In Vitro Effect of Copper (I) Complex [Cu(NN1)2](ClO4) on Vibrio harveyi BB170 Biofilm Formation
Abstract
1. Introduction
2. Materials and Methods
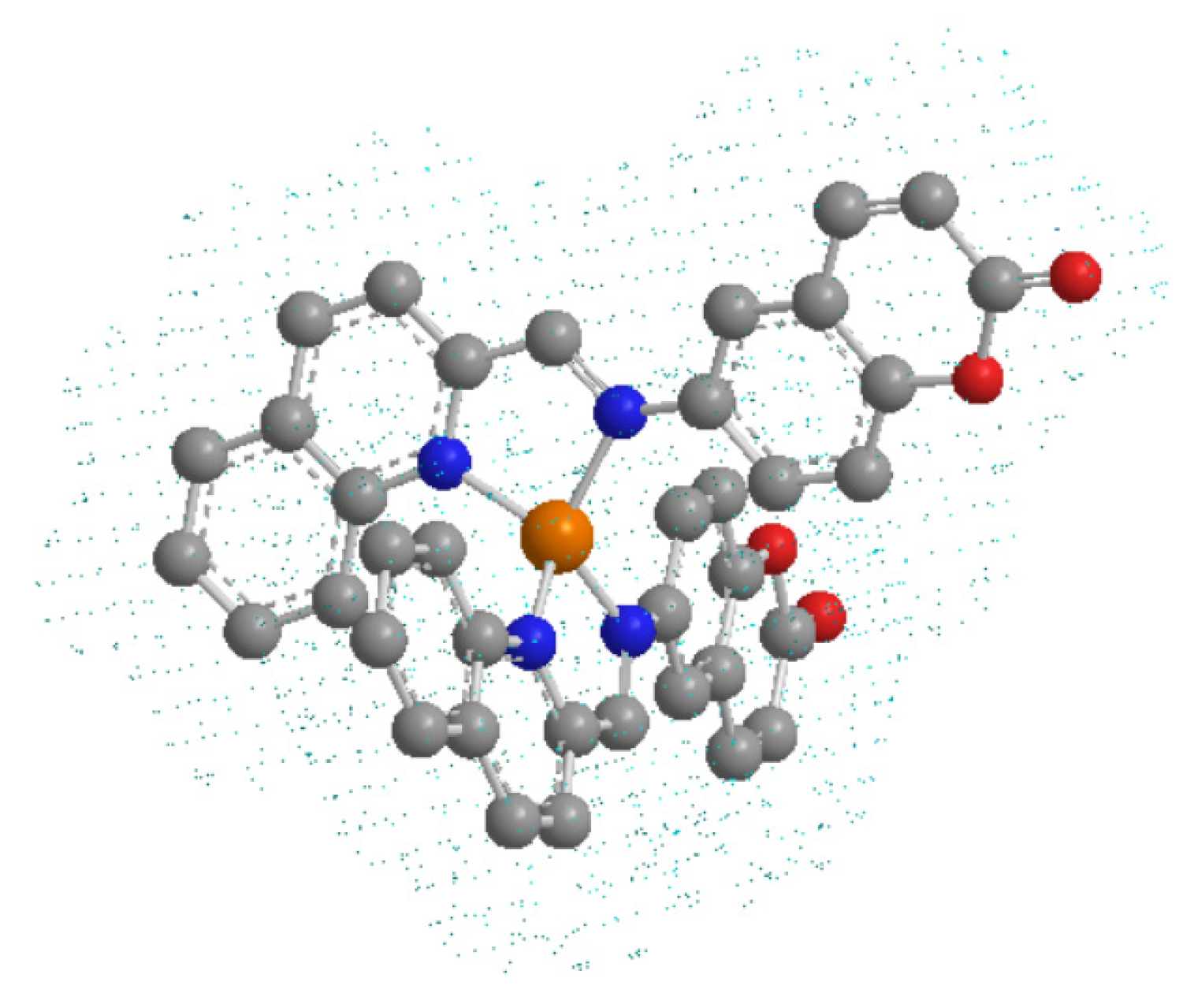
2.1. Chemical Compounds
2.2. Bacterial Strain and Growth Conditions
2.3. Measurement of Growth of V. harveyi BB170
2.4. Biofilm Quantification Assay
2.5. Bacterial Biofilm Viability
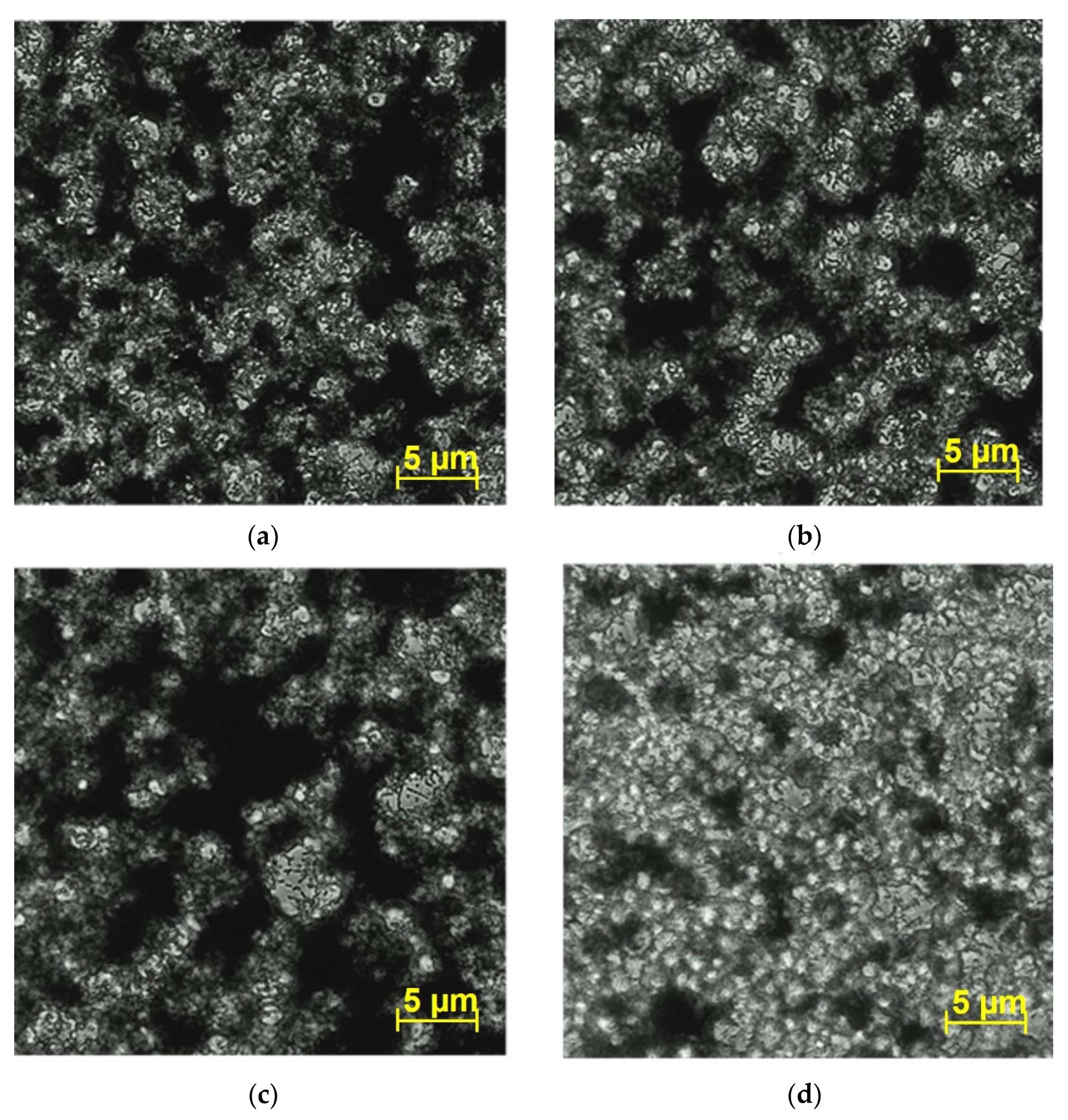
2.6. Biofilm Observation by Microscopy
3. Results
3.1. Antibacterial Effect of Compounds on V. harveyi BB170 Growth
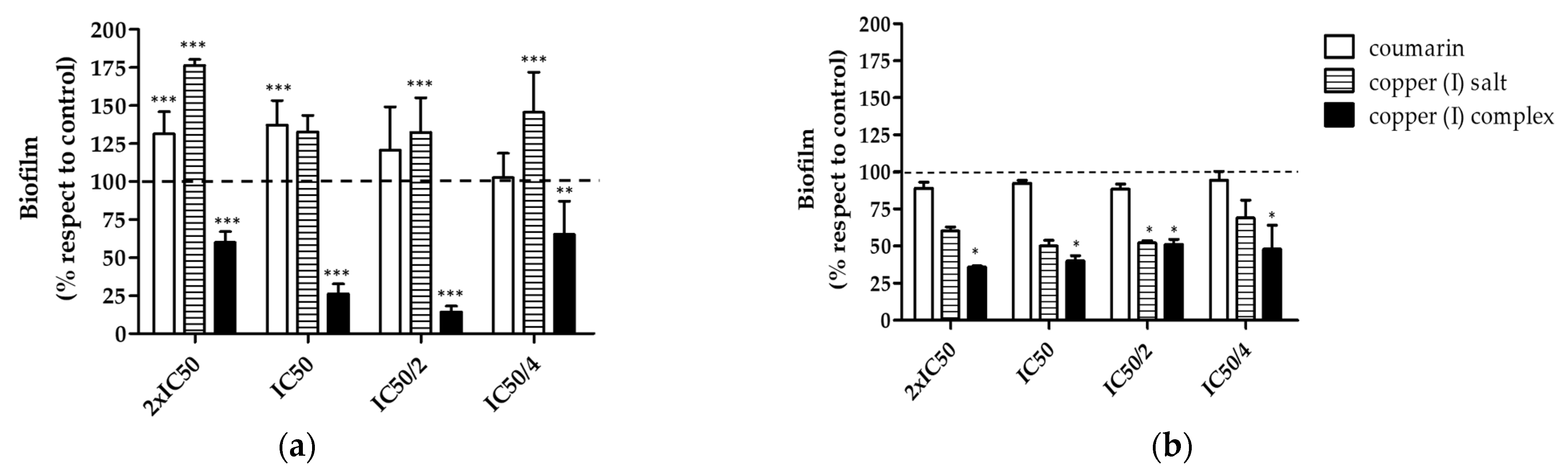
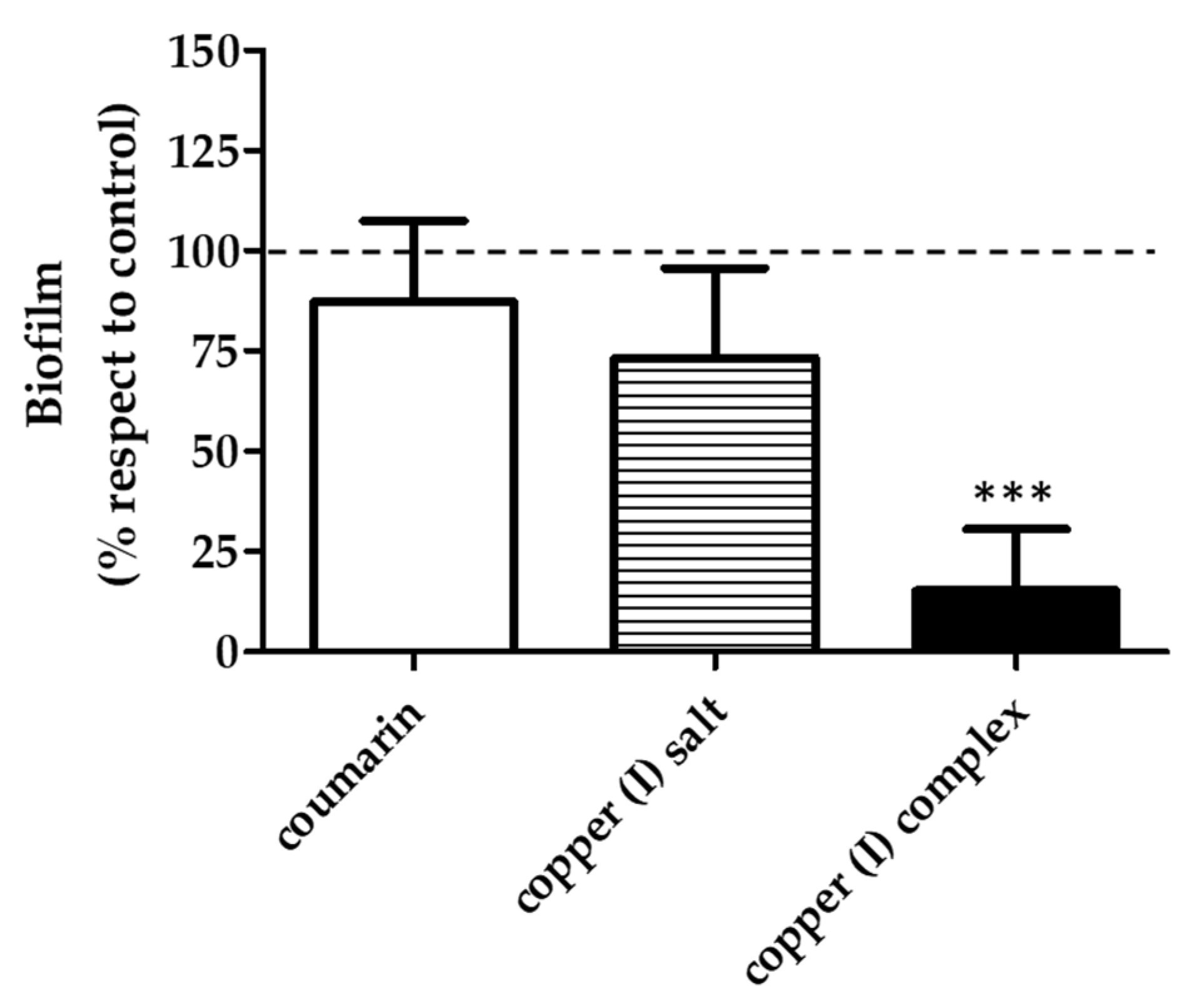
3.2. Antibiofilm Effect of Compounds in V. harveyi BB170
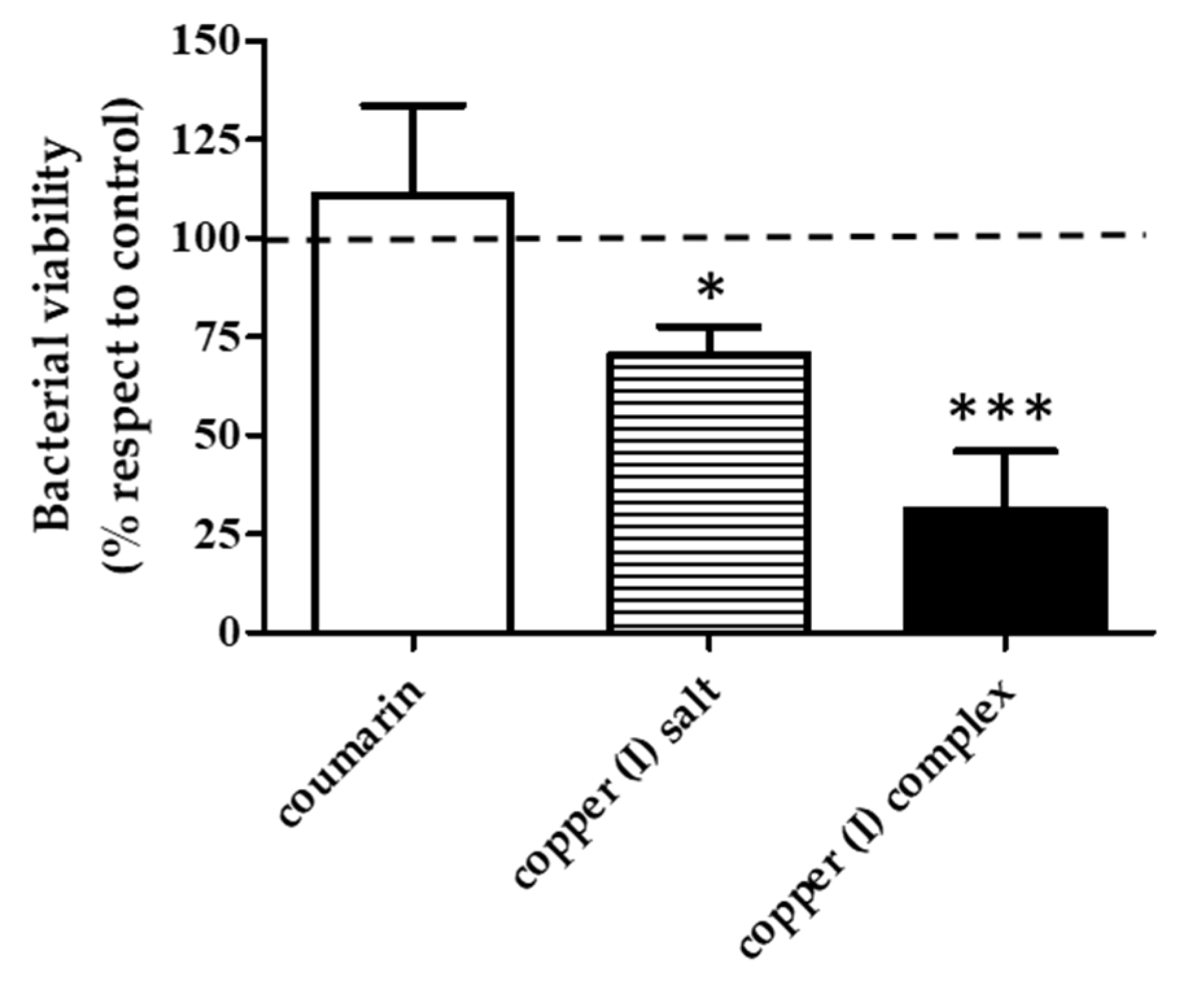
3.3. Effect of the Compounds on Bacterial Viability
3.4. Effect of the Compounds on V. harveyi BB170 Biofilm Structure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.-H.; Lee, J.-H. Antibiofilm Agents: A New Perspective for Antimicrobial Strategy. J. Microbiol. 2017, 55, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, H.; Charron-Mazenod, L.; Lewenza, S. Extracellular DNA Chelates Cations and Induces Antibiotic Resistance in Pseudomonas Aeruginosa Biofilms. PLoS Pathog. 2008, 4, e1000213. [Google Scholar] [CrossRef] [PubMed]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm Formation Mechanisms and Targets for Developing Antibiofilm Agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Campana, R.; Favi, G.; Baffone, W.; Lucarini, S. Marine Alkaloid 2,2-Bis(6-Bromo-3-Indolyl) Ethylamine and Its Synthetic Derivatives Inhibit Microbial Biofilms Formation and Disaggregate Developed Biofilms. Microorganisms 2019, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Buhlin, K.; Dufrêne, Y.F.; Gomelsky, M.; Moroni, A.; Ramstedt, M.; Rumbaugh, K.P.; Schulte, T.; Sun, L.; Åkerlund, B.; et al. Biofilm Formation-What We Can Learn from Recent Developments. J. Intern. Med. 2018, 284, 332–345. [Google Scholar] [CrossRef]
- Breidenstein, E.B.M.; de la Fuente-Núñez, C.; Hancock, R.E.W. Pseudomonas Aeruginosa: All Roads Lead to Resistance. Trends Microbiol. 2011, 19, 419–426. [Google Scholar] [CrossRef]
