Enrofloxacin Alters Fecal Microbiota and Resistome Irrespective of Its Dose in Calves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Collection
2.2. DNA Extraction and 16S rRNA Gene Sequencing
2.3. Bioinformatics and Statistical Analysis
2.4. Metagenomic Hi-C ProxiMeta
2.5. Quantification of Selected Antibiotic Resistance Determinants
3. Results
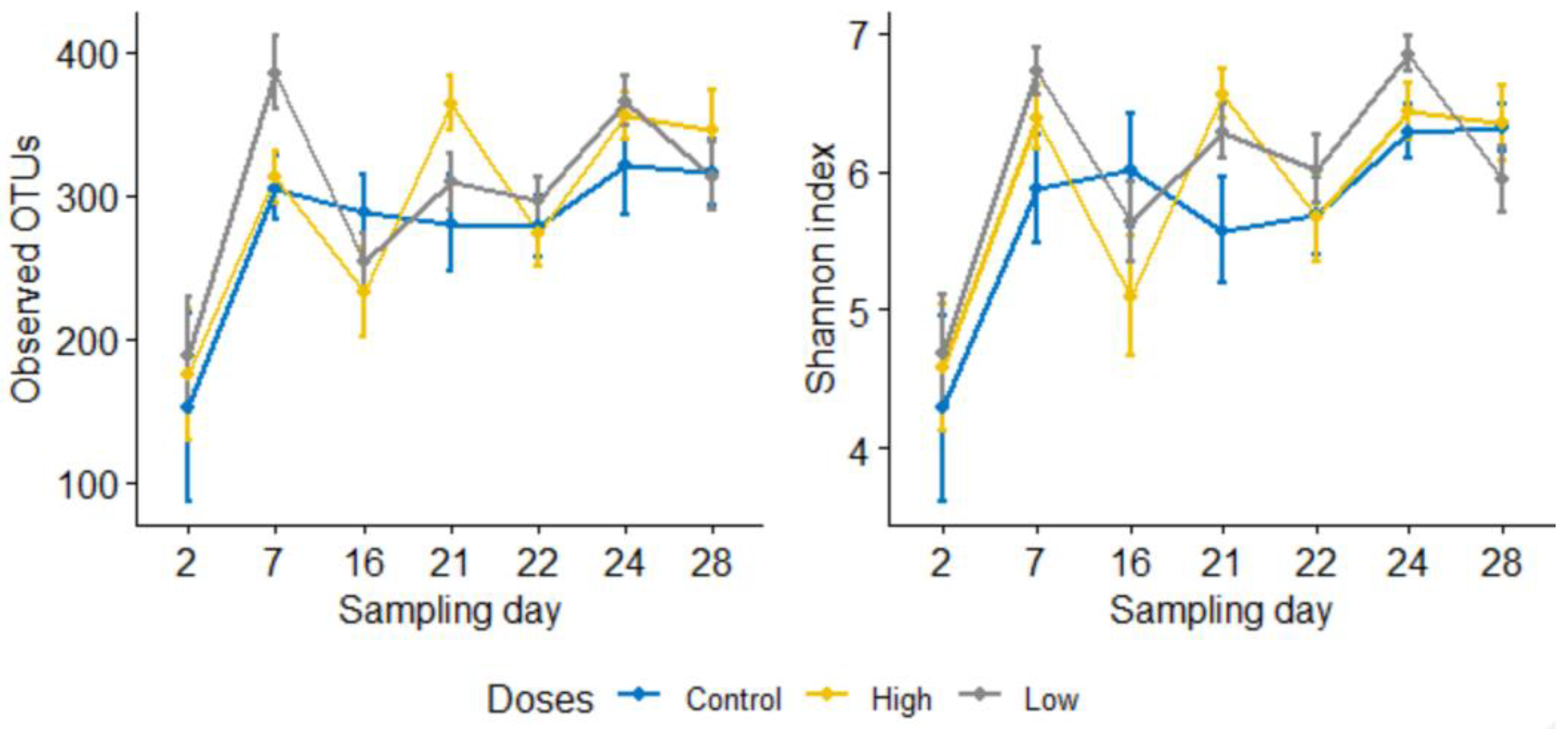
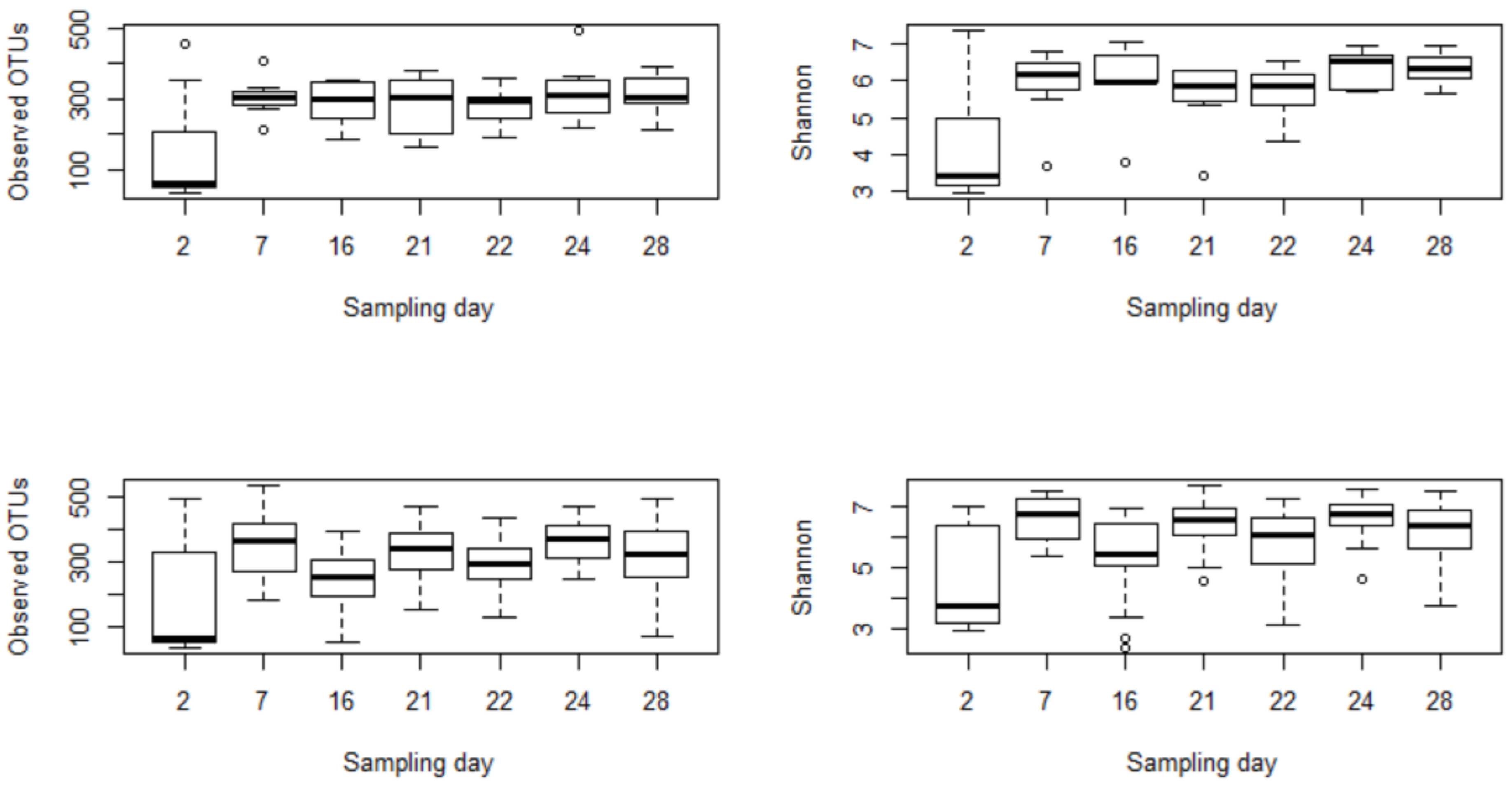
3.1. 16S rRNA Gene Sequencing Outputs
3.2. Microbial Profiling
3.2.1. Subsubsection Alpha and Beta Diversity Metrics Show a Significant Microbial Shift following Enrofloxacin Administration
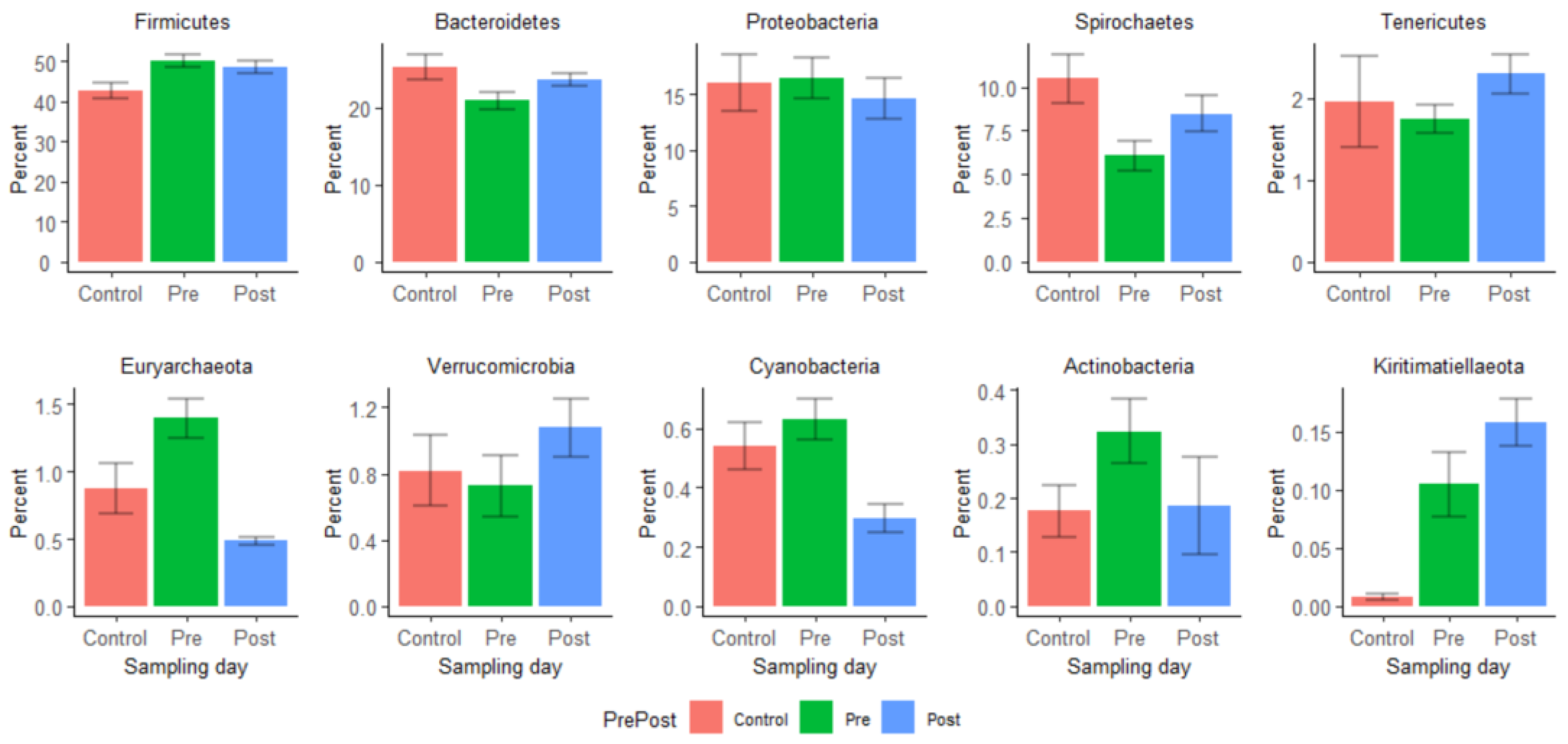
3.2.2. Relative Abundances of Certain Bacterial Taxa Varied Significantly between Pre- and Post-Treatment Samples
3.2.3. Analysis of Composition of Microbiomes (ANCOM) Shows Disruptions of Certain Bacterial Taxa by Enrofloxacin Injection
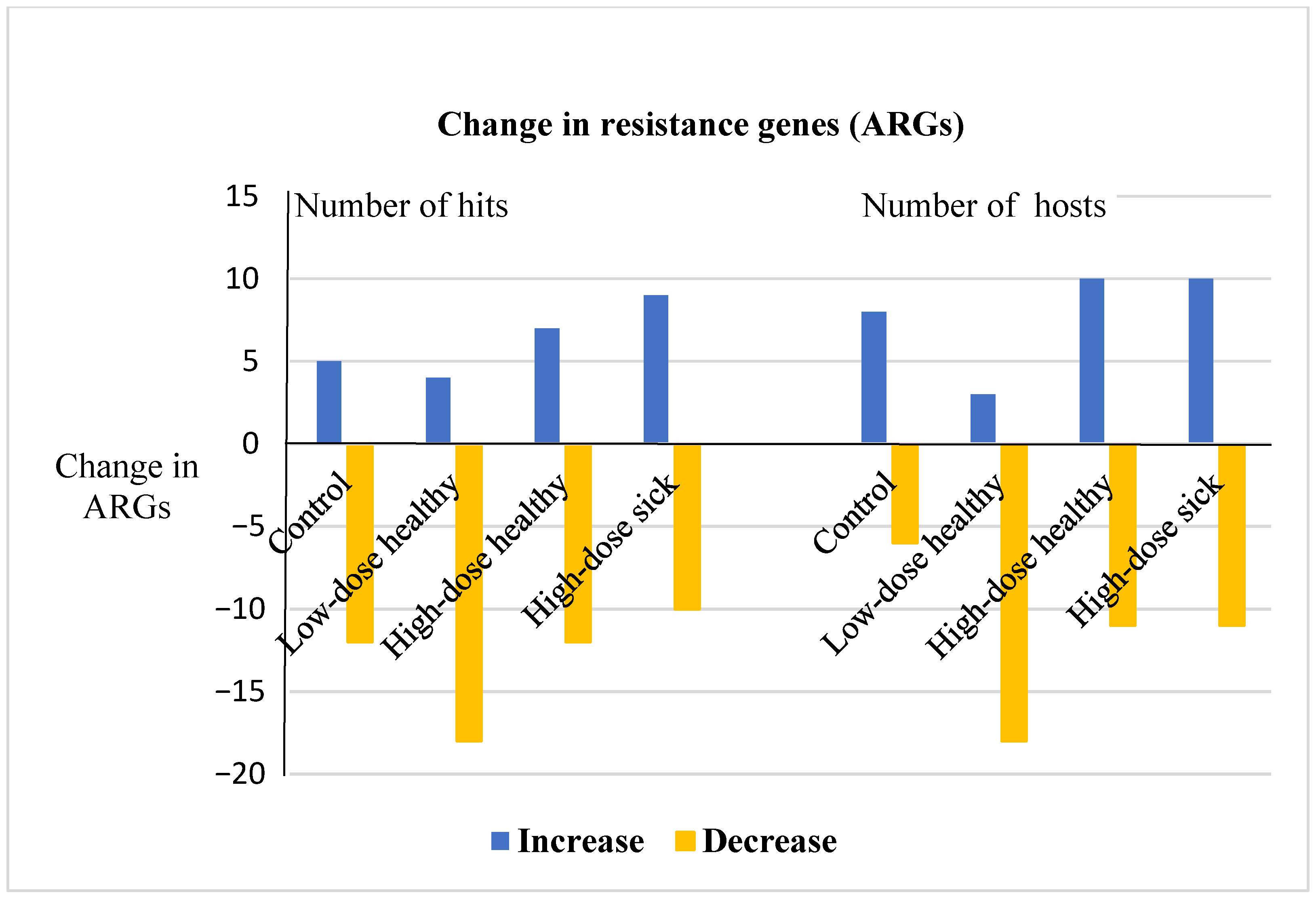
3.3. Metagenomic Hi-C Results Show Changes in Copy Numbers and Host Ranges of ARGs following Enrofloxacin Administration
3.4. Quantitative Alterations in Selected Resistance Determinants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomez, D.E.; Galvao, K.N.; Rodriguez-Lecompte, J.C.; Costa, M.C. The cattle microbiota and the immune system an evolving field. Vet. Clin. Food Anim. Pract. 2019, 35, 485–505. [Google Scholar] [CrossRef]
