Disinfectant and Antimicrobial Susceptibility Studies of Staphylococcus aureus Strains and ST398-MRSA and ST5-MRSA Strains from Swine Mandibular Lymph Node Tissue, Commercial Pork Sausage Meat and Swine Feces
Abstract
1. Introduction
2. Materials and Methods
2.1. Staphylococcus aureus Strains
2.2. Susceptibility Testing
2.3. Antimicrobial Susceptibility Testing
2.4. DNA Isolation from MRSA Strains for Molecular Analysis
2.5. Multilocus Sequence Typing of the Extracted DNA
2.6. Disinfectant Susceptibility Testing
2.7. Calculation of Theoretical MICs for Multiple Component Disinfectants
2.7.1. Calculation of theoMICs for the Active Components of the Complex Disinfectant DC&RCP against S. aureus Strains
2.7.2. Calculation of theoMICs for the Active Components of the Complex Disinfectant P-128CP against S. aureus Strains
3. Results
3.1. Antimicrobial Resistance
3.2. Multilocus Sequence Typing
3.3. Disinfectant Susceptibility
3.4. Calculation of Theoretical MICs for Multiple Component Disinfectants
3.5. Staphylococcus aureus Inhibition by Ammonium Chloride Disinfectant Components
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diekema, D.J.; Pfaller, M.A.; Schmitz, F.J.; Smayevsky, J.; Bell, J.; Jones, R.N.; Beach, M.; SENTRY Participants Group. Survey of infections due to Staphylococcus species: Frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin. Infect. Dis. 2001, 32 (Suppl. 2), S114–S132. [Google Scholar] [CrossRef]
- Morgan, M. Methicillin-resistant Staphylococcus aureus and animals: Zoonosis or humanosis? J. Antimicrob. Chemother. 2008, 62, 1181–1187. [Google Scholar] [CrossRef]
- Cuny, C.; Friedrich, A.; Kozytska, S.; Layer, F.; Nübel, U.; Ohlsen, K.; Strommenger, B.; Walther, B.; Wieler, L.; Witte, W. Emergence of methicillin-resistant Staphylococcus aureus (MRSA) in different animal species. Int. J. Med. Microbiol. 2010, 300, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Lassok, B.; Tenhagen, B.-A. From pig to pork: Methicillin-resistant Staphylococcus aureus in the pork production chain. J. Food Protect. 2013, 76, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Spahich, N.A.; Vitko, N.P.; Thurlow, L.R.; Temple, B.; Richardson, A.R. Staphylococcus aureus lactate-and malate-quinone oxidoreductases contribute to nitric oxide resistance and virulence. Mol. Microbiol. 2016, 100, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Saïd-Salim, B.; Dunman, P.M.; McAleese, F.M.; Macapagal, D.; Murphy, E.; McNamara, P.J.; Arvidson, S.; Foster, T.J.; Projan, S.J.; Kreiswirth, B.N. Global regulation of Staphylococcus aureus genes by Rot. J. Bacteriol. 2003, 185, 610–619. [Google Scholar] [CrossRef]
- Otero, M.C.; Morelli, L.; Nader-Macías, M.E. Probiotic properties of vaginal lactic acid bacteria to prevent metritis in cattle. Lett. Appl. Microbiol. 2006, 43, 91–97. [Google Scholar] [CrossRef]
- Kluytmans, J.; van Belkum, A.; Verbrugh, H. Nasal carriage of Staphylococcus aureus: Epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 1997, 10, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Noskin, G.A.; Rubin, R.J.; Schentag, J.J.; Kluytmans, J.; Hedblom, E.C.; Smulders, M.; Lapetina, E.; Gemmen, E. The burden of Staphylococcus aureus infections on hospitals in the United States: An analysis of the 2000 and 2001 Nationwide Inpatient Sample Database. Arch. Intern. Med. 2005, 165, 1756–1761. [Google Scholar] [CrossRef]
- McCaig, L.F.; McDonald, L.C.; Mandal, S.; Jernigan, B. Staphylococcus aureus–associated skin and soft tissue infections in ambulatory care. Emerg. Infect. Dis. 2006, 12, 1715–1723. [Google Scholar] [CrossRef]
- Gould, I.M. Antibiotics, skin and soft tissue infection and methicillin-resistant Staphylococcus aureus: Cause and effect. Int. J. Antimicrob. Agents 2009, 34 (Suppl. 1), S8–S11. [Google Scholar] [CrossRef]
- David, M.Z.; Daum, R.S. Community-associated methicillin-resistant Staphylococcus aureus: Epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 2010, 23, 616–687. [Google Scholar] [CrossRef]
- Mohanty, A.; Mohapaira, K.C.; Pal, B.B. Isolation and identification of Staphylococcus aureus from skin and soft tissue infection in sepsis cases, Odisha. J. Pure Appl. Microbiol. 2018, 12, 419–424. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Foodborne Germs and Illnesses. 2019. Available online: https://www.cdc.gov/foodsafety/foodborne-germs.html (accessed on 26 December 2020).
- Jevons, M. “Celberin”-resistant staphylococci. Br. Med. J. 1961, 1, 124–125. [Google Scholar] [CrossRef]
- Peacock, S.J.; Paterson, G.K. Mechanisms of methicillin resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015, 84, 577–601. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 31, 18033. [Google Scholar] [CrossRef] [PubMed]
- National Center for Case Methicillin resistant Staphylococcus aureus (MRSA). 2020. Available online: https://sciencecases.lib.buffalo.edu/files/Supplemental/UploadFolder/skin_graft.pdf (accessed on 26 December 2020).
- Hryniewicz, M.M.; Garbacz, K. Borderline oxacillin-resistant Staphylococcus aureus (BORSA)–a more common problem than expected? J. Med. Microbiol. 2017, 66, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.; Linden, P.K.; Friedman, B. Incidence, prevalence, and management of MRSA bacteremia across patient populations—A review of recent developments in MRSA management and treatment. Crit. Care 2017, 21, 211. [Google Scholar] [CrossRef]
- Kluytmans, J.; van Leeuwen, W.; Goessens, W.; Hollis, R.; Messer, S.; Herwaldt, L.; Bruining, H.; Heck, M.; Rost, J.; van Leeuwen, N.; et al. Food-initiated outbreak of methicillin-resistant Staphylococcus aureus analyzed by pheno-and genotyping. J. Clin. Microbiol. 1995, 33, 1121–1128. [Google Scholar] [CrossRef]
- Jones, T.F.; Kellum, M.E.; Porter, S.S.; Bell, M.; Schaffner, W. An outbreak of community-acquired foodborne illness caused by methicillin-resistant Staphylococcus aureus. Emerg. Infect. Dis. 2002, 8, 82–84. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Staphylococcal (Staph) Food Poisoning. 2018. Available online: https://www.cdc.gov/foodsafety/diseases/staphylococcal.html (accessed on 26 December 2020).
