Seasonal Changes in Soil Microbial Community and Co-Occurrence Network of Species of the Genus Corylus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Soil Sampling
2.2. Soil Physicochemical Properties
2.3. DNA Extraction and PCR Quantification
2.4. Ecological Niche Modeling
2.5. Statistical Analysis
3. Results
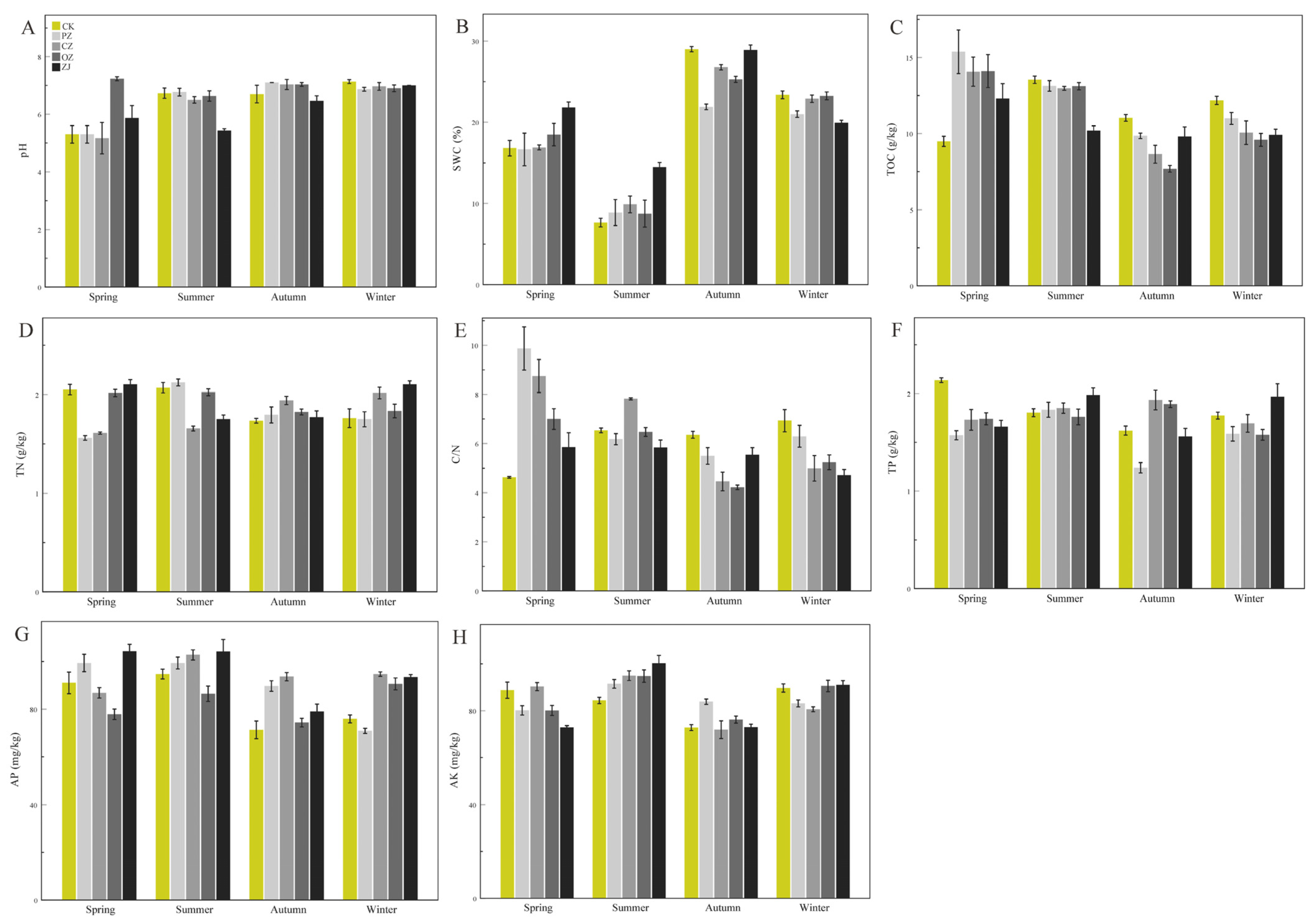
3.1. Soil Physicochemical Properties
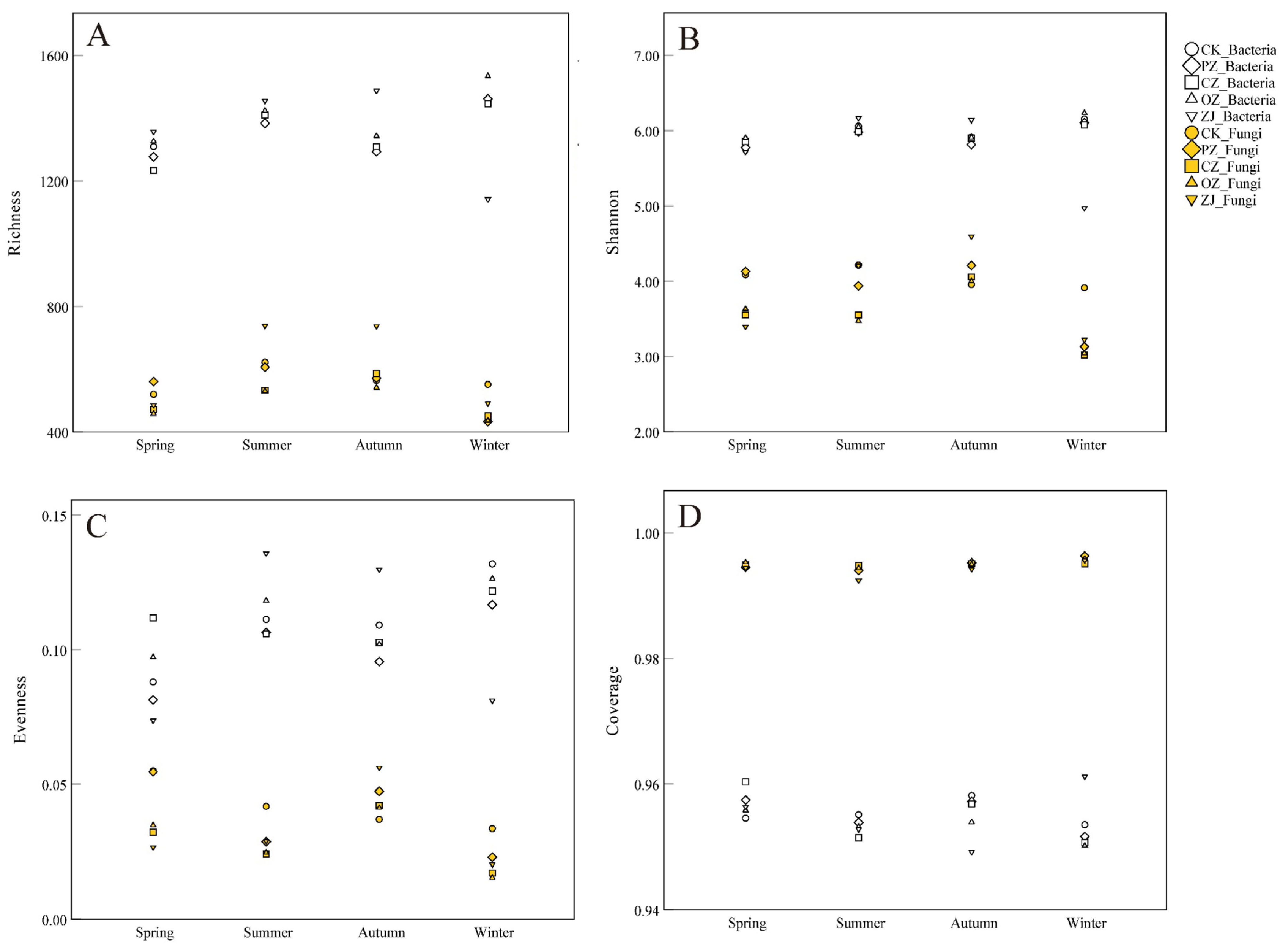
3.2. Soil Microbial Diversity
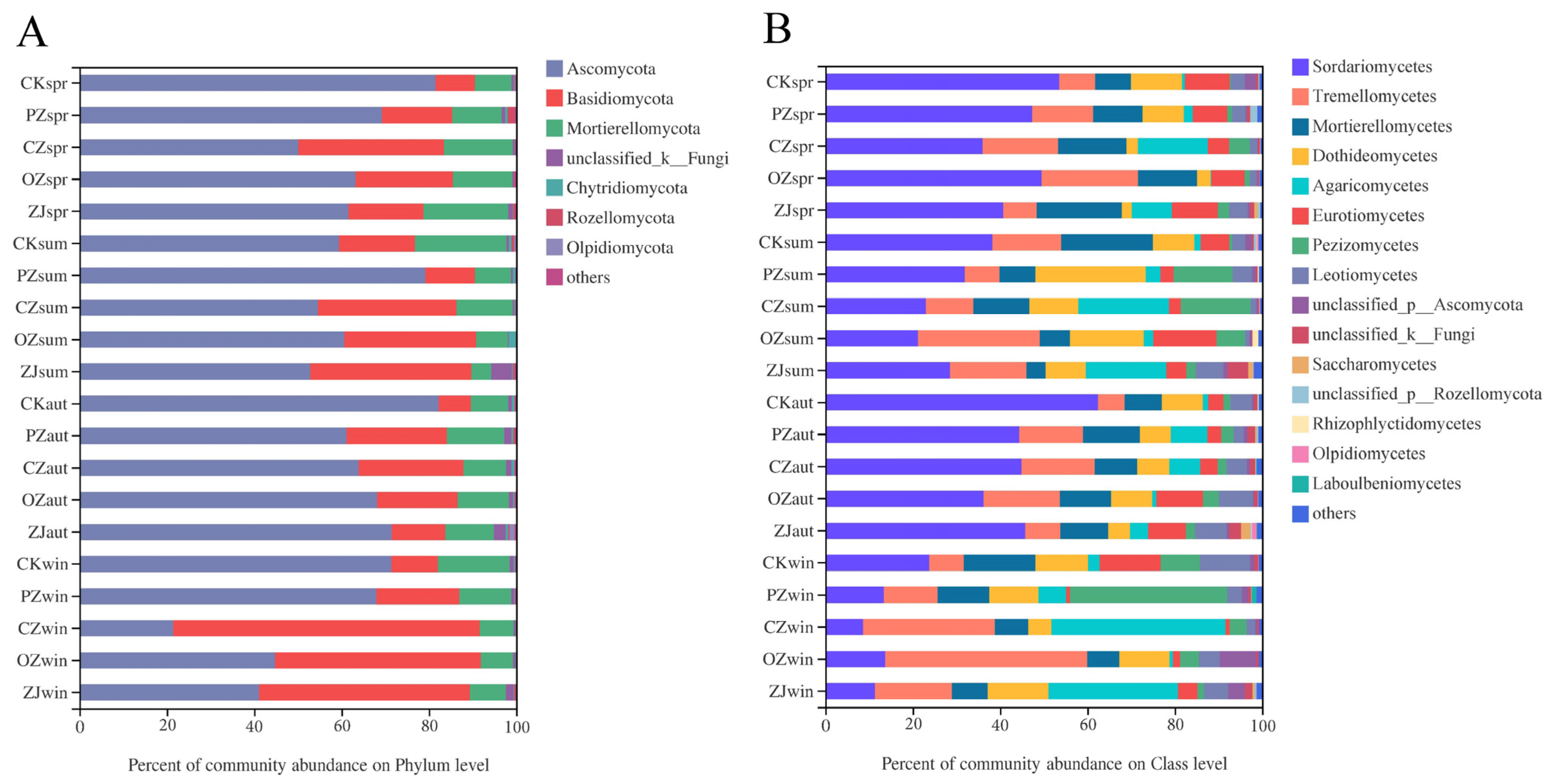
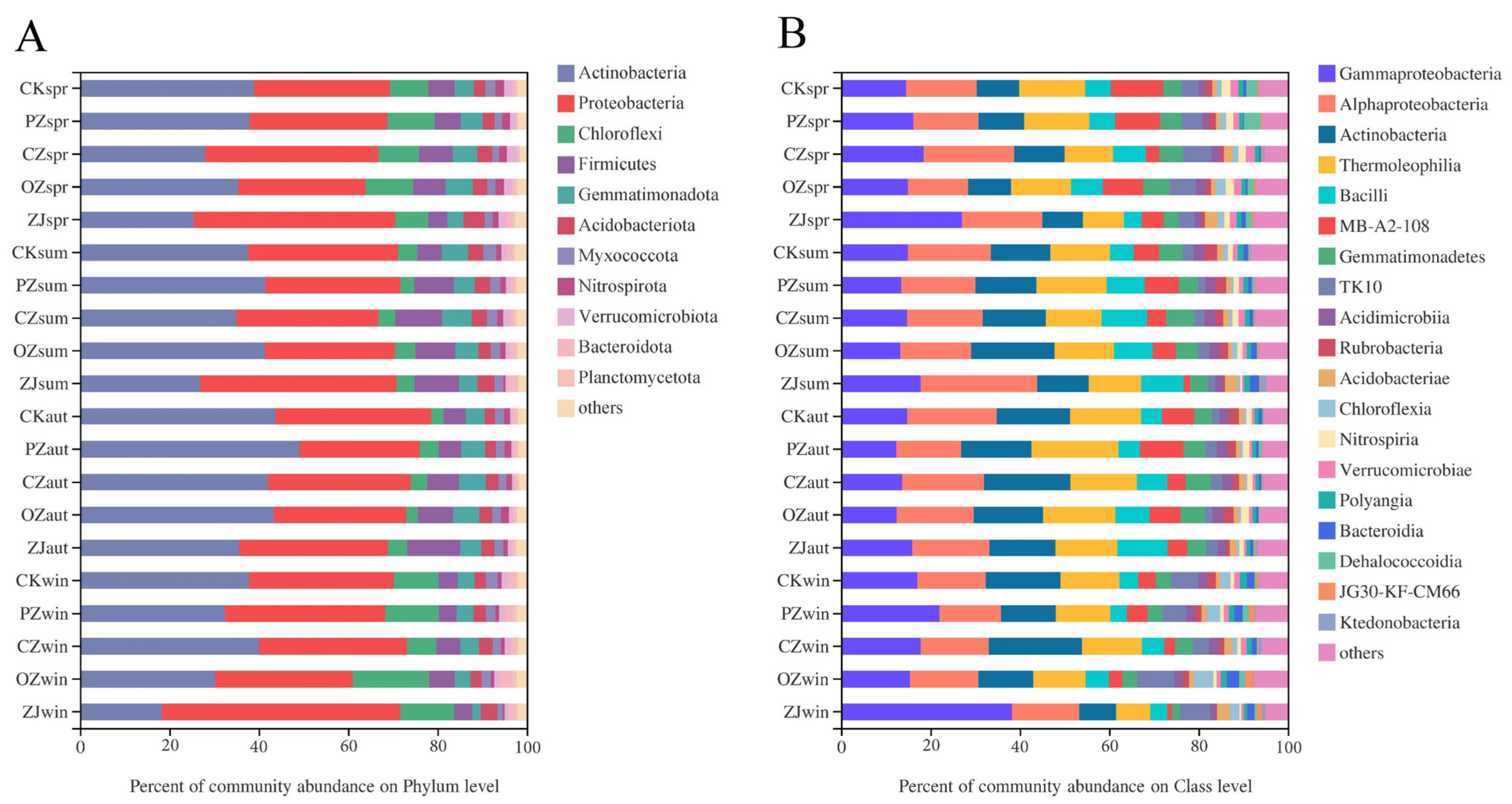
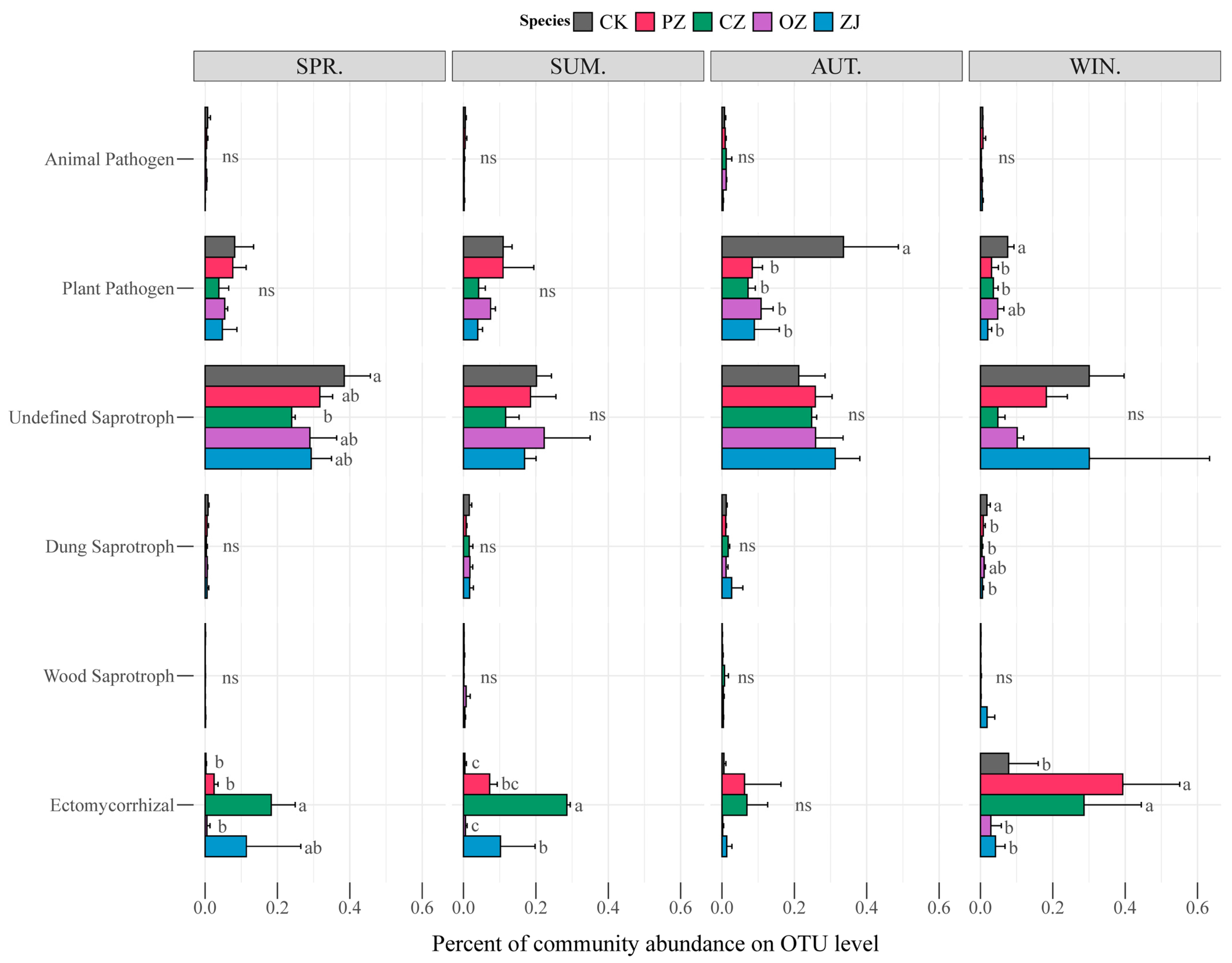
3.3. Soil Microbial Community Structure and Function
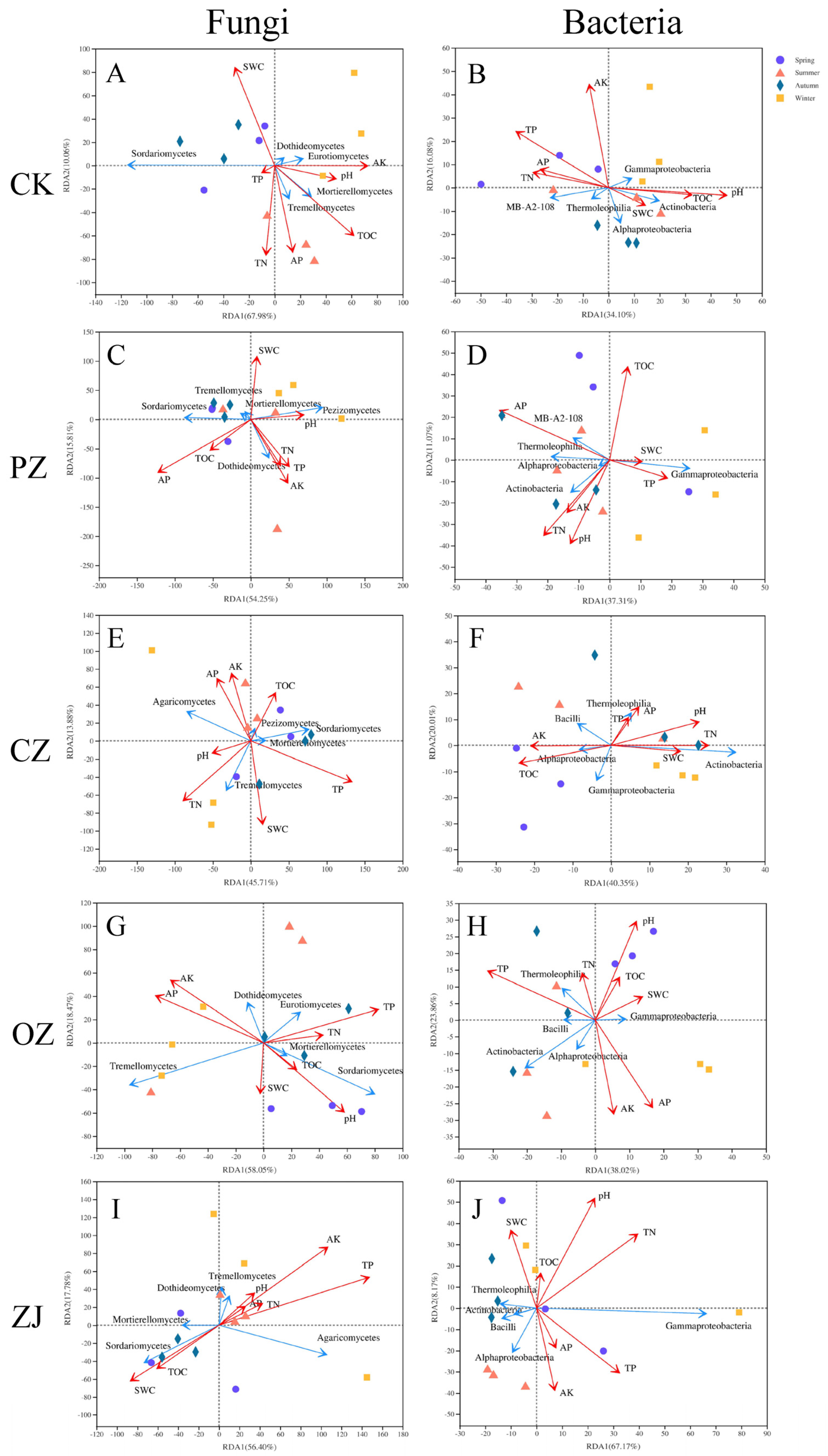
3.4. Relationships of Microbial Communities and Soil Properties
3.5. Co-Occurrence Network Characteristics
3.6. Future Distribution of C. kweichowensis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Z.; Wang, G.; Ma, Q.; Ma, W.; Liang, L.; Zhao, T. The complete chloroplast genomes of three Betulaceae species: Implications for molecular phylogeny and historical biogeography. PeerJ 2019, 2019, e6320. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Ma, W.; Ma, Q.; Yang, Z.; Liang, L.; Wang, G.; Wang, L. Genetic diversity and population structure of chinese corylus heterophylla and corylus kweichowensis using simple sequence repeat markers. J. Am. Soc. Hortic Sci. 2020, 145, 289–298. [Google Scholar] [CrossRef]
- Wang, G. Progress in Cultivation and Utilization of Corylus L. Resources in China (IV)—Nutrition, Comprehensive Utilization and Industry Development. For. Res. 2018, 31, 130–136. [Google Scholar]
- Zhang, Y.; Liu, L.; Liang, W. China Fruit’s Monograph: Chinese Chestnut and Chinese Hazelnut Volume; China Forestry Press: Beijing, China, 2005. [Google Scholar]
- Liang, W.; Xie, M. Study on breeding of new hazelnut varieties. China Fruits 2000, 2, 4–6. [Google Scholar]
- Habekost, M.; Eisenhauer, N.; Scheu, S.; Steinbeiss, S.; Weigelt, A.; Gleixner, G. Seasonal changes in the soil microbial community in a grassland plant diversity gradient four years after establishment. Soil Biol. Biochem. 2008, 40, 2588–2595. [Google Scholar] [CrossRef]
- Landesman, W.J.; Freedman, Z.B.; Nelson, D.M. Seasonal, sub-seasonal and diurnal variation of soil bacterial community composition in a temperate deciduous forest. FEMS Microbiol. Ecol. 2019, 95. [Google Scholar] [CrossRef] [Green Version]