- Hennessy, M.L.; Indugu, N.; Vecchiarelli, B.; Bender, J.; Pappalardo, C.; Leibstein, M.; Toth, J.; Katepalli, A.; Garapati, S.; Pitta, D. Temporal changes in the fecal bacterial community in Holstein dairy calves from birth through the transition to a solid diet. PLoS ONE 2020, 15, e0238882. [Google Scholar] [CrossRef]
- Jeong, S.J.; Lee, K.H.; Kim, J.-H.; Park, S.Y.; Song, Y.G. Efficacy and gut dysbiosis of gentamicin-intercalated smectite as a new therapeutic agent against Helicobacter pyloriin a mouse model. Antibiotics 2020, 9, 502. [Google Scholar] [CrossRef]
- Schokker, D.; Zhang, J.; Zhang, L.-L.; Vastenhouw, S.A.; Heilig, H.G.H.J.; Smidt, H.; Rebel, J.M.J.; Smits, M.A. Early-life environmental variation affects intestinal microbiota and immune development in new-born piglets. PLoS ONE 2014, 9, e100040. [Google Scholar] [CrossRef]
- Panda, S.; El Khader, I.; Casellas, F.; Vivancos, J.L.; Cors, M.G.; Santiago, A.; Cuenca, S.; Guarner, F.; Manichanh, C. Short-term effect of antibiotics on human gut microbiota. PLoS ONE 2014, 9, e95476. [Google Scholar] [CrossRef]
- Kirchhelle, C. Pharming animals: A global history of antibiotics in food production (1935–2017). Palgrave Commun. 