Agronomic Biofortification of Cayenne Pepper Cultivars with Plant Growth-Promoting Rhizobacteria and Chili Residue in a Chinese Solar Greenhouse
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site Description
2.2. Experimental Design and Crop Management
2.3. Agronomic Parameters
2.4. Determination of Mineral and Nitrate Contents in Long Cayenne Pepper Fruits
2.5. Determination of Folate in Long Cayenne Pepper Fruits
2.6. Determination of Moisture Content
2.7. Statistical Analysis
3. Results and Discussions
3.1. Leaf–Gas Exchange of Cayenne Pepper
3.2. Yield Attributes and Plant Biomass of Cayenne Pepper Cultivars
3.3. Quality Attributes of Cayenne Pepper Fruits
3.3.1. Total Soluble Solids, Mineral Contents, and Nitrate Accumulation
3.3.2. Folate Derivatives
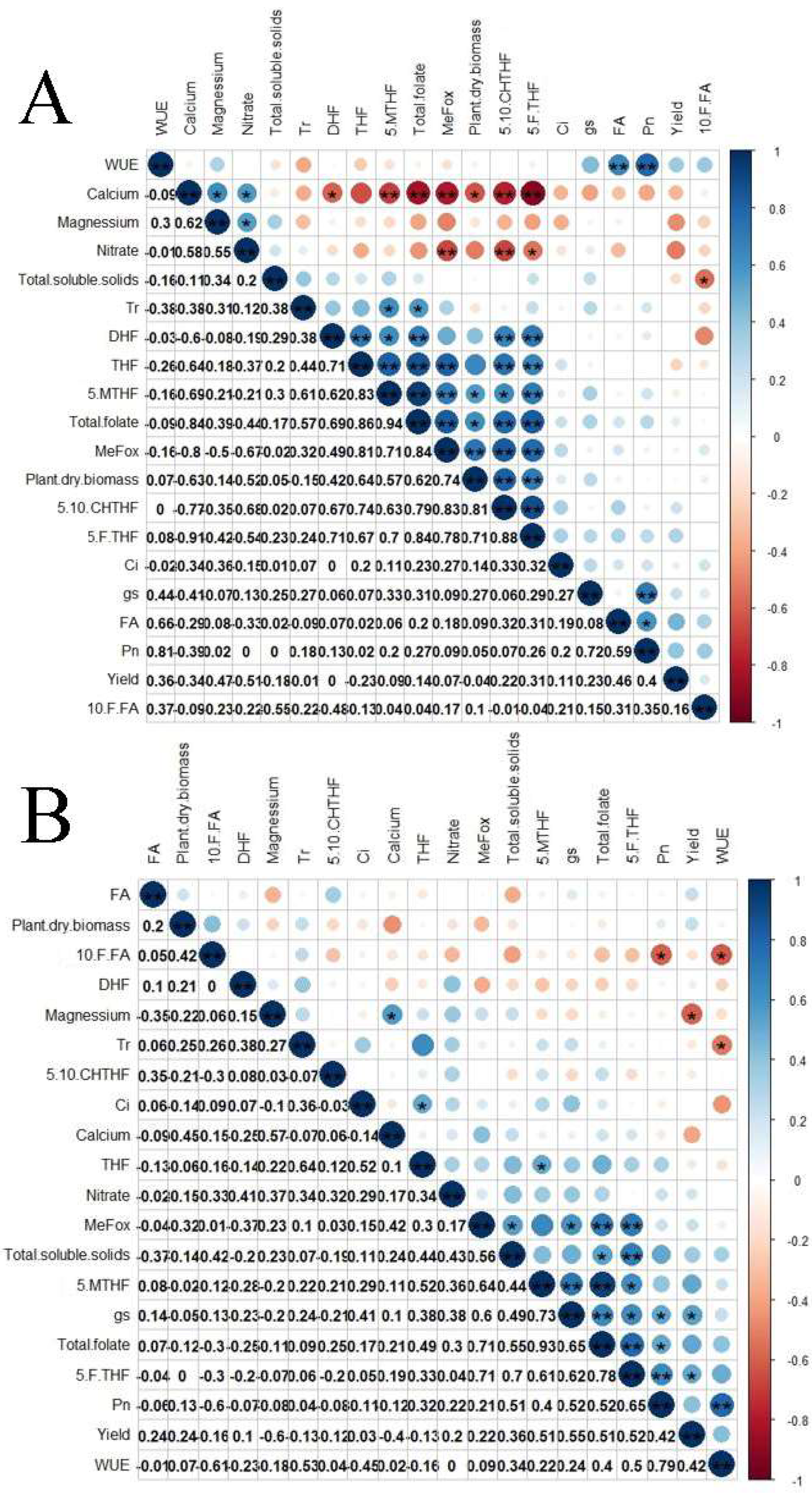
3.4. Correlations between Folate Derivatives and Mineral Contents of Cayenne Pepper
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Der Straeten, D.; Fitzpatrick, T.B.; De Steur, H. Editorial overview: Biofortification of crops: Achievements, future challenges, socio-economic, health and ethical aspects. Curr. Opin. Biotechnol. 2017, 44, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). Combating Micronutrient Deficiencies: Food-Based Approaches; Thompson, B., Amoroso, L., Eds.; FAO: Rome, Italy; CABI: Oxfordshire, UK, 2011; ISBN 9781845937140. [Google Scholar]
- Blancquaert, D.; De Steur, H.; Gellynck, X.; Van Der Straeten, D. Present and future of folate biofortification of crop plants. J. Exp. Bot. 2014, 65, 895–906. [Google Scholar] [CrossRef] [Green Version]
- Randhawa, M.A.; Khan, A.A.; Javed, M.S.; Sajid, M.W. Green Leafy Vegetables: A Health Promoting Source. In Handbook of Fertility: Nutrition, Diet, Lifestyle and Reproductive Health; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 205–220. ISBN 9780128009932. [Google Scholar]
- Oguntoyinbo, F.A.; Fusco, V.; Cho, G.S.; Kabisch, J.; Neve, H.; Bockelmann, W.; Huch, M.; Frommherz, L.; Trierweiler, B.; Becker, B.; et al. Produce from Africa’s gardens: Potential for leafy vegetable and fruit fermentations. Front. Microbiol. 2016, 7, 981. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Mehmood, S.; Zhang, C.; Liang, Q. Identification of the prepared foods promising for dietary folate intake in Beijing, China. Food Sci. Nutr. 2020, 8, 6557–6567. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Wang, K.; Shariful, I.; Ye, X.; Zhang, C. Folate content and retention in wheat grains and wheat-based foods: Effects of storage, processing, and cooking methods. Food Chem. 2020, 333, 127459. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Casal, M.N.; Peña-Rosas, J.P.; Giyose, B.; Groups, C. Working Staple crops biofortified with increased vitamins and minerals: Considerations for a public health strategy. Ann. N. Y. Acad. Sci. 2017, 1390, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Puthusseri, B.; Divya, P.; Lokesh, V.; Neelwarne, B. Enhancement of Folate Content and Its Stability Using Food Grade Elicitors in Coriander (Coriandrum sativum L.). Plant Foods Hum. Nutr. 2012, 67, 162–170. [Google Scholar] [CrossRef]
