radiata | Cone | BC114 | Barbour County, West Virginia USA | Pinus sylvestris | Needle | W171 |
| Cantábrica Alavesa, Spain | Pinus radiata | Cone | BC115 | Black Hills, South Dakota USA | Pinus ponderosa | Stem | W172 |
| Cantábrica Alavesa, Spain | Pinus radiata | Cone | BC116 | Idaho USA | Pinus ponderosa | Unknown | W174 |
| Valles Alaveses, Spain | Pinus halepensis | Cone | BC120 | Grant County, Wisconsin USA | Pinus resinosa | Unknown | W175 |
| Valles Alaveses, Spain | Pinus sylvestris | Cone | BC121 | Oktibbeha County, Mississippi USA | Pinus palustris | Unknown | W177 |
| Valles Alaveses, Spain | Pinus contorta | Cone | BC123 | Pennsylvania USA | Pinus sylvestris | Unknown | W180 |
| Estribaciones del Gorbea, Spain | Pinus radiata | Cone | BC124 | Riverside County, California USA | Pinus jeffreyi | Unknown | W185 |
| Cantábrica Alavesa, Spain | Pinus radiata | Cone | BC125 | Florida USA | Pinus elliottii | Cone-seed | W186 |
| Arratia Nervión, Spain | Pinus radiata | Cone | BC127 | Bennington County, Vermont USA | Pinus resinosa | Cone | W190 |
| Llanada Alavesa, Spain | Pinus radiata | Cone | BC128 | Maui, Hawaii USA | Pinus radiata | Unknown | W191 |
| Llanada Alavesa, Spain | Pinus radiata | Cone | BC129 | Itasca St. Park, Minnesota USA | Pinus resinosa | Unknown | W192 |
| Spain | Pinus nigra | Cone | BC131 | Dallas County, Texas USA | Pinus eldarica | Cone | W194 |
| Llanada Alavesa, Spain | Pinus nigra | Cone | BC132 | Georgia USA | Pinus taeda | Needle | W206 |
| Llanada Alavesa, Spain | Pinus radiata | Cone | BC133 | Wisconsin USA | Pinus banksiana | Needle | W210 |
| Llanada Alavesa, Spain | Pinus radiata | Cone | BC134 | Wisconsin USA | Pinus banksiana | Needle | W211 |
| Llanada Alavesa, Spain | Pinus radiata | Cone | BC135 | Waushara County, Wisconsin USA | Pinus resinosa | Needle | W212 |
| Llanada Alavesa, Spain | Pinus radiata | Cone | BC137 | Portage County, Wisconsin USA | Pinus banksiana | Needle | W213 |
| Llanada Alavesa, Spain | Pinus radiata | Cone | BC138 | Portage County, Wisconsin USA | Pinus resinosa | Needle | W214 |
| Montaña Alavesa, Spain | Pinus radiata | Cone | BC141 | Wood County, Wisconsin USA | Pinus resinosa | Needle | W215 |
| Alto Deba, Spain | Pinus radiata | Cone | BC142 | Adams County, Wisconsin USA | Pinus resinosa | Needle | W216 |
| Bajo Deba, Spain | Pinus radiata | Cone | BC143 | Marathon County, Wisconsin USA | Pinus resinosa | Needle | W217 |
| Bajo Deba, Spain | Pinus radiata | Cone | BC144 | Wallowa County, Oregon USA | Pinus ponderosa | Cone | W218 |
| Bajo Deba, Spain | Pinus radiata | Cone | BC145 | Wallowa County, Oregon, USA | Pinus ponderosa | Needle | W219 |
| Markina Ondarroa, Spain | Pinus radiata | Cone | BC146 | Bennington County, Vermont USA | Pinus resinosa | Stem tip | W220 |
| Estribaciones del Gorbea, Spain | Pinus radiata | Cone | BC147 | Sawyer County, Wisconsin USA | Pinus banksiana | Stem | W221 |
| Arratia Nervión, Spain | Pinus radiata | Cone | BC148 | Wood County, Wisconsin USA | Pinus banksiana | Stem | W222 |
| Arratia Nervión, Spain | Pinus radiata | Cone | BC149 | Vilas County, Wisconsin USA | Pinus resinosa | Unknown | W223 |
| Encartaciones, Spain | Pinus radiata | Cone | BC150 | South Dakota | Pinus ponderosa | Unknown | W224 |
| Encartaciones, Spain | Pinus radiata | Cone | BC151 | Dallas County, Texas USA | Pinus nigra | Cone | W225 |
| Encartaciones, Spain | Pinus radiata | Cone | BC152 | Bayfield County, Wisconsin USA | Pinus resinosa | Needle | W226 |
| Duranguesado, Spain | Pinus radiata | Cone | BC154 | Sumter County, Alabama USA | Pinus taeda | Cone | W227 |
| Gernika Bermeo, Spain | Pinus radiata | Cone | BC155 | Adams County, Wisconsin USA | Pinus sylvestris | Bark | W228 |