2018, 4, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Looft, T.; Johnson, T.A.; Allen, H.K.; Bayles, D.O.; Alt, D.P.; Stedtfeld, R.D.; Sul, W.J.; Stedtfeld, T.M.; Chai, B.; Cole, J.R.; et al. In-feed antibiotic effects on the swine intestinal microbiome. Proc. Natl. Acad. Sci. USA 2012, 109, 1691–1696. [Google Scholar] [CrossRef] [Green Version]
- Catry, B.; Dewulf, J.; Maes, D.; Pardon, B.; Callens, B.; Vanrobaeys, M.; Opsomer, G.; de Kruif, A.; Haesebrouck, F. Effect of antimicrobial consumption and production type on antibacterial resistance in the bovine respiratory and digestive tract. PLoS ONE 2016, 11, e0146488. [Google Scholar] [CrossRef] [Green Version]
- Blaser, M.J. Antibiotic use and its consequences for the normal microbiome. Science 2016, 352, 544–545. [Google Scholar] [CrossRef] [Green Version]
- Blondeau, J.M.; Borsos, S.; Blondeau, L.D.; Blondeau, B.J.J.; Hesje, C.E. Comparative minimum inhibitory and mutant prevention drug concentrations of enrofloxacin, ceftiofur, florfenicol, tilmicosin and tulathromycin against bovine clinical isolates of Mannheimia haemolytica. Vet. Microbiol. 2012, 160, 85–90. [Google Scholar] [CrossRef]
- Speer, N.C.; Young, C.; Roeber, D. The importance of preventing bovine respiratory disease: A beef industry review. Bov. Pract. 2001, 35, 189–196. [Google Scholar]
- USDA. Feedlot 2011 Part I: Management Practices on U.S. Feedlots with a Capacity of 1000 or More Head; USDA-APHIS-VS-CEAH-NAHMS: Fort Collins, CO, USA, 2013.