- Fagerlund, A.; Heir, E.; Møretrø, T.; Langsrud, S. Listeria Monocytogenes Biofilm Removal Using Different Commercial Cleaning Agents. Molecules 2020, 25, 792. [Google Scholar] [CrossRef]
- Jun, L.; Woo, N.Y.S. Pathogenicity of Vibrios in Fish: An Overview. J. Ocean Univ. Qingdao 2003, 2, 117–128. [Google Scholar] [CrossRef]
- Ina-Salwany, M.Y.; Al-Saari, N.; Mohamad, A.; Mursidi, F.-A.; Mohd-Aris, A.; Amal, M.N.A.; Kasai, H.; Mino, S.; Sawabe, T.; Zamri-Saad, M. Vibriosis in Fish: A Review on Disease Development and Prevention. J. Aquat. Anim. Health 2019, 31, 3–22. [Google Scholar] [CrossRef]
- Karnjana, K.; Soowannayan, C.; Wongprasert, K. Ethanolic Extract of Red Seaweed Gracilaria Fisheri and Furanone Eradicate Vibrio Harveyi and Vibrio Parahaemolyticus Biofilms and Ameliorate the Bacterial Infection in Shrimp. Fish Shellfish Immunol. 2019, 88, 91–101. [Google Scholar] [CrossRef]
- Austin, B.; Zhang, X.-H. Vibrio Harveyi: A Significant Pathogen of Marine Vertebrates and Invertebrates. Lett. Appl. Microbiol. 2006, 43, 119–124. [Google Scholar] [CrossRef]
- Vinay, T.N.; Ray, A.K.; Avunje, S.; Thangaraj, S.K.; Krishnappa, H.; Viswanathan, B.; Reddy, M.A.; Vijayan, K.K.; Patil, P.K. Vibrio Harveyi Biofilm as Immunostimulant Candidate for High-Health Pacific White Shrimp, Penaeus Vannamei Farming. Fish Shellfish Immunol. 2019, 95, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.J.; Turner, R.J.; Joo, D.A.; Stan, M.A.; Chan, C.S.; Allan, N.D.; Vrionis, H.A.; Olson, M.E.; Ceri, H. Copper and Quaternary Ammonium Cations Exert Synergistic Bactericidal and Antibiofilm Activity against Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 2008, 52, 2870–2881. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-G.; Cho, H.S.; Ryu, S.Y.; Cho, M.H.; Lee, J. Coumarins Reduce Biofilm Formation and the Virulence of Escherichia Coli O157:H7. Phytomedicine Int. J. Phytother. Phytopharm. 2014, 21, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Das, M.C.; Das, A.; Bhowmik, S.; Sandhu, P.; Akhter, Y.; Bhattacharjee, S.; De, U.C. Modulation of S. Aureus and P. Aeruginosa Biofilm: An in Vitro Study with New Coumarin Derivatives. World J. Microbiol. Biotechnol. 2018, 34, 170. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Ray, S.; Jhunjhunwala, S.; Nandi, D. Insights into Coumarin-Mediated Inhibition of Biofilm Formation in Salmonella Typhimurium. Biofouling 2020, 36, 479–491. [Google Scholar] [CrossRef]
