Succession of Microbial Communities in Waste Soils of an Iron Mine in Eastern China
Abstract
:1. Introduction
2. Materials and Methods
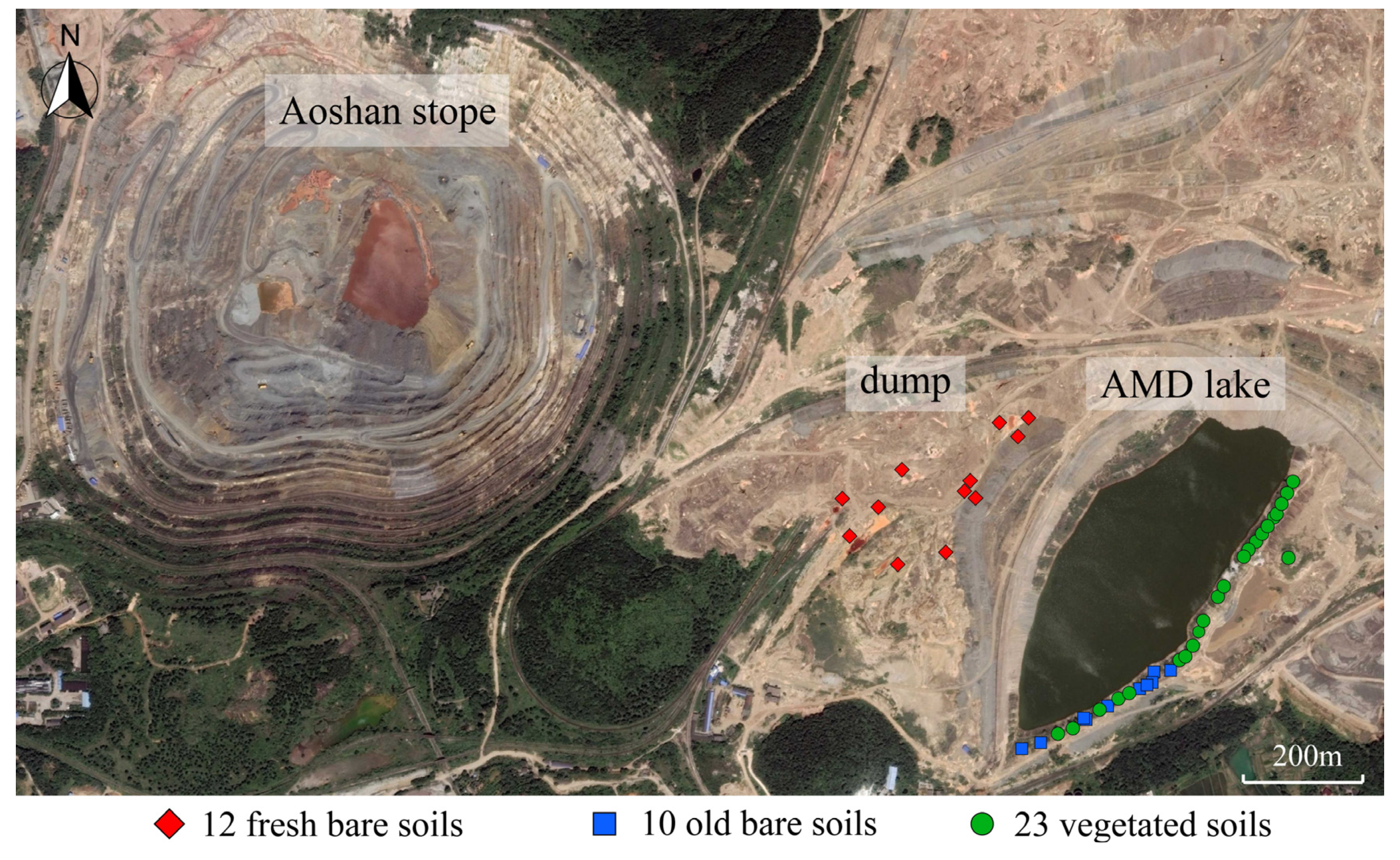
2.1. Sampling Site Description and Sample Collection
2.2. Determination of Physicochemical Parameters
2.3. DNA Extraction, PCR Amplification and High-Throughput Sequencing
2.4. Data Analysis
3. Results
3.1. Physicochemical Properties
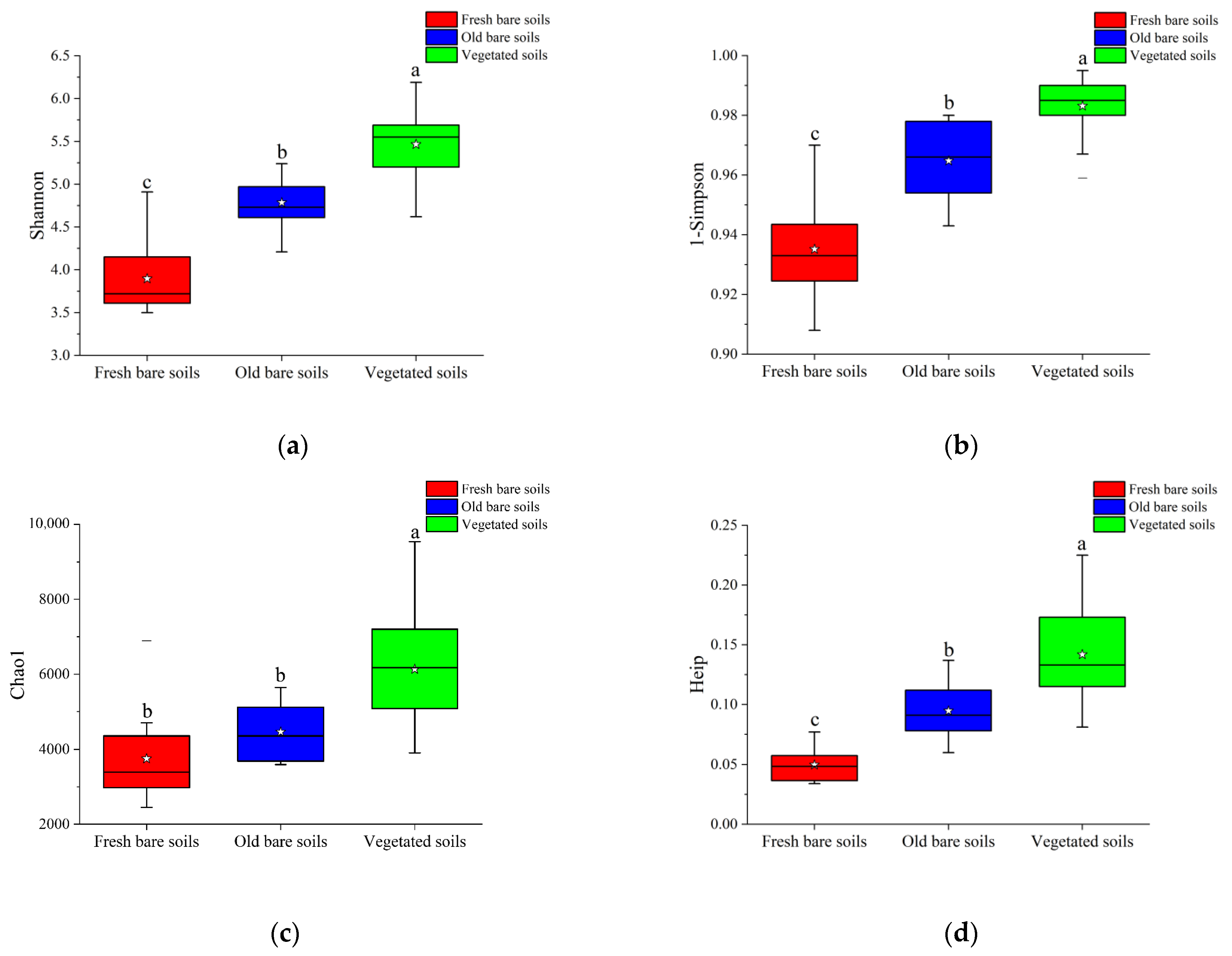
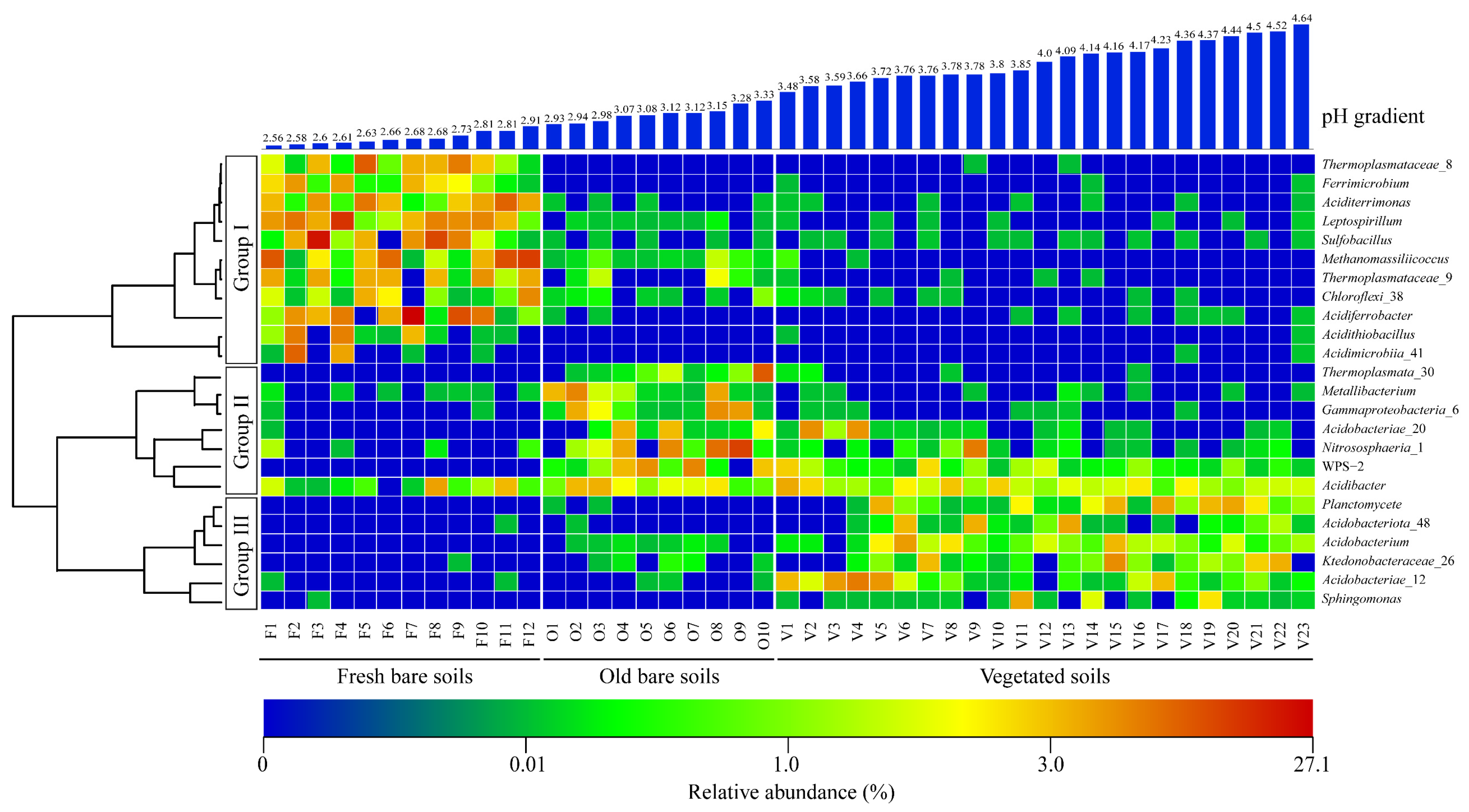
3.2. Alpha Diversity and Microbial Community Composition in Three Types of Mine Soil Samples
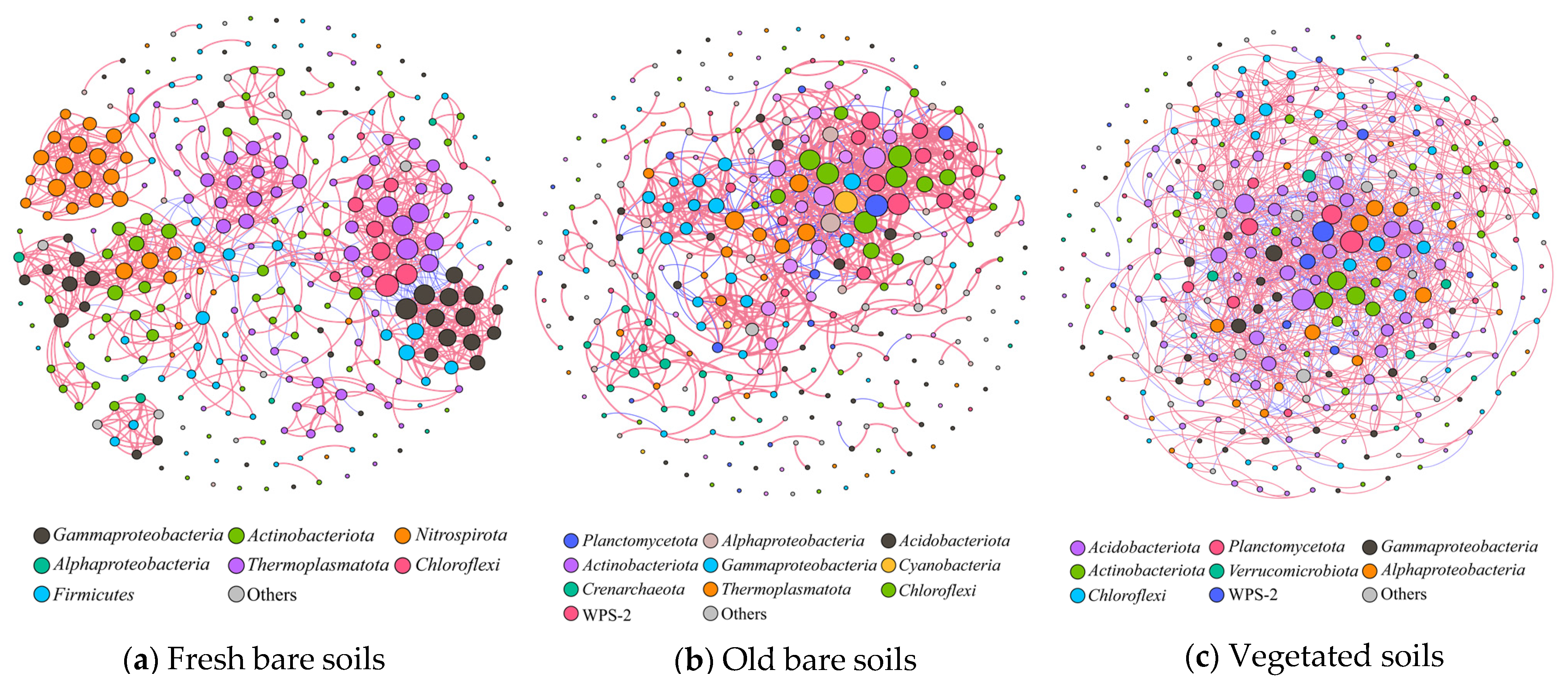
3.3. Microbial Co-Occurrence Network and Community Stability of the Mine Soils
3.4. Effects of Environmental Factors on the Microbial Diversity and Community Composition in the Mine Soils
4. Discussion
4.1. Succession of the Physicochemical Properties in the Mine Soils
4.2. Succession of Microbial Communities in Different Mine Soils
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ussiri, D.A.; Lal, R. Carbon Sequestration in Reclaimed Minesoils. Chem. Rev. Plant Sci. 2005, 24, 151–165. [Google Scholar] [CrossRef]
- Asensio, V.; Vega, F.; Andrade, M.; Covelo, E. Tree vegetation and waste amendments to improve the physical condition of copper mine soils. Chemosphere 2013, 90, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Li, M. Ecological restoration of mineland with particular reference to the metalliferous mine wasteland in China: A review of research and practice. Sci. Total. Environ. 2006, 357, 38–53. [Google Scholar] [CrossRef]
- Cánovas, C.; Peiffer, S.; Macias, F.; Olías, M.; Nieto, J.M. Geochemical processes in a highly acidic pit lake of the Iberian Pyrite Belt (SW Spain). Chem. Geol. 2015, 395, 144–153. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Dangi, S.R.; Stahl, P.D.; Wick, A.F.; Ingram, L.J.; Buyer, J.S. Soil Microbial Community Recovery in Reclaimed Soils on a Surface Coal Mine Site. Soil Sci. Soc. Am. J. 2012, 76, 915–924. [Google Scholar] [CrossRef]