- Davis, J.L.; Foster, D.M.; Papich, M.G. Pharmacokinetics and tissue distribution of enrofloxacin and its active metabolite ciprofloxacin in calves. J. Vet. Pharmacol. Ther. 2007, 30, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hao, H.; Cheng, G.; Liu, C.; Ahmed, S.; Shabbir, M.A.B.; Hussain, H.I.; Dai, M.; Yuan, Z. Microbial shifts in the intestinal microbiota of Salmonella infected chickens in response to enrofloxacin. Front. Microbiol. 2017, 8, 564–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferran, A.A.; Bibbal, D.; Pellet, T.; Laurentie, M.; Gicquel-Bruneau, M.; Sanders, P.; Schneider, M.; Toutain, P.L.; Bousquet-Melou, A. Pharmacokinetic/pharmacodynamic assessment of the effects of parenteral administration of a fluoroquinolone on the intestinal microbiota: Comparison of bactericidal activity at the gut versus the systemic level in a pig model. Int. J. Antimicrob. Agents 2013, 42, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.M.; Jacob, M.E.; Warren, C.D.; Papich, M.G. Pharmacokinetics of enrofloxacin and ceftiofur in plasma, interstitial fluid, and gastrointestinal tract of calves after subcutaneous injection, and bactericidal impacts on representative enteric bacteria. J. Vet. Pharmacol. Ther. 2016, 39, 62–71. [Google Scholar] [CrossRef]
- Ferguson, K.M.; Jacob, M.E.; Theriot, C.M.; Callahan, B.J.; Prange, T.; Papich, M.G.; Foster, D.M. Dosing regimen of enrofloxacin impacts intestinal pharmacokinetics and the fecal microbiota in steers. Front. Microbiol. 2018, 9, 2190. [Google Scholar] [CrossRef]
- Hanthorn, C.J.; Dewell, R.D.; Cooper, V.L.; Frana, T.S.; Plummer, P.J.; Wang, C.; Dewell, G.A. Randomized clinical trial to evaluate the pathogenicity of bibersteinia trehalosi in respiratory disease among calves. BMC Vet. Res. 2014, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- AVMA. AVMA Guidelines for the Euthanasia of Animals, Version 2020.0.1. 2020. Available online: https://www.avma.org/sites/default/files/2020-01/2020-Euthanasia-Final-1-17-20.pdf (accessed on 10 January 2021).
- Mandal, S.; Van Treuren, W.; White, R.A.; Eggesbo, M.; Knight, R.; Peddada, S.D. Analysis of composition of microbiomes: A novel method for studying microbial composition. Microb. Ecol. Health Dis. 2015, 26, 27663. [Google Scholar] [CrossRef] [Green Version]
- Stewart, R.D.; Auffret, M.D.; Warr, A.; Wiser, A.H.; Press, M.O.; Langford, K.W.; Liachko, I.; Snelling, T.J.; Dewhurst, R.J.; Walker, A.W.; et al. Assembly of 913 microbial genomes from metagenomic sequencing of the cow rumen. Nat. Commun. 2018, 9, 870. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Reyes, A.; Breton-Deval, L.; Mangelson, H.; Salinas-Peralta, I.; Sanchez-Flores, A. Hi-C deconvolution of a textile dye-related microbiome reveals novel taxonomic landscapes and links phenotypic potential to individual genomes. Int. Microbiol. 2021. [Google Scholar] [CrossRef]
- Beyi, A.F.; Hassall, A.; Phillips, G.J.; Plummer, P.J. Tracking reservoirs of antimicrobial resistance genes in a complex microbial community using metagenomic Hi-C: The case of bovine digital dermatitis. Antibiotics 2021, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Holman, D.B.; Gzyl, K.E. A meta-analysis of the bovine gastrointestinal tract microbiota. FEMS Microbiol. Ecol. 2019, 95, fiz072. [Google Scholar] [CrossRef]
- Hamm, M.; Wollen, T.; Highland, R.; Davidson, J.; TerHune, T.; Lechtenberg, K.; Apley, M.; Miles, D.; Wray, M.; Bechtol, D.; et al. Clinical efficacy of enrofloxacin against bovine respiratory disease comparing different treatment regimens. Bov. Pract. 1999, 33, 56–59. [Google Scholar]
- Dudek, K.; Bednarek, D.; Ayling, R.D.; Kycko, A.; Reichert, M. Preliminary study on the effects of enrofloxacin, flunixin meglumine and pegbovigrastim on Mycoplasma bovis pneumonia. BMC Vet. Res. 2019, 15, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holman, D.B.; Yang, W.; Alexander, T.W. Antibiotic treatment in feedlot cattle: A longitudinal study of the effect of oxytetracycline and tulathromycin on the fecal and nasopharyngeal microbiota. Microbiome 2019, 7, 86. [Google Scholar] [CrossRef] [Green Version]