- do Carmo, L.S.; Dias, R.S.; Linardi, V.R.; de Sena, M.J.; dos Santos, D.A.; de Faria, M.E.; Pena, E.C.; Jett, M.; Heneine, L.G. Food poisoning due to enterotoxigenic strains of Staphylococcus present in Minas cheese and raw milk in Brazil. Food Microbiol. 2002, 19, 9–14. [Google Scholar] [CrossRef]
- Normanno, G.; Firinu, A.; Virgilio, S.; Mula, G.; Dambrosio, A.; Poggiu, A.; Decastelli, L.; Mioni, R.; Scuota, S.; Bolzoni, G.; et al. Coagulase-positive Staphylococci and Staphylococcus aureus in food products marked in Italy. Int. J. Food Microbiol. 2005, 98, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Kérouanton, A.; Hennekinne, J.A.; Letertre, C.; Petit, L.; Chesneau, O.; Brisabois, A.; De Buyer, M.L. Characterization of Staphylococcus aureus strains associated with food poisoning outbreaks in France. Int. J. Food Microbiol. 2007, 115, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Tsegmed, U.; Normanno, G.; Pringle, M.; Krovacek, K. Occurrence of enterotoxic Staphylococcus aureus in raw milk from yaks and cattle in Mongolia. J. Food Protect. 2007, 70, 1726–1729. [Google Scholar] [CrossRef] [PubMed]
- Medveďová, A.; Valík, Ľ.; Sirotná, Z.; Liptáková, D. Growth characterisation of Staphylococcus aureus in milk: A quantitative approach. Czech. J. Food Sci. 2009, 27, 443–453. [Google Scholar] [CrossRef]
- Medveďová, A.; Valík, Ľ. Staphylococcus aureus: Characterisation and quantitative growth description in milk and artisanal raw milk cheese production. In Structure and Function of Food Engineering; Eissa, A.A., Ed.; IntechOpen: London, UK, 2012; Available online: https://www.intechopen.com/books/structure-and-function-of-food-engineering/staphylococcus-aureus-characterisation-and-quantitative-growth-description-in-milk-and-artisanal-raw (accessed on 4 February 2021). [CrossRef]
- Halpin-Dohnalek, M.I.; Marth, E.H. Staphylococcus aureus: Production of extracellular compounds and behavior in foods–A review. J. Food Protect. 1989, 52, 267–282. [Google Scholar] [CrossRef]
- Baird-Parker, T.C. Staphylococcus aureus. Chapter 47. In The Microbiological Safety and Quality of Food; Lund, B.M., Baird-Parker, T.C., Gould, G.W., Gaithersburg, M.D., Eds.; Aspen Publishers Inc.: New York, NY, USA, 2000; pp. 1317–1330. ISBN 0-8342-1323-0. [Google Scholar]
- Ananou, S.; Maqueda, M.; Martínez-Bueno, M.; Gálvez, A.; Valdivia, E. Control of Staphylococcus aureus in sausages by enterocin AS-48. Meat Sci. 2005, 71, 549–556. [Google Scholar] [CrossRef]
- Azimirad, M.; Dezfulian, A.; Alebouyeh, M.; Esfehani, R.B.; Shahroskh, S.; Zali, M.R. Infection with enterotoxigenic Staphylococcus aureus as a concern in patients with gastroenteritis. J. Glob. Antimicrob. Res. 2017, 9, 111–114. [Google Scholar] [CrossRef]
- Pexara, A.; Bourriel, A.; Govaris, A. Staphylococcus aureus and Staphylococcal enterotoxins in foodborne diseases. J. Hell. Vet. Med. Soc. 2018, 61, 316–322. [Google Scholar] [CrossRef][Green Version]
- Seo, K.S.; Bohach, G.A. Staphylococcus aureus, Chapter 21. In Food Microbiology: Fundamentals and Frontiers, 4th ed.; Doyle, M.P., Buchanan, R.L., Eds.; ASM Press: Washington, DC, USA, 2012; pp. 547–573. [Google Scholar] [CrossRef]
- Craven, S.E.; Blankenship, L.C.; Mercuri, A.J. Growth and production of enterotoxin by Staphylococcus aureus S-6 in soy proteins and soy-supplemented beef and pork sausage. J. Food Protect. 1978, 41, 794–797. [Google Scholar] [CrossRef]
- Verstappen, K.M.; Willems, E.; Fluit, A.C.; Duim, B.; Martens, M.; Wagenaar, J.A. Staphylococcus aureus nasal colonization differs among pig lineages and is associated with the presence of other staphylococcal species. Front. Vet. Sci. 2017, 4, 97. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S. Methicillin-resistant Staphylococcus aureus in animals. ILAR J. 2010, 51, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Carrel, M.; Schweizer, M.L.; Sarrazin, M.V.; Smith, T.C.; Perencevich, E.N. Residential proximity to large numbers of swine in feeding operations is associated with increased risk of methicillin-resistant Staphylococcus aureus colonization at time of hospital admission in rural Iowa veterans. Infect. Control Hosp. Epidemiol. 2014, 35, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Hau, S.J.; Sun, J.; Davies, P.R.; Frana, T.S.; Nicholson, T.L. Comparative prevalence of immune evasion complex genes associated with β-hemolysin converting bacteriophages in MRSA ST5 isolates from swine, swine facilities, humans with swine contact, and humans with no swine contact. PLoS ONE 2015, 10, e0142832. [Google Scholar] [CrossRef]
- Kadlec, K.; Feßler, A.T.; Hauscheld, T.; Schwarz, S. Novel and uncommon antimicrobial resistance genes in livestock-associated methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 2012, 18, 745–755. [Google Scholar] [CrossRef]
- Akwar, T.H.; Poppe, C.; Wilson, J.; Reid-Smith, R.J.; Dyck, M.; Waddington, J.; Shang, N.; Dassie, S.A.; McEwen, S.A. Risk factors for antimicrobial resistance among fecal Escherichia coli from residents on forty-three swine farms. Microb. Drug Resist. 2007, 13, 69–76. [Google Scholar] [CrossRef]
- Silbergeld, E.K.; Graham, J.; Price, L.B. Industrial food animal production, antimicrobial resistance, and human health. Ann. Rev. Pub. Health 2008, 29, 51–169. [Google Scholar] [CrossRef]
- Voss, A.; Loeffen, F.; Bakker, J.; Klaassen, C.; Wulf, M. Methicillin-resistant Staphylococcus aureus in pig farming. Emerg. Infect. Dis. 2005, 11, 1965–1966. [Google Scholar] [CrossRef]
- de Neeling, A.J.; van den Broek, M.J.M.; Spalburg, E.C.; van Santen-Verheuvel, M.G.; Dam-Deisz, W.D.C.; Boshuizen, H.C.; van de Giessen, A.W.; van Duijkeren, E.; Huijsdens, X.W. High prevalence of methicillin resistant Staphylococcus aureus in pigs. Vet. Microbiol. 2007, 122, 366–372. [Google Scholar] [CrossRef]
- Lewis, H.C.; Mølbak, K.; Reese, C.; Aarestrup, F.M.; Selchau, M.; Sørum, M.; Skov, R.L. Pigs as source of methicillin-resistant Staphylococcus aureus CC398 infections in humans, Denmark. Emerg. Infect. Dis. 2008, 14, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.C.; Male, M.J.; Harper, A.L.; Kroeger, J.S.; Tinkler, G.P.; Moritz, E.D.; Capuano, A.W.; Herwaldt, L.A.; Diekema, D.J. Methicillin-resistant Staphylococcus aureus (MRSA) strain ST398 is present in midwestern U.S. swine and swine workers. PLoS ONE 2009, 4, e4258. [Google Scholar] [CrossRef] [PubMed]
- Rinsky, J.L.; Nadimpalli, M.; Wing, S.; Hall, D.; Baron, D.; Price, L.B.; Larsen, J.; Stegger, M.; Stewart, J.; Heaney, C.A. Livestock-associated methicillin and multidrug resistant Staphylococcus aureus is present among industrial, not antibiotic-free livestock operation workers in North Carolina. PLoS ONE 2013, 8, e67641. [Google Scholar] [CrossRef]
- Nadimpalli, M.; Rinsky, J.L.; Wing, S.; Hall, D.; Stewart, J.; Larsen, J.; Nachman, K.E.; Love, D.C.; Pierce, E.; Pisanic, N.; et al. Persistence of livestock-associated antibiotic-resistant Staphylococcus aureus among industrial hog operation workers in North Carolina over 14 days. Occup. Environ. Med. 2015, 72, 90–99. [Google Scholar] [CrossRef][Green Version]