- Suresh, M.K.; Biswas, R.; Biswas, L. An Update on Recent Developments in the Prevention and Treatment of Staphylococcus Aureus Biofilms. Int. J. Med. Microbiol. 2019, 309, 1–12. [Google Scholar] [CrossRef]
- Konieczny, J.; Rdzawski, Z. Antibacterial Properties of Copper and its Alloys. Arch. Mater. Sci. Eng. 2012, 56, 53–60. [Google Scholar]
- Pontin, K.P.; Borges, K.A.; Furian, T.Q.; Carvalho, D.; Wilsmann, D.E.; Cardoso, H.R.P.; Alves, A.K.; Chitolina, G.Z.; Salle, C.T.P.; Moraes, H.L. de S.; et al. Antimicrobial Activity of Copper Surfaces against Biofilm Formation by Salmonella Enteritidis and its Potential Application in the Poultry Industry. Food Microbiol. 2021, 94, 103645. [Google Scholar] [CrossRef]
- O’Gorman, J.; Humphreys, H. Application of Copper to Prevent and Control Infection. Where Are We Now? J. Hosp. Infect. 2012, 81, 217–223. [Google Scholar] [CrossRef]
- Sumrra, S.H.; Hassan, A.U.; Imran, M.; Khalid, M.; Mughal, E.U.; Zafar, M.N.; Tahir, M.N.; Raza, M.A.; Braga, A.A.C. Synthesis, Characterization and Biological Screening of Metal Complexes of Novel Sulfonamide Derivatives: Experimental and Theoretical Analysis of Sulfonamide Crystal. Appl. Organomet. Chem. 2020, 34, e5623. [Google Scholar] [CrossRef]
- Călinescu, M.; Fiastru, M.; Bala, D.; Mihailciuc, C.; Negreanu-Pîrjol, T.; Jurcă, B. Synthesis, Characterization, Electrochemical Behavior and Antioxidant Activity of New Copper(II) Coordination Compounds with Curcumin Derivatives. J. Saudi Chem. Soc. 2019, 23, 817–827. [Google Scholar] [CrossRef]
- El-Halim, H.; Nour El-Dien, F.; Mohamed, G.; Mohamed, N. Synthesis, Spectroscopic, Thermal Characterization, and Antimicrobial Activity of Miconazole Drug and Its Metal Complexes. J. Therm. Anal. Calorim. 2011, 109, 1–10. [Google Scholar] [CrossRef]
