Clostridial Neurotoxins: Structure, Function and Implications to Other Bacterial Toxins
Abstract
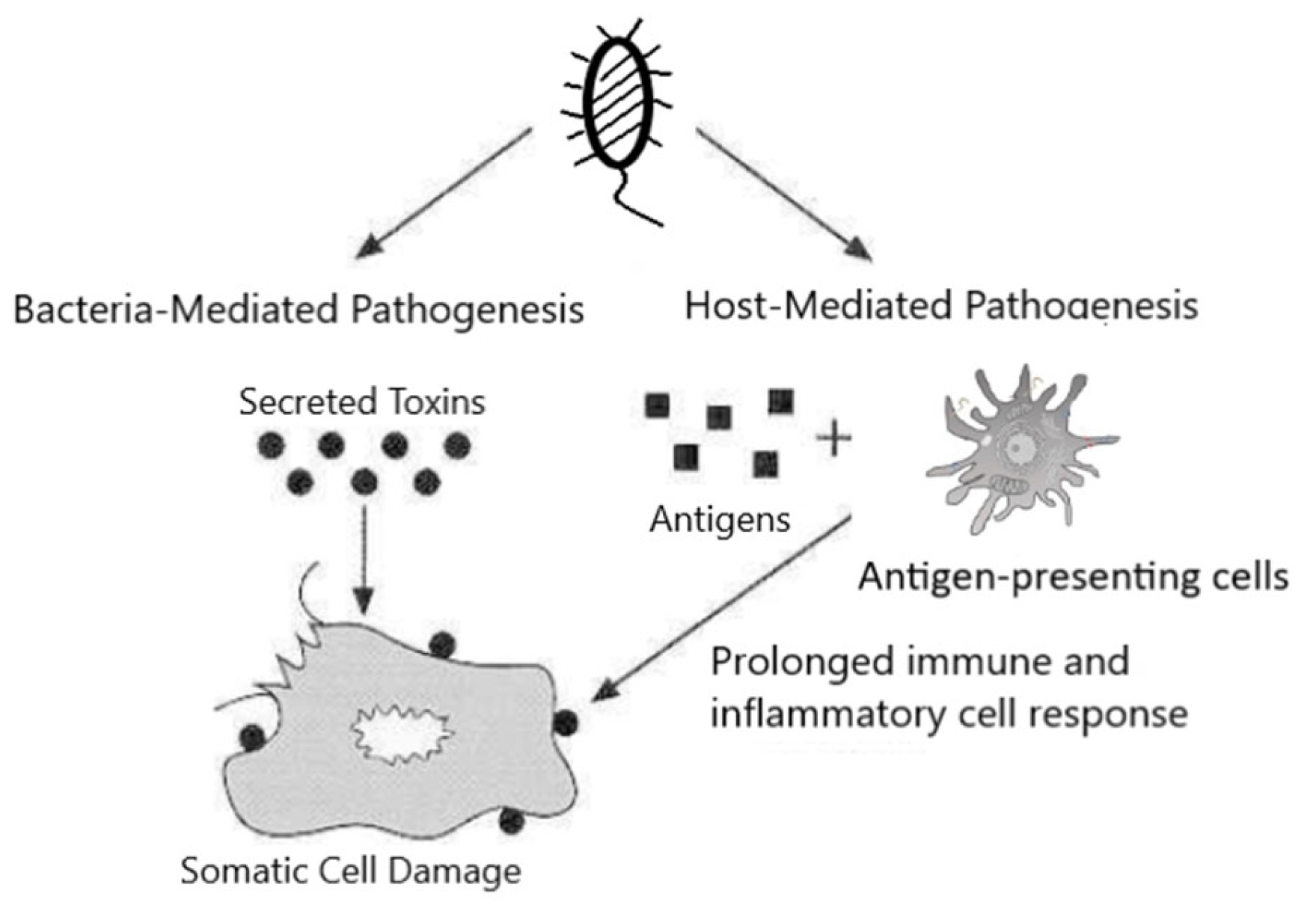
1. Introduction
2. Clostridia Neurotoxins
2.1. Diversity of Clostridial Neurotoxins
2.1.1. Tetanus Neurotoxin
2.1.2. Botulinum Neurotoxin
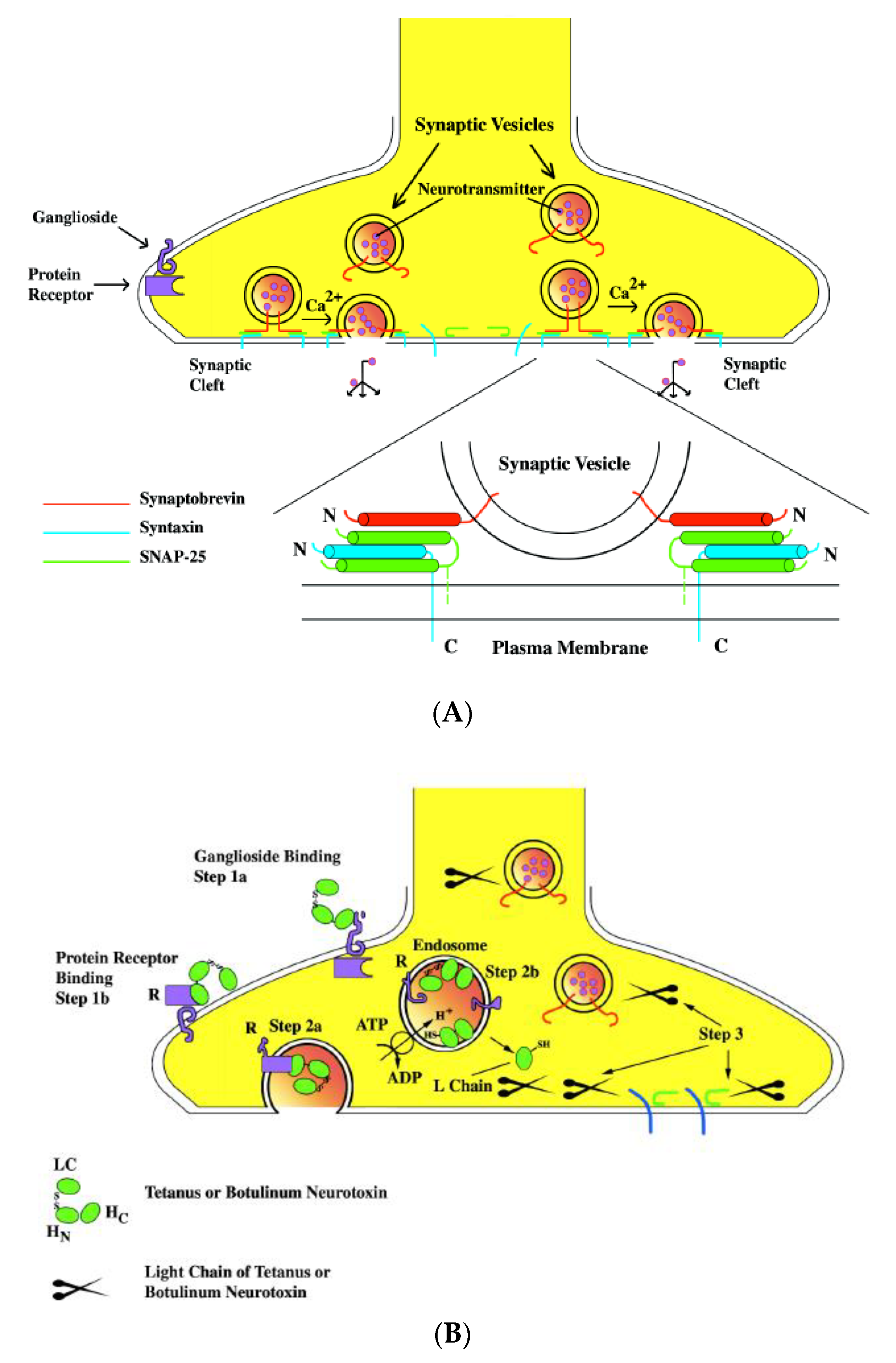
2.2. Mode of Action of Clostridial Neurotoxins
2.2.1. Uptake of Botulinum Neurotoxins in the GI Tract
Progenitor Toxin Complexes of BoNT
Protection of BoNT in GI Tract by Nontoxic Proteins
Uptake of BoNT through the Intestinal Epithelial Barrier
2.2.2. Molecular Mechanisms of Clostridial Neurotoxins toward the Targeted Neuronal Cells
Binding to the Neural Cells
Internalization and Translocation
Retrograde of Clostridial Neurotoxins to the CNS
Enzymatic Activity
2.3. Molten Globule-Type Structures Are Critical for the Action of Clostridial Neurotoxins
3. Flexible Structures Are Utilized as Active Structures beyond Clostridial Neurotoxins
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, R.; Feltrup, T.M.; Kukreja, R.V.; Patel, K.B.; Cai, S.; Singh, B.R. Evolutionary Features in the Structure and Function of Bacterial Toxins. Toxins 2019, 11, 15. [Google Scholar] [CrossRef]
- Peterson, J.W. Bacterial Pathogenesis. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; ISBN 978-0-9631172-1-2. [Google Scholar]
- Keusch, G.T. Bacterial toxins as virulence factors: Shiga bacillus dysentery viewed as a toxinosis. Mt. Sinai J. Med. N. Y. 1977, 44, 33–41. [Google Scholar]
- Todar, Kenneth Bacterial Protein Toxins. Available online: http://textbookofbacteriology.net/proteintoxins.html (accessed on 15 October 2021).
- Ramachandran, G. Gram-positive and gram-negative bacterial toxins in sepsis. Virulence 2014, 5, 213–218. [Google Scholar] [CrossRef]
- Balfanz, J.; Rautenberg, P.; Ullmann, U. Molecular Mechanisms of Action of Bacterial Exotoxins. Zent. Bakteriol. 1996, 284, 170–206. [Google Scholar] [CrossRef]
- Popoff, M.R.; Bouvet, P. Clostridial toxins. Future Microbiol. 2009, 4, 1021–1064. [Google Scholar] [CrossRef]
- Singh, B.R. Botulinum neurotoxin structure, engineering, and novel cellular trafficking and targeting. Neurotox. Res. 2006, 9, 73–92. [Google Scholar] [CrossRef]
- Murphy, J.R. Mechanism of Diphtheria Toxin Catalytic Domain Delivery to the Eukaryotic Cell Cytosol and the Cellular Factors that Directly Participate in the Process. Toxins 2011, 3, 294–308. [Google Scholar] [CrossRef]
- Dorlands Medical Dictionary: Enterotoxin. Available online: https://web.archive.org/web/20091207052257/http://www.mercksource.com/pp/us/cns/cns_hl_dorlands_split.jsp?pg=/ppdocs/us/common/dorlands/dorland/three/000035767.htm (accessed on 24 September 2021).