- García-Salinas, C.; Ramos-Parra, P.A.; Díaz de la Garza, R.I. Ethylene treatment induces changes in folate profiles in climacteric fruit during postharvest ripening. Postharvest Biol. Technol. 2016, 118, 43–50. [Google Scholar] [CrossRef]
- Nazki, F.H.; Sameer, A.S.; Ganaie, B.A. Folate: Metabolism, genes, polymorphisms and the associated diseases. Gene 2014, 533, 11–20. [Google Scholar] [CrossRef]
- Herrera-Araujo, D. Folic acid advisories: A public health challenge? Health Econ. 2016, 25, 1104–1122. [Google Scholar] [CrossRef] [Green Version]
- Choumenkovitch, S.F.; Selhub, J.; Wilson, P.W.F.; Rader, J.I.; Rosenberg, I.H.; Jacques, P.F. Folic acid intake from fortification in United States exceeds predictions. J. Nutr. 2002, 132, 2792–2798. [Google Scholar] [CrossRef] [PubMed]
- De Wals, P.; Tairou, F.; Van Allen, M.I.; Uh, S.H.; Lowry, R.B.; Sibbald, B.; Evans, J.A.; Van den Hof, M.C.; Zimmer, P.; Crowley, M.; et al. Reduction in neural-tube defects after folic acid fortification in Canada. N. Engl. J. Med. 2007, 28, 135–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marean, A.; Graf, A.; Zhang, Y.; Niswander, L. Folic acid supplementation can adversely affect murine neural tube closure and embryonic survival. Hum. Mol. Genet. 2011, 20, 3678–3683. [Google Scholar] [CrossRef] [PubMed]
- Blancquaert, D.; Van Daele, J.; Strobbe, S.; Kiekens, F.; Storozhenko, S.; De Steur, H.; Gellynck, X.; Lambert, W.; Stove, C.; Van Der Straeten, D. Improving folate (Vitamin B 9) stability in biofortified rice through metabolic engineering. Nat. Biotechnol. 2015, 33, 1076–1078. [Google Scholar] [CrossRef]
- Kwon, Y.-I.; Apostolidis, E.; Shetty, K. Evaluation of pepper (Capsicum anuum) for management of diabetes and hypertension. J. Food Biochem. 2007, 31, 370–385. [Google Scholar] [CrossRef]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant activity of spices and their impact on human health: A review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef] [Green Version]
- Olatunji, T.L.; Afolayan, A.J. The suitability of chili pepper (Capsicum annuum L.) for alleviating human micronutrient dietary deficiencies: A review. Food Sci. Nutr. 2018, 6, 2239–2251. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Pérez, T.; del Rocío Gómez-García, M.; Valverde, M.E.; Paredes-López, O. Capsicum annuum (hot pepper): An ancient Latin-American crop with outstanding bioactive compounds and nutraceutical potential. A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2972–2993. [Google Scholar] [CrossRef]
- Kantar, M.B.; Anderson, J.E.; Lucht, S.A.; Mercer, K.; Bernau, V.; Case, K.A.; Le, N.C.; Frederiksen, M.K.; DeKeyser, H.C.; Wong, Z.Z.; et al. Vitamin variation in Capsicum spp. Provides opportunities to improve nutritional value of human diets. PLoS ONE 2016, 11, e0161464. [Google Scholar] [CrossRef] [PubMed]
- Hickman, G.W. World Greenhouse Vegetable Statistics. Available online: https://www.cuestaroble.com/statistics.html (accessed on 13 October 2021).