- Lennon, J.T.; Jones, S.E. Microbial seed banks: The ecological and evolutionary implications of dormancy. Nat. Rev. Microbiol. 2011, 9, 119–130. [Google Scholar] [CrossRef]
- López-Mondéjar, R.; Voříšková, J.; Větrovský, T.; Baldrian, P. The bacterial community inhabiting temperate deciduous forests is vertically stratified and undergoes seasonal dynamics. Soil Biol. Biochem. 2015, 87, 43–50. [Google Scholar] [CrossRef]
- Thoms, C.; Gleixner, G. Seasonal differences in tree species’ influence on soil microbial communities. Soil Biol. Biochem. 2013, 66, 239–248. [Google Scholar] [CrossRef]
- Koranda, M.; Kaiser, C.; Fuchslueger, L.; Kitzler, B.; Sessitsch, A.; Zechmeister-Boltenstern, S.; Richter, A. Seasonal variation in functional properties of microbial communities in beech forest soil. Soil Biol. Biochem. 2013, 60, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Yao, M.; Rui, J.; Niu, H.; Heděnec, P.; Li, J.; He, Z.; Wang, J.; Cao, W.; Li, X. The differentiation of soil bacterial communities along a precipitation and temperature gradient in the eastern Inner Mongolia steppe. Catena 2017, 152, 47–56. [Google Scholar] [CrossRef]
- Irmak Yilmaz, F. Seasonal changes of some microbiological properties of soils in a field of hazelnut (Corylus avellana L.) growing. Appl. Ecol. Environ. Res. 2020, 18, 253–262. [Google Scholar] [CrossRef]
- Bargali, K.; Manral, V.; Padalia, K.; Bargali, S.S.; Upadhyay, V.P. Effect of vegetation type and season on microbial biomass carbon in Central Himalayan forest soils, India. Catena 2018, 171, 125–135. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Hester, A.J.; Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Hewison, R.L.; Potts, J.M. Is vegetation composition or soil chemistry the best predictor of the soil microbial community? Plant Soil 2010, 333, 417–430. [Google Scholar] [CrossRef]
- Nielsen, U.N.; Osler, G.H.R.; Campbell, C.D.; Burslem, D.F.R.P.; van der Wal, R. The influence of vegetation type, soil properties and precipitation on the composition of soil mite and microbial communities at the landscape scale. J. Biogeogr. 2010, 37, 1317–1328. [Google Scholar] [CrossRef]
- Huang, X.F.; Chaparro, J.M.; Reardon, K.F.; Zhang, R.; Shen, Q.; Vivanco, J.M. Rhizosphere interactions: Root exudates, microbes, and microbial communities 1. Botany 2014, 92, 267–275. [Google Scholar] [CrossRef]