- Hermans, S.M.; Buckley, H.L.; Case, B.S.; Curran-Cournane, F.; Taylor, M.; Lear, G. Using soil bacterial communities to predict physico-chemical variables and soil quality. Microbiome 2020, 8, 1–13. [Google Scholar] [CrossRef]
- Srivastava, S.C.; Jha, A.K.; Singh, J.S. Changes with time in soil Biomass C, N and P of Mine spoils in a dry tropical environment. Can. J. Soil Sci. 1989, 69, 849–855. [Google Scholar] [CrossRef]
- Lei, H.; Peng, Z.; Yigang, H.; Yang, Z. Vegetation and soil restoration in refuse dumps from open pit coal mines. Ecol. Eng. 2016, 94, 638–646. [Google Scholar] [CrossRef]
- Li, X.R.; Kong, D.S.; Tan, H.J.; Wang, X.P. Changes in soil and vegetation following stabilisation of dunes in the southeastern fringe of the Tengger Desert, China. Plant Soil 2007, 300, 221–231. [Google Scholar] [CrossRef]
- Sparling, G.; Ross, D.; Trustrum, N.; Arnold, G.; West, A.; Speir, T.; Schipper, L. Recovery of topsoil characteristics after landslip erosion in dry hill country of New Zealand, and a test of the space-for-time hypothesis. Soil Biol. Biochem. 2003, 35, 1575–1586. [Google Scholar] [CrossRef]
- Brooks, J.P.; Adeli, A.; Smith, R.K.; McGrew, R.; Lang, D.J.; Read, J.J. Bacterial Community Structure Recovery in Reclaimed Coal Mined Soil under Two Vegetative Regimes. J. Environ. Qual. 2019, 48, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Environmental Protection. Water and Wastewater Monitoring and Analysis Methods, 4th ed.; Chinese Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Jiang, Y.