- Hertz, F.B.; Budding, A.E.; van der Lugt-Degen, M.; Savelkoul, P.H.; Lobner-Olesen, A.; Frimodt-Moller, N. Effects of Antibiotics on the Intestinal Microbiota of Mice. Antibiotics 2020, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Drusano, G.L. Antimicrobial pharmacodynamics: Critical interactions of ‘bug and drug’. Nat. Rev. Microbiol. 2004, 2, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, S.; van Leeuwe, T.M.; Qachach, O.; Zhang, Z.; van Wezel, G.P.; Rozen, D.E. The evolution of no-cost resistance at sub-MIC concentrations of streptomycin in Streptomyces coelicolor. ISME J. 2017, 11, 1168–1178. [Google Scholar] [CrossRef] [Green Version]
- Keijser, B.J.F.; Agamennone, V.; van den Broek, T.J.; Caspers, M.; van de Braak, A.; Bomers, R.; Havekes, M.; Schoen, E.; van Baak, M.; Mioch, D.; et al. Dose-dependent impact of oxytetracycline on the veal calf microbiome and resistome. BMC Genom. 2019, 20, 65. [Google Scholar] [CrossRef] [Green Version]
- Holman, D.B.; Chenier, M.R. Antimicrobial use in swine production and its effect on the swine gut microbiota and antimicrobial resistance. Can. J. Microbiol. 2015, 61, 785–798. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Huse, S.; Sogin, M.L.; Relman, D.A. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008, 6, 2383–2400. [Google Scholar] [CrossRef] [PubMed]
- Valerio de Oliveira, M.N.; Jewell, K.A.; Freitas, F.S.; Benjamin, L.A.; Totola, M.R.; Borges, A.C.; Moraes, C.A.; Suen, G. Characterizing the microbiota across the gastrointestinal tract of a Brazilian Nelore steer. Vet. Microbiol. 2013, 164, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Alexander, T.W.; Yanke, L.J.; Topp, E.; Olson, M.E.; Read, R.R.; Morck, D.W.; McAllister, T.A. Effect of subtherapeutic administration of antibiotics on the prevalence of antibiotic-resistant Escherichia coli bacteria in feedlot cattle. Appl. Environ. Microb. 2008, 74, 4405–4416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, R.V.; Altier, C.; Siler, J.D.; Mann, S.; Jordan, D.; Warnick, L.D. Longitudinal effects of enrofloxacin or tulathromycin use in preweaned calves at high risk of bovine respiratory disease on the shedding of antimicrobial-resistant fecal Escherichia coli. J. Dairy Sci. 2020, 103, 10547–10559. [Google Scholar] [CrossRef]
- Weinroth, M.D.; Scott, H.M.; Norby, B.; Loneragan, G.H.; Noyes, N.R.; Rovira, P.; Doster, E.; Yang, X.; Woerner, D.R.; Morley, P.S.; et al. Effects of Ceftiofur and Chlortetracycline on the Resistomes of Feedlot Cattle. Appl. Environ. Microb. 2018, 84, e00610-18. [Google Scholar] [CrossRef] [Green Version]
- Brenciani, A.; Bacciaglia, A.; Vecchi, M.; Vitali, L.A.; Varaldo, P.E.; Giovanettil, E. Genetic elements carrying erm(B) in Streptococcus pyogenes and association with tet(M) tetracycline resistance gene. Antimicrob. Agents Chemother. 2007, 51, 1209–1216. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.W.; Shin, M.K.; Jung, M.; Belaynehe, K.M.; Yoo, H.S. Prevalence of antimicrobial resistance and transfer of tetracycline resistance genes in Escherichia coli isolates from beef cattle. Appl. Environ. Microb. 2015, 81, 5560–5566. [Google Scholar] [CrossRef] [Green Version]
- Beukers, A.G.; Zaheer, R.; Cook, S.R.; Chaves, A.V.; Ward, M.P.; Tymensen, L.; Morley, P.S.; Hannon, S.; Booker, C.W.; Read, R.R.; et al. Comparison of antimicrobial resistance genes in feedlots and urban wastewater. Can. J. Vet. Res. 2018, 82, 24–38. [Google Scholar]
- FDA. 2019 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals. 2020. Available online: https://www.fda.gov/media/144427/download (accessed on 30 October 2020).
