The Effect of an Adsorbent Matrix on Recovery of Microorganisms from Hydrocarbon-Contaminated Groundwater
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey of Adsorbent Materials
2.2. Experimental Microcosms
2.3. Analytical Procedures
2.4. Microbial Community Analysis by 16S rRNA Gene Sequencing
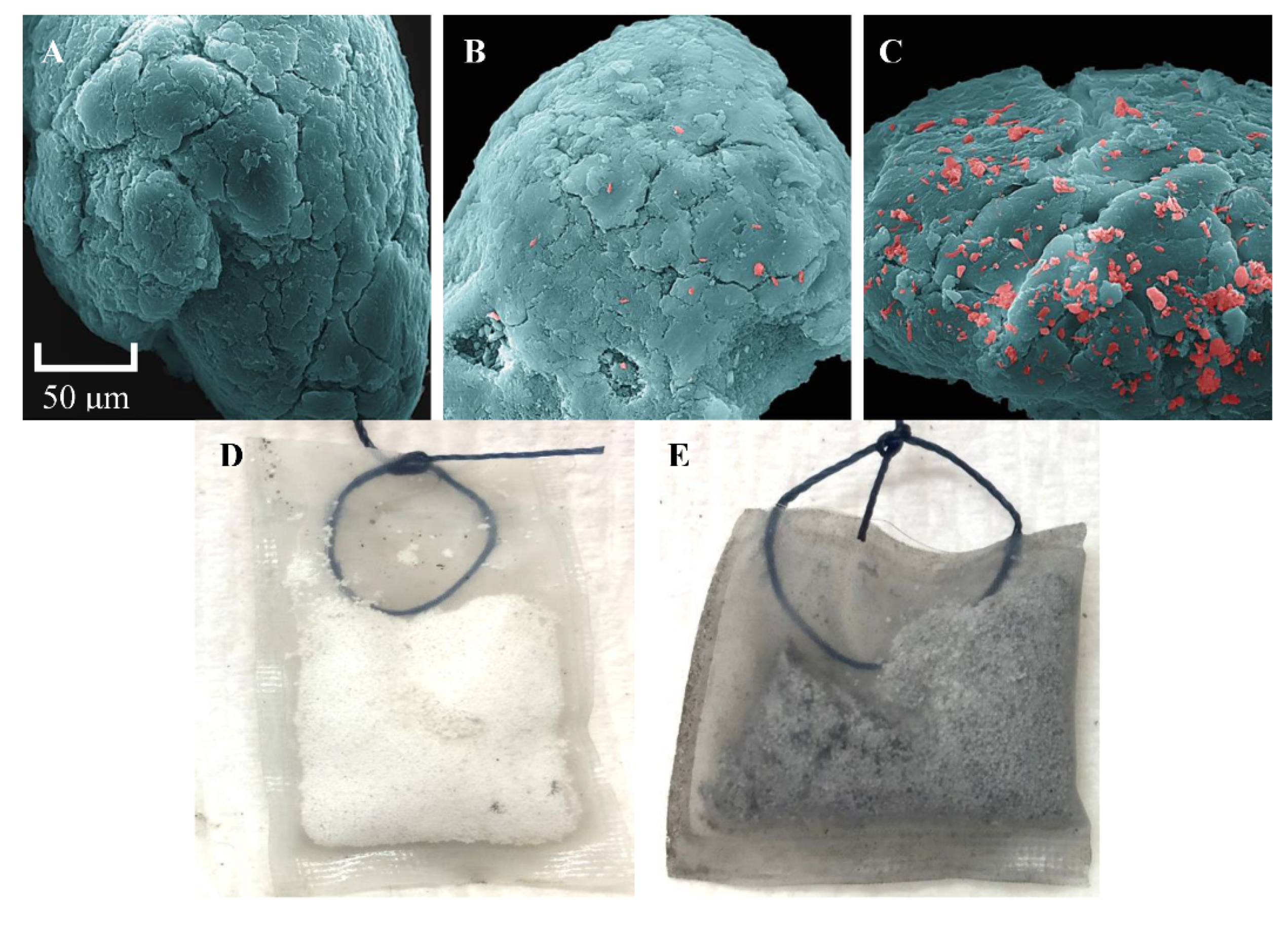
2.5. Scanning Electron Microscopy
3. Results
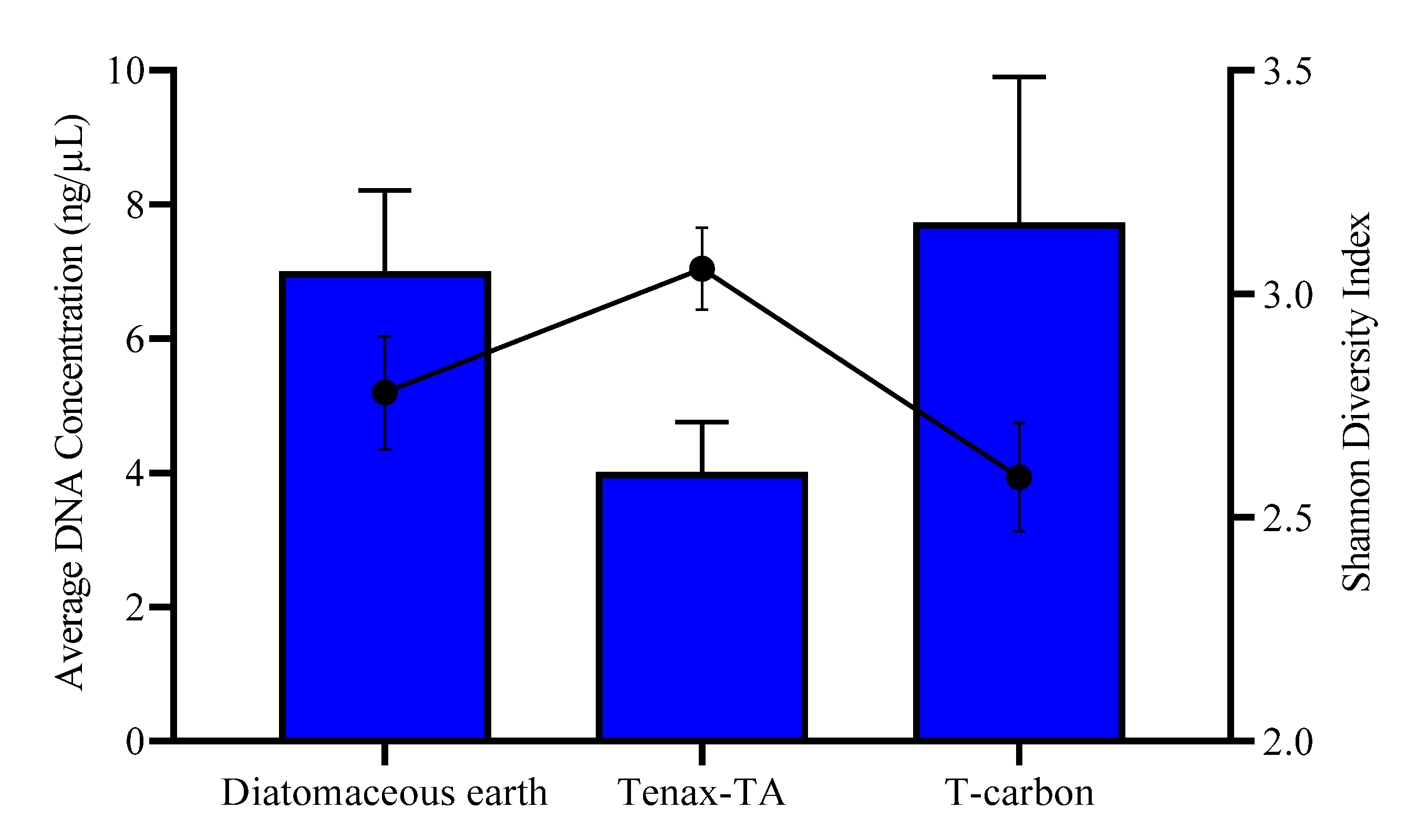
3.1. Field Testing of Microbial Trapping Matrices
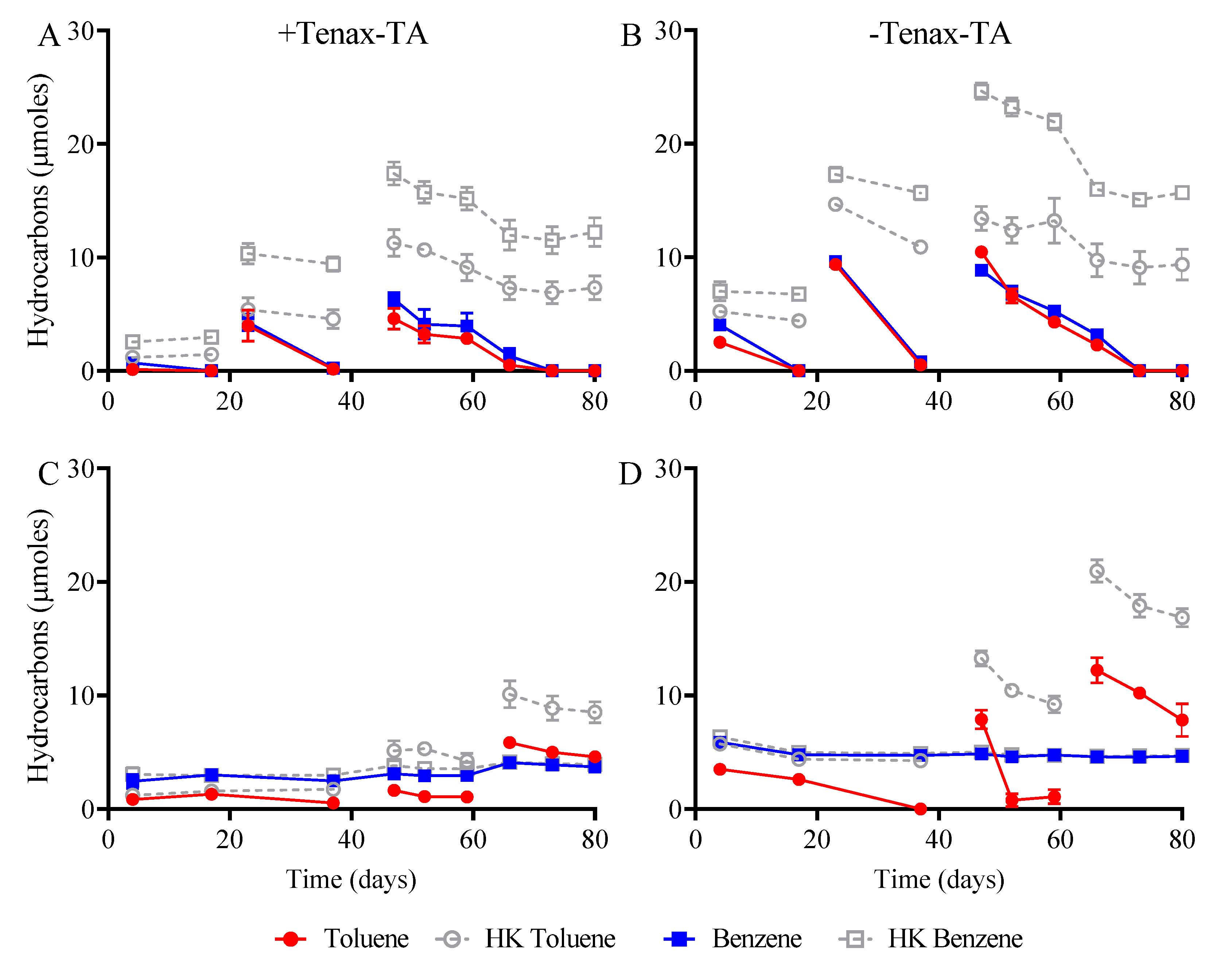
3.2. Hydrocarbon Biodegradation and Mineralization of Electron Acceptors
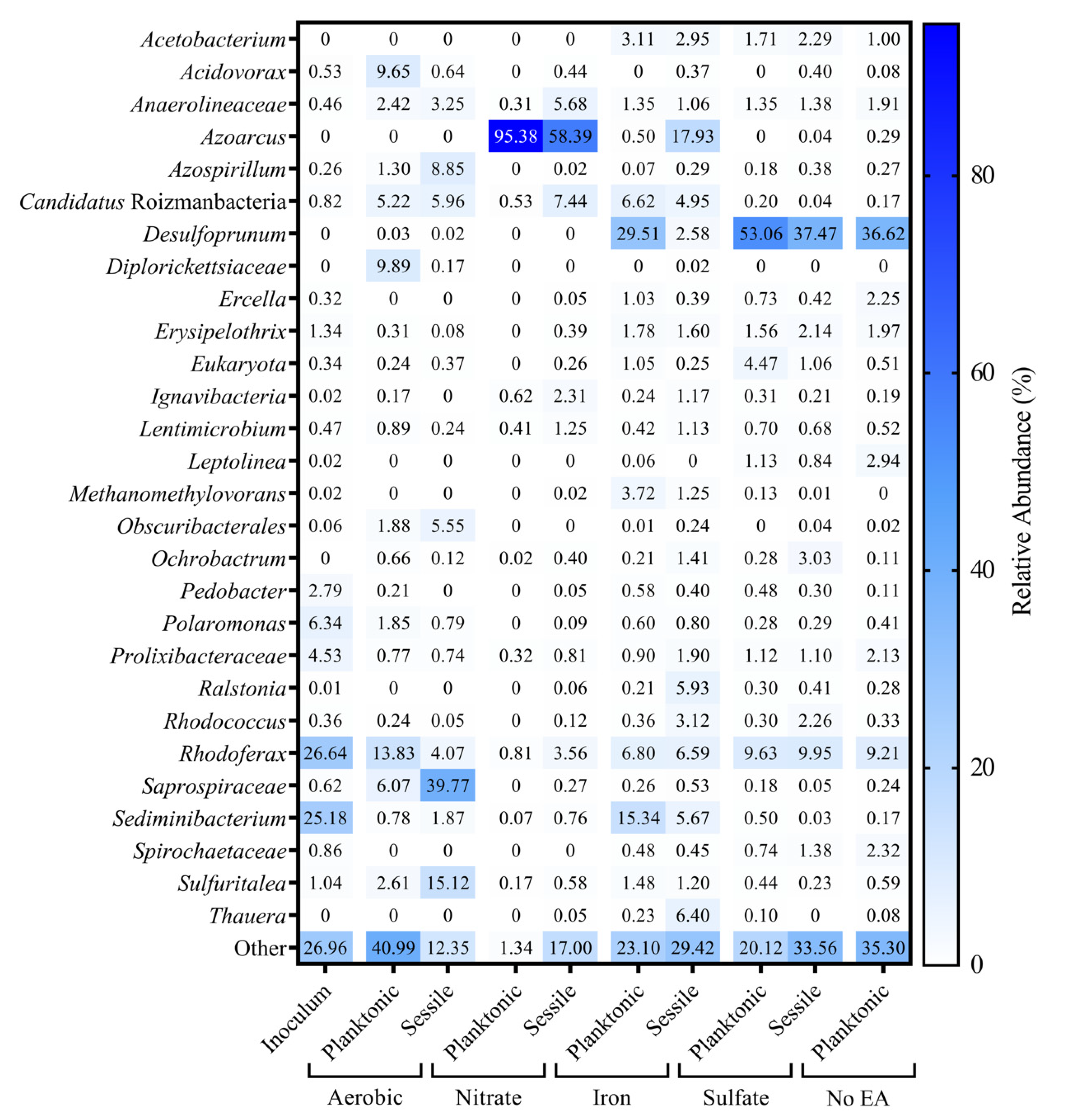
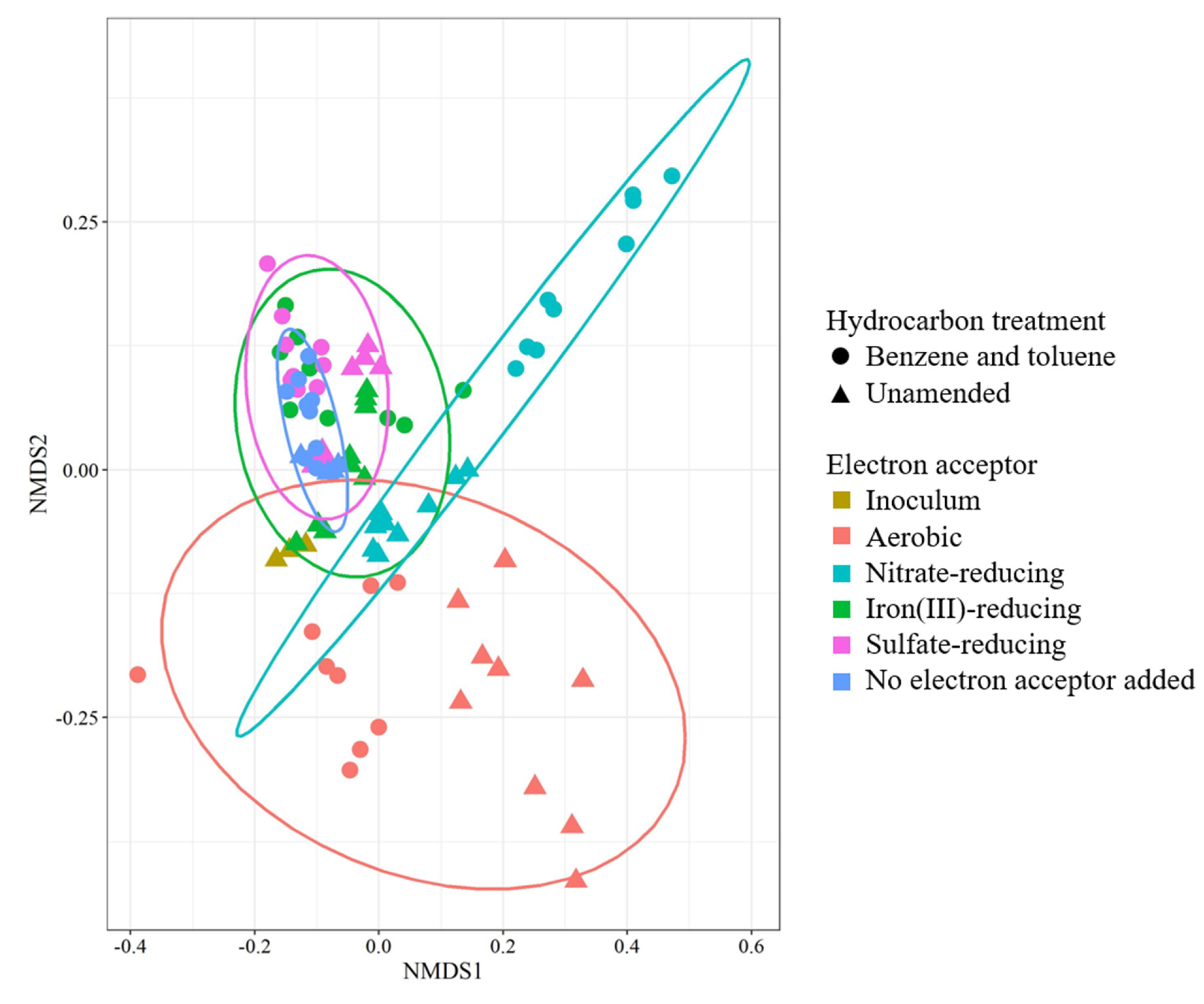
3.3. Microbial Community Analysis
3.4. Visualization of Tenax-TA
4.1. Matrix Effects
4.2. Biodegradation of Hydrocarbons
4.3. Mineralization Analysis
4.4. Microbial Community Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Widdel, F.; Musat, F. Diversity and common principles in enzymatic activation of hydrocarbons. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 981–1009. [Google Scholar]