-H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Yang, Y.; Chen, S.; Zhao, M.; Zhu, Z.; Yang, S.; Qu, Y.; Ma, Q.; He, Z.; Zhou, J.; et al. Long-term successional dynamics of microbial association networks in anaerobic digestion processes. Water Res. 2016, 104, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Xun, W.; Liu, Y.; Li, W.; Ren, Y.; Xiong, W.; Xu, Z.; Zhang, N.; Miao, Y.; Shen, Q.; Zhang, R. Specialized metabolic functions of keystone taxa sustain soil microbiome stability. Microbiome 2021, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.; Banfield, J. Microbial communities in acid mine drainage. FEMS Microbiol. Ecol. 2003, 44, 139–152. [Google Scholar] [CrossRef] [Green Version]
- Gagnon, V.; Rodrigue-Morin, M.; Tremblay, J.; Wasserscheid, J.; Champagne, J.; Bellenger, J.-P.; Greer, C.W.; Roy, S. Vegetation drives the structure of active microbial communities on an acidogenic mine tailings deposit. PeerJ 2020, 8, e10109. [Google Scholar] [CrossRef]
- Lammel, D.R.; Barth, G.; Ovaskainen, O.; Cruz, L.M.; Zanatta, J.A.; Ryo, M.; De Souza, E.M.; Pedrosa, F.O.; Lammel, D.R.; Barth, G.; et al. Direct and indirect effects of a pH gradient bring insights into the mechanisms driving prokaryotic community structures. Microbiome 2018, 6, 106. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Hallberg, K.B.; Hedrich, S.; Johnson, D.B. Acidiferrobacter thiooxydans, gen. nov. sp. nov.; an acidophilic, thermo-tolerant, facultatively anaerobic iron- and sulfur-oxidizer of the family Ectothiorhodospiraceae. Extremophiles 2011, 15, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Hippe, H. Leptospirillum gen. nov. (ex Markosyan 1972), nom. rev., including Leptospirillum ferrooxidans sp. nov. (ex Markosyan 1972), nom. rev. and Leptospirillum thermoferrooxidans sp. nov. (Golovacheva et al. 1992). Int. J. Syst. Evol. Microbiol. 