| Gernika Bermeo, Spain | Pinus radiata | Cone | BC156 | Vilas County, Wisconsin USA | Pinus ponderosa | Unknown | W229 |
| Markina-Ondarroa, Spain | Pinus radiata | Cone | BC157 | Vilas County, Wisconsin USA | Pinus resinosa | Unknown | W231 |
| Markina-Ondarroa, Spain | Pinus radiata | Cone | BC158 | Pine County, Minnesota USA | Pinus resinosa | Unknown | W232 |
| Duranguesado, Spain | Pinus radiata | Cone | BC159 | Morgantown, WV | Pinus nigra | Needle | W233 |
| Gernika Bermeo, Spain | Pinus radiata | Cone | BC160 | Jackson County, Wisconsin USA | Pinus resinosa | Needle | W234 |
| Spain | Pinus radiata | Cone | BC161 | Dane County, Wisconsin USA | Pinus nigra | Needle | W235 |
| Plentzia-Munguia, Spain | Pinus radiata | Cone | BC162 | Northern Highland American Legion State Forest, Wisconsin USA | Pinus banksiana | Twig | W236 |
| Goierri, Spain | Pinus radiata | Cone | BC163 | Marquette County, Wisconsin USA | Pinus resinosa | Needle | W238 |
| Tolosa, Spain | Pinus radiata | Cone | BC164 | Trempealeau County, Wisconsin USA | Pinus resinosa | Needle | W239 |
| Tolosa, Spain | Pinus radiata | Cone | BC165 | Fairfield County, Connecticut USA | Pinus sylvestris | Needle | W240 |
| Urola Costa, Spain | Pinus radiata | Cone | BC166 | Adair County, Iowa USA | Pinus nigra | Needle | W241 |
| Markina-Ondarroa, Spain | Pinus radiata | Cone | BC169 | Codington County, South Dakota USA | Pinus sylvestris | Needle | W242 |
| Cantábrica Alavesa, Spain | Pinus radiata | Cone | BC196 | Polk County, Iowa USA | Pinus nigra | Needle | W243 |
| Arratia Nervión, Spain | Pinus radiata | Cone | BC197 | Lacrosse County, Wisconsin USA | Pinus resinosa | Needle | W244 |
| Arratia Nervión, Spain | Pinus radiata | Cone | BC198 | Wood County, Wisconsin USA | Pinus resinosa | Stem | W245 |
| Arratia Nervión, Spain | Pinus radiata | Cone | BC199 | Centre County, Pennsylvania USA | Pinus nigra | Needle | W246 |
| Arratia Nervión, Spain | Pinus radiata | Cone | BC200 | Upshur County, West Virginia USA | Pinus sylvestris | Needle | W247 |
| Duranguesado, Spain | Pinus radiata | Cone | BC201 | Lafayette County, Wisconsin USA | Pinus resinosa | Needle | W248 |
| Gran Bilbao, Spain | Pinus radiata | Cone | BC202 | Monroe County, Wisconsin USA | Pinus banksiana | Needle | W250 |
| Plentzia-Munguia, Spain | Pinus radiata | Cone | BC203 | Cheboygan County, Michigan USA | Pinus banksiana | Needle | W251 |
| Plentzia-Munguia, Spain | Pinus radiata | Stem | BC208 | Manistee National Forest, Michigan USA | Pinus resinosa | Unknown | W252 |
| Plentzia-Munguia, Spain | Pinus radiata | Stem | BC209 | Jefferson County, West Virginia USA | Pinus nigra | Needle | W253 |
| P1 (Laukiniz), Spain | Pinus radiata | Cone | BC2 | Stanislaus National Forest, California USA | Pinus ponderosa | Unknown | W254 |
| P1 (Laukiniz), Spain | Pinus radiata | Cone | BC3 | Morgan County, West Virginia USA | Pinus sylvestris | Needle | W255 |
| P1 (Laukiniz), Spain | Pinus radiata | Cone | BC4 | Marion County, Indiana USA | Pinus nigra | Cone | W256 |
| P1 (Laukiniz), Spain | Pinus radiata | Cone | BC5 | Foxborough, Massachusetts, USA | Pinus resinosa | Cone | U1 |
| P1 (Laukiniz), Spain | Pinus radiata | Cone | BC6 | Washington, Vermont, USA | Pinus resinosa | Cone | U2 |
| P1 (Laukiniz), Spain | Pinus radiata | Cone | BC7 | Hancock County, Maine, USA | Pinus resinosa (natural) | Cone | U3 |
| P1 (Laukiniz), Spain | Pinus radiata | Cone | BC8 | Hancock County, Maine, USA | Pinus resinosa (natural) | Cone | U4 |
| P1 (Laukiniz), Spain | Pinus radiata | Cone | BC9 | Andover, Massachusetts, USA | Pinus resinosa | Cone | U5 |
| P1 (Laukiniz), Spain | Pinus radiata | Cone | BC10 | Hancock County, Maine, USA | Pinus resinosa (natural) | Cone | U6 |