- Köck, R.; Harlizius, J.; Bressan, N.; Laerberg, R.; Wieler, L.H.; Witte, W.; Deurenberg, R.H.; Voss, A.; Becker, K.; Friedrich, A.W. Prevalence and molecular characteristics of methicillin-resistant Staphylococcus aureus (MRSA) among pigs on German farms and import of livestock-related MRSA into hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1375–1382. [Google Scholar] [CrossRef]
- Köck, R.; Siam, K.; Al-Malat, S.; Christmann, J.; Schaumburg, F.; Becker, K.; Friedrich, A.W. Characteristics of hospital patients colonized with livestock-associated methicillin-resistant Staphylococcus aureus (MRSA) CC398 versus other MRSA clones. J. Hosp. Infect. 2011, 79, 292–296. [Google Scholar] [CrossRef]
- Wulf, M.W.H.; Verduin, C.M.; van Nes, A.; Huijsdens, X.; Voss, A. Infection and colonization with methicillin resistant Staphylococcus aureus ST398 versus other MRSA in an area with a high density of pig farms. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 61–65. [Google Scholar] [CrossRef]
- Smith, T.C.; Gebreyes, W.A.; Abley, M.J.; Harper, A.L.; Forshey, B.M.; Male, M.J.; Martin, H.W.; Molla, B.Z.; Sreevatsan, S.; Thakur, S.; et al. Methicillin-resistant Staphylococcus aureus in pigs and farm workers on conventional and antibiotic-free swine farms in the USA. PLoS ONE 2013, 8, e63704. [Google Scholar] [CrossRef]
- O’Brien, A.M.; Hanson, B.M.; Farina, S.A.; Wu, J.Y.; Simmering, J.E.; Wardyn, S.E.; Forshey, B.M.; Kulick, M.E.; Wallinga, D.B.; Smith, T.C. MRSA in conventional and alternative retail pork products. PLoS ONE 2012, 7, e30092. [Google Scholar] [CrossRef]
- Verkade, E.; Kluytmans, J. Livestock-associated Staphylococcus aureus CC398: Animal reservoirs and human infections. Infect. Genet. Evol. 2014, 21, 523–530. [Google Scholar] [CrossRef]
- Anjum, M.F.; Marco-Jimenez, F.; Duncan, D.; Marín, C.; Smith, R.P.; Evans, S.J. Livestock-associated methicillin-resistant Staphylococcus aureus from animals and animal products in the UK. Front. Microbiol. 2019, 10, 2136. [Google Scholar] [CrossRef]
- Parisi, A.; Caruso, M.; Normanno, G.; Latorre, L.; Miccolupo, A.; Fraccalvieri, R.; Intini, F.; Manginelli, T.; Santagada, G. MRSA in swine, farmers and abattoir workers in Southern Italy. Food Microbiol. 2019, 82, 287–293. [Google Scholar] [CrossRef]
- Cuny, C.; Wieler, L.H.; Witte, W. Livestock-associated MRSA: The impact on humans. Antibiotics 2015, 4, 521–543. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.C.; Wardyn, S.E. Human infections with Staphylococcus aureus CC398. Cur. Environ. Health Rpt. 2015, 2, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, S.E.; Forshey, B.M.; Farina, S.A.; Kates, A.E.; Nair, R.; Quick, M.K.; Wu, J.Y.; Hanson, B.M.; O’Malley, S.M.; Shows, H.W.; et al. Swine farming is a risk factor for infection with high prevalence of carriage of multidrug-resistant Staphylococcus aureus. Clin. Infect. Dis. 2015, 61, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Nadimpalli, M.; Stewart, J.R.; Pierce, E.; Pisanic, N.; Love, D.C.; Hall, D.; Larsen, J.; Carroll, D.C.; Tekle, T.; Perl, T.M.; et al. Livestock-associated, antibiotic-resistant Staphylococcus aureus nasal carriage and recent skin and soft tissue infection among industrial hog operation workers. PLoS ONE 2016, 11, e0165713. [Google Scholar] [CrossRef]
- Ye, X.; Fan, Y.; Wang, X.; Liu, W.; Yu, H.; Zhou, J.; Chen, S.; Yao, Z. Livestock-associated methicillin and multidrug resistant S. aureus in humans is associated with occupational pig contact, and pet contact. Sci. Rep. 2016, 6, 19184. [Google Scholar] [CrossRef]
- Hatcher, S.M.; Rhodes, S.M.; Stewart, J.R.; Silbergweld, E.; Pisanic, N.; Larsen, J.; Jiang, S.; Krosche, A.; Hall, D.; Carroll, K.C.; et al. The prevalence of antibiotic-resistant Staphylococcus aureus nasal carriage among industrial hog operation workers, community residents, and children living in their households: North Carolina, USA. Environ. Health Perspect. 2017, 125, 560–569. [Google Scholar] [CrossRef]
- Davis, M.F.; Pisanic, N.; Rhodes, S.M.; Brown, A.; Keller, H.; Nadimpalli, M.; Christ, A.; Ludwig, S.; Ordak, C.; Spicer, K.; et al. Occurrence of Staphylococcus aureus in swine and swine workplace environments on industrial and antibiotic-free hog operations in North Carolina, USA: A one health pilot study. Environ. Res. 2018, 163, 88–96. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus—Minnesota and North Dakota, 1997–1999. Morb. Mortal. Wkly. Rep. 1999, 48, 707–710. [Google Scholar] [PubMed]
- Bens, C.C.; Voss, A.; Klaassen, C.H. Presence of a novel DNA methylation enzyme in methicillin-resistant Staphylococcus aureus isolates associated with pig farming leads to uninterpretable results in standard pulsed-field gel electrophoresis analysis. J. Clin. Microbiol. 2006, 44, 1875–1876. [Google Scholar] [CrossRef][Green Version]
- Enright, M.C.; Day, N.P.J.; Davies, C.E.; Peacock, S.J.; Spratt, B.G. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 2000, 38, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Beier, R.C.; Bischoff, K.M.; Ziprin, R.L.; Poole, T.L.; Nisbet, D.J. Chlorhexidine susceptibility, virulence factors, and antibiotic resistance of beta-hemolytic Escherichia coli isolated from neonatal swine with diarrhea. Bull. Environ. Contam. Toxicol. 2005, 75, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Beier, R.C.; Byrd, J.A.; Andrews, K.; Caldwell, D.; Crippen, T.L.; Anderson, R.C.; Nisbet, D.J. Disinfectant and antimicrobial susceptibility studies of the foodborne pathogen Campylobacter jejuni isolated from the litter of broiler chicken houses. Poult. Sci. 2021, 100, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- White, D.G.; McDermott, P.F. Biocides, drug resistance and microbial evolution. Curr. Opin. Microbiol. 2001, 4, 313–317. [Google Scholar] [CrossRef]
- Maris, P. Resistance of 700 gram-negative bacterial strains to antiseptics and antibiotics. Ann. Rech. Vet. 1991, 22, 11–23. [Google Scholar]
- Sidhu, M.S.; Heir, E.; Leegaard, T.K.; Wiger, K.; Holck, A. Frequency of disinfectant resistance genes and genetic linkage with β-lactamase transposon Tn552 among clinical staphylococci. Antimicrob. Agents Chemother. 2002, 46, 2797–2803. [Google Scholar] [CrossRef]
- Braoudaki, M.; Hilton, A.C. Adaptive resistance to biocides in Salmonella enterica and Escherichia coli O157 and cross-resistance to antimicrobial agents. J. Clin. Microbiol. 2004, 42, 73–78. [Google Scholar] [CrossRef]
- Davin-Regli, A.; Pagès, J.-M. Cross-resistance between biocides and antimicrobials: An emerging question. Rev. Sci. Tech. Int. Off. Epiz. 2012, 31, 89–104. [Google Scholar] [CrossRef]
- Al-Jailawi, M.H.; Ameen, R.S.; Al-Jeboori, M.R. Effect of disinfectants on antibiotics susceptibility of Pseudomonas aeruginosa. J. Appl. Biotechnol. 2013, 1, 54–63. [Google Scholar] [CrossRef]
- Gnanadhas, D.P.; Marathe, S.A.; Chakravortty, D. Biocides—Resistance, cross-resistance mechanisms and assessment. Expert Opin. Investig. Drugs 2013, 22, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.G.; Saye, E.J.; Jimenez-Truque, N.; Soper, N.; Thomsen, I.; Talbot, T.R.; Creech, C.B. Frequency of disinfectant resistance genes in pediatric strains of methicillin-resistant Staphylococcus aureus. Infect. Control Hosp. Epidemiol. 2013, 34, 1326–1327. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wales, A.D.; Davies, R.H. Co-selection of resistance to antibiotics, biocides and heavy metals, and its relevance to foodborne pathogens. Antibiotics 2015, 4, 567–604. [Google Scholar] [CrossRef]