- Hope, K.J.; Apley, M.D.; Schrag, N.F.D.; Lubbers, B.V.; Singer, R.S. Antimicrobial use in 22 US beef feedyards: 2016–2017. Zoonoses Public Health 2020, 67, 94–110. [Google Scholar] [CrossRef]
- Evans, B.A.; Amyes, S.G.B. OXA beta-Lactamases. Clin. Microbiol. Rev. 2014, 27, 241–263. [Google Scholar] [CrossRef] [Green Version]
- Amudhan, S.M.; Sekar, U.; Arunagiri, K.; Sekar, B. OXA beta-lactamase-mediated carbapenem resistance in Acinetobacter baumannii. Indian J. Med. Microbiol. 2011, 29, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Potron, A.; Poirel, L.; Rondinaud, E.; Nordmann, P. Intercontinental spread of OXA-48 beta-lactamase-producing Enterobacteriaceae over a 11-year period, 2001 to 2011. Eurosurveillance 2013, 18, 20549. [Google Scholar] [CrossRef] [PubMed]
- Walther-Rasmussen, J.; Hoiby, N. OXA-type carbapenemases. J. Antimicrob. Chemother. 2006, 57, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Ellabaan, M.M.H.; Charusanti, P.; Munck, C.; Blin, K.; Tong, Y.; Weber, T.; Sommer, M.O.A.; Lee, S.Y. Dissemination of antibiotic resistance genes from antibiotic producers to pathogens. Nat. Commun. 2017, 8, 15784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, M.C.; Schwarz, S. Tetracycline and Phenicol Resistance Genes and Mechanisms: Importance for Agriculture, the Environment, and Humans. J. Environ. Qual. 2016, 45, 576–592. [Google Scholar] [CrossRef]
- Poole, K. Efflux pumps as antimicrobial resistance mechanisms. Ann. Med. 2007, 39, 162–176. [Google Scholar] [CrossRef]
- Kyselkova, M.; Jirout, J.; Vrchotova, N.; Schmitt, H.; Elhottova, D. Spread of tetracycline resistance genes at a conventional dairy farm. Front. Microbiol. 2015, 6, 536. [Google Scholar] [CrossRef] [Green Version]
- Francino, M.P. Antibiotics and the human gut microbiome: Dysbioses and accumulation of resistances. Front. Microbiol. 2016, 7, 1543. [Google Scholar] [CrossRef] [Green Version]
- Slizovskiy, I.B.; Mukherjee, K.; Dean, C.J.; Boucher, C.; Noyes, N.R. Mobilization of antibiotic resistance: Are current approaches for colocalizing resistomes and mobilomes useful? Front. Microbiol. 2020, 11, 1376. [Google Scholar] [CrossRef]
- Levison, M.E.; Levison, J.H. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect. Dis. Clin. 2009, 23, 791–815. [Google Scholar] [CrossRef] [Green Version]
- De Smet, J.; Boyen, F.; Croubels, S.; Rasschaert, G.; Haesebrouck, F.; Temmerman, R.; Rutjens, S.; De Backer, P.; Devreese, M. The impact of therapeutic-dose induced intestinal enrofloxacin concentrations in healthy pigs on fecal Escherichia coli populations. BMC Vet. Res. 2020, 16, 382. [Google Scholar] [CrossRef]
- Sarkozy, G. Quinolones: A class of antimicrobial agents. Vet. Med.-Czech 2001, 46, 257–274. [Google Scholar] [CrossRef] [Green Version]
- Day, T.; Read, A.F. Does high-dose antimicrobial chemotherapy prevent the evolution of resistance? PLoS Comput. Biol. 2016, 12, e1004689. [Google Scholar] [CrossRef] [PubMed]
- Raymond, B. Five rules for resistance management in the antibiotic apocalypse, a road map for integrated microbial management. Evol. Appl. 2019, 12, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Lan, W.; Wang, Y.; Jiang, L.; Jiang, Y.; Wang, Z. Comparative pharmacokinetics of danofloxacin in healthy and Pasteurella multocida infected ducks. J. Vet. Pharmacol. Ther. 2018, 41, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Day, D.N.; Sparks, J.W.; Karriker, L.A.; Stalder, K.J.; Wulf, L.W.; Zhang, J.; Kinyon, J.M.; Stock, M.L.; Gehring, R.; Wang, C.; et al. Impact of an experimental PRRSV and Streptococcus suis coinfection on the pharmacokinetics of ceftiofur hydrochloride after intramuscular injection in pigs. J. Vet. Pharmacol. Ther. 2015, 38, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Su, J.-Q.; An, X.-L.; Huang, F.-Y.; Rensing, C.; Brandt, K.K.; Zhu, Y.-G. Feed additives shift gut microbiota and enrich antibiotic resistance in swine gut. Sci. Total Environ. 2018, 621, 1224–1232. [Google Scholar] [CrossRef]
- Silverman, J.D.; Bloom, R.J.; Jiang, S.; Durand, H.K.; Dallow, E.; Mukherjee, S.; David, L.A. Measuring and mitigating PCR bias in microbiota datasets. PLoS Comput. Biol. 2021, 17, e1009113. [Google Scholar] [CrossRef]
- Stalder, T.; Press, M.O.; Sullivan, S.; Liachko, I.; Top, E.M. Linking the resistome and plasmidome to the microbiome. ISME J. 2019, 13, 2437–2446. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Activities | Pre-Treatment | Post-Treatment | Involved Group * | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | 4 | 7 | 13 | 16 | 21 | 22 | 24 | 28 | ||
| 1. Calf examination, weighing, and room assignment | All groups | |||||||||
| 2. C. jejuni inoculation | All | |||||||||
| 3. M. haemolytica inoculation | Low and high- dose BRD groups | |||||||||
| 4. Enrofloxacin injection | All, except the control | |||||||||