| P1 (Laukiniz), Spain | Pinus radiata | Cone | BC11 | Washington, Vermont, USA | Pinus resinosa | Cone | U7 |
| P1 (Laukiniz), Spain | Pinus radiata | Core | BCI1 | Washington, Vermont, USA | Pinus resinosa | Cone | U8 |
| P1 (Laukiniz), Spain | Pinus radiata | Core | BCI3 | Andover, Massachusetts, USA | Pinus resinosa | Cone | U9 |
| P1 (Laukiniz), Spain | Pinus radiata | Core | BCI4 | Foxborough, Massachusetts, USA | Pinus resinosa | Cone | U10 |
| P1 (Laukiniz), Spain | Pinus radiata | Core | BCI6 | Shrewsbury, Vermont, USA | Pinus resinosa | Cone | U11 |
| P1 (Laukiniz), Spain | Pinus radiata | Core | BCI7 | Hancock County, Maine, USA | Pinus resinosa (natural) | Cone | U12 |
| P1 (Laukiniz), Spain | Pinus radiata | Core | BCI8 | Shrewsbury, Vermont, USA | Pinus resinosa | Cone | U13 |
| P1 (Laukiniz), Spain | Pinus radiata | Core | BCI9 | Hancock County, Maine, USA | Pinus resinosa (natural) | Cone | U14 |
| P1 (Laukiniz), Spain | Pinus radiata | Core | BCI10 | Hudson, Massachusetts, USA | Pinus resinosa | Cone | U15 |
| P1 (Laukiniz), Spain | Pinus radiata | Core | BCI11 | Shrewsbury, Vermont, USA | Pinus resinosa | Cone | U16 |
| P1 (Laukiniz), Spain | Pinus radiata | Core | BCI12 | Hancock County, Maine, USA | Pinus resinosa (natural) | Cone | U17 |
| P1 (Laukiniz), Spain | Pinus radiata | Core | BCI13 | Hancock County, Maine, USA | Pinus resinosa (natural) | Cone | U18 |
| P1 (Laukiniz), Spain | Pinus radiata | Core | BCI14 | Hancock County, Maine, USA | Pinus resinosa (natural) | Cone | U19 |
| P1 (Laukiniz), Spain | Pinus radiata | Core | BCI15 | Shrewsbury, Vermont, USA | Pinus resinosa | Cone | U20 |
| P1 (Laukiniz), Spain | Pinus radiata | Core | BCI16 | Hudson, Massachusetts, USA | Pinus resinosa | Cone | U21 |
| P1 (Laukiniz), Spain | Pinus radiata | Core | BCI17 | Washington, Vermont, USA | Pinus resinosa | Cone | U22 |
| P1 (Laukiniz), Spain | Pinus radiata | Core | BCI18 | Foxborough, Massachusetts, USA | Pinus resinosa | Cone | U23 |
| P1 (Laukiniz), Spain | Pinus radiata | Core | BCI19 | Hudson, Massachusetts, USA | Pinus resinosa | Cone | U24 |
| P1 (Laukiniz), Spain | Pinus radiata | Core | BCI20 | Hudson, Massachusetts, USA | Pinus resinosa | Cone | U25 |
| P1 (Laukiniz), Spain | Pinus radiata | Core | BCI21 | Washington, Vermont, USA | Pinus resinosa | Cone | U26 |
| P1 (Laukiniz), Spain | Pinus radiata | Core | BCI22 | Andover, Massachusetts, USA | Pinus resinosa | Cone | U27 |
| P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM161 | Washington, Vermont, USA | Pinus resinosa | Cone | U28 |
| P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM165 | Foxborough, Massachusetts, USA | Pinus resinosa | Cone | U29 |
| P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM167 | Washington, Vermont, USA | Pinus resinosa | Cone | U30 |
| P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM168 | Hancock County, Maine, USA | Pinus resinosa (natural) | Cone | U31 |
| P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM169 | Hudson, Massachusetts, USA | Pinus resinosa | Cone | U32 |
| P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM170 | Hancock County, Maine, USA | Pinus resinosa (natural) | Cone | U33 |
| P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM171 | Andover, Massachusetts, USA | Pinus resinosa | Cone | U34 |
| P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM172 | Hancock County, Maine, USA | Pinus resinosa (natural) | Cone | U35 |
| P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM174 | Andover, Massachusetts, USA | Pinus resinosa | Cone | U36 |
| P1 (Laukiniz), Spain | Pinus radiata | Root | BCM182 | Hancock County, Maine, USA | Pinus resinosa (natural) | Cone | U37 |