- Romaro, J.L.; Burgos, M.J.G.; Pérez-Pulido, R.; Gálvez, A.; Lucas, R. Resistance to antibiotics, biocides, preservatives and metals in bacteria isolated from seafoods: Co-selection of strains resistant or tolerant to different classes of compounds. Front. Microbiol. 2017, 8, 1650. [Google Scholar] [CrossRef] [PubMed]
- Wand, M.E.; Bock, L.J.; Bonney, L.C.; Sutton, J.M. Mechanisms of increased resistance to chlorhexidine and cross-resistance to colistin following exposure of Klebsiella pneumoniae clinical isolates to chlorhexidine. Antimicrob. Agents Chemother. 2017, 61, 1–31. [Google Scholar] [CrossRef]
- Cadena, M.; Kelman, T.; Marco, M.L.; Pitesky, M. Understanding antimicrobial resistance (AMR) profiles of Salmonella biofilm and planktonic bacteria challenged with disinfectants commonly used during poultry processing. Foods 2019, 8, 275. [Google Scholar] [CrossRef]
- Morente, E.O.; Fernández-Fuentes, M.A.; Grande Burgos, M.J.; Abriouel, H.; Pulido, R.P.; Gálvez, A. Biocide tolerance in bacteria. Int. J. Food Microbiol. 2013, 162, 13–25. [Google Scholar] [CrossRef]
- Capita, R.; Riesco-Peláez, F.; Alonso-Hernando, A.; Alonso-Calleja, C. Exposure of Escherichia coli ATCC 12806 to sublethal concentrations of food-grade biocides influences its ability to form biofilm, resistance to antimicrobials, and ultrastructure. Appl. Environ. Microbiol. 2014, 80, 1268–1280. [Google Scholar] [CrossRef]
- Ziech, R.E.; Perin, A.P.; Lampurnani, C.; Sereno, M.J.; Viana, C.; Soares, V.M.; Pereira, J.G.; Pinto, J.P.d.A.N.; Bersot, L.d.S. Biofilm-processing ability and tolerance to industrial sanitizers in Salmonella spp. isolated from Brazilian poultry processing plants. LWT Food Sci. Technol. 2016, 68, 85–90. [Google Scholar] [CrossRef]
- Takasaki, A.; Hashida, T.; Fujiwara, S.; Kato, K.-I.; Nishihara, T. Bactericidal action of a quaternary ammonium disinfectant, didecyldimethyl ammonium chloride, against Staphylococcus aureus. Jpn. J. Toxicol. Environ. Health 1994, 40, 344–350. [Google Scholar] [CrossRef][Green Version]
- Ioannou, C.J.; Hanlon, G.W.; Denyer, S.P. Action of disinfectant quaternary ammonium compounds against Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Heir, E.; Sundheim, G.; Holck, A.L. Resistance to quaternary ammonium compounds in Staphylococcus spp. isolated from the food industry and nucleotide sequence of the resistance plasmid pST827. J. Appl. Bacteriol. 1995, 79, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Barbee, S.L.; Aguiar, N.C.; Sobsey, M.D.; Weber, D.J. Antimicrobial activity of home disinfectants and natural products against potential human pathogens. Infect. Control Hosp. Epidemiol. 2000, 21, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Aboualizadeh, E.; Bumah, V.V.; Masson-Meyers, D.S.; Eells, J.T.; Hirschmugl, C.J.; Enwermeka, C.S. Understanding the antimicrobial activity of selected disinfectants against methicillin-resistant Staphylococcus aureus (MRSA). PLoS ONE 2017, 12, e0186375. [Google Scholar] [CrossRef]
- Lineback, C.B.; Nkemngong, C.A.; Wu, S.T.; Li, X.; Teska, P.J.; Oliver, H.F. Hydrogen peroxide and sodium hypochlorite disinfectants are more effective against Staphylococcus aureus and Pseudomonas aeruginosa biofilms than quaternary ammonium compounds. Antimicrob. Resist. Infect. Control 2018, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Beier, R.C.; Poole, T.L.; Brichta-Harhay, D.M.; Anderson, R.C.; Bischoff, K.M.; Hernandez, C.A.; Bono, J.L.; Arthur, T.M.; Nagaraja, T.G.; Crippen, T.L.; et al. Disinfectant and antibiotic susceptibility profiles of Escherichia coli O157:H7 strains from cattle carcasses, feces, and hides and ground beef from the United States. J. Food Protect. 2013, 76, 6–17. [Google Scholar] [CrossRef]
- Beier, R.C.; Foley, S.L.; Davidson, M.K.; White, D.G.; McDermott, P.F.; Bodeis-Jones, S.; Zhao, S.; Andrews, K.; Crippen, T.L.; Sheffield, C.L.; et al. Characterization of antibiotic and disinfectant susceptibility profiles among Pseudomonas aeruginosa veterinary isolates recovered during 1994–2003. J. Appl. Microbiol. 2014, 118, 326–342. [Google Scholar] [CrossRef]
- Beier, R.C.; Franz, E.; Bono, J.L.; Mandrell, R.E.; Fratamico, P.M.; Callaway, T.R.; Andrews, K.; Poole, T.L.; Crippen, T.L.; Sheffield, C.L.; et al. Disinfectant and antimicrobial susceptibility profiles of the big six non-O157 Shiga toxin-producing Escherichia coli strains from food animals and humans. J. Food Protect. 2016, 79, 1355–1370. [Google Scholar] [CrossRef]
- Beier, R.C.; Callaway, T.R.; Andrews, K.; Poole, T.L.; Crippen, T.L.; Anderson, R.C.; Nisbet, D.J. Disinfectant and antimicrobial susceptibility profiles of Salmonella strains from feedlot water-sprinkled cattle: Hides and feces. J. Food Chem. Nanotechnol. 2017, 3, 50–59. [Google Scholar] [CrossRef]
- Beier, R.C.; Harvey, R.B.; Hernandez, C.A.; Andrews, K.; Droleskey, R.E.; Hume, M.E.; Davidson, M.K.; Bodeis-Jones, S.; Young, S.; Anderson, R.C.; et al. Disinfectant and antimicrobial susceptibility profiles of Campylobacter coli isolated in 1998 to 1999 and 2015 from swine and commercial pork chops. J. Food Sci. 2019, 84, 1501–1512. [Google Scholar] [CrossRef]
- Beier, R.C.; Duke, S.E.; Ziprin, R.L.; Harvey, R.B.; Hume, M.E.; Poole, T.L.; Scott, H.M.; Highfield, L.D.; Alali, W.Q.; Andrews, K.; et al. Antibiotic and disinfectant susceptibility profiles of vancomycin-resistant Enterococcus faecium (VRE) isolated from community wastewater in Texas. Bull. Environ. Contam. Toxicol. 2008, 80, 188–194. [Google Scholar] [CrossRef]
- Beier, R.C.; Andrews, K.; Poole, T.L.; Harvey, R.B.; Crippen, T.L.; Anderson, R.C.; Nisbet, D.J. Interactions of organic acids with Staphylococcus aureus and MRSA strains from swine mandibular lymph node tissue, commercial pork sausage meat and feces. Int. J. Microbiol. Biotechnol. 2020, 5, 165–183. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. In Approved Standard—3rd Edition—Document VET01S; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. In Approved Standard—11th Edition—Document M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Langsrud, S.; Sundheim, G. Factors influencing a suspension test method for antimicrobial activity of disinfectants. J. Appl. Microbiol. 1998, 85, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), 5–16. [Google Scholar] [CrossRef]
- Beier, R.C.; Byrd, J.A.; Caldwell, D.; Andrews, K.; Crippen, T.L.; Anderson, R.C.; Nisbet, D.J. Inhibition and interactions of Camplylobactder jejuni from broiler chicken houses with organic acids. Microorganisms 2019, 7, 223. [Google Scholar] [CrossRef]
- Galaxy Australia. Galaxy Training Materials. 2021. Available online: https://usegalaxy.org.au/ (accessed on 26 April 2021).
- CaviCide™: Surface Disinfectants. 2021. Available online: https://www.metrex.com/en-us/products/surface-disinfectants/cavicide/ (accessed on 15 February 2021).
- Triclocarban Information. 2021. Available online: https://www.cosmeticsinfo.org/triclocarban-information (accessed on 15 February 2021).