2000, 50, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, M.S.; Rainey, F.A.; Albuquerque, L. Sulfobacillus. In Bergey’s Manual of Systematics of Archaea and Bacteria; Trujillo, M.E., Dedysh, S., DeVos, P., Hedlund, B., Kämpfer, P., Rainey, F.A., Whitman, W.B., Eds.; John Wiley & Sons: New York, NY, USA, 2015; pp. 1–6. [Google Scholar] [CrossRef]
- Feng, K.; Zhang, Z.; Cai, W.; Liu, W.; Xu, M.; Yin, H.; Wang, A.; He, Z.; Deng, Y. Biodiversity and species competition regulate the resilience of microbial biofilm community. Mol. Ecol. 2017, 26, 6170–6182. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.M.; Guo, X.; Wu, L.; Zhang, Y.; Xiao, N.; Ning, D.; Shi, Z.; Zhou, X.; Wu, L.; Yang, Y.; et al. Climate warming enhances microbial network complexity and stability. Nat. Clim. Chang. 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Hernandez, D.J.; David, A.S.; Menges, E.S.; Searcy, C.A.; Afkhami, M.E. Environmental stress destabilizes microbial networks. ISME J. 2021, 1–13. [Google Scholar] [CrossRef]
- Falagan´-Rodriguez, C.; Johnson, D.B. Acidibacter ferrireducens gen. nov., sp. nov.: An acidophilic ferric iron-reducing gammaproteobacterium. Extremophiles 2014, 18, 1067–1073. [Google Scholar] [CrossRef]
- Bartsch, S.; Gensch, A.; Stephan, S.; Doetsch, A.; Gescher, J. Metallibacterium scheffleri: Genomic data reveal a versatile metabolism. FEMS Microbiol. Ecol. 2017, 93, fix011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, M.; Williams, T.J.; Montgomery, K.; Wong, H.L.; Zaugg, J.; Berengut, J.F.; Bissett, A.; Chuvochina, M.; Hugenholtz, P.; Ferrari, B.C. Candidatus Eremiobacterota, a metabolically and phylogenetically diverse terrestrial phylum with acid-tolerant adaptations. ISME J. 2021, 15, 2692–2707. [Google Scholar] [CrossRef]