- Hu, W.; Zhang, Y.; Huang, B.; Teng, Y. Soil environmental quality in greenhouse vegetable production systems in eastern China: Current status and management strategies. Chemosphere 2017, 170, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yu, J.; Chen, B.; Feng, Z.; Li, J.; Zhao, C.; Lyu, J.; Hu, L.; Gan, Y.; Siddique, K.H.M. Facility Cultivation Systems “She Shi Nong Ye”: A Chinese Model for the Planet, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; 145p. [Google Scholar]
- Chang, J.; Wu, X.; Wang, Y.; Meyerson, L.A.; Gu, B.; Min, Y.; Xue, H.; Peng, C.; Ge, Y. Does growing vegetables in plastic greenhouses enhance regional ecosystem services beyond the food supply? Front. Ecol. Environ. 2013, 11, 43–49. [Google Scholar] [CrossRef]
- Sánchez, Ó.J.; Ospina, D.A.; Montoya, S. Compost supplementation with nutrients and microorganisms in composting process. Waste Manag. 2017, 69, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Sonbarse, P.P.; Sharma, P.; Parvatam, G. PGPR’s mix treatment to moringa improved plant growth and iron content in foliage as substantiated by biochemical and molecular methods. J. Plant Interact. 2017, 12, 526–532. [Google Scholar] [CrossRef] [Green Version]
- Rosier, A.; Medeiros, F.H.V.; Bais, H.P. Defining plant growth promoting rhizobacteria molecular and biochemical networks in beneficial plant-microbe interactions. Plant Soil 2018, 428, 35–55. [Google Scholar] [CrossRef] [Green Version]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Meena, R.S.; Meena, V.S.; Meena, S.K.; Verma, J.P. The needs of healthy soils for a healthy world. J. Clean. Prod. 2015, 102, 560–561. [Google Scholar] [CrossRef]
- Yao, Z.; Xing, J.; Gu, H.; Wang, H.; Wu, J.; Xu, J.; Brookes, P.C. Development of microbial community structure in vegetable-growing soils from open-field to plastic-greenhouse cultivation based on the PLFA analysis. J. Soils Sediments 2016, 16, 2041–2049. [Google Scholar] [CrossRef]
- Kianpoor Kalkhajeh, Y.; Huang, B.; Hu, W.; Ma, C.; Gao, H.; Thompson, M.L.; Bruun Hansen, H.C. Environmental soil quality and vegetable safety under current greenhouse vegetable production management in China. Agric. Ecosyst. Environ. 2021, 307, 107230. [Google Scholar] [CrossRef]
- Olasupo, I.O.; Aiyelaagbe, I.O.O.; Makinde, E.A.; Afolabi, W.A.O. Growth, Yield, and Nutritional Composition of Plastic Tunnel-Grown Lettuce in Response to Poultry Manure. Int. J. Veg. Sci. 2018, 24, 526–538. [Google Scholar] [CrossRef]
- Wei, X.; Li, Y.; Fan, X.; He, C.; Yan, Y.; Sun, M.; Ding, C.; Wang, J.; Yu, X. Techno-economic feasibility of in-situ vegetable residue return in the Chinese solar greenhouse. Agronomy 2021, 11, 1828. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, X.; Zhao, H.; Sun, B.; Lu, F.; Hu, L. Extension of residue retention increases net greenhouse gas mitigation in China’s croplands. J. Clean. Prod. 2017, 165, 1–12. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, L.; Huang, X.; Zhang, Y.; Yin, C.; Lebailly, P. Incentive mechanism to promote corn stalk return sustainably in Henan, China. Sci. Total Environ. 2020, 738, 139775. [Google Scholar] [CrossRef]
- Jiang, W.; Yan, T.; Chen, B. Impact of media channels and social interactions on the adoption of straw return by Chinese farmers. Sci. Total Environ. 2021, 756, 144078. [Google Scholar] [CrossRef]