- Lucas, F.; Popoff, M.; Corthier, G. Bacterial enterotoxins: Structure, mode of action. Ann. Rech. Vet. Ann. Vet. Res. 1991, 22, 147–162. [Google Scholar]
- Farrar, J.J.; Yen, L.M.; Cook, T.; Fairweather, N.; Binh, N.; Parry, J.; Parry, C.M. Tetanus. J. Neurol. Neurosurg. Psychiatry 2000, 69, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Singh, B.R.; Sharma, S. Botulism diagnostics: From clinical symptoms to in vitro assays. Crit. Rev. Microbiol. 2007, 33, 109–125. [Google Scholar] [CrossRef]
- Sobel, J. Botulism. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2005, 41, 1167–1173. [Google Scholar] [CrossRef]
- Arnon, S.S.; Schechter, R.; Inglesby, T.V.; Henderson, D.A.; Bartlett, J.G.; Ascher, M.S.; Eitzen, E.; Fine, A.D.; Hauer, J.; Layton, M.; et al. Botulinum toxin as a biological weapon: Medical and public health management. JAMA 2001, 285, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Rashid, E.A.M.A.; El-Mahdy, N.M.; Kharoub, H.S.; Gouda, A.S.; ElNabarawy, N.A.; Mégarbane, B. Iatrogenic Botulism Outbreak in Egypt due to a Counterfeit Botulinum Toxin A Preparation—A Descriptive Series of Patient Features and Outcome. Basic Clin. Pharmacol. Toxicol. 2018, 123, 622–627. [Google Scholar] [CrossRef]
- Harris, R.A.; Anniballi, F.; Austin, J.W. Adult Intestinal Toxemia Botulism. Toxins 2020, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Ibatullin, R.A.; Magjanov, R.V. Case of iatrogenic botulism after botulinotherapy in clinical practice. Ter. Arkh. 2018, 90, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, I.A.; Karim, S. Botulism. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Garrigues, L.; Do, T.D.; Bideaux, C.; Guillouet, S.E.; Meynial-Salles, I. Insights into Clostridium tetani: From genome to bioreactors. Biotechnol. Adv. 2021, 107781. [Google Scholar] [CrossRef] [PubMed]
- Genome List—Genome—NCBI. Available online: https://www.ncbi.nlm.nih.gov/genome/browse/#!/prokaryotes/1098/ (accessed on 19 July 2021).
- Cohen, J.E.; Wang, R.; Shen, R.-F.; Wu, W.W.; Keller, J.E. Comparative pathogenomics of Clostridium tetani. PLoS ONE 2017, 12, e0182909. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Hill, K.K.; Raphael, B.H. Historical and current perspectives on Clostridium botulinum diversity. Res. Microbiol. 2015, 166, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Masuyer, G.; Stenmark, P. Botulinum and Tetanus Neurotoxins. Annu. Rev. Biochem. 2019, 88, 811–837. [Google Scholar] [CrossRef] [PubMed]
- Barash, J.R.; Arnon, S.S. A Novel Strain of Clostridium botulinum That Produces Type B and Type H Botulinum Toxins. J. Infect. Dis. 2014, 209, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Dover, N.; Barash, J.R.; Hill, K.K.; Xie, G.; Arnon, S.S. Molecular characterization of a novel botulinum neurotoxin type H gene. J. Infect. Dis. 2014, 209, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Maslanka, S.E.; Lúquez, C.; Dykes, J.K.; Tepp, W.H.; Pier, C.L.; Pellett, S.; Raphael, B.H.; Kalb, S.R.; Barr, J.R.; Rao, A.; et al. A Novel Botulinum Neurotoxin, Previously Reported as Serotype H, Has a Hybrid-Like Structure With Regions of Similarity to the Structures of Serotypes A and F and Is Neutralized With Serotype A Antitoxin. J. Infect. Dis. 2016, 213, 379–385. [Google Scholar] [CrossRef]
- Pellett, S.; Tepp, W.H.; Bradshaw, M.; Kalb, S.R.; Dykes, J.K.; Lin, G.; Nawrocki, E.M.; Pier, C.L.; Barr, J.R.; Maslanka, S.E.; et al. Purification and Characterization of Botulinum Neurotoxin FA from a Genetically Modified Clostridium botulinum Strain. mSphere 2016, 1, e00100-15. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Barash, J.R.; Lou, J.; Conrad, F.; Marks, J.D.; Arnon, S.S. Immunological Characterization and Neutralizing Ability of Monoclonal Antibodies Directed Against Botulinum Neurotoxin Type H. J. Infect. Dis. 2016, 213, 1606–1614. [Google Scholar] [CrossRef]
- Kalb, S.R.; Baudys, J.; Raphael, B.H.; Dykes, J.K.; Lúquez, C.; Maslanka, S.E.; Barr, J.R. Functional characterization of botulinum neurotoxin serotype H as a hybrid of known serotypes F and A (BoNT F/A). Anal. Chem. 2015, 87, 3911–3917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Berntsson, R.P.-A.; Tepp, W.H.; Tao, L.; Johnson, E.A.; Stenmark, P.; Dong, M. Structural basis for the unique ganglioside and cell membrane recognition mechanism of botulinum neurotoxin DC. Nat. Commun. 2017, 8, 1637. [Google Scholar] [CrossRef] [PubMed]
- Masuyer, G.; Zhang, S.; Barkho, S.; Shen, Y.; Henriksson, L.; Košenina, S.; Dong, M.; Stenmark, P. Structural characterisation of the catalytic domain of botulinum neurotoxin X—High activity and unique substrate specificity. Sci. Rep. 2018, 8, 4518. [Google Scholar] [CrossRef] [PubMed]
- Peck, M.W.; Smith, T.J.; Anniballi, F.; Austin, J.W.; Bano, L.; Bradshaw, M.; Cuervo, P.; Cheng, L.W.; Derman, Y.; Dorner, B.G.; et al. Historical Perspectives and Guidelines for Botulinum Neurotoxin Subtype Nomenclature. Toxins 2017, 9, 38. [Google Scholar] [CrossRef]
- Hill, K.K.; Smith, T.J.; Helma, C.H.; Ticknor, L.O.; Foley, B.T.; Svensson, R.T.; Brown, J.L.; Johnson, E.A.; Smith, L.A.; Okinaka, R.T.; et al. Genetic diversity among Botulinum Neurotoxin-producing clostridial strains. J. Bacteriol. 2007, 189, 818–832. [Google Scholar] [CrossRef]
- Smith, T.J.; Hill, K.K.; Foley, B.T.; Detter, J.C.; Munk, A.C.; Bruce, D.C.; Doggett, N.A.; Smith, L.A.; Marks, J.D.; Xie, G.; et al. Analysis of the neurotoxin complex genes in Clostridium botulinum A1-A4 and B1 strains: BoNT/A3, /Ba4 and /B1 clusters are located within plasmids. PLoS ONE 2007, 2, e1271. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Lou, J.; Geren, I.N.; Forsyth, C.M.; Tsai, R.; LaPorte, S.L.; Tepp, W.H.; Bradshaw, M.; Johnson, E.A.; Smith, L.A.; et al. Sequence Variation within Botulinum Neurotoxin Serotypes Impacts Antibody Binding and Neutralization. Infect. Immun. 2005, 73, 5450–5457. [Google Scholar] [CrossRef] [PubMed]
- Arndt, J.W.; Jacobson, M.J.; Abola, E.E.; Forsyth, C.M.; Tepp, W.H.; Marks, J.D.; Johnson, E.A.; Stevens, R.C. A structural perspective of the sequence variability within botulinum neurotoxin subtypes A1-A4. J. Mol. Biol. 2006, 362, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Korkeala, H.; Aarnikunnas, J.; Lindström, M. Sequencing the botulinum neurotoxin gene and related genes in Clostridium botulinum type E strains reveals orfx3 and a novel type E neurotoxin subtype. J. Bacteriol. 2007, 189, 8643–8650. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.T.; Paul, C.J.; Mason, D.R.; Twine, S.M.; Alston, M.J.; Logan, S.M.; Austin, J.W.; Peck, M.W. Independent evolution of neurotoxin and flagellar genetic loci in proteolytic Clostridium botulinum. BMC Genom. 2009, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Peck, M.W. Biology and Genomic Analysis of Clostridium botulinum. In Advances in Microbial Physiology; Poole, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2009; Volume 55, pp. 183–320. [Google Scholar]
- Peck, M.W.; Stringer, S.C.; Carter, A.T. Clostridium botulinum in the post-genomic era. Food Microbiol. 2011, 28, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Brunt, J.; van Vliet, A.H.M.; van den Bos, F.; Carter, A.T.; Peck, M.W. Diversity of the Germination Apparatus in Clostridium botulinum Groups I, II, III, and IV. Front. Microbiol. 2016, 7, 1702. [Google Scholar] [CrossRef]
- Kakinuma, H.; Maruyama, H.; Takahashi, H.; Yamakawa, K.; Nakamura, S. The first case of type B infant botulism in Japan. Acta Paediatr. Jpn. Overseas Ed. 1996, 38, 541–543. [Google Scholar] [CrossRef]
- Kozaki, S.; Kamata, Y.; Nishiki, T.; Kakinuma, H.; Maruyama, H.; Takahashi, H.; Karasawa, T.; Yamakawa, K.; Nakamura, S. Characterization of Clostridium botulinum type B neurotoxin associated with infant botulism in japan. Infect. Immun. 1998, 66, 4811–4816. [Google Scholar] [CrossRef] [PubMed]
- Ihara, H.; Kohda, T.; Morimoto, F.; Tsukamoto, K.; Karasawa, T.; Nakamura, S.; Mukamoto, M.; Kozaki, S. Sequence of the gene for Clostridium botulinum type B neurotoxin associated with infant botulism, expression of the C-terminal half of heavy chain and its binding activity. Biochim. Biophys. Acta 2003, 1625, 19–26. [Google Scholar] [CrossRef]
- Umeda, K.; Seto, Y.; Kohda, T.; Mukamoto, M.; Kozaki, S. Stability of toxigenicity in proteolytic Clostridium botulinum type B upon serial passage. Microbiol. Immunol. 2012, 56, 338–341. [Google Scholar] [CrossRef]