- Satyro, S.; Saggioro, E.M.; Veríssimo, F.; Buss, D.F.; de Magalhães, D.P.; Oliveira, A. Triclocarban: UV photolysis, wastewater disinfection, and ecotoxicity assessment using molecular biomarkers. Environ. Sci. Pollut. Res. 2017, 24, 16077–16085. [Google Scholar] [CrossRef]
- Chen, J.; Ahn, K.C.; Gee, N.A.; Ahmed, M.I.; Duleba, A.J.; Zhao, L.; Gee, S.J.; Hammock, B.D.; Lasley, B.L. Triclocarban enhances testosterone action: A new type of endocrine disruptor? Endocrinology 2008, 149, 1173–1179. [Google Scholar] [CrossRef]
- Tarnow, P.; Tralau, T.; Hunecke, D.; Luch, A. Effects of triclocarban on the transcription of estrogen, androgen and aryl hydrocarbon receptor responsive genes in hman breast cancer cells. Toxicol. Vitr. 2013, 27, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.J.; Rock, C.O. A triclosan-resistant bacterial enzyme. Nature 2000, 406, 145–146. [Google Scholar] [CrossRef]
- Leelaporn, A.; Paulsen, I.T.; Tennent, J.M.; Littlejohn, T.G.; Skurray, R.A. Multidrug resistance to antiseptics and disinfectants in coagulase-negative staphylococci. J. Med. Microbiol. 1994, 40, 214–220. [Google Scholar] [CrossRef]
- Sidhu, M.S.; Sørum, H.; Holck, A. Resistance to quaternary ammonium compounds in food-related bacteria. Microb. Drug Resist. 2002, 8, 393–399. [Google Scholar] [CrossRef]
- Tdenover, F.C. Mechanisms of antimicrobial resistance in bacteria. Am. J. Infect. Control 2006, 34 (5 Suppl. 1), S3–S10. [Google Scholar] [CrossRef]
- Piątkowska, E.; Piątkowski, J.; Przondo-Mordarska, A. The strongest resistance of Staphylococcus aureus to erythromycin is caused by decreasing uptake of the antibiotic into the cells. Cell. Molecul. Biol. Lett. 2012, 17, 633–645. [Google Scholar] [CrossRef]
- Feßler, A.T.; Li, J.; Kadlec, K.; Wang, Y.; Schwarz, S. Antimicrobial resistance properties of Staphylococcus aureus, Chapter 4. In Staphylococcus aureus; Fetsch, A., Cambridge, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 57–85. [Google Scholar] [CrossRef]
- Graveland, H.; Wagenaar, J.A.; Heesterbeek, H.; Mevius, D.; van Duijkeren, E.; Heederik, D. Methicillin resistant Staphylococcus aureus ST398 in veal calf farming: Human MRSA carriage related with animal antimicrobial usage and farm hygiene. PLoS ONE 2010, 5, e10990. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Analysis of the baseline survey on the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in holdings with breeding pigs, in the EU, 2008. Part A: MRSA prevalence estimates. EFSA J. 2009, 7, 1376. [Google Scholar] [CrossRef]
- Wagenaar, J.A.; Yue, H.; Pritchard, J.; Broekhuizen-Stins, M.; Huijsdens, X.; Mevius, D.J.; Bosch, T.; Van Duijkeren, E. Unexpected sequence types in livestock associated methicillin-resistant Staphylococcus aureus (MRSA): MRSA ST9 and a single locus variant of ST9 in pig farming in China. Vet. Microbiol. 2009, 139, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Hasman, H.; Moodley, A.; Guardabassi, L.; Stegger, M.; Skov, R.L.; Aarestrup, F.M. spa type distribution in Staphylococcus aureus originating from pigs, cattle and poultry. Vet. Microbiol. 2010, 141, 326–331. [Google Scholar] [CrossRef]
- Khanna, T.; Friendship, R.; Dewey, C.; Weese, J.S. Methicillin resistant Staphylococcus aureus colonization in pigs and pig farmers. Vet. Microbiol. 2008, 128, 298–303. [Google Scholar] [CrossRef]
- Witte, W.; Strommenger, B.; Stanek, C.; Cuny, C. Methicillin-resistant Staphylococcus aureus ST398 in humans and animals, Central Europe. Emerg. Infect. Dis. 2007, 13, 255–258. [Google Scholar] [CrossRef]
- Krziwanek, K.; Metz-Gercek, S.; Mittermayer, H. Methicillin-resistant Staphylococcus aureus ST398 from human patients, upper Austria. Emerg. Infect. Dis. 2009, 15, 766–769. [Google Scholar] [CrossRef]
- Golding, G.R.; Bryden, L.; Levett, P.N.; McDonald, R.R.; Wong, A.; Wylie, J.; Graham, M.R.; Tyler, S.; Van Domselaar, G.; Simor, A.E.; et al. Livestock-associated methicillin-resistant Staphylococcus aureus sequence type 398 in humans, Canada. Emerg. Infect. Dis. 2010, 16, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Dumortier, C.; Taylor, B.S.; Miller, M.; Vasquez, G.; Yunen, J.; Brudney, K.; Sánchez-E, J.; Rodriguez-Taveras, C.; Rojas, R.; et al. Staphylococcus aureus ST398, New York City and Dominican Republic. Emerg. Infect. Dis. 2009, 15, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Beneke, B.; Klees, S.; Stührenberg, B.; Fetsch, A.; Kraushaar, B.; Tenhagen, B.-A. Prevalence of methicillin-resistant Staphylococcus aureus in a fresh meat pork production chain. J. Food Protect. 2011, 74, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Ekkclcnkamp, M.B.; Sckkar, M.; Carpaij, N.; Troelstra, A.; Bonten, M.J.M. Endocarditis due to methicillin-resistant Staphylococcus aureus originating from pigs. Ned. Tijdschn. Geneeskd. 2006, 150, 2442–2447. [Google Scholar]
- Hartmeyer, G.N.; Gahrn-Hansen, B.; Skov, R.L.; Kolmos, H.J. Pig-associated methicillin-resistant Staphylococcus aureus: Family transmission and severe pneumonia in a newborn. Scand. J. Infect. Dis. 2010, 42, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Mammina, C.; Bonura, C.; di Carlo, P.; Calà, C.; Aleo, A.; Monastero, R.; Palma, D.M. Daptomycin non-susceptible, vancomycin intermediate methicillin-resistant Staphylococcus aureus ST398 from a chronic leg ulcer, Italy. Scand. J. Infect. Dis. 2010, 42, 955–957. [Google Scholar] [CrossRef]
- Valentin-Domelier, A.-S.; Girard, M.; Bertrand, X.; Violette, J.; François, P.; Donnio, P.-Y.; Talon, D.; Quentin, R.; Schrenzel, J.; van der Mee-Marquet, N.; et al. Methicillin-susceptible ST398 Staphylococcus aureus responsible for bloodstream infections: An emerging human-adapted subclone? PLoS ONE 2011, 6, e28369. [Google Scholar] [CrossRef]
- Coombs, G.W.; Pang, S.; Daley, D.A.; Lee, Y.T.; Abraham, S.; Leroi, M. Severe disease caused by community-associated MRSA ST398 Type V, Australia, 2017. Emerg. Infect. Dis. 2019, 25, 190–192. [Google Scholar] [CrossRef]
- Park, J.Y.; Jin, J.S.; Kang, H.Y.; Jeong, E.H.; Lee, J.C.; Lee, Y.C.; Seol, S.Y.; Cho, D.T.; Kim, J. A comparison of adult and pediatric methicillin-resistant Staphylococcus aureus isolates collected from patients at a university hospital in Korea. J. Microbiol. 2007, 45, 447–452. [Google Scholar]
- Beier, R.C.; Anderson, P.N.; Hume, M.E.; Poole, T.L.; Duke, S.E.; Crippen, T.L.; Sheffield, C.L.; Caldwell, D.J.; Byrd, J.A.; Anderson, R.C. Characterization of Salmonella enterica isolates from turkeys in commercial processing plants for resistance to antibiotics, disinfectdants, and a growth promoter. Foodborne Pathog. Dis. 2011, 8, 593–600. [Google Scholar] [CrossRef]
- Webber, M.A.; Buckner, M.M.C.; Redgrave, L.S.; Ifill, G.; Mitchenall, L.A.; Webb, C.; Iddles, R.; Maxwell, A.; Maxwell, A.; Piddock, L.J.V. Quinolone-resistant gyrase mutants demonstrate decreased susceptibility to triclosan. J. Antimicrob. Chemother. 2017, 72, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Jin, M.; Nguyen, S.H.; Mao, L.; Li, J.; Coin, L.J.M.; Yuan, Z.; Guo, J. Non-antibiotic antimicrobial triclosan induces multiple antibiotic resistance through genetic mutation. Environ. Int. 2018, 118, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Pacific Texchem Private Limited. Benzalkonium Chloride-Benzalkonium Chloride 80 Manufacturer from Mumbai. 2021. Available online: pacifictexchem.com (accessed on 16 March 2021).
- Sharkey, J.W. Benzalkonium Chloride (BZK) Hand Sanitizers During Covid-19. 2020. Available online: linkedin.com (accessed on 16 March 2021).