- Jindrová, E.; Chocová, M.; Demnerová, K.; Brenner, V. Bacterial aerobic degradation of benzene, toluene, ethylbenzene and xylene. Folia Microbiol. 2002, 47, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Fahy, A.; McGenity, T.J.; Timmis, K.N.; Ball, A.S. Heterogeneous aerobic benzene-degrading communities in oxygen-depleted groundwaters. FEMS Microbiol. Ecol. 2006, 58, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Foght, J. Anaerobic biodegradation of aromatic hydrocarbons: Pathways and prospects. J. Mol. Microbiol. Biotechnol. 2008, 15, 93–120. [Google Scholar] [CrossRef] [PubMed]
- Meckenstock, R.U.; Boll, M.; Mouttaki, H.; Koelschbach, J.S.; Tarouco, P.C.; Weyrauch, P.; Dong, X.; Himmelberg, A.M. Anaerobic degradation of benzene and polycyclic aromatic hydrocarbons. J. Mol. Microbiol. Biotechnol. 2016, 26, 92–118. [Google Scholar] [CrossRef] [PubMed]
- Toth, C.R.; Berdugo-Clavijo, C.; O’Farrell, C.M.; Jones, G.M.; Sheremet, A.; Dunfield, P.F.; Gieg, L.M. Stable isotope and metagenomic profiling of a methanogenic naphthalene-degrading enrichment culture. Microorganisms 2018, 6, 65. [Google Scholar] [CrossRef]
- Lovely, D.R.; Lonergan, D.J. Anaerobic oxidation of toluene, phenol, and p-cresol by the dissimilatory iron-reducing organism, GS-15. Appl. Environ. Microbiol. 1990, 56, 1858–1864. [Google Scholar] [CrossRef]
- Beller, H.R.; Grbić-Galić, D.; Reinhard, M. Microbial degradation of toluene under sulfate-reducing conditions and the influence of iron on the process. Appl. Environ. Microbiol. 1992, 58, 786–793. [Google Scholar] [CrossRef]
- Biegert, T.; Fuchs, G.; Heider, J. Evidence that anaerobic oxidation of toluene in the denitrifying bacterium Thauera aromatica is initiated by formation of benzylsuccinate from toluene and fumarate. Eur. J. Biochem. 1996, 238, 661–668. [Google Scholar] [CrossRef]