- Iqbal, A.; Amanullah; Song, M.; Shah, Z.; Alamzeb, M.; Iqbal, M. Integrated use of plant residues, phosphorus and beneficial microbes improve hybrid maize productivity in semiarid climates. Acta Ecol. Sin. 2019, 39, 348–355. [Google Scholar] [CrossRef]
- Qin, S.; Jiao, K.; Lyu, D.; Shi, L.; Liu, L. Effects of maize residue and cellulose-decomposing bacteria inocula on soil microbial community, functional diversity, organic fractions, and growth of Malus hupehensis Rehd. Arch. Agron. Soil Sci. 2015, 61, 173–184. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, Q.; Li, S.; Wang, P.; Dong, L.; Su, Z.; Zhang, X.; Lu, X.; Ma, P. Effects of Bacillus subtilis NCD-2 and broccoli residues return on potato Verticillium wilt and soil fungal community structure. Biol. Control 2021, 159, 104628. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, J.; Bai, Z.; Wu, S.; Li, X.; Wang, N.; Du, X.; Fan, H.; Zhuang, G.; Bohu, T.; et al. Unraveling mechanisms and impact of microbial recruitment on oilseed rape (Brassica napus L.) and the rhizosphere mediated by plant growth-promoting rhizobacteria. Microorganisms 2021, 9, 161. [Google Scholar] [CrossRef]
- Han, W.; He, M. The application of exogenous cellulase to improve soil fertility and plant growth due to acceleration of straw decomposition. Bioresour. Technol. 2010, 101, 3724–3731. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kaushal, R.; Spehia, R.S.; Pathania, S.S.; Sharma, V.; Kaushal, R.; Spehia, R.S.; Pathania, S.S.; Sharma, V. Productivity of capsicum influenced by conjoint application of isolated indigenous PGPR and chemical fertilizers. J. Plant Nutr. 2017, 40, 921–927. [Google Scholar] [CrossRef]
- Nielsen, S.S. Food Analysis Laboratory Manual, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 9781441914620. [Google Scholar]
- Pinto, E.; Almeida, A.A.; Aguiar, A.A.; Ferreira, I.M.P.L.V.O. Comparison between the mineral profile and nitrate content of microgreens and mature lettuces. J. Food Compos. Anal. 2015, 37, 38–43. [Google Scholar] [CrossRef]
- Wan, X.; Han, L.-D.; Yang, M.; Zhang, H.-Y.; Zhang, C.-Y.; Hu, P. Simultaneous extraction and determination of mono-/polyglutamyl folates using high-performance liquid chromatography-tandem mass spectrometry and its applications in starchy crops. Anal. Bioanal. Chem. 2019, 411, 2891–2904. [Google Scholar] [CrossRef]
- Fazili, Z.; Sternberg, M.R.; Potischman, N.; Wang, C.Y.; Storandt, R.J.; Yeung, L.; Yamini, S.; Gahche, J.J.; Juan, W.; Qi, Y.P.; et al. Demographic, Physiologic, and Lifestyle Characteristics Observed with Serum Total Folate Differ among Folate Forms: Cross-Sectional Data from Fasting Samples in the NHANES 2011-2016. J. Nutr. 2019, 150, 851–860. [Google Scholar] [CrossRef] [Green Version]