- Stringer, S.C.; Carter, A.T.; Webb, M.D.; Wachnicka, E.; Crossman, L.C.; Sebaihia, M.; Peck, M.W. Genomic and physiological variability within Group II (non-proteolytic) Clostridium botulinum. BMC Genom. 2013, 14, 333. [Google Scholar] [CrossRef]
- Carter, A.T.; Stringer, S.C.; Webb, M.D.; Peck, M.W. The type F6 neurotoxin gene cluster locus of group II Clostridium botulinum has evolved by successive disruption of two different ancestral precursors. Genome Biol. Evol. 2013, 5, 1032–1037. [Google Scholar] [CrossRef]
- Lindström, M.; Nevas, M.; Kurki, J.; Sauna-aho, R.; Latvala-Kiesilä, A.; Pölönen, I.; Korkeala, H. Type C botulism due to toxic feed affecting 52,000 farmed foxes and minks in Finland. J. Clin. Microbiol. 2004, 42, 4718–4725. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, A.E.; Brady, C.P.; Byrne, W.; Moriarty, J.; O’Neill, P.; McLaughlin, J.G. Major outbreak of suspected botulism in a dairy herd in the Republic of Ireland. Vet. Rec. 2008, 162, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Woudstra, C.; Le Maréchal, C.; Souillard, R.; Bayon-Auboyer, M.-H.; Mermoud, I.; Desoutter, D.; Fach, P. Draft Genome Sequences of 17 French Clostridium botulinum Group III Strains. Genome Announc. 2015, 3, e01105-15. [Google Scholar] [CrossRef]
- Sonnabend, O.; Sonnabend, W.; Heinzle, R.; Sigrist, T.; Dirnhofer, R.; Krech, U. Isolation of Clostridium botulinum type G and identification of type G botulinal toxin in humans: Report of five sudden unexpected deaths. J. Infect. Dis. 1981, 143, 22–27. [Google Scholar] [CrossRef]
- Suen, J.C.; Hatheway, C.L.; Steigerwalt, A.G.; Brenner, D.J. Genetic confirmation of identities of neurotoxigenic Clostridium baratii and Clostridium butyricum implicated as agents of infant botulism. J. Clin. Microbiol. 1988, 26, 2191–2192. [Google Scholar] [CrossRef] [PubMed]
- Mazuet, C.; Legeay, C.; Sautereau, J.; Ma, L.; Bouchier, C.; Bouvet, P.; Popoff, M.R. Diversity of Group I and II Clostridium botulinum Strains from France Including Recently Identified Subtypes. Genome Biol. Evol. 2016, 8, 1643–1660. [Google Scholar] [CrossRef] [PubMed]
- Brüggemann, H. Genomics of clostridial pathogens: Implication of extrachromosomal elements in pathogenicity. Curr. Opin. Microbiol. 2005, 8, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, M.J.; Adams, J.B.; Doxey, A.C. Botulinum neurotoxin homologs in non-Clostridium species. FEBS Lett. 2015, 589, 342–348. [Google Scholar] [CrossRef]
- Brunt, J.; Carter, A.T.; Stringer, S.C.; Peck, M.W. Identification of a novel botulinum neurotoxin gene cluster in Enterococcus. FEBS Lett. 2018, 592, 310–317. [Google Scholar] [CrossRef]
- Mansfield, M.J.; Wentz, T.G.; Zhang, S.; Lee, E.J.; Dong, M.; Sharma, S.K.; Doxey, A.C. Bioinformatic discovery of a toxin family in Chryseobacterium piperi with sequence similarity to botulinum neurotoxins. Sci. Rep. 2019, 9, 1634. [Google Scholar] [CrossRef] [PubMed]
- Zornetta, I.; Azarnia Tehran, D.; Arrigoni, G.; Anniballi, F.; Bano, L.; Leka, O.; Zanotti, G.; Binz, T.; Montecucco, C. The first non Clostridial botulinum-like toxin cleaves VAMP within the juxtamembrane domain. Sci. Rep. 2016, 6, 30257. [Google Scholar] [CrossRef]
- Zhang, S.; Lebreton, F.; Mansfield, M.J.; Miyashita, S.-I.; Zhang, J.; Schwartzman, J.A.; Tao, L.; Masuyer, G.; Carranza, M.M.; Stenmark, P.; et al. Identification of a botulinum neurotoxin-like toxin in a commensal strain of Enterococcus faecium. Cell Host Microbe 2018, 23, 169–176.e6. [Google Scholar] [CrossRef]
- Brunt, J.; van Vliet, A.H.M.; Carter, A.T.; Stringer, S.C.; Amar, C.; Grant, K.A.; Godbole, G.; Peck, M.W. Diversity of the Genomes and Neurotoxins of Strains of Clostridium botulinum Group I and Clostridium sporogenes Associated with Foodborne, Infant and Wound Botulism. Toxins 2020, 12, 586. [Google Scholar] [CrossRef]
- Padda, I.S.; Tadi, P. Botulinum Toxin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Hafeez, M.U.; Moore, M.; Hafeez, K.; Jankovic, J. Exploring the role of botulinum toxin in critical care. Expert Rev. Neurother. 2021, 21, 881–894. [Google Scholar] [CrossRef]
- Call, J.E.; Cooke, P.H.; Miller, A.J. In situ characterization of Clostridium botulinum neurotoxin synthesis and export. J. Appl. Bacteriol. 1995, 79, 257–263. [Google Scholar] [CrossRef]
- Popoff, M.R.; Poulain, B. Bacterial Toxins and the Nervous System: Neurotoxins and Multipotential Toxins Interacting with Neuronal Cells. Toxins 2010, 2, 683–737. [Google Scholar] [CrossRef]
- Lacy, D.B.; Tepp, W.; Cohen, A.C.; DasGupta, B.R.; Stevens, R.C. Crystal structure of botulinum neurotoxin type A and implications for toxicity. Nat. Struct. Biol. 1998, 5, 898–902. [Google Scholar] [CrossRef]
- Sakaguchi, G. Clostridium botulinum toxins. Pharmacol. Ther. 1982, 19, 165–194. [Google Scholar] [CrossRef]
- Smith, T.J.; Williamson, C.H.D.; Hill, K.K.; Johnson, S.L.; Xie, G.; Anniballi, F.; Auricchio, B.; Fernández, R.A.; Caballero, P.A.; Keim, P.; et al. The Distinctive Evolution of orfX Clostridium parabotulinum Strains and Their Botulinum Neurotoxin Type A and F Gene Clusters Is Influenced by Environmental Factors and Gene Interactions via Mobile Genetic Elements. Front. Microbiol. 2021, 12, 566908. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Fujinaga, Y.; Watanabe, T.; Ohyama, T.; Takeshi, K.; Moriishi, K.; Nakajima, H.; Inoue, K.; Oguma, K. Molecular composition of Clostridium botulinum type A progenitor toxins. Infect. Immun. 1996, 64, 1589–1594. [Google Scholar] [CrossRef]
- Minton, N.P. Molecular Genetics of Clostridial Neurotoxins. In Clostridial Neurotoxins: The Molecular Pathogenesis of Tetanus and Botulism; Montecucco, C., Ed.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 1995; pp. 161–194. ISBN 978-3-642-85173-5. [Google Scholar]
- Fujita, R.; Fujinaga, Y.; Inoue, K.; Nakajima, H.; Kumon, H.; Oguma, K. Molecular characterization of two forms of nontoxic-nonhemagglutinin components of Clostridium botulinum type A progenitor toxins. FEBS Lett. 1995, 376, 41–44. [Google Scholar] [CrossRef]
- Mazuet, C.; Ezan, E.; Volland, H.; Popoff, M.R.; Becher, F. Toxin Detection in Patients’ Sera by Mass Spectrometry during Two Outbreaks of Type A Botulism in France. J. Clin. Microbiol. 2012, 50, 4091–4094. [Google Scholar] [CrossRef] [PubMed]
- Kalb, S.R.; Baudys, J.; Smith, T.J.; Smith, L.A.; Barr, J.R. Characterization of Hemagglutinin Negative Botulinum Progenitor Toxins. Toxins 2017, 9, 193. [Google Scholar] [CrossRef]
- Gustafsson, R.; Zhang, S.; Masuyer, G.; Dong, M.; Stenmark, P. Crystal Structure of Botulinum Neurotoxin A2 in Complex with the Human Protein Receptor SV2C Reveals Plasticity in Receptor Binding. Toxins 2018, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.-H.; Qi, R.; Liu, S.; Kroh, A.; Yao, G.; Perry, K.; Rummel, A.; Jin, R. The hypothetical protein P47 of Clostridium botulinum E1 strain Beluga has a structural topology similar to bactericidal/permeability-increasing protein. Toxicon Off. J. Int. Soc. Toxinol. 2018, 147, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, R.; Berntsson, R.P.-A.; Martínez-Carranza, M.; El Tekle, G.; Odegrip, R.; Johnson, E.A.; Stenmark, P. Crystal structures of OrfX2 and P47 from a Botulinum neurotoxin OrfX-type gene cluster. FEBS Lett. 2017, 591, 3781–3792. [Google Scholar] [CrossRef]
- Fujinaga, Y.; Sugawara, Y.; Matsumura, T. Uptake of Botulinum Neurotoxin in the Intestine. In Botulinum Neurotoxins; Rummel, A., Binz, T., Eds.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 364, pp. 45–59. ISBN 978-3-642-33569-3. [Google Scholar]
- Benefield, D.A.; Dessain, S.K.; Shine, N.; Ohi, M.D.; Lacy, D.B. Molecular assembly of botulinum neurotoxin progenitor complexes. Proc. Natl. Acad. Sci. USA 2013, 110, 5630–5635. [Google Scholar] [CrossRef]
- Carter, A.T.; Peck, M.W. Genomes, neurotoxins and biology of Clostridium botulinum Group I and Group II. Res. Microbiol. 2015, 166, 303–317. [Google Scholar] [CrossRef]
- Li, B.; Qian, X.; Sarkar, H.K.; Singh, B.R. Molecular characterization of type E Clostridium botulinum and comparison to other types of Clostridium botulinum. Biochim. Biophys. Acta 1998, 1395, 21–27. [Google Scholar] [PubMed]