- Fowler, S.J.; Dong, X.; Sensen, C.W.; Suflita, J.M.; Gieg, L.M. Methanogenic toluene metabolism: Community structure and intermediates. Environ. Microbiol. 2012, 14, 754–764. [Google Scholar] [CrossRef]
- Fowler, S.J.; Gutierrez-Zamora, M.-L.; Manefield, M.; Gieg, L.M. Identification of toluene degraders in a methanogenic enrichment culture. FEMS Microbiol. Ecol. 2014, 89, 625–636. [Google Scholar] [CrossRef]
- Lovely, D.R.; Coates, J.D.; Woodward, J.C.; Phillips, E.J.P. Benzene oxidation coupled to sulfate reduction. Appl. Environ. Microbiol. 1995, 61, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Burland, S.M.; Edwards, E.A. Anaerobic benzene biodegradation linked to nitrate reduction. Appl. Environ. Microbiol. 1999, 65, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, A.C.; Edwards, E.A. Physiological and molecular characterization of anaerobic benzene-degrading mixed cultures. Environ. Microbiol. 2003, 5, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Abu Laban, N.; Selesi, D.; Rattei, T.; Tischler, P.; Meckenstock, R.U. Identification of enzymes involved in anaerobic benzene degradation by a strictly anaerobic iron-reducing enrichment culture. Environ. Microbiol. 