- Jangid, K.; Williams, M.A.; Franzluebbers, A.J.; Schmidt, T.M.; Coleman, D.C.; Whitman, W.B. Land-use history has a stronger impact on soil microbial community composition than aboveground vegetation and soil properties. Soil Biol. Biochem. 2011, 43, 2184–2193. [Google Scholar] [CrossRef]
- Wu, A.L.; Jiao, X.Y.; Fan, F.F.; Wang, J.S.; Guo, J.; Dong, E.W.; Wang, L.G.; Shen, X.M. Effect of continuous sorghum cropping on the rhizosphere microbial community and the role of Bacillus amyloliquefaciens in altering the microbial composition. Plant Growth Regul. 2019, 89, 299–308. [Google Scholar] [CrossRef]
- He, D.; Shen, W.; Eberwein, J.; Zhao, Q.; Ren, L.; Wu, Q.L. Diversity and co-occurrence network of soil fungi are more responsive than those of bacteria to shifts in precipitation seasonality in a subtropical forest. Soil Biol. Biochem. 2017, 115, 499–510. [Google Scholar] [CrossRef]
- Barberán, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2012, 6, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Greenblum, S.; Turnbaugh, P.J.; Borenstein, E. Metagenomic systems biology of the human gut microbiome reveals topological shifts associated with obesity and inflammatory bowel disease. Proc. Natl. Acad. Sci. USA 2012, 109, 594–599. [Google Scholar] [CrossRef] [Green Version]
- FAO. World Reference Base for Soil Resources. In World Reference Base for Soil Resources 2014 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2015. [Google Scholar]
- Qian, X.; Gu, J.; Pan, H.J.; Zhang, K.Y.; Sun, W.; Wang, X.J.; Gao, H. Effects of living mulches on the soil nutrient contents, enzyme activities, and bacterial community diversities of apple orchard soils. Eur. J. Soil Biol. 2015, 70, 23–30. [Google Scholar] [CrossRef]
- Institute of Soil Science Chinese Academy of Sciences. Soil Physical and Chemical Analysis; Shanghai Scientific & Technical Publishers: Shanghai, China, 1978. [Google Scholar]
- Qian, X.; Gu, J.; Sun, W.; Li, Y.D.; Fu, Q.X.; Wang, X.J.; Gao, H. Changes in the soil nutrient levels, enzyme activities, microbial community function, and structure during apple orchard maturation. Appl. Soil Ecol. 2014, 77, 18–25. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Garrido-Oter, R.; Münch, P.C.; Weiman, A.; Dröge, J.; Pan, Y.; McHardy, A.C.; Schulze-Lefert, P. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 2015, 17, 392–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, W.D.; Bitz, C.M.; Blackmon, M.L.; Bonan, G.B.; Bretherton, C.S.; Carton, J.A.; Chang, P.; Doney, S.C.; Hack, J.J.; Henderson, T.B.; et al. The Community Climate System Model Version 3 (CCSM3). J. Clim. 2006, 19, 2122–2143. [Google Scholar] [CrossRef]