- Hines, H.B.; Lebeda, F.; Hale, M.; Brueggemann, E.E. Characterization of botulinum progenitor toxins by mass spectrometry. Appl. Environ. Microbiol. 2005, 71, 4478–4486. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Tepp, W.H.; Bradshaw, M.; Fredrick, C.M.; Johnson, E.A. Immunoprecipitation of native botulinum neurotoxin complexes from Clostridium botulinum subtype A strains. Appl. Environ. Microbiol. 2015, 81, 481–491. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singh, B.R.; Zhang, Z. Proteins within the Type E Botulinum Neurotoxin Complex. U.S. Patent 7,981,432, 19 July 2011. [Google Scholar]
- Fu, F.N.; Sharma, S.K.; Singh, B.R. A protease-resistant novel hemagglutinin purified from type A Clostridium botulinum. J. Protein Chem. 1998, 17, 53–60. [Google Scholar] [CrossRef]
- Sharma, S.K.; Singh, B.R. Hemagglutinin binding mediated protection of botulinum neurotoxin from proteolysis. J. Nat. Toxins 1998, 7, 239–253. [Google Scholar]
- Gu, S.; Jin, R. Assembly and Function of the Botulinum Neurotoxin Progenitor Complex. Curr. Top. Microbiol. Immunol. 2013, 364, 21–44. [Google Scholar] [CrossRef]
- Ahsan, C.R.; Hajnóczky, G.; Maksymowych, A.B.; Simpson, L.L. Visualization of binding and transcytosis of botulinum toxin by human intestinal epithelial cells. J. Pharmacol. Exp. Ther. 2005, 315, 1028–1035. [Google Scholar] [CrossRef]
- Maksymowych, A.B.; Simpson, L.L. Structural Features of the Botulinum Neurotoxin Molecule That Govern Binding and Transcytosis across Polarized Human Intestinal Epithelial Cells. J. Pharmacol. Exp. Ther. 2004, 310, 633–641. [Google Scholar] [CrossRef]
- Maksymowych, A.B.; Simpson, L.L. Binding and transcytosis of botulinum neurotoxin by polarized human colon carcinoma cells. J. Biol. Chem. 1998, 273, 21950–21957. [Google Scholar] [CrossRef]
- Couesnon, A.; Pereira, Y.; Popoff, M.R. Receptor-mediated transcytosis of botulinum neurotoxin A through intestinal cell monolayers. Cell. Microbiol. 2008, 10, 375–387. [Google Scholar] [CrossRef]
- Couesnon, A.; Shimizu, T.; Popoff, M.R. Differential entry of botulinum neurotoxin A into neuronal and intestinal cells. Cell. Microbiol. 2009, 11, 289–308. [Google Scholar] [CrossRef] [PubMed]
- Couesnon, A.; Molgó, J.; Connan, C.; Popoff, M.R. Preferential entry of botulinum neurotoxin A Hc domain through intestinal crypt cells and targeting to cholinergic neurons of the mouse intestine. PLoS Pathog. 2012, 8, e1002583. [Google Scholar] [CrossRef] [PubMed]
- Fujinaga, Y.; Inoue, K.; Watanabe, S.; Yokota, K.; Hirai, Y.; Nagamachi, E.; Oguma, K. The haemagglutinin of Clostridium botulinum type C progenitor toxin plays an essential role in binding of toxin to the epithelial cells of guinea pig small intestine, leading to the efficient absorption of the toxin. Microbiology 1997, 143, 3841–3847. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, K.J.; Patel, K.; Singh, B.R.; Hale, M.L. Role of critical elements in botulinum neurotoxin complex in toxin routing across intestinal and bronchial barriers. PLoS ONE 2018, 13, e0199524. [Google Scholar] [CrossRef]
- Mukkavalli, S.V. Cellular and Molecular Mechanism of Bio-Enhancing Properties of Biological Macromolecules and Herbal Nanoparticles: A Dissertation in BIOMEDICAL Engineering and Biotechnology. Ph.D. Thesis, University of Massachusetts Dartmouth, North Dartmouth, MA, USA, 2016. [Google Scholar]
- Matsumura, T.; Jin, Y.; Kabumoto, Y.; Takegahara, Y.; Oguma, K.; Lencer, W.I.; Fujinaga, Y. The HA proteins of botulinum toxin disrupt intestinal epithelial intercellular junctions to increase toxin absorption. Cell. Microbiol. 2008, 10, 355–364. [Google Scholar] [CrossRef]
- Jin, Y.; Takegahara, Y.; Sugawara, Y.; Matsumura, T.; Fujinaga, Y. Disruption of the epithelial barrier by botulinum haemagglutinin (HA) proteins—Differences in cell tropism and the mechanism of action between HA proteins of types A or B, and HA proteins of type C. Microbiol. Read. Engl. 2009, 155, 35–45. [Google Scholar] [CrossRef]
- Sugawara, Y.; Matsumura, T.; Takegahara, Y.; Jin, Y.; Tsukasaki, Y.; Takeichi, M.; Fujinaga, Y. Botulinum hemagglutinin disrupts the intercellular epithelial barrier by directly binding E-cadherin. J. Cell Biol. 2010, 189, 691–700. [Google Scholar] [CrossRef]
- Connan, C.; Popoff, M.R. Uptake of Clostridial Neurotoxins into Cells and Dissemination. In Uptake and Trafficking of Protein Toxins; Barth, H., Ed.; Current Topics in Microbiology and Immunology; Springer International Publishing: Cham, Switzerland, 2017; Volume 406, pp. 39–78. ISBN 978-3-319-58891-9. [Google Scholar]
- Cheng, L.W.; Stanker, L.H.; Henderson, T.D.; Lou, J.; Marks, J.D. Antibody protection against botulinum neurotoxin intoxication in mice. Infect. Immun. 2009, 77, 4305–4313. [Google Scholar] [CrossRef]
- Bagramyan, K.; Kaplan, B.E.; Cheng, L.W.; Strotmeier, J.; Rummel, A.; Kalkum, M. Substrates and controls for the quantitative detection of active botulinum neurotoxin in protease-containing samples. Anal. Chem. 2013, 85, 5569–5576. [Google Scholar] [CrossRef]
- Fujinaga, Y.; Popoff, M.R. Translocation and dissemination of botulinum neurotoxin from the intestinal tract. Toxicon 2018, 147, 13–18. [Google Scholar] [CrossRef]
- Connan, C.; Varela-Chavez, C.; Mazuet, C.; Molgó, J.; Haustant, G.M.; Disson, O.; Lecuit, M.; Vandewalle, A.; Popoff, M.R. Translocation and dissemination to target neurons of botulinum neurotoxin type B in the mouse intestinal wall. Cell. Microbiol. 2016, 18, 282–301. [Google Scholar] [CrossRef] [PubMed]
- Simpson, L. The life history of a botulinum toxin molecule. Toxicon 2013, 68, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Poulain, B.; Popoff, M. Why Are Botulinum Neurotoxin-Producing Bacteria So Diverse and Botulinum Neurotoxins So Toxic? Toxins 2019, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, F.; Rossi, C.; Gianfranceschi, L.; Rossetto, O.; Caleo, M. Long-Distance Retrograde Effects of Botulinum Neurotoxin A. J. Neurosci. 2008, 28, 3689–3696. [Google Scholar] [CrossRef]
- Montecucco, C.; Schiavo, G. Structure and function of tetanus and botulinum neurotoxins. Q. Rev. Biophys. 1995, 28, 423–472. [Google Scholar] [CrossRef]
- Singh, B.R. Intimate details of the most poisonous poison. Nat. Struct. Biol. 2000, 7, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Umland, T.C.; Wingert, L.M.; Swaminathan, S.; Furey, W.F.; Schmidt, J.J.; Sax, M. Structure of the receptor binding fragment HC of tetanus neurotoxin. Nat. Struct. Biol. 1997, 4, 788–792. [Google Scholar] [CrossRef]
- Swaminathan, S.; Eswaramoorthy, S. Structural analysis of the catalytic and binding sites of Clostridium botulinum neurotoxin B. Nat. Struct. Biol. 2000, 7, 693–699. [Google Scholar] [CrossRef]
- Berntsson, R.P.-A.; Peng, L.; Dong, M.; Stenmark, P. Structure of dual receptor binding to botulinum neurotoxin B. Nat. Commun. 2013, 4, 2058. [Google Scholar] [CrossRef]
- Davies, J.R.; Liu, S.M.; Acharya, K.R. Variations in the Botulinum Neurotoxin Binding Domain and the Potential for Novel Therapeutics. Toxins 2018, 10, 421. [Google Scholar] [CrossRef]
- Montecucco, C. How do tetanus and botulinum toxins bind to neuronal membranes? Trends Biochem. Sci. 1986, 11, 314–317. [Google Scholar] [CrossRef]
- Binz, T.; Rummel, A. Cell entry strategy of clostridial neurotoxins. J. Neurochem. 2009, 109, 1584–1595. [Google Scholar] [CrossRef]
- Swaminathan, S. Molecular structures and functional relationships in clostridial neurotoxins. FEBS J. 2011, 278, 4467–4485. [Google Scholar] [CrossRef]
- Karalewitz, A.P.-A.; Fu, Z.; Baldwin, M.R.; Kim, J.-J.P.; Barbieri, J.T. Botulinum Neurotoxin Serotype C Associates with Dual Ganglioside Receptors to Facilitate Cell Entry. J. Biol. Chem. 2012, 287, 40806–40816. [Google Scholar] [CrossRef] [PubMed]
- Ginalski, K.; Venclovas, C.; Lesyng, B.; Fidelis, K. Structure-based sequence alignment for the β-trefoil subdomain of the clostridial neurotoxin family provides residue level information about the putative ganglioside binding site. FEBS Lett. 2000, 482, 119–124. [Google Scholar] [CrossRef]
- Rummel, A.; Häfner, K.; Mahrhold, S.; Darashchonak, N.; Holt, M.; Jahn, R.; Beermann, S.; Karnath, T.; Bigalke, H.; Binz, T. Botulinum neurotoxins C, E and F bind gangliosides via a conserved binding site prior to stimulation-dependent uptake with botulinum neurotoxin F utilising the three isoforms of SV2 as second receptor. J. Neurochem. 2009, 110, 1942–1954. [Google Scholar] [CrossRef] [PubMed]