- Dalecki, A.G.; Crawford, C.L.; Wolschendorf, F. Copper and Antibiotics: Discovery, Modes of Action, and Opportunities for Medicinal Applications. Adv. Microb. Physiol. 2017, 70, 193–260. [Google Scholar] [CrossRef]
- Aldabaldetrecu, M.; Parra, M.; Soto, S.; Arce, P.; Tello, M.; Guerrero, J.; Modak, B. New Copper(I) Complex with a Coumarin as Ligand with Antibacterial Activity against Flavobacterium Psychrophilum. Molecules 2020, 25, 3183. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.F.; Henriques, I.S.; Correia, A.; Santos, E.B.H.; Esteves, V.I. Antibacterial Activity of Oxytetracycline Photoproducts in Marine Aquaculture’s Water. Environ. Pollut. 2017, 220, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, E.P.; Hastings, J.W.; Ulitzur, S. Induction of Luciferase Synthesis in Beneckea Harveyi by Other Marine Bacteria. Arch. Microbiol. 1979, 120, 87–91. [Google Scholar] [CrossRef]
- Weinstein, M.P.; Patel, J.B. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: M07-A11, 11th ed.; Documents/Clinical and Laboratory Standards Institute; Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2018; ISBN 978-1-56238-836-2. [Google Scholar]
- Barry, A.L.; Craig, W.A.; Reller, L.B.; Sanders, C.C.; Swenson, J.M.; Nadler, H. CLSI Methods for determining bactericidal activity of antimicrobial agents: Approved guideline M26-A; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 1999; ISBN 1-56238-384-1. [Google Scholar]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. J. Vis. Exp. 2011, 2437. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting Microbial Biofilms: Current and Prospective Therapeutic Strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic Resistance in Pseudomonas Aeruginosa: Mechanisms and Alternative Therapeutic Strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Warnes, S.L.; Keevil, C.W. Mechanism of Copper Surface Toxicity in Vancomycin-Resistant Enterococci Following Wet or Dry Surface Contact. Appl. Environ. Microbiol. 2011, 77, 6049–6059. [Google Scholar] [CrossRef]
- Fu, Y.; Chang, F.-M.J.; Giedroc, D.P. Copper Transport and Trafficking at the Host-Bacterial Pathogen Interface. Acc. Chem. Res. 2014, 47, 3605–3613. [Google Scholar] [CrossRef]