- Huo, H.; Ma, Q.; Li, J.; Zhao, T.; Wang, G. Study on the Distribution of Corylus L. in China and the Climatic Evaluation of the Suitable Areas. J. Plant Genet. Resour. 2016, 17, 801–808. [Google Scholar]
- Ma, Q.; Huo, H.; Chen, X.; Zhao, T.; Liang, W.; Wang, G. Study on the Taxonomy, Distribution, Development and Utilization of Corylus kweichowensis Hu. J. Plant Genet. Resour. 2014, 15, 1223–1231. [Google Scholar]
- Wang, L.; Zhao, T.; Ma, Q.; Xiao, Z.; Wang, G. Analysis on relationship between geographical distribution pattern of Chinese endemic species Corylus kweichowensis and climatic environmental factors. J. Plant Resour. Environ. 2017, 26, 77–83. [Google Scholar]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Ren, C.; Sun, Z. Effects of thinning on soil saprotrophic and ectomycorrhizal fungi in a Korean larch plantation. For. Ecol. Manag. 2020, 461, 117920. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2: An improved and extensible approach for metagenome inference. bioRxiv 2019, 2019, 672295. [Google Scholar] [CrossRef] [Green Version]
- Mandakovic, D.; Rojas, C.; Maldonado, J.; Latorre, M.; Travisany, D.; Delage, E.; Bihouée, A.; Jean, G.; Díaz, F.P.; Fernández-Gómez, B.; et al. Structure and co-occurrence patterns in microbial communities under acute environmental stress reveal ecological factors fostering resilience. Sci. Rep. 2018, 8, 5875. [Google Scholar] [CrossRef]
- Leite, M.F.A.; Kuramae, E.E. You must choose, but choose wisely: Model-based approaches for microbial community analysis. Soil Biol. Biochem. 2020, 151, 108042. [Google Scholar] [CrossRef]
- Chu, H.; Neufeld, J.D.; Walker, V.K.; Grogan, P. The Influence of Vegetation Type on the Dominant Soil Bacteria, Archaea, and Fungi in a Low Arctic Tundra Landscape. Soil Sci. Soc. Am. J. 2011, 75, 1756–1765. [Google Scholar] [CrossRef] [Green Version]
- Fierer, N.; Schimel, J.P.; Holden, P.A. Influence of drying-rewetting frequency on soil bacterial community structure. Microb. Ecol. 2003, 45, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Schimel, J.P. Effects of drying–rewetting frequency on soil carbon and nitrogen transformations. Soil Biol. Biochem. 2002, 34, 777–787. [Google Scholar] [CrossRef]