- Ringling, C.; Rychlik, M. Origins of the difference between food folate analysis results obtained by LC–MS/MS and microbiological assays. Anal. Bioanal. Chem. 2017, 409, 1815–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix 2021; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; Al-Huqail, A.A.; Wirth, S.; Egamberdieva, D. The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Front. Microbiol. 2016, 7, 1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Verma, J.P. Does plant—Microbe interaction confer stress tolerance in plants: A review? Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Maheshwari, D.K. Ecotoxicology and Environmental Safety Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef]
- Kennisnet, G. Effects of Cultivation Practices on the Nutritional Value of Crops. Available online: https://wiki.groenkennisnet.nl/display/CPC (accessed on 10 October 2021).
- Paque, S.; Weijers, D. Q&A: Auxin: The plant molecule that influences almost anything. BMC Biol. 2016, 14, 67. [Google Scholar] [CrossRef] [Green Version]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef] [PubMed]
- Helaly, A.A.; Hassan, S.M.; Craker, L.E.; Mady, E. Effects of growth-promoting bacteria on growth, yield and nutritional value of collard plants. Ann. Agric. Sci. 2020, 65, 77–82. [Google Scholar] [CrossRef]
- Pérez-Rodriguez, M.M.; Piccoli, P.; Anzuay, M.S.; Baraldi, R.; Neri, L.; Taurian, T.; Lobato Ureche, M.A.; Segura, D.M.; Cohen, A.C. Native bacteria isolated from roots and rhizosphere of Solanum lycopersicum L. increase tomato seedling growth under a reduced fertilization regime. Sci. Rep. 2020, 10, 15642. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Zhang, Z. Reducing the nitrate content in vegetables through joint regulation of short-distance distribution and long-distance transport. Front. Plant Sci. 2020, 11, 1079. [Google Scholar] [CrossRef] [PubMed]
- Salehzadeh, H.; Maleki, A.; Rezaee, R.; Shahmoradi, B.; Ponnet, K. The nitrate content of fresh and cooked vegetables and their health-related risks. PLoS ONE 2020, 15, e0227551. [Google Scholar] [CrossRef]
- Dawwam, G.E.; Elbeltagy, A.; Emara, H.M.; Abbas, I.H.; Hassan, M.M. Beneficial effect of plant growth promoting bacteria isolated from the roots of potato plant. Ann. Agric. Sci. 2013, 58, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Galieni, A.; Stagnari, F.; Speca, S.; D’Egidio, S.; Pagnani, G.; Pisante, M. Management of crop residues to improve quality traits of tomato (Solanum lycopersicum L.) fruits. Ital. J. Agron. 2017, 12, 59–62. [Google Scholar] [CrossRef] [Green Version]
- Strobbe, S.; Van Der Straeten, D. Folate biofortification in food crops. Curr. Opin. Biotechnol. 2017, 44, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Sonbarse, P.P.; Kiran, K.; Sharma, P.; Parvatam, G. Biochemical and molecular insights of PGPR application for the augmentation of carotenoids, tocopherols, and folate in the foliage of Moringa oleifera. Phytochemistry 2020, 179, 112506. [Google Scholar] [CrossRef]
- Prasad, R.; Shivay, Y.S. Agronomic biofortification of plant foods with minerals, vitamins and metabolites with chemical fertilizers and liming. J. Plant Nutr. 2020, 43, 1534–1554. [Google Scholar] [CrossRef]
- National Research Council (US) Subcommittee on the Tenth Edition of the Recommended Dietary Allowances Recommended Dietary Allowances. 8, Water-Soluble Vitamins. Available online: https://www.ncbi.nlm.nih.gov/books/NBK234924/ (accessed on 5 October 2021).