| P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM184 | Hancock County, Maine, USA | Pinus resinosa (natural) | Cone | U38 |
| P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM185 | Foxborough, Massachusetts, USA | Pinus resinosa | Cone | U39 |
| P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM186 | Andover, Massachusetts, USA | Pinus resinosa | Cone | U40 |
| P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM188 | Washington, Vermont, USA | Pinus resinosa | Cone | U41 |
| P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM189 | Washington, Vermont, USA | Pinus resinosa | Cone | U42 |
| P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM190 | Hancock County, Maine, USA | Pinus resinosa (natural) | Cone | U43 |
| P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM191 | Hancock County, Maine, USA | Pinus resinosa (natural) | Cone | U44 |
| P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM192 | Washington, Vermont, USA | Pinus resinosa | Cone | U45 |
| P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM193 | Hudson, Massachusetts, USA | Pinus resinosa | Cone | U46 |
| P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM194 | Washington, Vermont, USA | Pinus resinosa | Cone | U47 |
| P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM195 | Washington, Vermont, USA | Pinus resinosa | Cone | U48 |
| P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM196 | Shrewsbury, Vermont, USA | Pinus resinosa | Cone | U49 |
| P1 (Laukiniz), Spain | Pinus radiata | Canker | BCM197 | Shrewsbury, Vermont, USA | Pinus resinosa | Cone | U50 |
| Parametersb | Country | Nursery Location in the Basque Country | Sample Type | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spain (Basque Country) | USA | Laukiniz | Sollano | Hernani | Luyando | Oiarztun | Canker | Root | Cone | Core | |
| Sample size (N)c | 216 | 106 | 58 | 14 | 16 | 9 | 12 | 28 | 13 | 145 | 30 |
| MLG/Diversity (G) | 19 | 34 | 6 | 4 | 8 | 4 | 4 | 5 | 5 | 19 | 4 |
| eMLG | 19 | 19 | 6 | 4 | 8 | 4 | 4 | 5 | 5 | 10 | 4 |
| Evenness (E5) | 1 | 1 | 0.427 | 0.3 | 0.407 | 0.333 | 0.3 | 1 | 1 | 1 | 1 |
| Diversity (H) | 2.94 | 3.53 | 1.79 | 1.39 | 2.08 | 1.39 | 1.39 | 1.61 | 1.61 | 2.94 | 1.39 |
| rbarD | −0.074 | 0.087 | −0.080 | −0.316 | −0.066 | −0.115 | −0.333 | −0.056 | −0.194 | −0.074 | −0.115 |
| p-value | 0.999 | 0.001 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragonés, A.; Manzanos, T.; Stanosz, G.; Munck, I.A.; Raposo, R.; Elvira-Recuenco, M.; Berbegal, M.; Mesanza, N.; Smith, D.R.; Simmons, M.; et al. Comparison of Diplodia Tip Blight Pathogens in Spanish and North American Pine Ecosystems. Microorganisms 2021, 9, 2565. https://doi.org/10.3390/microorganisms9122565
Aragonés A, Manzanos T, Stanosz G, Munck IA, Raposo R, Elvira-Recuenco M, Berbegal M, Mesanza N, Smith DR, Simmons M, et al. Comparison of Diplodia Tip Blight Pathogens in Spanish and North American Pine Ecosystems. Microorganisms. 2021; 9(12):2565. https://doi.org/10.3390/microorganisms9122565
Chicago/Turabian StyleAragonés, Ana, Tania Manzanos, Glen Stanosz, Isabel A. Munck, Rosa Raposo, Margarita Elvira-Recuenco, Mónica Berbegal, Nebai Mesanza, Denise R. Smith, Michael Simmons, and et al. 2021. "Comparison of Diplodia Tip Blight Pathogens in Spanish and North American Pine Ecosystems" Microorganisms 9, no. 12: 2565. https://doi.org/10.3390/microorganisms9122565
APA StyleAragonés, A., Manzanos, T., Stanosz, G., Munck, I. A., Raposo, R., Elvira-Recuenco, M., Berbegal, M., Mesanza, N., Smith, D. R., Simmons, M., Wyka, S., & Iturritxa, E. (2021). Comparison of Diplodia Tip Blight Pathogens in Spanish and North American Pine Ecosystems. Microorganisms, 9(12), 2565. https://doi.org/10.3390/microorganisms9122565