- Sun, S.; Li, S.; Avera, B.N.; Strahm, B.D.; Badgley, B.D. Soil bacterial and fungal communities show distinct recovery patterns during forest ecosystem restoration. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, L.; Ren, H.; Li, S.; Leng, X.; Yao, X. Soil bacterial community structure and co-occurrence pattern during vegetation restoration in karst rocky desertification area. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.; Lv, L.; Liu, G. Research on Soil Acidification and Acidic Soil’s Melioration. J. South China Univ. Trop. Agric. 2006, 12, 23–28. [Google Scholar]
- Hartmann, M.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef] [Green Version]
- Ren, C.; Zhang, W.; Zhong, Z.K.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Differential responses of soil microbial biomass, diversity, and compositions to altitudinal gradients depend on plant and soil characteristics. Sci. Total Environ. 2018, 610–611, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Bossio, D.A.; Scow, K.M.; Gunapala, N.; Graham, K.J. Determinants of Soil Microbial Communities: Effects of Agricultural Management, Season, and Soil Type on Phospholipid Fatty Acid Profiles. Microb. Ecol. 1998, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Keet, J.H.; Ellis, A.G.; Hui, C.; Le Roux, J.J. Strong spatial and temporal turnover of soil bacterial communities in South Africa’s hyperdiverse fynbos biome. Soil Biol. Biochem. 2019, 136. [Google Scholar] [CrossRef]
- Mergel, A.; Kloos, K.; Bothe, H. Seasonal fluctuations in the population of denitrifying and N2-fixing bacteria in an acid soil of a norway spruce forest. Plant Soil 2001, 230, 145–160. [Google Scholar] [CrossRef]
- Samaritani, E.; Mitchell, E.A.D.; Rich, J.; Shrestha, J.; Fournier, B.; Frey, B. Soil bacterial communities and ecosystem functioning change more strongly with season than habitat in a restored floodplain. Appl. Soil Ecol. 2017, 112, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Wang, G.; Liu, J.; Ni, J. Effects of vegetation type, season, and soil properties on soil microbial community in subtropical forests. Appl. Soil Ecol. 2021, 158. [Google Scholar] [CrossRef]