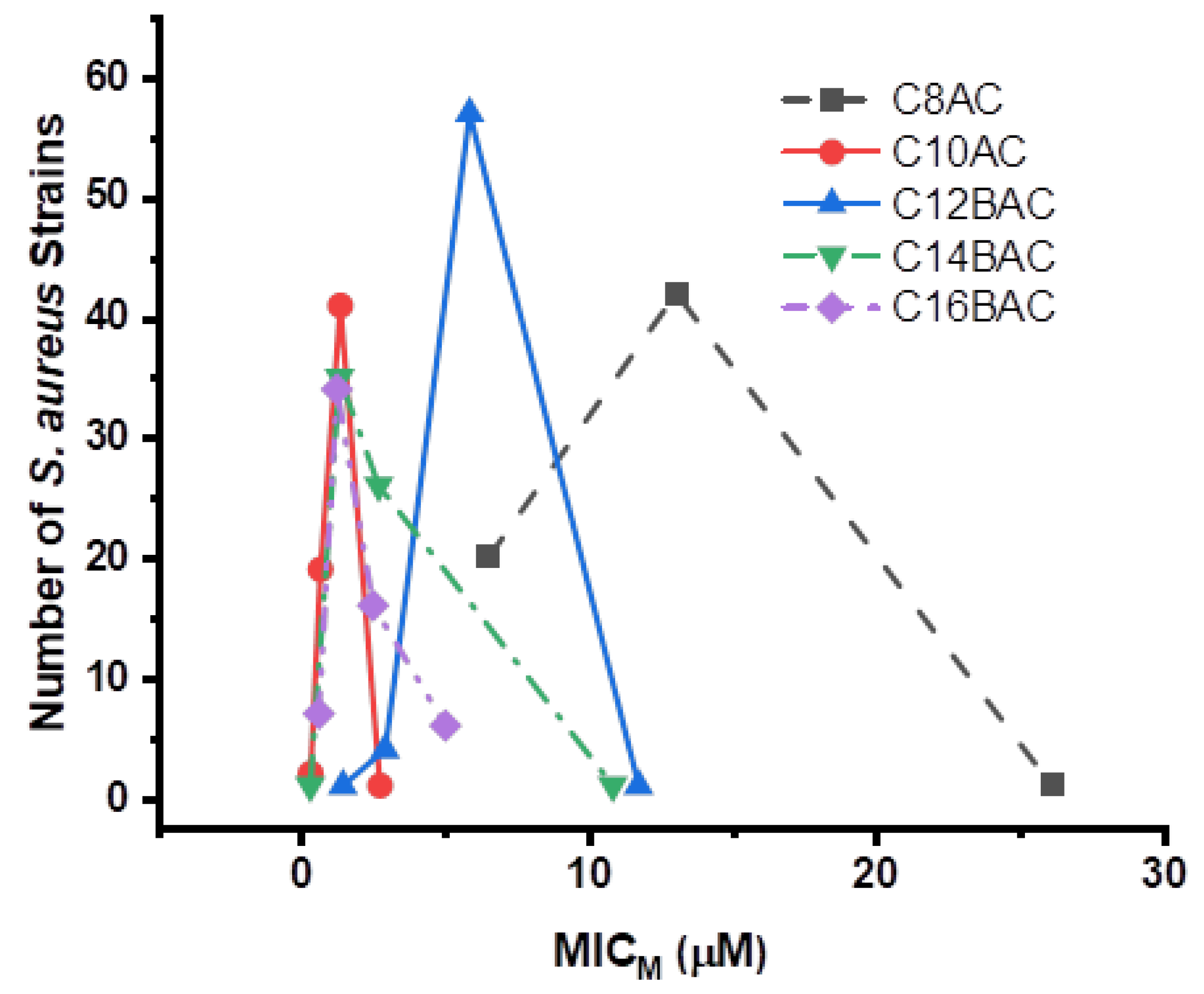
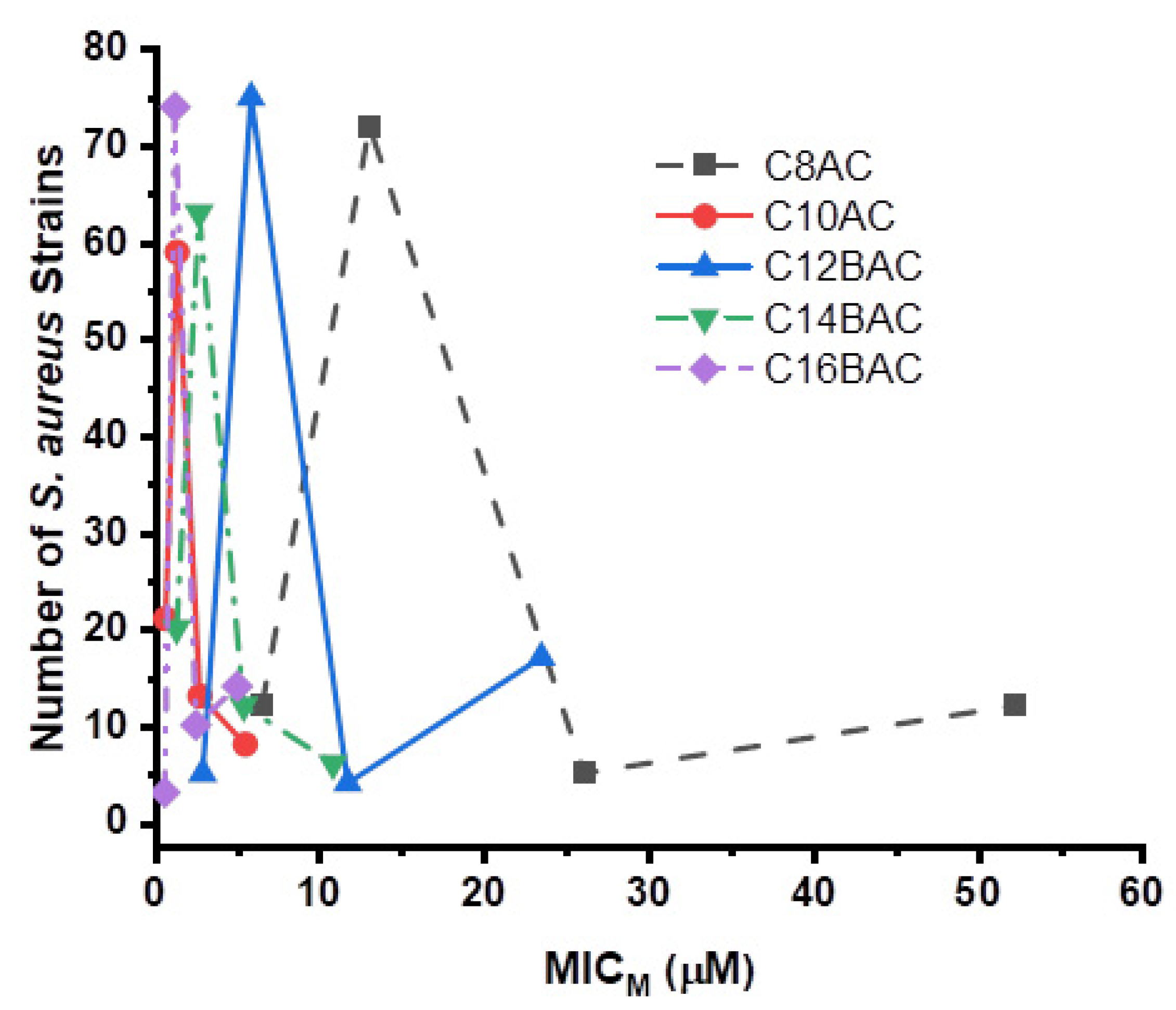
| Antimicrobial | MIC50 (µg/mL) | MIC90 (µg/mL) | MIC Range (µg/mL) | No. (%) Resistant | Breakpoint (µg/mL) | ||
|---|---|---|---|---|---|---|---|
| Aminoglycosides | |||||||
| Gentamicin | ≤128 | ≤128 | ≤128–1028 | 1 (0.6) | >500 | ||
| Kanamycin | ≤128 | ≤128 | ≤128 | CDR * | ≥64 | ||
| Streptomycin | ≤512 | ≤512 | ≤512–2048 | 4 (2.4) | ≥1000 | ||
| Amphenicols | |||||||
| Chloramphenicol | 8 | 16 | 8–>32 | 2 (3.8) | ≥32 | ||
| Cyclic Lipopeptides | |||||||
| Daptomycin | ≤0.25 | 0.5 | ≤0.25–1 | 0 (0) | >1 | ||
| Fluoroquinolones | |||||||
| Ciprofloxacin | 0.5 | >4 | 0.12–>4 | 21 (12.8) | ≥1 | ||
| Glycopeptides | |||||||
| Vancomycin | 0.5 | 1 | 0.5–32 | 1 (0.6) | ≥16 | ||
| Lincosamides | |||||||
| Lincomycin | >8 | >8 | ≤1–>8 | CDR * | ≥32 | ||
| Macrolides | |||||||
| Erythromycin | >8 | >8 | ≤0.25–>8 | 83 (50.6) | ≥8 | ||
| Tylosin Tartrate | 2 | >32 | 0.5–>32 | 70 (42.7) | ≥20 | ||
| Nitrofurans | |||||||
| Nitrofurantoin | 16 | 16 | ≤0.25–16 | 0 (0) | ≥128 | ||
| Oxazolidinones | |||||||
| Linezolid | 2 | 4 | ≤0.5–4 | 0 (0) | ≥8 | ||
| Penicillins | |||||||
| Penicillin | >16 | >16 | ≤0.25–>16 | 118 (72) | ≥16 | ||
| Streptogramins | |||||||
| Quinupristin/Dalfopristin | ≤0.5 | 1 | ≤0.5–32 | 6 (3.7) | ≥4 | ||
| Tetracyclines | |||||||
| Tetracycline | >32 | >32 | ≤1–>32 | 113 (68.9) | ≥16 | ||
| Tigecycline | 0.25 | 0.5 | 0.06–0.5 | 0 (0) | >0.5 | ||
| Number of Strains with Resistance to the Number of Antibiotics | |||||||
| No. of Antibiotics | 0 | 1 | 2 | 3 | 4 | 5 | 8 |
| No. of Strains (%) | 6 (3.6) | 49 (29.9) | 28 (17.1) | 28 (17.1) | 39 (23.8) | 13 (7.9) | 1 (0.6) |
| Sample Type | No. (%) S. aureus Strains Isolated | No. Resistant Strains | Resistance Profiles |
|---|---|---|---|
| Swine Feces | 63 | 18 | PEN |
| 3 | TET | ||
| 7 | TET-PEN | ||
| 3 | ERY-TET-TYLT | ||
| MDR = 32 (50.8%) | 31 | ERY-TET-PEN-TYLT | |
| Strains | 1 | ERY-TET-CHL-PEN-TYLT | |
| Lymph Node Tissue | 49 | 10 | PEN |
| 5 | TET | ||
| 2 | ERY-TET | ||
| 3 | ERY-PEN | ||
| 1 | TET-CIP | ||
| 1 | TET-PEN | ||
| 10 | ERY-TET-TYLT | ||
| 1 | ERY-PEN-TYLT | ||
| Strains: 147L, 150L | 2 | TET-PEN MRSA * | |
| 3 | ERY-TET-PEN | ||
| 25.0% MDR Strains | Strains: 21L, 38L | 2 | TET-CIP-PEN MRSA * |
| were MRSA | 1 | ERY-TET-CIP-PEN | |
| MDR = 16 (32.7%) | 3 | ERY-TET-PEN-TYLT | |
| Strains | 2 | ERY-TET-CIP-PEN-STR | |
| 3 | ERY-TET-CIP-PEN-TYLT | ||
| Pork Sausage Meat | 52 | 6 | None |
| 1 | CIP | ||
| 5 | TET | ||
| 7 | PEN | ||
| 1 | ERY-TYLT | ||
| 1 | TET-CHL | ||
| 3 | TET-CIP | ||
| 7 | TET-PEN | ||
| 4 | ERY-PEN-TYLT | ||
| 2 | ERY-TET-TYLT | ||
| 1 | ERY-TET-PEN | ||
| 20.0% MDR Strains | Strain: D16a | 1 | TET-CIP-PEN MRSA * |
| were MRSA | 1 | TET-CIP-PEN | |
| MDR = 15 (28.8%) | 1 | ERY-TET-PEN-TYLT | |
| Strains | 1 | ERY-TET-TYLT-GEN | |
| 2 | ERY-TET-TYLT-SYN | ||
| Strains: D15, D16 | 2 | ERY-TET-CIP-PEN-TYLT MRSA * | |
| 2 | ERY-TET-CIP-PEN-TYLT | ||
| 1 | ERY-TET-CIP-PEN-STR | ||
| 2 | ERY-TET-PEN-TYLT-SYN | ||
| 1 | ERY-TET-CIP-CHL-PEN-STR-TYLT-SYN |
| Sample | Bacteria | MLST Number (Allelic Profile) |
|---|---|---|
| 21L | Wildtype S. aureus | 398 (03-35-19-02-20-26-39) |
| 38L | Wildtype S. aureus | 398 (03-35-19-02-20-26-39) |
| 147L | Wildtype S. aureus | 398 (03-35-19-02-20-26-39) |
| 150L | Wildtype S. aureus | 398 (03-35-19-02-20-26-39) |
| D15 | Wildtype S. aureus | 5 (01-04-01-04-12-01-10) |
| D16a | Wildtype S. aureus | 398 (03-35-19-02-20-26-39) |
| D16 | Wildtype S. aureus | 398 (03-35-19-02-20-26-39) |
| Control | S. aureus ATCC® 43300 | 39 (02-02-02-02-02-02-02) |
| Control | S. aureus ATCC® 29213 | 5 (01-04-01-04-12-01-10) |