- Evangelinou, O.; Hatzidimitriou, A.G.; Velali, E.; Pantazaki, A.A.; Voulgarakis, N.; Aslanidis, P. Mixed-Ligand Copper(I) Halide Complexes Bearing 4,5-Bis(Diphenylphosphano)-9,9-Dimethyl-Xanthene and N-Methylbenzothiazole-2-Thione: Synthesis, Structures, Luminescence and Antibacterial Activity Mediated by DNA and Membrane Damage. Polyhedron 2014, 72, 122–129. [Google Scholar] [CrossRef]
- Gomes da Silva Dantas, F.; Araújo de Almeida-Apolonio, A.; Pires de Araújo, R.; Regiane Vizolli Favarin, L.; Fukuda de Castilho, P.; de Oliveira Galvão, F.; Inez Estivalet Svidzinski, T.; Antônio Casagrande, G.; Mari Pires de Oliveira, K. A Promising Copper(II) Complex as Antifungal and Antibiofilm Drug against Yeast Infection. Mol. J. Synth. Chem. Nat. Prod. Chem. 2018, 23, 1856. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, F.; Haghi, F.; Bikas, R.; Heidari, A.; Gholami, M.; Kozakiewicz, A.; Zeighami, H. Synthesis, Characterization and Assessment of Anti-Quorum Sensing Activity of Copper(II)-Ciprofloxacin Complex against Pseudomonas Aeruginosa PAO1. AMB Express 2020, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, P.d.P.M.; Ruviaro, A.R.; Martins, I.M.; Macedo, J.A.; LaPointe, G.; Macedo, G.A. Enzyme-Assisted Extraction of Flavanones from Citrus Pomace: Obtention of Natural Compounds with Anti-Virulence and Anti-Adhesive Effect against Salmonella Enterica Subsp. Enterica Serovar Typhimurium. Food Control 2021, 120, 107525. [Google Scholar] [CrossRef]
- Esteban-Fernández, A.; Zorraquín-Peña, I.; Ferrer, M.D.; Mira, A.; Bartolomé, B.; González de Llano, D.; Moreno-Arribas, M.V. Inhibition of Oral Pathogens Adhesion to Human Gingival Fibroblasts by Wine Polyphenols Alone and in Combination with an Oral Probiotic. J. Agric. Food Chem. 2018, 66, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Chang, I.-M.; Oh, K.-B. Inhibition of the Bacterial Surface Protein Anchoring Transpeptidase Sortase by Medicinal Plants. Biosci. Biotechnol. Biochem. 2002, 66, 2751–2754. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Patel, M.; Deshpande, S.; Alreshidi, M.; Siddiqui, A.J.; Reddy, M.N.; Emira, N.; De Feo, V. Effect of Adiantum Philippense Extract on Biofilm Formation, Adhesion With Its Antibacterial Activities Against Foodborne Pathogens, and Characterization of Bioactive Metabolites: An in Vitro-in Silico Approach. Front. Microbiol. 2020, 11, 823. [Google Scholar] [CrossRef]