- Strotmeier, J.; Lee, K.; Völker, A.K.; Mahrhold, S.; Zong, Y.; Zeiser, J.; Zhou, J.; Pich, A.; Bigalke, H.; Binz, T.; et al. Botulinum neurotoxin serotype D attacks neurons via two carbohydrate-binding sites in a ganglioside-dependent manner. Biochem. J. 2010, 431, 207–216. [Google Scholar] [CrossRef]
- Zhang, Y.; Varnum, S.M. The receptor binding domain of botulinum neurotoxin serotype C binds phosphoinositides. Biochimie 2012, 94, 920–923. [Google Scholar] [CrossRef][Green Version]
- Tsukamoto, K.; Kohda, T.; Mukamoto, M.; Takeuchi, K.; Ihara, H.; Saito, M.; Kozaki, S. Binding of Clostridium botulinum type C and D neurotoxins to ganglioside and phospholipid. Novel insights into the receptor for clostridial neurotoxins. J. Biol. Chem. 2005, 280, 35164–35171. [Google Scholar] [CrossRef]
- Muraro, L.; Tosatto, S.; Motterlini, L.; Rossetto, O.; Montecucco, C. The N-terminal half of the receptor domain of botulinum neurotoxin A binds to microdomains of the plasma membrane. Biochem. Biophys. Res. Commun. 2009, 380, 76–80. [Google Scholar] [CrossRef]
- Dong, M.; Yeh, F.; Tepp, W.H.; Dean, C.; Johnson, E.A.; Janz, R.; Chapman, E.R. SV2 is the protein receptor for botulinum neurotoxin A. Science 2006, 312, 592–596. [Google Scholar] [CrossRef]
- Mahrhold, S.; Rummel, A.; Bigalke, H.; Davletov, B.; Binz, T. The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS Lett. 2006, 580, 2011–2014. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Richards, D.A.; Goodnough, M.C.; Tepp, W.H.; Johnson, E.A.; Chapman, E.R. Synaptotagmins I and II mediate entry of botulinum neurotoxin B into cells. J. Cell Biol. 2003, 162, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Rummel, A.; Eichner, T.; Weil, T.; Karnath, T.; Gutcaits, A.; Mahrhold, S.; Sandhoff, K.; Proia, R.L.; Acharya, K.R.; Bigalke, H.; et al. Identification of the protein receptor binding site of botulinum neurotoxins B and G proves the double-receptor concept. Proc. Natl. Acad. Sci. USA 2007, 104, 359–364. [Google Scholar] [CrossRef]
- Stenmark, P.; Dong, M.; Dupuy, J.; Chapman, E.R.; Stevens, R.C. Crystal structure of the botulinum neurotoxin type G binding domain: Insight into cell surface binding. J. Mol. Biol. 2010, 397, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Tepp, W.H.; Johnson, E.A.; Dong, M. Botulinum Neurotoxin D Uses Synaptic Vesicle Protein SV2 and Gangliosides as Receptors. PLoS Pathog. 2011, 7, e1002008. [Google Scholar] [CrossRef]
- Dong, M.; Liu, H.; Tepp, W.H.; Johnson, E.A.; Janz, R.; Chapman, E.R. Glycosylated SV2A and SV2B Mediate the Entry of Botulinum Neurotoxin E into Neurons. Mol. Biol. Cell 2008, 19, 5226–5237. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, C.; Barbieri, J.T.; Kim, J.-J.P.; Baldwin, M.R. Glycosylated SV2 and Gangliosides as Dual Receptors for Botulinum Neurotoxin Serotype F. Biochemistry 2009, 48, 5631–5641. [Google Scholar] [CrossRef]
- Rummel, A.; Karnath, T.; Henke, T.; Bigalke, H.; Binz, T. Synaptotagmins I and II act as nerve cell receptors for botulinum neurotoxin G. J. Biol. Chem. 2004, 279, 30865–30870. [Google Scholar] [CrossRef]
- Schmitt, J.; Karalewitz, A.; Benefield, D.A.; Mushrush, D.J.; Pruitt, R.N.; Spiller, B.W.; Barbieri, J.T.; Lacy, D.B. Structural analysis of botulinum neurotoxin type G receptor binding. Biochemistry 2010, 49, 5200–5205. [Google Scholar] [CrossRef]
- Willjes, G.; Mahrhold, S.; Strotmeier, J.; Eichner, T.; Rummel, A.; Binz, T. Botulinum neurotoxin G binds synaptotagmin-II in a mode similar to that of serotype B: Tyrosine 1186 and lysine 1191 cause its lower affinity. Biochemistry 2013, 52, 3930–3938. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Lam, K.-H.; Perry, K.; Weisemann, J.; Rummel, A.; Jin, R. Crystal Structure of the Receptor-Binding Domain of Botulinum Neurotoxin Type HA, Also Known as Type FA or H. Toxins 2017, 9, 93. [Google Scholar] [CrossRef]
- Montecucco, C.; Rossetto, O.; Schiavo, G. Presynaptic receptor arrays for clostridial neurotoxins. Trends Microbiol. 2004, 12, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.L.; Dong, M.; Yao, J.; Tepp, W.H.; Lin, G.; Johnson, E.A.; Chapman, E.R. SV2 mediates entry of tetanus neurotoxin into central neurons. PLoS Pathog. 2010, 6, e1001207. [Google Scholar] [CrossRef]
- Matteoli, M.; Verderio, C.; Rossetto, O.; Iezzi, N.; Coco, S.; Schiavo, G.; Montecucco, C. Synaptic vesicle endocytosis mediates the entry of tetanus neurotoxin into hippocampal neurons. Proc. Natl. Acad. Sci. USA 1996, 93, 13310–13315. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G.; Ockleford, C.D.; Critchley, D.R. A study of the mechanism of internalisation of tetanus toxin by primary mouse spinal cord cultures. J. Neurochem. 1987, 49, 1057–1068. [Google Scholar] [CrossRef]
- Staub, G.C.; Walton, K.M.; Schnaar, R.L.; Nichols, T.; Baichwal, R.; Sandberg, K.; Rogers, T.B. Characterization of the binding and internalization of tetanus toxin in a neuroblastoma hybrid cell line. J. Neurosci. 1986, 6, 1443–1451. [Google Scholar] [CrossRef]
- Lalli, G.; Herreros, J.; Osborne, S.L.; Montecucco, C.; Rossetto, O.; Schiavo, G. Functional characterisation of tetanus and botulinum neurotoxins binding domains. J. Cell Sci. 1999, 112, 2715–2724. [Google Scholar] [CrossRef]
- Simpson, L.L.; Coffield, J.A.; Bakry, N. Inhibition of vacuolar adenosine triphosphatase antagonizes the effects of clostridial neurotoxins but not phospholipase A2 neurotoxins. J. Pharmacol. Exp. Ther. 1994, 269, 256–262. [Google Scholar]
- Williamson, L.C.; Neale, E.A. Bafilomycin A1 inhibits the action of tetanus toxin in spinal cord neurons in cell culture. J. Neurochem. 1994, 63, 2342–2345. [Google Scholar] [CrossRef]
- Masuyer, G.; Davies, J.R.; Moore, K.; Chaddock, J.A.; Ravi Acharya, K. Structural analysis of Clostridium botulinum neurotoxin type D as a platform for the development of targeted secretion inhibitors. Sci. Rep. 2015, 5, 13397. [Google Scholar] [CrossRef]
- Kumaran, D.; Eswaramoorthy, S.; Furey, W.; Navaza, J.; Sax, M.; Swaminathan, S. Domain organization in Clostridium botulinum neurotoxin type E is unique: Its implication in faster translocation. J. Mol. Biol. 2009, 386, 233–245. [Google Scholar] [CrossRef]
- Fischer, A.; Montal, M. Molecular dissection of botulinum neurotoxin reveals interdomain chaperone function. Toxicon Off. J. Int. Soc. Toxinol. 2013, 75, 101–107. [Google Scholar] [CrossRef]
- Austin, C.D.; Wen, X.; Gazzard, L.; Nelson, C.; Scheller, R.H.; Scales, S.J. Oxidizing potential of endosomes and lysosomes limits intracellular cleavage of disulfide-based antibody-drug conjugates. Proc. Natl. Acad. Sci. USA 2005, 102, 17987–17992. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Kukreja, R.; Shoesmith, S.; Chang, T.-W.; Singh, B.R. Botulinum neurotoxin light chain refolds at endosomal pH for its translocation. Protein J. 2006, 25, 455–462. [Google Scholar] [CrossRef]
- Chellappan, G.; Kumar, R.; Santos, E.; Goyal, D.; Cai, S.; Singh, B.R. Structural and Functional Analysis of Botulinum Neurotoxin subunits for pH-dependent Membrane Channel Formation and Translocation. Biochim. Biophys. Acta 2015, 1854, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, H.K.; Thiruvanakarasu, N.; Kumar, R.; Patel, K.; Ambrin, G.; Cai, S.; Singh, B.R. High Yield Preparation of Functionally Active Catalytic-Translocation Domain Module of Botulinum Neurotoxin Type A That Exhibits Uniquely Different Enzyme Kinetics. Protein J. 2017, 36, 489–501. [Google Scholar] [CrossRef]
- Montal, M. Tetanus neurotoxin: Conformational plasticity as an adaptive strategy. EMBO Rep. 2017, 18, 1268–1270. [Google Scholar] [CrossRef] [PubMed]
- Surana, S.; Tosolini, A.P.; Meyer, I.F.G.; Fellows, A.D.; Novoselov, S.S.; Schiavo, G. The travel diaries of tetanus and botulinum neurotoxins. Toxicon 2018, 147, 58–67. [Google Scholar] [CrossRef]
- Blum, F.C.; Chen, C.; Kroken, A.R.; Barbieri, J.T. Tetanus Toxin and Botulinum Toxin A Utilize Unique Mechanisms To Enter Neurons of the Central Nervous System. Infect. Immun. 2012, 80, 1662–1669. [Google Scholar] [CrossRef]
- Bohnert, S.; Schiavo, G. Tetanus toxin is transported in a novel neuronal compartment characterized by a specialized pH regulation. J. Biol. Chem. 2005, 280, 42336–42344. [Google Scholar] [CrossRef]
- Li, Y.; Foran, P.; Lawrence, G.; Mohammed, N.; Chan-Kwo-Chion, C.K.; Lisk, G.; Aoki, R.; Dolly, O. Recombinant forms of tetanus toxin engineered for examining and exploiting neuronal trafficking pathways. J. Biol. Chem. 2001, 276, 31394–31401. [Google Scholar] [CrossRef] [PubMed]