- Ward, L.M.; Cardona, T.; Holland-Moritz, H. Evolutionary Implications of Anoxygenic Phototrophy in the Bacterial Phylum Candidatus Eremiobacterota (WPS-2). Front. Microbiol. 2019, 10, 1658. [Google Scholar] [CrossRef] [Green Version]
- Holland-Moritz, H.; Stuart, J.; Lewis, L.R.; Miller, S.; Mack, M.C.; McDaniel, S.F.; Fierer, N. Novel bacterial lineages associated with boreal moss species. Environ. Microbiol. 2018, 20, 2625–2638. [Google Scholar] [CrossRef]
- Sheremet, A.; Jones, G.M.; Jarett, J.; Bowers, R.M.; Bedard, I.; Culham, C.; Eloe-Fadrosh, E.A.; Ivanova, N.; Malmstrom, R.R.; Grasby, S.E.; et al. Ecological and genomic analyses of candidate phylum WPS -2 bacteria in an unvegetated soil. Environ. Microbiol. 2020, 22, 3143–3157. [Google Scholar] [CrossRef]
- Ji, M.; Greening, C.; Vanwonterghem, I.; Carere, C.R.; Bay, S.K.; Steen, J.; Montgomery, K.; Lines, T.; Beardall, J.; van Dorst, J.; et al. Atmospheric trace gases support primary production in Antarctic desert surface soil. Nature 2017, 552, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Kong, W.; Wang, F.; Long, X.-E.; Guo, C.; Yue, L.; Yao, H.; Dong, X. Desert and steppe soils exhibit lower autotrophic microbial abundance but higher atmospheric CO2 fixation capacity than meadow soils. Soil Biol. Biochem. 2018, 127, 230–238. [Google Scholar] [CrossRef]
- Li, Y.; Wen, H.; Chen, L.; Yin, T. Succession of Bacterial Community Structure and Diversity in Soil along a Chronosequence of Reclamation and Re-Vegetation on Coal Mine Spoils in China. PLoS ONE 2014, 9, e115024. [Google Scholar] [CrossRef]
- Huber, K.J.; Geppert, A.M.; Wanner, G.; Fösel, B.U.; Wüst, P.K.; Overmann, J. The first representative of the globally widespread subdivision 6 Acidobacteria, Vicinamibacter silvestris gen. nov., sp. nov., isolated from subtropical savannah soil. Int. J. Syst. Evol. Microbiol. 2016, 66, 2971–2979. [Google Scholar] [CrossRef]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent Understanding of Soil Acidobacteria and Their Ecological Significance: A Critical Review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef]