- Shahid, M.; Lian, T.; Wan, X.; Jiang, L.; Han, L.; Zhang, C.; Liang, Q. Folate monoglutamate in cereal grains: Evaluation of extraction techniques and determination by LC-MS/MS. J. Food Compos. Anal. 2020, 91, 103510. [Google Scholar] [CrossRef]
- Zhang, H.; Jha, A.B.; Warkentin, T.D.; Vandenberg, A.; Purves, R.W. Folate stability and method optimization for folate extraction from seeds of pulse crops using LC-SRM MS. J. Food Compos. Anal. 2018, 71, 44–55. [Google Scholar] [CrossRef]
| Sample | pH | EC (µS cm−1) | OM (%) | TC (%) | TN (%) | C:N | TP (g kg−1) | TK (g kg−1) | Ca (mg kg−1) | Mg (mg kg−1) | Sand (%) | Silt (%) | Clay (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil | 8.0 | 394 | 4.3 | 4.7 | 0.24 | 19.1 | 2.1 | 17.4 | 186.4 | 31 | 35 | 40 | 25 |
| Residue | NA | NA | NA | 37.5 | 3.2 | 11.7 | 12 | 0.28 | 100.7 | 42.4 | NA | NA | NA |
| Cultivar | Pn (µmol m−2 s−1 CO2) | gs (mmol m−2 s−1 H2O) | Tr (mmol m−2 s−1 H2O) | WUE (µmolCO2/mmolH2O) |
|---|---|---|---|---|
| V43 | 23.65a | 2.067a | 12.03a | 1.992a |
| V6 | 24.12a | 1.843a | 11.63a | 2.088a |
| p ≤ 0.05 | ns | ns | ns | ns |
| Treatment | ||||
| B1 | 26.24a | 1.928a | 11.09b | 2.382a |
| B2 | 25.08ab | 2.124a | 12.63a | 2.038ab |
| B3 | 25.28ab | 2.371a | 12.61a | 2.02ab |
| NP | 20.82c | 1.475a | 10.89b | 1.917b |
| p ≤ 0.05 | ** | ns | * | * |
| Cultivar | Fruit Length (cm) | Number of Fruits (ha−1) | Shoot Dry Weight (gplant−1) | Root Dry Weight (gplant−1) | Plant Dry Weight (gplant−1) | Fruit Yield (tha−1) |
|---|---|---|---|---|---|---|
| V43 | 41.08a | 651,066a | 59.9a | 9.694a | 69.61a | 22.79a |
| V6 | 33.68b | 505,125b | 43.6b | 9.982a | 53.63b | 17.68b |
| p ≤ 0.05 | ** | ** | ** | ns | ** | ** |
| Treatment | ||||||
| B1 | 37.87a | 674,376a | 58.5a | 9.31a | 67.80a | 23.60a |
| B2 | 36.75a | 489,116b | 56.9a | 11.14a | 68.06a | 17.12b |
| B3 | 37.20a | 771,655a | 54.9ab | 9.73a | 64.58ab | 27.01a |
| NP | 37.56a | 490,023b | 51.9ab | 10.26a | 62.18ab | 17.15b |
| p ≤ 0.05 | ns | ** | * | ns | * | ** |
| Cultivar | Calcium (mg kg−1) | Magnesium (mg kg−1) | Potassium (mg kg−1) | TSS (%) | Nitrate (mg kg−1) |
|---|---|---|---|---|---|
| V43 | 106.75b | 144.13a | 2370.3a | 4.595a | 266.0a |
| V6 | 116.21a | 147.37a | 2191.4b | 4.022b | 250.3a |
| p ≤ 0.05 | ** | ns | * | ** | ns |
| Treatment | |||||
| B1 | 100.78b | 138.00bc | 2144.2bc | 4.238a | 204.2c |
| B2 | 108.50b | 155.25a | 2288.3ab | 4.710a | 288.3a |