- Kaisermann, A.; Maron, P.A.; Beaumelle, L.; Lata, J.C. Fungal communities are more sensitive indicators to non-extreme soil moisture variations than bacterial communities. Appl. Soil Ecol. 2015, 86, 158–164. [Google Scholar] [CrossRef]
- Luo, X.; Wang, M.K.; Hu, G.; Weng, B. Seasonal change in microbial diversity and its relationship with soil chemical properties in an orchard. PLoS ONE 2019, 14, e0215556. [Google Scholar] [CrossRef]
- Schutter, M.E.; Sandeno, J.M.; Dick, R.P. Seasonal, soil type, and alternative management influences on microbial communities of vegetable cropping systems. Biol. Fertil. Soils 2001, 34, 397–410. [Google Scholar] [CrossRef]
- Smit, E.; Leeflang, P.; Gommans, S.; Van Den Broek, J.; Van Mil, S.; Wernars, K. Diversity and Seasonal Fluctuations of the Dominant Members of the Bacterial Soil Community in a Wheat Field as Determined by Cultivation and Molecular Methods. Appl. Environ. Microbiol. 2001, 67, 2284–2291. [Google Scholar] [CrossRef] [Green Version]
- Högberg, P.; Nordgren, A.; Buchmann, N.; Taylor, A.F.S.; Ekblad, A.; Högberg, M.N.; Nyberg, G.; Ottosson-Löfvenius, M.; Read, D.J. Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 2001, 411, 789–792. [Google Scholar] [CrossRef]
- Berg, M.P.; Kniese, J.P.; Verhoef, H.A. Dynamics and stratification of bacteria and fungi in the organic layers of a scots pine forest soil. Biol. Fertil. Soils 1998, 26, 313–322. [Google Scholar] [CrossRef]
- Griffiths, R.P.; Caldwell, B.A.; Cromack, K.; Morita, R.Y. Douglas-fir forest soils colonized by ectomycorrhizal mats. I. Seasonal variation in nitrogen chemistry and nitrogen cycle transformation rates. Can. J. For. Res. 1990, 20, 211–218. [Google Scholar] [CrossRef]
- Shigyo, N.; Umeki, K.; Hirao, T. Seasonal dynamics of soil fungal and bacterial communities in cool-temperate montane forests. Front. Microbiol. 2019, 10, 1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.L.; Dell, B.; Malajczuk, N. Effect of Scleroderma spore density and age on mycorrhiza formation and growth of containerized Eucalyptus globulus and E. urophylla seedlings. New For. 2006, 31, 453–467. [Google Scholar] [CrossRef]
- De Román, M.; Boa, E.; Woodward, S. Wild-gathered fungi for health and rural livelihoods. Proc. Nutr. Soc. 2006, 65, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Benucci, G.M.N.; Gógán Csorbai, A.; Baciarelli Falini, L.; Bencivenga, M.; Di Massimo, G.; Donnini, D. Mycorrhization of Quercus robur L., Quercus cerris L. and Corylus avellana L. seedlings with Tuber macrosporum Vittad. Mycorrhiza 2012, 22, 639–646. [Google Scholar] [CrossRef]