| MIC (µg/mL) | MIC50 | MIC90 | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disinfectant * | 0.008 | 0.0156 | 0.031 | 0.0625 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | 2048 | 4096 | 8192 | µg/mL | µg/mL |
| DC&RCP | 6 † | 29 | 28 | 4 | 8 | ||||||||||||||||||
| Tek-TrolCP | 15 | 48 | 64 | 64 | |||||||||||||||||||
| CaviCideCP | 25 | 38 | 128 | 128 | |||||||||||||||||||
| Chlorhexidine ‡ | 52 | 10 § | 1 | 0.5 | 1 § | ||||||||||||||||||
| Triclosan | 1 | 15 | 26 | 17 | 3 | 1 | 0.062 | 0.125 | |||||||||||||||
| TCC | 3 | 53 | 7 | 0.25 | 0.5 | ||||||||||||||||||
| P-128CP | 3 | 54 | 6 | 0.5 | 0.5 | ||||||||||||||||||
| BKC | 12 | 44 | 6 | 1 | 1 | 2 | |||||||||||||||||
| P-I | 1 | 14 | 22 | 24 | 2 | 2048 | 4096 | ||||||||||||||||
| FSS | 3 | 45 | 15 | 0.5 | 1 | ||||||||||||||||||
| F25 | 1 | 43 | 18 | 1 | 0.5 | 1 | |||||||||||||||||
| FS512 | 5 | 44 | 14 | 0.5 | 1 | ||||||||||||||||||
| OdoBanCP | 32 | 31 | 0.5 | 1 | |||||||||||||||||||
| CPB | 3 | 29 | 27 | 4 | 0.25 | 0.5 | |||||||||||||||||
| CPC | 1 | 3 | 31 | 24 | 4 | 0.25 | 0.5 | ||||||||||||||||
| CDEAB | 1 | 7 | 34 | 21 | 0.5 | 1 | |||||||||||||||||
| CTAB | 1 | 30 | 28 | 4 | 1 | 1 | |||||||||||||||||
| C8AC ¶ | 20 | 42 | 1 | 4 | 4 | ||||||||||||||||||
| C10AC ¶ | 2 | 19 | 41 | 1 | 0.5 | 0.5 | |||||||||||||||||
| C12BAC ¶ | 1 | 4 | 57 | 1 | 2 | 2 | |||||||||||||||||
| C14BAC ¶ | 1 | 35 | 26 | 1 | 0.5 | 1 | |||||||||||||||||
| C16BAC ¶ | 7 | 34 | 16 | 6 | 0.5 | 1 | |||||||||||||||||
| THN ¶ | 1 | 60 | 2 | 256 | 256 | ||||||||||||||||||
| Formaldehyde ¶ | 1 | 18 | 43 | 1 | 64 | 64 | |||||||||||||||||
| MIC (µg/mL) | MIC50 | MIC90 | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disinfectant * | 0.008 | 0.0156 | 0.031 | 0.0625 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | 2048 | 4096 | 8192 | µg/mL | µg/mL |
| DC&RCP | 2 † | 26 | 53 | 6 ‡ | 14 | 8 | 32 | ||||||||||||||||
| Tek-TrolCP | 34 | 53 | 14 | 64 | 128 | ||||||||||||||||||
| CaviCideCP | 2 | 27 | 55 | 12 | 5 | 128 | 256 | ||||||||||||||||
| Chlorhexidine § | 59 | 42 ¶ | 0.5 | 1 ¶ | |||||||||||||||||||
| Triclosan | 1 | 4 | 11 | 44 | 34 | 7 | 0.125 | 0.25 | |||||||||||||||
| TCC | 1 | 15 | 72 | 13 | 0.25 | 0.5 | |||||||||||||||||
| P-128CP | 1 | 64 | 23 | 13 | 0.5 | 2 | |||||||||||||||||
| BKC | 3 | 54 | 26 | 16 | 2 | 1 | 4 | ||||||||||||||||
| P-I | 1 | 44 | 56 | 4096 | 4096 | ||||||||||||||||||
| FSS | 32 | 49 | 16 | 2 | 2 | 1 | 2 | ||||||||||||||||
| F25 | 42 | 41 | 16 | 2 | 1 | 2 | |||||||||||||||||
| FS512 | 31 | 53 | 16 | 1 | 1 | 2 | |||||||||||||||||
| OdoBanCP | 19 | 63 | 11 | 6 | 1 | 1 | 1 | 2 | |||||||||||||||
| CPB | 1 | 33 | 49 | 1 | 1 | 16 | 0.5 | 4 | |||||||||||||||
| CPC | 2 | 32 | 45 | 5 | 17 | 0.5 | 4 | ||||||||||||||||
| CDEAB | 1 | 1 | 3 | 51 | 28 | 16 | 1 | 0.5 | 4 | ||||||||||||||
| CTAB | 2 | 16 | 64 | 2 | 12 | 5 | 1 | 4 | |||||||||||||||
| C8AC ** | 12 | 72 | 5 | 12 | 4 | 16 | |||||||||||||||||
| C10AC ** | 21 | 59 | 13 | 8 | 0.5 | 1 | |||||||||||||||||
| C12BAC ** | 5 | 75 | 4 | 17 | 2 | 8 | |||||||||||||||||
| C14BAC ** | 20 | 63 | 12 | 6 | 1 | 2 | |||||||||||||||||
| C16BAC ** | 3 | 74 | 10 | 14 | 0.5 | 2 | |||||||||||||||||
| THN ** | 2 | 78 | 20 | 1 | 256 | 512 | |||||||||||||||||
| Formaldehyde ** | 2 | 99 | 64 | 64 | |||||||||||||||||||
| Sample Type | No. of MDR * S. aureus Strains | No. of MRSA Strains † | No. of Strains with Elevated Disinfectant Susceptibility ‡ |
|---|---|---|---|
| Swine Feces | None | None | None |
| Lymph Node Tissue | + | + | − |
| + | + | + §, ¶ | |
| + | + | + §, ** | |
| + | + | + §, ¶, ** | |
| + | −§§ | + § | |
| + | − | + ¶ | |
| 2 −‡‡ | − | 2 + § | |
| Semi-totals | 6 (12.3%) | 4 strains | 7 strains |
| Pork Sausage Meat | + | + | + § |
| + | + | + †† | |
| + | + | + §, **, †† | |