| B3 | 98.97b | 122.00c | 1950.0c | 4.228a | 266.7ab |
| NP | 118.67a | 146.83a | 2441.0a | 4.005a | 221.7bc |
| p ≤ 0.05 | ** | ** | ** | ns | ** |
| Cultivar | Moisture (%) | THF (µg/100 g) | 5-MTHF (µg/100 g) | 5,10-CHTHF (µg/100 g) | 10-F-FA (µg/100 g) | 5-F-THF (µg/100 g) | DHF (µg/100 g) | FA (µg/100 g) | MeFox (µg/100 g) | Total Folate α (µg/100 g) |
|---|---|---|---|---|---|---|---|---|---|---|
| V43 | 94.4 | 0.400a | 7.057a | 0.574b | 0.282a | 2.166a | 0.069b | 0.002b | 32.23a | 10.55a |
| V6 | 94.1 | 0.315b | 6.686a | 0.795a | 0.258a | 1.379b | 0.105a | 0.024a | 25.23b | 9.56b |
| p ≤ 0.05 | ** | ns | ** | ns | ** | * | * | ** | * | |
| Treatment | ||||||||||
| B1 | 94.6 | 0.304b | 6.077b | 0.636b | 0.248a | 1.600bc | 0.056a | 0.024a | 24.31c | 8.94b |
| B2 | 93.9 | 0.504a | 7.771a | 1.048a | 0.15a | 2.345a | 0.113a | 0.004a | 31.59a | 11.94a |
| B3 | 93.4 | 0.359b | 8.026a | 0.775ab | 0.316a | 1.794b | 0.137a | 0.015a | 30.42ab | 11.42a |
| NP | 95 | 0.259b | 5.817b | 0.501b | 0.35a | 1.317c | 0.056a | 0.006a | 27.03abc | 8.31b |
| p ≤ 0.05 | ** | ** | ** | ns | ** | ns | ns | * | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olasupo, I.O.; Liang, Q.; Zhang, C.; Islam, M.S.; Li, Y.; Yu, X.; He, C. Agronomic Biofortification of Cayenne Pepper Cultivars with Plant Growth-Promoting Rhizobacteria and Chili Residue in a Chinese Solar Greenhouse. Microorganisms 2021, 9, 2398. https://doi.org/10.3390/microorganisms9112398
Olasupo IO, Liang Q, Zhang C, Islam MS, Li Y, Yu X, He C. Agronomic Biofortification of Cayenne Pepper Cultivars with Plant Growth-Promoting Rhizobacteria and Chili Residue in a Chinese Solar Greenhouse. Microorganisms. 2021; 9(11):2398. https://doi.org/10.3390/microorganisms9112398
Chicago/Turabian StyleOlasupo, Ibraheem Olamide, Qiuju Liang, Chunyi Zhang, Md Shariful Islam, Yansu Li, Xianchang Yu, and Chaoxing He. 2021. "Agronomic Biofortification of Cayenne Pepper Cultivars with Plant Growth-Promoting Rhizobacteria and Chili Residue in a Chinese Solar Greenhouse" Microorganisms 9, no. 11: 2398. https://doi.org/10.3390/microorganisms9112398
APA StyleOlasupo, I. O., Liang, Q., Zhang, C., Islam, M. S., Li, Y., Yu, X., & He, C. (2021). Agronomic Biofortification of Cayenne Pepper Cultivars with Plant Growth-Promoting Rhizobacteria and Chili Residue in a Chinese Solar Greenhouse. Microorganisms, 9(11), 2398. https://doi.org/10.3390/microorganisms9112398