| 5. Fecal sample collection | All | |||||||||
| 6. Lung examination | All | |||||||||
| ARGs | Forward Primer | Reverse Primer | Reference |
|---|---|---|---|
| ermB | TGAAAGCCATGCGTCTGACA | CCCTAGTGTTCGGTGAATATCCA | Looft et al., 2012 [9] |
| ermF | TTTCAAAGTGGTGTCAAATATTCCTT | GGACAATGGAACCTCCCAGAA | |
| tetO | ATGTGGATACTACAACGCATGAGATT | TGCCTCCACATGATATTTTTCCT | |
| tetW | TCCTTCCAGTGGCACAGATGT | GCCCCATCTAAAACAGCCAAA | |
| tetX | AAATTTGTTACCGACACGGAAGTT | CATAGCTGAAAAAATCCAGGACAGTT |
| Comparisons | Bray–Curtis (Adjusted p-Value) | |
|---|---|---|
| 1 | Low dose: pre- vs. post-treatment | 0.002 |
| 2 | High dose: pre- vs. post-treatment | 0.002 |
| 3 | Low dose pre-treatment vs. high dose pre-treatment | 0.272 |
| 4 | Low dose post-treatment vs. high dose post-treatment | 0.003 |
| 5 | Pre- and post-treatment (low and high dose groups combined) | 0.001 |
| Families | Control vs. Pre vs. Post (W) | Pre vs. Post (W) | Pre vs. Post (Change) | |
|---|---|---|---|---|
| 1 | Bacteroidetes_Bacteroidia_Bacteroidales_uncultured | 153 | 143 | Increased |
| 2 | Proteobacteria_Gammaproteobacteria_Enterobacteriales_Enterobacteriaceae | 139 | 140 | Decreased |
| 3 | Actinobacteria_Actinobacteria_Bifidobacteriales_Bifidobacteriaceae | 145 | 140 | Decreased |
| 4 | Bacteroidetes_Bacteroidia_Bacteroidales_p-251-o5 | 147 | 140 | Increased |
| 5 | Bacteroidetes_Bacteroidia_Bacteroidales_Bacteroidales_RF16_group | 147 | 138 | Increased |
| 6 | Kiritimatiellaeota_Kiritimatiellae_WCHB1-41_uncultured_rumen_bacterium | 151 | 131 | Decreased |
| 7 | Spirochaetes_Spirochaetia_Spirochaetales_Spirochaetaceae | 143 | 130 | Decreased |
| 8 | Tenericutes_Mollicutes_Anaeroplasmatales_Anaeroplasmataceae | 144 | 130 | Increased |
| 9 | Epsilonbacteraeota_Campylobacteria_Campylobacterales_Campylobacteraceae | not significant | 129 | Decreased |
| 10 | Tenericutes_Mollicutes_EMP-G18_uncultured_bacterium | 129 | 129 | Increased |
| 11 | Verrucomicrobia_Verrucomicrobiae_Verrucomicrobiales_Akkermansiaceae | 127 | 128 | Decreased |
| 12 | Tenericutes_Mollicutes_Izimaplasmatales_gut_metagenome | 141 | 127 | Increased |
| 13 | Bacteroidetes_Bacteroidia_Bacteroidales_Bacteroidaceae | 141 | 126 | Decreased |
| 14 | Bacteroidetes_Bacteroidia_Bacteroidales_Bacteroidales_UCG-001 | 125 | 118 | Increased |
| 15 | Tenericutes_Mollicutes_Izimaplasmatales_uncultured_bacterium | 138 | 117 | Decreased |
| 16 | Bacteroidetes_Bacteroidia_Bacteroidales_Prevotellaceae | No significant | 116 | Increased |
| 17 | Firmicutes_Clostridia_Clostridiales_Eubacteriaceae | 116 | 113 | Decreased |
| Family | Genus | Group * | Sampling Days (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 7 | 16 | 21 * | 22 | 24 | 28 | |||
| Enterobacteriaceae | Escherichia-Shigella | Control | 7.63 | 0.00 | 0.07 | 0.02 | 0.05 | 0.02 | 0.03 |
| Trt | 0.08 | 0.03 | 0.02 | 0.06 | 0.00 | 0.00 | 0.03 | ||
| Clostridiaceae_1 | Clostridium_sensu_stricto_1 | Control | 0.03 | 1.05 | 1.19 | 0.24 | 0.15 | 0.37 | 0.14 |
| Trt | 0.14 | 1.55 | 0.81 | 1.34 | 0.03 | 0.64 | 0.63 | ||
| Acidaminococcaceae | Phascolarctobacterium | Control | 0.00 | 0.08 | 0.09 | 0.42 | 0.16 | 0.20 | 0.12 |
| Trt | 0.00 | 0.13 | 0.07 | 0.53 | 0.12 | 0.12 | 0.10 | ||
| Rhodospirillales | Unassigned | Control | 0.00 | 0.09 | 0.11 | 0.02 | 0.00 | 0.02 | 0.04 |
| Trt | 0.00 | 0.20 | 0.03 | 0.03 | 0.00 | 0.00 | 0.00 | ||
| Ruminococcaceae | Ruminococcaceae_UCG-010 | Control | 0.00 | 0.50 | 0.11 | 0.57 | 0.69 | 0.30 | 0.17 |
| Trt | 0.00 | 0.11 | 0.06 | 0.41 | 0.23 | 0.28 | 0.16 | ||
| Erysipelotrichaceae | Turicibacter | Control | 0.00 | 0.15 | 0.12 | 0.04 | 0.01 | 0.03 | 0.06 |
| Trt | 0.00 | 0.37 | 0.10 | 0.29 | 0.00 | 0.18 | 0.15 | ||
| Phylum | Class | Genus | Order | W | Pre vs. Post (Change) |
|---|---|---|---|---|---|
| 1. Low dose | |||||
| Bacteroidetes | Bacteroidia | uncultured_Bacteroidales_bacterium | Bacteroidales | 325 | Increased |
| Bacteroidetes | Bacteroidia | uncultured_bacterium | Bacteroidales | 298 | Increased |
| 2. High dose | |||||
| Proteobacteria | Alphaproteobacteria | uncultured_bacterium | Rhodospirillales | 344 | Decreased |
| Bacteroidetes | Bacteroidia | Prevotellaceae_UCG-003 | Bacteroidales | 335 | Increased |
| Firmicutes | Clostridia | Lachnospiraceae_FCS020_group | Clostridiales | 316 | Increased |
| Kiritimatiellaeota | Kiritimatiellae | uncultured_rumen_bacterium | WCHB1-41 | 313 | Decreased |
| Antibiotic Class | Control | Low Dose Healthy | High Dose Healthy | High Dose BRD | ||||
|---|---|---|---|---|---|---|---|---|
| Pre (60 a, 9 b) | Post (43, 5) | Pre (80, 7) | Post (118, 25) | Pre (90, 22) | Post (67, 18) | Pre (39, 5) | Post (123, 22) | |