- da Cunha, M.G.; de Cássia Orlandi Sardi, J.; Freires, I.A.; Franchin, M.; Rosalen, P.L. Antimicrobial, Anti-Adherence and Antibiofilm Activity against Staphylococcus Aureus of a 4-Phenyl Coumarin Derivative Isolated from Brazilian Geopropolis. Microb. Pathog. 2020, 139, 103855. [Google Scholar] [CrossRef] [PubMed]
- Yeu, J.-E.; Lee, H.-G.; Park, G.-Y.; Lee, J.; Kang, M.-S. Antimicrobial and Antibiofilm Activities of Weissella Cibaria against Pathogens of Upper Respiratory Tract Infections. Microorganisms 2021, 9, 1181. [Google Scholar] [CrossRef]
- Okshevsky, M.; Meyer, R.L. The Role of Extracellular DNA in the Establishment, Maintenance and Perpetuation of Bacterial Biofilms. Crit. Rev. Microbiol. 2015, 41, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Kanampalliwar, A.; Singh, D.V. Extracellular DNA Builds and Interacts with Vibrio Polysaccharide in the Biofilm Matrix Formed by Vibrio Cholerae. Environ. Microbiol. Rep. 2020, 12, 594–606. [Google Scholar] [CrossRef]
- O’Shaughnessy, M.; McCarron, P.; Viganor, L.; McCann, M.; Devereux, M.; Howe, O. The Antibacterial and Anti-Biofilm Activity of Metal Complexes Incorporating 3,6,9-Trioxaundecanedioate and 1,10-Phenanthroline Ligands in Clinical Isolates of Pseudomonas Aeruginosa from Irish Cystic Fibrosis Patients. Antibiotics 2020, 9, 674. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhang, C.; Mu, Y.; Shen, Q.; Feng, Y. Indole Affects Biofilm Formation in Bacteria. Indian J. Microbiol. 2010, 50, 362–368. [Google Scholar] [CrossRef]
- Mueller, R.S.; Beyhan, S.; Saini, S.G.; Yildiz, F.H.; Bartlett, D.H. Indole Acts as an Extracellular Cue Regulating Gene Expression in Vibrio Cholerae. J. Bacteriol. 2009, 191, 3504–3516. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, Y.; Zhao, X.; Li, C.; Guo, M.; Lv, Z.; Zhang, W. Indole Contributes to Tetracycline Resistance via the Outer Membrane Protein OmpN in Vibrio Splendidus. World J. Microbiol. Biotechnol. 