- Falagán, C.; Foesel, B.; Johnson, B. Acidicapsa ferrireducens sp. nov., Acidicapsa acidiphila sp. nov., and Granulicella acidiphila sp. nov.: Novel acidobacteria isolated from metal-rich acidic waters. Extremophiles 2017, 21, 459–469. [Google Scholar] [CrossRef]
- Kishimoto, N.; Kosako, Y.; Tano, T. Acidobacterium capsulatum gen. nov., sp. nov. An acidophilic chemoorganotrophic bacterium containing menaquinone from acidic mineral environment. Curr. Microbiol. 1991, 22, 1–7. [Google Scholar] [CrossRef]
- Männistö, M.K.; Rawat, S.; Starovoytov, V.; Haggblom, M. Granulicella arctica sp. nov., Granulicella mallensis sp. nov., Granulicella tundricola sp. nov. and Granulicella sapmiensis sp. nov., novel acidobacteria from tundra soil. Int. J. Syst. Evol. Microbiol. 2012, 62, 2097–2106. [Google Scholar] [CrossRef] [Green Version]
- Okamura, K.; Kawai, A.; Yamada, T.; Hiraishi, A. Acidipila rosea gen. nov., sp. nov., an acidophilic chemoorganotrophic bacterium belonging to the phylum Acidobacteria. FEMS Microbiol. Lett. 2011, 317, 138–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kielak, A.M.; Barreto, C.; Kowalchuk, G.A.; Van Veen, J.A.; Kuramae, E.E. The Ecology of Acidobacteria: Moving beyond Genes and Genomes. Front. Microbiol. 2016, 7, 744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolksdorf, B.; Nie, C.; Niemeyer, D.; Röhrs, V.; Berg, J.; Lauster, D.; Adler, J.M.; Haag, R.; Trimpert, J.; Kaufer, B.; et al. Inhibition of SARS-CoV-2 replication by a small interfering RNA targeting the leader sequence. Viruses 2021, 13, 2030. [Google Scholar] [CrossRef] [PubMed]