- Maskos, U.; Kissa, K.; St Cloment, C.; Brûlet, P. Retrograde trans-synaptic transfer of green fluorescent protein allows the genetic mapping of neuronal circuits in transgenic mice. Proc. Natl. Acad. Sci. USA 2002, 99, 10120–10125. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zurawski, T.H.; Meng, J.; Lawrence, G.W.; Aoki, K.R.; Wheeler, L.; Dolly, J.O. Novel chimeras of botulinum and tetanus neurotoxins yield insights into their distinct sites of neuroparalysis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2012, 26, 5035–5048. [Google Scholar] [CrossRef]
- Blum, F.C.; Przedpelski, A.; Tepp, W.H.; Johnson, E.A.; Barbieri, J.T. Entry of a recombinant, full-length, atoxic tetanus neurotoxin into Neuro-2a cells. Infect. Immun. 2014, 82, 873–881. [Google Scholar] [CrossRef]
- Deinhardt, K.; Salinas, S.; Verastegui, C.; Watson, R.; Worth, D.; Hanrahan, S.; Bucci, C.; Schiavo, G. Rab5 and Rab7 Control Endocytic Sorting along the Axonal Retrograde Transport Pathway. Neuron 2006, 52, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Schmieg, N.; Menendez, G.; Schiavo, G.; Terenzio, M. Signalling endosomes in axonal transport: Travel updates on the molecular highway. Semin. Cell Dev. Biol. 2014, 27, 32–43. [Google Scholar] [CrossRef]
- Sleigh, J.N.; Tosolini, A.P.; Schiavo, G. In Vivo Imaging of Anterograde and Retrograde Axonal Transport in Rodent Peripheral Nerves. Methods Mol. Biol. 2020, 2143, 271–292. [Google Scholar] [CrossRef]
- Matak, I.; Riederer, P.; Lacković, Z. Botulinum toxin’s axonal transport from periphery to the spinal cord. Neurochem. Int. 2012, 61, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Restani, L.; Novelli, E.; Bottari, D.; Leone, P.; Barone, I.; Galli-Resta, L.; Strettoi, E.; Caleo, M. Botulinum neurotoxin A impairs neurotransmission following retrograde transynaptic transport. Traffic Cph. Den. 2012, 13, 1083–1089. [Google Scholar] [CrossRef]
- Restani, L.; Antonucci, F.; Gianfranceschi, L.; Rossi, C.; Rossetto, O.; Caleo, M. Evidence for anterograde transport and transcytosis of botulinum neurotoxin A (BoNT/A). J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 15650–15659. [Google Scholar] [CrossRef] [PubMed]
- Restani, L.; Giribaldi, F.; Manich, M.; Bercsenyi, K.; Menendez, G.; Rossetto, O.; Caleo, M.; Schiavo, G. Botulinum neurotoxins A and E undergo retrograde axonal transport in primary motor neurons. PLoS Pathog. 2012, 8, e1003087. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Singh, B.R. Strategies to design inhibitors of Clostridium botulinum neurotoxins. Infect. Disord. Drug Targets 2007, 7, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Cai, S.; Singh, B.R. Current strategies for designing antidotes against botulinum neurotoxins. Expert Opin. Drug Discov. 2014, 9, 319–333. [Google Scholar] [CrossRef]
- Lebeda, F.J.; Cer, R.Z.; Mudunuri, U.; Stephens, R.; Singh, B.R.; Adler, M. The zinc-dependent protease activity of the botulinum neurotoxins. Toxins 2010, 2, 978–997. [Google Scholar] [CrossRef]
- Hanig, J.P.; Lamanna, C. Toxicity of botulinum toxin: A stoichiometric model for the locus of its extraordinary potency and persistence at the neuromuscular junction. J. Theor. Biol. 1979, 77, 107–113. [Google Scholar] [CrossRef]
- Foran, P.G.; Mohammed, N.; Lisk, G.O.; Nagwaney, S.; Lawrence, G.W.; Johnson, E.; Smith, L.; Aoki, K.R.; Dolly, J.O. Evaluation of the therapeutic usefulness of botulinum neurotoxin B, C1, E, and F compared with the long lasting type A. Basis for distinct durations of inhibition of exocytosis in central neurons. J. Biol. Chem. 2003, 278, 1363–1371. [Google Scholar] [CrossRef]
- Eleopra, R.; Tugnoli, V.; Quatrale, R.; Rossetto, O.; Montecucco, C. Different types of botulinum toxin in humans. Mov. Disord. Off. J. Mov. Disord. Soc. 2004, 19, S53–S59. [Google Scholar] [CrossRef]
- Morbiato, L.; Carli, L.; Johnson, E.A.; Montecucco, C.; Molgó, J.; Rossetto, O. Neuromuscular paralysis and recovery in mice injected with botulinum neurotoxins A and C. Eur. J. Neurosci. 2007, 25, 2697–2704. [Google Scholar] [CrossRef]
- Brashear, A.; Lew, M.F.; Dykstra, D.D.; Comella, C.L.; Factor, S.A.; Rodnitzky, R.L.; Trosch, R.; Singer, C.; Brin, M.F.; Murray, J.J.; et al. Safety and efficacy of NeuroBloc (Botulinum toxin type B) in type A-responsive cervical dystonia. Neurology 1999, 53, 1439–1446. [Google Scholar] [CrossRef]
- Pons, L.; Vilain, C.; Volteau, M.; Picaut, P. Safety and pharmacodynamics of a novel recombinant botulinum toxin E (rBoNT-E): Results of a phase 1 study in healthy male subjects compared with abobotulinumtoxinA (Dysport®). J. Neurol. Sci. 2019, 407, 116516. [Google Scholar] [CrossRef]
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum Neurotoxins: Biology, Pharmacology, and Toxicology. Pharmacol. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef] [PubMed]
- Belle, A.; Tanay, A.; Bitincka, L.; Shamir, R.; O’Shea, E.K. Quantification of protein half-lives in the budding yeast proteome. Proc. Natl. Acad. Sci. USA 2006, 103, 13004–13009. [Google Scholar] [CrossRef]
- Cambridge, S.B.; Gnad, F.; Nguyen, C.; Bermejo, J.L.; Krüger, M.; Mann, M. Systems-wide proteomic analysis in mammalian cells reveals conserved, functional protein turnover. J. Proteome Res. 2011, 10, 5275–5284. [Google Scholar] [CrossRef]
- Price, J.C.; Guan, S.; Burlingame, A.; Prusiner, S.B.; Ghaemmaghami, S. Analysis of proteome dynamics in the mouse brain. Proc. Natl. Acad. Sci. USA 2010, 107, 14508–14513. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.P.; Barbieri, J.T. Light Chain Diversity among the Botulinum Neurotoxins. Toxins 2018, 10, 268. [Google Scholar] [CrossRef]
- Blasi, J.; Chapman, E.R.; Link, E.; Binz, T.; Yamasaki, S.; De Camilli, P.; Südhof, T.C.; Niemann, H.; Jahn, R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature 1993, 365, 160–163. [Google Scholar] [CrossRef]
- Schiavo, G.; Benfenati, F.; Poulain, B.; Rossetto, O.; Polverino de Laureto, P.; DasGupta, B.R.; Montecucco, C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature 1992, 359, 832–835. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, G.; Shone, C.C.; Bennett, M.K.; Scheller, R.H.; Montecucco, C. Botulinum neurotoxin type C cleaves a single Lys-Ala bond within the carboxyl-terminal region of syntaxins. J. Biol. Chem. 1995, 270, 10566–10570. [Google Scholar] [CrossRef]
- Yamamoto, H.; Ida, T.; Tsutsuki, H.; Mori, M.; Matsumoto, T.; Kohda, T.; Mukamoto, M.; Goshima, N.; Kozaki, S.; Ihara, H. Specificity of botulinum protease for human VAMP family proteins. Microbiol. Immunol. 2012, 56, 245–253. [Google Scholar] [CrossRef]
- Yamasaki, S.; Baumeister, A.; Binz, T.; Blasi, J.; Link, E.; Cornille, F.; Roques, B.; Fykse, E.M.; Südhof, T.C.; Jahn, R. Cleavage of members of the synaptobrevin/VAMP family by types D and F botulinal neurotoxins and tetanus toxin. J. Biol. Chem. 1994, 269, 12764–12772. [Google Scholar] [CrossRef]
- Binz, T.; Blasi, J.; Yamasaki, S.; Baumeister, A.; Link, E.; Südhof, T.C.; Jahn, R.; Niemann, H. Proteolysis of SNAP-25 by types E and A botulinal neurotoxins. J. Biol. Chem. 1994, 269, 1617–1620. [Google Scholar] [CrossRef]
- Schiavo, G.; Santucci, A.; Dasgupta, B.R.; Mehta, P.P.; Jontes, J.; Benfenati, F.; Wilson, M.C.; Montecucco, C. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 1993, 335, 99–103. [Google Scholar] [CrossRef]
- Schiavo, G.; Malizio, C.; Trimble, W.S.; Polverino de Laureto, P.; Milan, G.; Sugiyama, H.; Johnson, E.A.; Montecucco, C. Botulinum G neurotoxin cleaves VAMP/synaptobrevin at a single Ala-Ala peptide bond. J. Biol. Chem. 1994, 269, 20213–20216. [Google Scholar] [CrossRef]
- Yamasaki, S.; Binz, T.; Hayashi, T.; Szabo, E.; Yamasaki, N.; Eklund, M.; Jahn, R.; Niemann, H. Botulinum neurotoxin type G proteolyses the Ala81-Ala82 bond of rat synaptobrevin 2. Biochem. Biophys. Res. Commun. 1994, 200, 829–835. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Maditz, R.; Kuo, C.; Fishman, P.S.; Shoemaker, C.B.; Oyler, G.A.; Weissman, A.M. Targeting botulinum neurotoxin persistence by the ubiquitin-proteasome system. Proc. Natl. Acad. Sci. USA 2010, 107, 16554–16559. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, C.B.; Oyler, G.A. Persistence of Botulinum neurotoxin inactivation of nerve function. Curr. Top. Microbiol. Immunol. 2013, 364, 179–196. [Google Scholar] [CrossRef]