- Santelices, R.; Palfner, G. Controlled Rhizogenesis and Mycorrhization of Hazelnut (Corylus avellana L.) Cuttings with Black Truffle (Tuber melanosporum Vitt.). Chil. J. Agric. Res. 2010, 70, 204–212. [Google Scholar] [CrossRef]
- Wedén, C.; Pettersson, L.; Danell, E. Truffle cultivation in Sweden: Results from Quercus robur and Corylus avellana field trials on the island of Gotland. Scand. J. For. Res. 2009, 24, 37–53. [Google Scholar] [CrossRef]
- Kuffner, M.; Hai, B.; Rattei, T.; Melodelima, C.; Schloter, M.; Zechmeister-Boltenstern, S.; Jandl, R.; Schindlbacher, A.; Sessitsch, A. Effects of season and experimental warming on the bacterial community in a temperate mountain forest soil assessed by 16S rRNA gene pyrosequencing. FEMS Microbiol. Ecol. 2012, 82, 551–562. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Ritz, K.; Ebblewhite, N.; Dobson, G. Soil microbial community structure: Effects of substrate loading rates. Soil Biol. Biochem. 1998, 31, 145–153. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; Da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Liang, W. A survey on the introduction of Corylus Avellan. Econ. For. Res. 1986, 4, 59–64. [Google Scholar] [CrossRef]
- Fan, K.; Weisenhorn, P.; Gilbert, J.A.; Chu, H. Wheat rhizosphere harbors a less complex and more stable microbial co-occurrence pattern than bulk soil. Soil Biol. Biochem. 2018, 125, 251–260. [Google Scholar] [CrossRef]
- Zhang, L.; Adams, J.M.; Dumont, M.G.; Li, Y.; Shi, Y.; He, D.; He, J.S.; Chu, H. Distinct methanotrophic communities exist in habitats with different soil water contents. Soil Biol. Biochem. 2019, 132, 143–152. [Google Scholar] [CrossRef]
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; van der Heijden, M.G.A. Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef] [Green Version]
- Santolini, M.; Barabási, A.L. Predicting perturbation patterns from the topology of biological networks. Proc. Natl. Acad. Sci. USA 2018, 115, E6375–E6383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
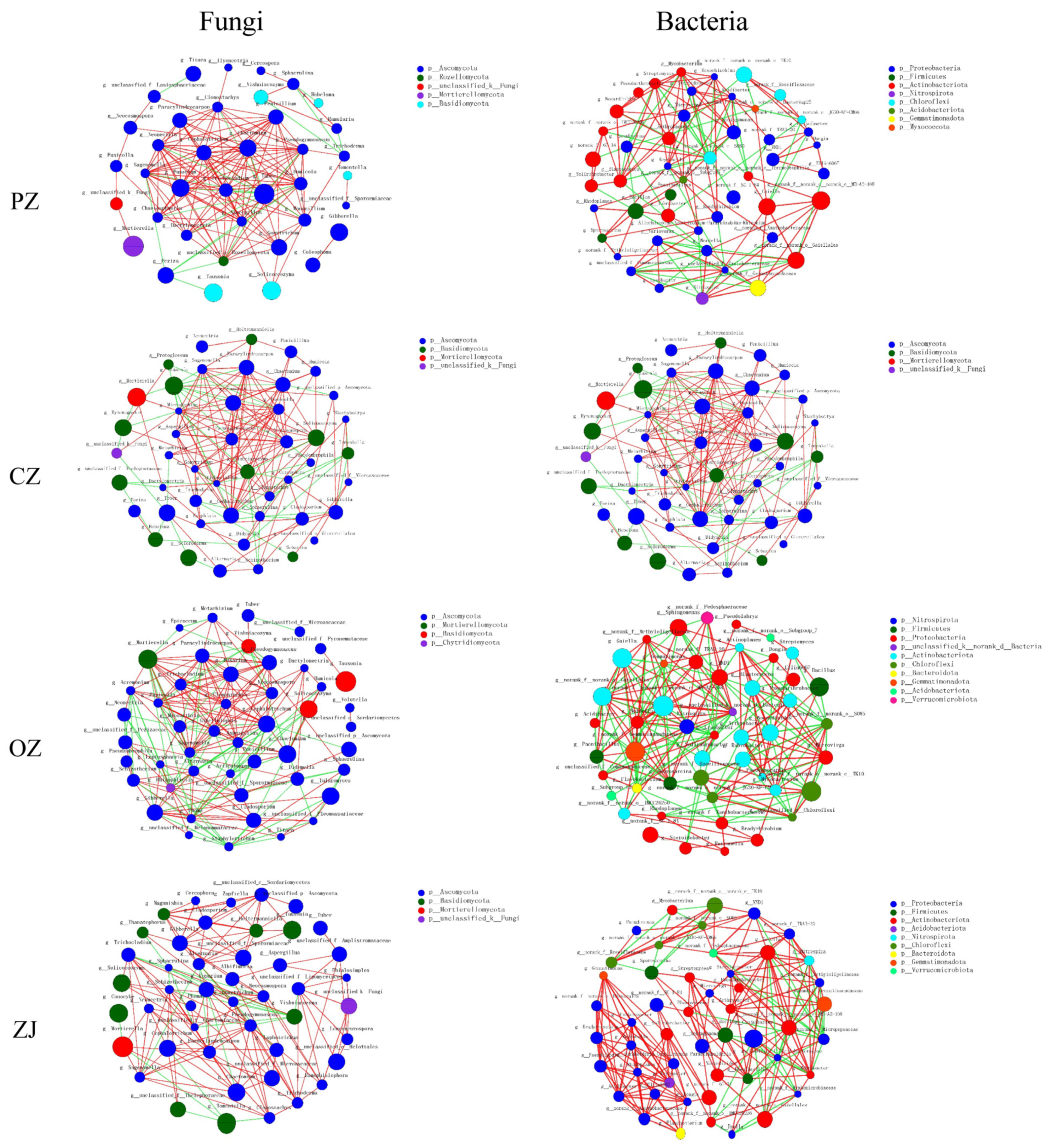
| Network Metrics | CK | PZ | CZ | OZ | ZJ | |
|---|---|---|---|---|---|---|
| Fungi | Number of nodes | 46 | 48 | 47 | 48 | 46 |