| 2+ | −§§ | 2 + | |
| 4 − | − | 4 + | |
| − | − | + §, ¶, ** | |
| Semi-totals | 5 (9.6%) | 3 strains | 10 strains |
| Totals | 11 strains | 7 strains | 17 strains |
| DC&R Component MICs—Feces Strains | DC&R Component MICs—Tissue & Sausage | ||||
|---|---|---|---|---|---|
| Component | Calculated theoMICs | Actual MICs from Table 3 * | Component | Calculated theoMICs | Actual MICs from Table 4 † |
| theoBACsDC&R | 0.25 (9.6%) ‡ | 0.125 (0.5%) ‡ | theoBACsDC&R | 0.25 (2.0%) ‡ | 0.25 (1.0%) ‡ |
| 0.5 (46.0%) | 0.25 (3.7%) | 0.5 (25.7%) | 0.5 (31.0%) | ||
| 1.0 (44.4%) | 0.5 (37.0%) | 1.0 (52.5%) | 1.0 (25.8%) | ||
| 1.0 (24.3%) | 2.0 (5.9%) | 2.0 (33.3) | |||
| 2.0 (33.3%) | 4.0 (13.9%) | 4.0 (3.3%) | |||
| 4.0 (1.1%) | 8.0 (5.6%) | ||||
| theoFormDC&R | 0.19 (9.5%) ‡ | 16.0 (1.6%) ‡ | theoFormDC&R | 0.19 (2.0%) ‡ | 32.0 (2.0%) ‡ |
| 0.37 (46.0%) | 32.0 (28.6%) | 0.37 (25.7%) | 64.0 (98.0%) | ||
| 0.74 (44.4%) | 64.0 (68.2%) | 0.74 (52.5%) | |||
| 128.0 (1.6%) | 1.49 (5.9%) | ||||
| 2.97 (13.9%) | |||||
| theoTHNDC&R | 1.56 (9.5%) ‡ | 128.0 (1.6%) ‡ | theoTHNDC&R | 1.56 (2.0%) ‡ | 128.0 (2.0%) ‡ |
| 3.13 (46.0%) | 256.0 (95.2%) | 3.13 (25.7%) | 256.0 (77.2%) | ||
| 6.25 (44.4%) | 512.0 (3.2%) | 6.25 (52.5%) | 512.0 (19.8%) | ||
| 12.51 (5.9%) | 1024.0 (1.0%) | ||||
| 25.0 (13.9%) | |||||
| P-128 Component MICs—Feces Strains | P-128 Component MICs—Tissue & Sausage Strains | ||||
| theoBACsP-128 | 0.1 (4.8%) ‡ | 0.125 (0.5%) ‡ | theoBACsP-128 | 0.1 (1.0%) ‡ | 0.25 (1.0%) ‡ |
| 0.2 (85.7%) | 0.25 (3.7%) | 0.2 (63.4%) | 0.5 (31.0%) | ||
| 0.4 (9.5%) | 0.5 (37.0%) | 0.4 (22.8%) | 1.0 (25.7%) | ||
| 1.0 (24.3%) | 0.8 (12.9%) | 2.0 (33.3%) | |||
| 2.0 (33.3%) | 4.0 (3.3%) | ||||
| 4.0 (1.1%) | 8.0 (5.6%) | ||||
| theoC10ACP-128 | 0.15 (4.8%) ‡ | 0.125 (3.2%) ‡ | theoC10ACP-128 | 0.15 (1.0%) ‡ | 0.25 (20.8%) ‡ |
| 0.3 (85.7%) | 0.25 (30.1%) | 0.3 (63.4%) | 0.5 (58.4%) | ||
| 0.6 (9.5%) | 0.5 (65.1%) | 0.6 (22.8%) | 1.0 (12.9%) | ||
| 1.0 (1.6%) | 1.2 (12.9%) | 2.0 (7.9%) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beier, R.C.; Andrews, K.; Hume, M.E.; Sohail, M.U.; Harvey, R.B.; Poole, T.L.; Crippen, T.L.; Anderson, R.C. Disinfectant and Antimicrobial Susceptibility Studies of Staphylococcus aureus Strains and ST398-MRSA and ST5-MRSA Strains from Swine Mandibular Lymph Node Tissue, Commercial Pork Sausage Meat and Swine Feces. Microorganisms 2021, 9, 2401. https://doi.org/10.3390/microorganisms9112401
Beier RC, Andrews K, Hume ME, Sohail MU, Harvey RB, Poole TL, Crippen TL, Anderson RC. Disinfectant and Antimicrobial Susceptibility Studies of Staphylococcus aureus Strains and ST398-MRSA and ST5-MRSA Strains from Swine Mandibular Lymph Node Tissue, Commercial Pork Sausage Meat and Swine Feces. Microorganisms. 2021; 9(11):2401. https://doi.org/10.3390/microorganisms9112401
Chicago/Turabian StyleBeier, Ross C., Kathleen Andrews, Michael E. Hume, Muhammad Umar Sohail, Roger B. Harvey, Toni L. Poole, Tawni L. Crippen, and Robin C. Anderson. 2021. "Disinfectant and Antimicrobial Susceptibility Studies of Staphylococcus aureus Strains and ST398-MRSA and ST5-MRSA Strains from Swine Mandibular Lymph Node Tissue, Commercial Pork Sausage Meat and Swine Feces" Microorganisms 9, no. 11: 2401. https://doi.org/10.3390/microorganisms9112401
APA StyleBeier, R. C., Andrews, K., Hume, M. E., Sohail, M. U., Harvey, R. B., Poole, T. L., Crippen, T. L., & Anderson, R. C. (2021). Disinfectant and Antimicrobial Susceptibility Studies of Staphylococcus aureus Strains and ST398-MRSA and ST5-MRSA Strains from Swine Mandibular Lymph Node Tissue, Commercial Pork Sausage Meat and Swine Feces. Microorganisms, 9(11), 2401. https://doi.org/10.3390/microorganisms9112401