- Vagin, O.; Tokhtaeva, E.; Garay, P.E.; Souda, P.; Bassilian, S.; Whitelegge, J.P.; Lewis, R.; Sachs, G.; Wheeler, L.; Aoki, R.; et al. Recruitment of septin cytoskeletal proteins by botulinum toxin A protease determines its remarkable stability. J. Cell Sci. 2014, 127, 3294–3308. [Google Scholar] [CrossRef]
- Whitemarsh, R.C.M.; Tepp, W.H.; Johnson, E.A.; Pellett, S. Persistence of Botulinum Neurotoxin A Subtypes 1-5 in Primary Rat Spinal Cord Cells. PLoS ONE 2014, 9, e90252. [Google Scholar] [CrossRef]
- Banerjee, A.; Kowalchyk, J.A.; DasGupta, B.R.; Martin, T.F. SNAP-25 is required for a late postdocking step in Ca2+-dependent exocytosis. J. Biol. Chem. 1996, 271, 20227–20230. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Berberian, K.; Gong, L.-W.; Hafez, I.; Sørensen, J.B.; Lindau, M. The role of the C terminus of the SNARE protein SNAP-25 in fusion pore opening and a model for fusion pore mechanics. Proc. Natl. Acad. Sci. USA 2008, 105, 15388–15392. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Salas, E.; Ho, H.; Garay, P.; Steward, L.E.; Aoki, K.R. Is the light chain subcellular localization an important factor in botulinum toxin duration of action? Mov. Disord. Off. J. Mov. Disord. Soc. 2004, 19, S23–S34. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Salas, E.; Steward, L.E.; Ho, H.; Garay, P.E.; Sun, S.W.; Gilmore, M.A.; Ordas, J.V.; Wang, J.; Francis, J.; Aoki, K.R. Plasma membrane localization signals in the light chain of botulinum neurotoxin. Proc. Natl. Acad. Sci. USA 2004, 101, 3208–3213. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, G.; Papini, E.; Genna, G.; Montecucco, C. An intact interchain disulfide bond is required for the neurotoxicity of tetanus toxin. Infect. Immun. 1990, 58, 4136–4141. [Google Scholar] [CrossRef]
- de Paiva, A.; Poulain, B.; Lawrence, G.W.; Shone, C.C.; Tauc, L.; Dolly, J.O. A role for the interchain disulfide or its participating thiols in the internalization of botulinum neurotoxin A revealed by a toxin derivative that binds to ecto-acceptors and inhibits transmitter release intracellularly. J. Biol. Chem. 1993, 268, 20838–20844. [Google Scholar] [CrossRef]
- Fischer, A.; Montal, M. Crucial role of the disulfide bridge between botulinum neurotoxin light and heavy chains in protease translocation across membranes. J. Biol. Chem. 2007, 282, 29604–29611. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Rossetto, O.; Bolognese, P.; Shone, C.C.; Montecucco, C. Double anchorage to the membrane and intact inter-chain disulfide bond are required for the low pH induced entry of tetanus and botulinum neurotoxins into neurons. Cell. Microbiol. 2011, 13, 1731–1743. [Google Scholar] [CrossRef]
- Rossetto, O.; Schiavo, G.; Montecucco, C.; Poulain, B.; Deloye, F.; Lozzi, L.; Shone, C.C. SNARE motif and neurotoxins. Nature 1994, 372, 415–416. [Google Scholar] [CrossRef]
- Breidenbach, M.A.; Brunger, A.T. Substrate recognition strategy for botulinum neurotoxin serotype A. Nature 2004, 432, 925–929. [Google Scholar] [CrossRef]
- Agarwal, R.; Schmidt, J.J.; Stafford, R.G.; Swaminathan, S. Mode of VAMP substrate recognition and inhibition of Clostridium botulinum neurotoxin F. Nat. Struct. Mol. Biol. 2009, 16, 789–794. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Byrne, M.P.; Jensen, M.; Hines, H.B.; Brueggemann, E.; Smith, L.A. Enzymatic autocatalysis of botulinum A neurotoxin light chain. J. Protein Chem. 2001, 20, 221–231. [Google Scholar] [CrossRef]
- Rossetto, O.; Pirazzini, M.; Montecucco, C. Botulinum neurotoxins: Genetic, structural and mechanistic insights. Nat. Rev. Microbiol. 2014, 12, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Pellizzari, R.; Rossetto, O.; Lozzi, L.; Giovedi’, S.; Johnson, E.; Shone, C.C.; Montecucco, C. Structural determinants of the specificity for synaptic vesicle-associated membrane protein/synaptobrevin of tetanus and botulinum type B and G neurotoxins. J. Biol. Chem. 1996, 271, 20353–20358. [Google Scholar] [CrossRef]
- Washbourne, P.; Pellizzari, R.; Baldini, G.; Wilson, M.C.; Montecucco, C. Botulinum neurotoxin types A and E require the SNARE motif in SNAP-25 for proteolysis. FEBS Lett. 1997, 418, 1–5. [Google Scholar] [CrossRef]
- Cai, S.; Lindo, P.; Park, J.-B.; Vasa, K.; Singh, B.R. The identification and biochemical characterization of drug-like compounds that inhibit botulinum neurotoxin serotype A endopeptidase activity. Toxicon Off. J. Int. Soc. Toxinol. 2010, 55, 818–826. [Google Scholar] [CrossRef]
- Fasshauer, D.; Otto, H.; Eliason, W.K.; Jahn, R.; Brünger, A.T. Structural changes are associated with soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor complex formation. J. Biol. Chem. 1997, 272, 28036–28041. [Google Scholar] [CrossRef] [PubMed]
- Puffer, E.B.; Lomneth, R.B.; Sarkar, H.K.; Singh, B.R. Differential roles of developmentally distinct SNAP-25 isoforms in the neurotransmitter release process. Biochemistry 2001, 40, 9374–9378. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Maksudov, F.; Kononova, O.; Marx, K.A.; Barsegov, V.; Singh, B.R. Botulinum Endopeptidase: SAXS Experiments and MD Simulations Reveal Extended Solution Structures That Account for Its Biochemical Properties. J. Phys. Chem. B 2020, 124, 5801–5812. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.C.; Schmidt, J.J.; McGrath, C.F.; Nguyen, T.L.; Hermone, A.R.; Panchal, R.G.; Vennerstrom, J.L.; Kodukula, K.; Zaharevitz, D.W.; Gussio, R.; et al. Conformational sampling of the botulinum neurotoxin serotype A light chain: Implications for inhibitor binding. Bioorg. Med. Chem. 2005, 13, 333–341. [Google Scholar] [CrossRef]
- Cai, S.; Singh, B.R. Role of the disulfide cleavage induced molten globule state of type a botulinum neurotoxin in its endopeptidase activity. Biochemistry 2001, 40, 15327–15333. [Google Scholar] [CrossRef]
- Kukreja, R.V.; Sharma, S.K.; Singh, B.R. Molecular basis of activation of endopeptidase activity of botulinum neurotoxin type E. Biochemistry 2010, 49, 2510–2519. [Google Scholar] [CrossRef][Green Version]
- Kukreja, R.; Singh, B. Biologically active novel conformational state of botulinum, the most poisonous poison. J. Biol. Chem. 2005, 280, 39346–39352. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.B.; Kononova, O.; Cai, S.; Barsegov, V.; Parmar, V.S.; Kumar, R.; Singh, B.R. Botulinum neurotoxin inhibitor binding dynamics and kinetics relevant for drug design. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129933. [Google Scholar] [CrossRef]
- Kumar, R.; Kukreja, R.V.; Cai, S.; Singh, B.R. Differential role of molten globule and protein folding in distinguishing unique features of botulinum neurotoxin. Biochim. Biophys. Acta BBA-Proteins Proteomics 2014, 1844, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Silvaggi, N.R.; Wilson, D.; Tzipori, S.; Allen, K.N. Catalytic Features of the Botulinum Neurotoxin A Light Chain Revealed by High Resolution Structure of an Inhibitory Peptide Complex. Biochemistry 2008, 47, 5736–5745. [Google Scholar] [CrossRef] [PubMed]
- Feltrup, T.M.; Patel, K.; Kumar, R.; Cai, S.; Singh, B.R. A novel role of C-terminus in introducing a functionally flexible structure critical for the biological activity of botulinum neurotoxin. Sci. Rep. 2018, 8, 8884. [Google Scholar] [CrossRef]
- Cai, S.; Sarkar, H.K.; Singh, B.R. Enhancement of the endopeptidase activity of botulinum neurotoxin by its associated proteins and dithiothreitol. Biochemistry 1999, 38, 6903–6910. [Google Scholar] [CrossRef]
- Cai, S.; Singh, B.R. A correlation between differential structural features and the degree of endopeptidase activity of type A botulinum neurotoxin in aqueous solution. Biochemistry 2001, 40, 4693–4702. [Google Scholar] [CrossRef]
- Sharma, S.K.; Singh, B.R. Enhancement of the endopeptidase activity of purified botulinum neurotoxins A and E by an isolated component of the native neurotoxin associated proteins. Biochemistry 2004, 43, 4791–4798. [Google Scholar] [CrossRef]
- Hasegawa, K.; Watanabe, T.; Suzuki, T.; Yamano, A.; Oikawa, T.; Sato, Y.; Kouguchi, H.; Yoneyama, T.; Niwa, K.; Ikeda, T.; et al. A novel subunit structure of Clostridium botulinum serotype D toxin complex with three extended arms. J. Biol. Chem. 2007, 282, 24777–24783. [Google Scholar] [CrossRef]
- Lee, K.; Gu, S.; Jin, L.; Le, T.T.N.; Cheng, L.W.; Strotmeier, J.; Kruel, A.M.; Yao, G.; Perry, K.; Rummel, A.; et al. Structure of a bimodular botulinum neurotoxin complex provides insights into its oral toxicity. PLoS Pathog. 2013, 9, e1003690. [Google Scholar] [CrossRef] [PubMed]