2020, 36, 36. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.-W.; Schaffer, J.N.; Tu, K.C.; Mehta, P.; Lu, W.; Ong, N.P.; Bassler, B.L.; Wingreen, N.S. Active Regulation of Receptor Ratios Controls Integration of Quorum-Sensing Signals in Vibrio Harveyi. Mol. Syst. Biol. 2011, 7, 491. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.A.; Pomianek, M.E.; Kraml, C.M.; Taylor, R.K.; Semmelhack, M.F.; Bassler, B.L. The Major Vibrio Cholerae Autoinducer and Its Role in Virulence Factor Production. Nature 2007, 450, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Winzer, K.; Williams, P. Quorum Sensing and the Regulation of Virulence Gene Expression in Pathogenic Bacteria. Int. J. Med. Microbiol. 2001, 291, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Rémy, B.; Mion, S.; Plener, L.; Elias, M.; Chabrière, E.; Daudé, D. Interference in Bacterial Quorum Sensing: A Biopharmaceutical Perspective. Front. Pharmacol. 2018, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Lyon, G.J.; Muir, T.W. Chemical Signaling among Bacteria and Its Inhibition. Chem. Biol. 2003, 10, 1007–1021. [Google Scholar] [CrossRef][Green Version]
- Feng, L.; Wu, Z.; Yu, X. Quorum Sensing in Water and Wastewater Treatment Biofilms. J. Environ. Biol. 2013, 34, 437–444. [Google Scholar]
- Glišić, B.Đ.; Aleksic, I.; Comba, P.; Wadepohl, H.; Ilic-Tomic, T.; Nikodinovic-Runic, J.; Djuran, M.I. Copper(II) Complexes with Aromatic Nitrogen-Containing Heterocycles as Effective Inhibitors of Quorum Sensing Activity in Pseudomonas Aeruginosa. RSC Adv. 2016, 6, 86695–86709. [Google Scholar] [CrossRef]
- Zhang, Y.; Sass, A.; Van Acker, H.; Wille, J.; Verhasselt, B.; Van Nieuwerburgh, F.; Kaever, V.; Crabbé, A.; Coenye, T. Coumarin Reduces Virulence and Biofilm Formation in Pseudomonas Aeruginosa by Affecting Quorum Sensing, Type III Secretion and C-Di-GMP Levels. Front. Microbiol. 2018, 9, 1952. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Barranquero, J.A.; Reen, F.J.; McCarthy, R.R.; O’Gara, F. Deciphering the Role of Coumarin as a Novel Quorum Sensing Inhibitor Suppressing Virulence Phenotypes in Bacterial Pathogens. Appl. Microbiol. Biotechnol. 2015, 99, 3303–3316. [Google Scholar] [CrossRef]