- Bogino, P.C.; Oliva, M.D.L.M.; Sorroche, F.G.; Giordano, W. The Role of Bacterial Biofilms and Surface Components in Plant-Bacterial Associations. Int. J. Mol. Sci. 2013, 14, 15838–15859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianciotto, V.; Andreotti, S.; Balestrini, R.; Bonfante, P.; Perotto, S. Extracellular polysaccharides are involved in the attachment of Azospirillum brasilense and Rhizobium leguminosarum to arbuscular mycorrhizal structures. Eur. J. Histochem. 2009, 45, 39–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kielak, A.M.; Cipriano, M.A.; Kuramae, E.E. Acidobacteria strains from subdivision 1 act as plant growth-promoting bacteria. Arch. Microbiol. 2016, 198, 987–993. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Wei, P.; Banda, J.F.; Ma, L.; Mao, W.; Li, H.; Hao, C.; Dong, H. Succession of Microbial Communities in Waste Soils of an Iron Mine in Eastern China. Microorganisms 2021, 9, 2463. https://doi.org/10.3390/microorganisms9122463
Zhang Q, Wei P, Banda JF, Ma L, Mao W, Li H, Hao C, Dong H. Succession of Microbial Communities in Waste Soils of an Iron Mine in Eastern China. Microorganisms. 2021; 9(12):2463. https://doi.org/10.3390/microorganisms9122463
Chicago/Turabian StyleZhang, Qin, Pengfei Wei, Joseph Frazer Banda, Linqiang Ma, Weiao Mao, Hongyi Li, Chunbo Hao, and Hailiang Dong. 2021. "Succession of Microbial Communities in Waste Soils of an Iron Mine in Eastern China" Microorganisms 9, no. 12: 2463. https://doi.org/10.3390/microorganisms9122463
APA StyleZhang, Q., Wei, P., Banda, J. F., Ma, L., Mao, W., Li, H., Hao, C., & Dong, H. (2021). Succession of Microbial Communities in Waste Soils of an Iron Mine in Eastern China. Microorganisms, 9(12), 2463. https://doi.org/10.3390/microorganisms9122463