- Mizanur, R.M.; Frasca, V.; Swaminathan, S.; Bavari, S.; Webb, R.; Smith, L.A.; Ahmed, S.A. The C Terminus of the Catalytic Domain of Type A Botulinum Neurotoxin May Facilitate Product Release from the Active Site. J. Biol. Chem. 2013, 288, 24223–24233. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Cai, S.; Ojadi, E.; Singh, B.R. Resolution of sub-nanosecond motions in botulinum neurotoxin endopeptidase: An evidence of internal flexibility. Biochim. Biophys. Acta BBA-Proteins Proteomics 2015, 1854, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.; Pain, R.H. Molten globule intermediates and protein folding. Eur. Biophys. J. EBJ 1991, 19, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Ptitsyn, O.B. Molten globule and protein folding. Adv. Protein Chem. 1995, 47, 83–229. [Google Scholar] [CrossRef]
- Martin, J.; Langer, T.; Boteva, R.; Schramel, A.; Horwich, A.L.; Hartl, F.U. Chaperonin-mediated protein folding at the surface of groEL through a ’molten globule’-like intermediate. Nature 1991, 352, 36–42. [Google Scholar] [CrossRef]
- Baldwin, R.L.; Rose, G.D. Molten globules, entropy-driven conformational change and protein folding. Curr. Opin. Struct. Biol. 2013, 23, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Geny, B.; Popoff, M.R. Bacterial protein toxins and lipids: Pore formation or toxin entry into cells. Biol. Cell 2006, 98, 667–678. [Google Scholar] [CrossRef]
- Man, P.; Montagner, C.; Vitrac, H.; Kavan, D.; Pichard, S.; Gillet, D.; Forest, E.; Forge, V. Accessibility changes within diphtheria toxin T domain when in the functional molten globule state, as determined using hydrogen/deuterium exchange measurements. FEBS J. 2010, 277, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Kachel, K.; Kim, H.; Malenbaum, S.E.; Collier, R.J.; London, E. Interaction of diphtheria toxin T domain with molten globule-like proteins and its implications for translocation. Science 1999, 284, 955–957. [Google Scholar] [CrossRef]
- Collier, R.J. Membrane translocation by anthrax toxin. Mol. Aspects Med. 2009, 30, 413–422. [Google Scholar] [CrossRef]
- Noskov, A.N. Molecular model of anthrax toxin translocation into target-cells. Russ. J. Bioorganic Chem. 2014, 40, 399–404. [Google Scholar] [CrossRef]
- Felix, I.; Lomada, S.K.; Barth, H.; Wieland, T. Bacillus anthracis’ PA63 Delivers the Tumor Metastasis Suppressor Protein NDPK-A/NME1 into Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 3295. [Google Scholar] [CrossRef]
- Krantz, B.A.; Trivedi, A.D.; Cunningham, K.; Christensen, K.A.; Collier, R.J. Acid-induced unfolding of the amino-terminal domains of the lethal and edema factors of anthrax toxin. J. Mol. Biol. 2004, 344, 739–756. [Google Scholar] [CrossRef] [PubMed]
- Madden, J.C.; Ruiz, N.; Caparon, M. Cytolysin-mediated translocation (CMT): A functional equivalent of type III secretion in gram-positive bacteria. Cell 2001, 104, 143–152. [Google Scholar] [CrossRef]
- Seveau, S. Multifaceted Activity of Listeriolysin O, the Cholesterol-Dependent Cytolysin of Listeria monocytogenes. Subcell. Biochem. 2014, 80, 161–195. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.B.; Heuck, A.P. Perfringolysin O Structure and Mechanism of Pore Formation as a Paradigm for Cholesterol-Dependent Cytolysins. Subcell. Biochem. 2014, 80, 63–81. [Google Scholar] [CrossRef]
- Tilley, S.J.; Orlova, E.V.; Gilbert, R.J.C.; Andrew, P.W.; Saibil, H.R. Structural Basis of Pore Formation by the Bacterial Toxin Pneumolysin. Cell 2005, 121, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Peraro, M.D.; van der Goot, F.G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. Microbiol. 2016, 14, 77–92. [Google Scholar] [CrossRef]
- Vécsey-Semjén, B.; Möllby, R.; van der Goot, F.G. Partial C-terminal unfolding is required for channel formation by staphylococcal alpha-toxin. J. Biol. Chem. 1996, 271, 8655–8660. [Google Scholar] [CrossRef]
- Lesieur, C.; Vécsey-Semjén, B.; Abrami, L.; Fivaz, M.; Gisou van der Goot, F. Membrane insertion: The strategies of toxins (Review). Mol. Membr. Biol. 1997, 14, 45–64. [Google Scholar] [CrossRef]
- Chen, L.; Balabanidou, V.; Remeta, D.P.; Minetti, C.A.S.A.; Portaliou, A.G.; Economou, A.; Kalodimos, C.G. Structural Instability Tuning as a Regulatory Mechanism in Protein-Protein Interactions. Mol. Cell 2011, 44, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Vamvaca, K.; Vögeli, B.; Kast, P.; Pervushin, K.; Hilvert, D. An enzymatic molten globule: Efficient coupling of folding and catalysis. Proc. Natl. Acad. Sci. USA 2004, 101, 12860–12864. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Uversky, V.N.; Dunker, A.K. Understanding Protein Non-Folding. Biochim. Biophys. Acta 2010, 1804, 1231–1264. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Endo, S.; Nakamura, H. How a Novel Scientific Concept Was Coined the “Molten Globule State”. Biomolecules 2020, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Wada, A.; Tachibana, H.; Hayashi, H.; Saito, Y. Multidimensional spectroscopic data correlation in the conformation transition of biological macromolecules. J. Biochem. Biophys. Methods 1980, 2, 257–269. [Google Scholar] [CrossRef]
- Hsu, D.J.; Leshchev, D.; Kosheleva, I.; Kohlstedt, K.L.; Chen, L.X. Unfolding bovine α-lactalbumin with T-jump: Characterizing disordered intermediates via time-resolved x-ray solution scattering and molecular dynamics simulations. J. Chem. Phys. 2021, 154, 105101. [Google Scholar] [CrossRef]
- De Vivo, M.; Masetti, M.; Bottegoni, G.; Cavalli, A. Role of Molecular Dynamics and Related Methods in Drug Discovery. J. Med. Chem. 2016, 59, 4035–4061. [Google Scholar] [CrossRef] [PubMed]
- Platania, C.B.M.; Bucolo, C. Molecular Dynamics Simulation Techniques as Tools in Drug Discovery and Pharmacology: A Focus on Allosteric Drugs. Methods Mol. Biol. 2021, 2253, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Aci-Sèche, S.; Ziada, S.; Braka, A.; Arora, R.; Bonnet, P. Advanced molecular dynamics simulation methods for kinase drug discovery. Future Med. Chem. 2016, 8, 545–566. [Google Scholar] [CrossRef] [PubMed]
- Nasir, A.; Caetano-Anollés, G. A phylogenomic data-driven exploration of viral origins and evolution. Sci. Adv. 2015, 1, e1500527. [Google Scholar] [CrossRef] [PubMed]



| Toxin Type | Receptors | References |
|---|---|---|
| BoNT/A | SV2A, SV2B, SV2C | [123,124] |
| BoNT/B | SYT-I, SYT-II | [125,126,127] |
| BoNT/C | Not identified, unknown SV structures | [118,120,121] |
| BoNT/D | SV2A, SV2B, SV2C | [128] |
| BoNT/E | SV2A, SV2B | [118,129] |
| BoNT/F | SV2A, SV2B, SV2C | [130] |
| BoNT/G | SYT-I, SYT-II | [126,127,131,132,133] |
| BoNT/H (BoNT/FA) | SV2A, SV2B, SV2C | [134] |
| BoNT/X | not identified | |
| TeNT | GPI-anchored protein, nidogen SV2A/B? | [135,136] |
| Toxin Type | Target | Cleavage Site | References |
|---|---|---|---|
| BoNT/A | SNAP-25 | Ala-Ser-Gln197–Phe198-Glu-Thr | [179] |
| BoNT/B; TeNT | VAMP | Ala-Ser-Gln78–Phe79-Glu-Thr | [180] |
| BoNT/C | SNAP-25 | Ala-Ser-Gln-Phe198–Glu199-Thr | [169] |
| Syntaxin | Thr-Lys-Lys252–Ala253-Val-Lys | [181] | |
| BoNT/D | VAMP | Asp-Gln-Lys61–Leu62-Ser-Glu | [182,183] |
| BoNT/E | SNAP-25 | Ile-Asp-Arg180–Ile181-Met-Glu | [184,185] |
| BoNT/F | VAMP | Arg-Asp-Gln60–Lys61-Leu-Ser | [108] |
| BoNT/G | VAMP | Thr-Ser-Ala83–Ala84-Lys-Leu | [186,187] |
| BoNT/H (FA) | VAMP | Lys-Val-Ile56–Glu57-Arg-Asp | [27,30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, S.; Kumar, R.; Singh, B.R. Clostridial Neurotoxins: Structure, Function and Implications to Other Bacterial Toxins. Microorganisms 2021, 9, 2206. https://doi.org/10.3390/microorganisms9112206
Cai S, Kumar R, Singh BR. Clostridial Neurotoxins: Structure, Function and Implications to Other Bacterial Toxins. Microorganisms. 2021; 9(11):2206. https://doi.org/10.3390/microorganisms9112206
Chicago/Turabian StyleCai, Shuowei, Raj Kumar, and Bal Ram Singh. 2021. "Clostridial Neurotoxins: Structure, Function and Implications to Other Bacterial Toxins" Microorganisms 9, no. 11: 2206. https://doi.org/10.3390/microorganisms9112206
APA StyleCai, S., Kumar, R., & Singh, B. R. (2021). Clostridial Neurotoxins: Structure, Function and Implications to Other Bacterial Toxins. Microorganisms, 9(11), 2206. https://doi.org/10.3390/microorganisms9112206