| Aminoglycoside | aph2(161 c,8 d), aph3(163,9), ant6(132,10), ant9(6,4), sat(72,5) | aph2(47,15), aph3(47,15), ant6(45,8), ant9(40,9), sat(22,8) | aph2(141,23), aph3(153,26), ant6(193,33), ant9(48,13), sat(141,23) | aph2(7,6), aph3(68,8), ant6(3,2), ant9(1,1) | aph2(515,21), aph3(516,22), ant6(65,15), ant9(67,13), sat(115,15) | aph2(99,16), aph3(101,18), ant6(159,14), ant9(150,11), sat(53,11) | aph2(79,11), aph3(79,11), ant6(17,6), ant9(20,8), sat(79,11) | aph2(44,17), aph3(41,15), ant6(42,18), ant9(30,12), sat(8,5) |
| Beta-lactam | aci(2,1), rob(2,1) | aci(2,1) | aci(17,3), cfx(1,1), rob(12,3) | pbp2(1,1), rob(63,4) | rob(1,1) | aci(1,1), oxa(162,4), rob(2,2) | cfX(2,1) | aci(1,1),cmX(7,2) |
| Macrolide | ermB(1,1), ermF(1,1), ermG(7,5), ermQ(2,2), mefE(445,4) | ermF(11,1), ermG(2,2), mefE(109,3) | ermB(4,4), ermF(783,12), ermG(36,4), ermQ(1,1), mefE(442,9) | ermF(3,1), ermG(3,3), ermQ(3,2), mefE(86,2) | ermB(2,2), ermG(7,5), ermQ(47,8), ermX(1,1), mefE(11350,51) | ermF(2,2), ermG(63,2), ermQ(1,1), mefE(508,3) | ermG(4,3), ermQ(2,1), mefE(1752,5) | ermB(2,1), ermF(1,1), ermG(3,3), ermQ(87,12), ermX(2,1), mefE(4693,45) |
| Phenicol | cfr(16,5) | cfr(42,12) | cfr(34,7) | cfr(3,2) | cfr(52,7), floR(1,1) | cfr(13,4) | cfr(9,5) | cfr(29,3) |
| Sulfonamide | NA e | NA | NA | NA | sulII(1,1) | NA | NA | NA |
| Tetracycline | tet32(1,1), tet40(118,19), tet44(2,2), tetBP(2,2), tetL(1,1), tetO(14,4), tetQ(457,8), tetW(294,25) | tet32(1,1), tet40(157,18), tetA(5,2), tetB(9,1), tetO(4,3), tetW(248, 23), tetX(1,1) | tet32(2,2), tet40(371,31), tetA(4,3), tetL(1,1), tetM(2,2), tetO(121,23), tetQ(1833,31), tetW(721,56) | tet32(2,1), tet40(32,16), tet44(3,3), tetB(4,1), tetL(1,1), tetM(3,1), tetO(32,15), tetQ(67,4), tetW(186,36), tetX(2,1) | tet32(3,3), tet40(534,46), tet44(69,11), tetA(54,15), tetB(436,21), tetM(23,7), tetO(76,22), tetQ(647,27), tetW(718,49), tetX(1,1) | tet32(21,8), tet40(154,27), tetA(2,2), tetO(46,27), tetW(1371,79) | tet40(48,15), tetA(2,2),tetM(1,1),tetO(67,18), tetQ(27,3), tetW(185,30), tetX(1,1) | tet40(161,40), tet44(6,3), tetA(16,9), tetB(8,4), tetM(11,5), tetO(39,15), tetQ(414,17), tetW(440,63), tetX(1,1) |
| Group | Both Pre-and Post-Treatment | Only Pre-Treatment | Only Post-Treatment |
|---|---|---|---|
| Control | aph2, aph3, ant6, ant9, aci, ermF, ermG, mefE, cfr, sat, tet32, tet40, tetO, tetW (total ARGs 14) | rob, ermB, ermQ, tet44, tetBP, tetL, tetQ (total 7) | tetA, tetB, tetX (total 3) |
| Low dose healthy | aph2, aph3, ant6, ant9, ermF, ermG, ermQ, mefE, cfr, rob, tet32, tet40, tetL, tetM, tetO, tetQ, tetW (total 17) | aci, cfX, ermB, sat, tetA (total 5) | pbp2, tet44, tetB, tetX, emrD (total 5) |
| High dose healthy | aph2, aph3, ant6, ant9, rob, ermG, ermQ, mefE, cfr, sat, tet32, tet40, tetA, tetO, tetW, tetX (total 16) | ermB, ermX, sulII, floR, tet44, tetB, tetM, tetQ (total 8) | aci, oxa, ermF (total 3) |
| High dose BRD | aph2, aph3, ant6, ant9, ermG, ermQ, mefE, cfr, sat, tet40, tetA, tetM, tetO, tetQ, tetW, tetX (total 16) | cfx | aci, cmX, ermB, ermF, ermX, tet44, tetB, tetQ (total 8) |
| ARGs | Fold Change | Control | Low Dose Healthy | High Dose Healthy |
|---|---|---|---|---|
| tetW | Mean | −0.21 | 0.58 | 0.34 |
| SD | 0.017 | 0.065 | 0.015 | |
| p-value * | NA | 0.022 a | 0.359 | |
| tetO | Mean | −0.33 | 0.07 | −0.36 |
| SD | 0.040 | 0.209 | 0.081 | |
| p-value | NA | 0.147 | 0.655 | |
| tetX | Mean | −1.30 | −2.28 | 0.23 |
| SD | 0.100 | 0.095 | 0.084 | |
| p-value | NA | 0.180 | 0.359 | |
| ermB | Mean | 0.20 | −0.43 | −1.01 |
| SD | 0.026 | 0.162 | 0.018 | |
| p-value | NA | 0.359 | 0.022 b | |
| ermF | Mean | −1.06 | −1.80 | 0.28 |
| SD | 0.234 | 0.055 | 0.106 | |
| NA | 0.180 | 0.359 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beyi, A.F.; Brito-Goulart, D.; Hawbecker, T.; Ruddell, B.; Hassall, A.; Dewell, R.; Dewell, G.; Sahin, O.; Zhang, Q.; Plummer, P.J. Enrofloxacin Alters Fecal Microbiota and Resistome Irrespective of Its Dose in Calves. Microorganisms 2021, 9, 2162. https://doi.org/10.3390/microorganisms9102162
Beyi AF, Brito-Goulart D, Hawbecker T, Ruddell B, Hassall A, Dewell R, Dewell G, Sahin O, Zhang Q, Plummer PJ. Enrofloxacin Alters Fecal Microbiota and Resistome Irrespective of Its Dose in Calves. Microorganisms. 2021; 9(10):2162. https://doi.org/10.3390/microorganisms9102162
Chicago/Turabian StyleBeyi, Ashenafi Feyisa, Debora Brito-Goulart, Tyler Hawbecker, Brandon Ruddell, Alan Hassall, Renee Dewell, Grant Dewell, Orhan Sahin, Qijing Zhang, and Paul J. Plummer. 2021. "Enrofloxacin Alters Fecal Microbiota and Resistome Irrespective of Its Dose in Calves" Microorganisms 9, no. 10: 2162. https://doi.org/10.3390/microorganisms9102162
APA StyleBeyi, A. F., Brito-Goulart, D., Hawbecker, T., Ruddell, B., Hassall, A., Dewell, R., Dewell, G., Sahin, O., Zhang, Q., & Plummer, P. J. (2021). Enrofloxacin Alters Fecal Microbiota and Resistome Irrespective of Its Dose in Calves. Microorganisms, 9(10), 2162. https://doi.org/10.3390/microorganisms9102162