| Number of edges | 146 | 158 | 170 | 223 | 152 | |
| Average connectivity | 6.35 | 6.58 | 7.23 | 9.29 | 6.61 | |
| Clustering coefficient | 0.4 | 0.45 | 0.46 | 0.54 | 0.52 | |
| Positive interaction | 95 | 134 | 124 | 146 | 129 | |
| Negative interaction | 51 | 24 | 46 | 77 | 23 | |
| Bacteria | Number of nodes | 49 | 48 | 49 | 48 | 48 |
| Number of edges | 207 | 150 | 182 | 222 | 223 | |
| Average connectivity | 8.45 | 6.25 | 7.43 | 9.25 | 9.29 | |
| Clustering coefficient | 0.46 | 0.48 | 0.5 | 0.52 | 0.62 | |
| Positive interaction | 105 | 101 | 96 | 118 | 181 | |
| Negative interaction | 102 | 49 | 86 | 104 | 42 |
| Network Metrics | Spring | Summer | Autumn | Winter | |
|---|---|---|---|---|---|
| Fungi | Number of nodes | 48 | 49 | 47 | 46 |
| Number of edges | 127 | 188 | 116 | 94 | |
| Average connectivity | 5.29 | 7.67 | 4.94 | 4.09 | |
| Clustering coefficient | 0.36 | 0.4 | 0.45 | 0.33 | |
| Positive interaction | 79 | 98 | 57 | 68 | |
| Negative interaction | 48 | 90 | 59 | 26 | |
| Bacteria | Number of nodes | 49 | 48 | 45 | 50 |
| Number of edges | 173 | 235 | 126 | 183 | |
| Average connectivity | 7.06 | 9.79 | 5.6 | 7.32 | |
| Clustering coefficient | 0.48 | 0.52 | 0.37 | 0.53 | |
| Positive interaction | 116 | 126 | 76 | 158 | |
| Negative interaction | 57 | 109 | 50 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.; Yang, Z.; Liang, L.; Ma, Q.; Wang, G.; Zhao, T. Seasonal Changes in Soil Microbial Community and Co-Occurrence Network of Species of the Genus Corylus. Microorganisms 2021, 9, 2228. https://doi.org/10.3390/microorganisms9112228
Ma W, Yang Z, Liang L, Ma Q, Wang G, Zhao T. Seasonal Changes in Soil Microbial Community and Co-Occurrence Network of Species of the Genus Corylus. Microorganisms. 2021; 9(11):2228. https://doi.org/10.3390/microorganisms9112228
Chicago/Turabian StyleMa, Wenxu, Zhen Yang, Lisong Liang, Qinghua Ma, Guixi Wang, and Tiantian Zhao. 2021. "Seasonal Changes in Soil Microbial Community and Co-Occurrence Network of Species of the Genus Corylus" Microorganisms 9, no. 11: 2228. https://doi.org/10.3390/microorganisms9112228
APA StyleMa, W., Yang, Z., Liang, L., Ma, Q., Wang, G., & Zhao, T. (2021). Seasonal Changes in Soil Microbial Community and Co-Occurrence Network of Species of the Genus Corylus. Microorganisms, 9(11), 2228. https://doi.org/10.3390/microorganisms9112228