- Brahma, U.; Kothari, R.; Sharma, P.; Bhandari, V. Antimicrobial and Anti-Biofilm Activity of Hexadentated Macrocyclic Complex of Copper (II) Derived from Thiosemicarbazide against Staphylococcus Aureus. Sci. Rep. 2018, 8, 8050. [Google Scholar] [CrossRef]




| Vibrio harveyi BB170 | |||
|---|---|---|---|
| MIC µg/mL | MBC µg/mL | IC50 µg/mL | |
| Coumarin | 1024 | 1024 | 89.38 ± 0.05 a |
| Copper (I) salt [Cu(CH3CN)4]ClO4 | 256 | 512 | 71.90 ± 0.02 a |
| Copper (I) complex [Cu(NN1)2]ClO4 | 256 | 256 | 25.19 ± 0.04 b |
| IC50 (µg/mL) | ||||
|---|---|---|---|---|
| 2× IC50 | IC50 | IC50/2 | IC50/4 | |
| Coumarin | 178.76 | 89.38 | 44.69 | 22.35 |
| Copper (I) salt [Cu(CH3CN)4]ClO4 | 143.8 | 71.9 | 35.95 | 17.97 |
| Copper (I) complex [Cu(NN12]ClO4 | 50.38 | 25.19 | 12.6 | 6.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Aguilera, S.; Modak, B.; Aldabaldetrecu, M.; Lozano, C.P.; Guerrero, J.; Lefimil, C.; Parra, M. In Vitro Effect of Copper (I) Complex [Cu(NN1)2](ClO4) on Vibrio harveyi BB170 Biofilm Formation. Microorganisms 2021, 9, 2273. https://doi.org/10.3390/microorganisms9112273
Soto-Aguilera S, Modak B, Aldabaldetrecu M, Lozano CP, Guerrero J, Lefimil C, Parra M. In Vitro Effect of Copper (I) Complex [Cu(NN1)2](ClO4) on Vibrio harveyi BB170 Biofilm Formation. Microorganisms. 2021; 9(11):2273. https://doi.org/10.3390/microorganisms9112273
Chicago/Turabian StyleSoto-Aguilera, Sarita, Brenda Modak, Maialen Aldabaldetrecu, Carla P. Lozano, Juan Guerrero, Claudia Lefimil, and Mick Parra. 2021. "In Vitro Effect of Copper (I) Complex [Cu(NN1)2](ClO4) on Vibrio harveyi BB170 Biofilm Formation" Microorganisms 9, no. 11: 2273. https://doi.org/10.3390/microorganisms9112273
APA StyleSoto-Aguilera, S., Modak, B., Aldabaldetrecu, M., Lozano, C. P., Guerrero, J., Lefimil, C., & Parra, M. (2021). In Vitro Effect of Copper (I) Complex [Cu(NN1)2](ClO4) on Vibrio harveyi BB170 Biofilm Formation. Microorganisms, 9(11), 2273. https://doi.org/10.3390/microorganisms9112273