2010, 12, 2783–2796. [Google Scholar] [CrossRef]
- Van der Zaan, B.M.; Saia, F.T.; Stams, A.J.; Plugge, C.M.; de Vos, W.M.; Smidt, H.; Langenhoff, A.A.; Gerritse, J. Anaerobic benzene degradation under denitrifying conditions: Peptococcaceae as dominant benzene degraders and evidence for a syntrophic process. Environ. Microbiol. 2012, 14, 1171–1181. [Google Scholar] [CrossRef]
- Luo, F.; Devine, C.E.; Edwards, E.A. Cultivating microbial dark matter in benzene-degrading methanogenic consortia: Microbes involved in anaerobic benzene activation. Environ. Microbiol. 2016, 18, 2923–2936. [Google Scholar] [CrossRef]
- Keller, A.H.; Kleinsteuber, S.; Vogt, C. Anaerobic benzene mineralization by nitrate-reducing and sulfate-reducing microbial consortia enriched from the same site: Comparison of community composition and degradation characteristics. Microb. Ecol. 2018, 75, 941–953. [Google Scholar] [CrossRef]
- McMahon, S.; Parnell, J. Weighing the deep continental biosphere. FEMS Microbiol. Ecol. 2014, 87, 113–120. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef]
- Morris, B.E.L.; Henneberger, R.; Huber, H.; Moissl-Eichinger, C. Microbial syntrophy: Interaction for the common good. FEMS Microbiol. Rev. 2013, 37, 384–406. [Google Scholar] [CrossRef]
- Gieg, L.M.; Fowler, S.J.; Berdugo-Clavijo, C. Syntrophic biodegradation of hydrocarbon contaminants. Curr. Opin. Biotechnol. 2014, 27, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, M.; Hopkins, G.D.; Steinle-Darling, E.; LeBron, C.A. In situ biotransformation of BTEX compounds under methanogenic conditions. Ground Water Monit Remediat 2005, 25, 50–59. [Google Scholar] [CrossRef]
- Sublette, K.; Peacock, A.; White, D.; Davis, G.; Ogles, D.; Cook, D.; Kolhatkar, R.; Beckmann, D.; Yang, X. Monitoring subsurface microbial ecology in a sulfate-amended, gasoline-contaminated aquifer. Ground Water Monit. Remediat. 2006, 26, 70–78. [Google Scholar] [CrossRef]
- Ahad, J.M.; Pakdel, H.; Gammon, P.R.; Siddique, T.; Kuznetsova, A.; Savard, M.M. Evaluating in situ biodegradation of 13C-labelled naphthenic acids in groundwater near oil sands tailings ponds. Sci. Total Environ. 2018, 643, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Bombach, P.; Richnow, H.H.; Kästner, M.; Fischer, A. Current approaches for the assessment of in situ biodegradation. Appl. Microbiol. Biotechnol. 2010, 86, 839–852. [Google Scholar] [CrossRef]
- Williams, N.; Hyland, A.; Mitchener, R.; Sublette, K.; Key, K.C.; Davis, G.; Ogles, D.; Baldwin, B.; Biernacki, A. Demonstrating the in situ biodegradation potential of phenol using Bio-Sep® Bio-Traps® and stable isotope probing. Remediat. J. 2013, 23, 7–22. [Google Scholar] [CrossRef]
- Peacock, A.D.; Chang, Y.J.; Istok, J.D.; Krumholz, L.; Geyer, R.; Kinsall, B.; Watson, D.; Sublette, K.L.; White, D.C. Utilization of microbial biofilms as monitors of bioremediation. Microb. Ecol. 2004, 47, 284–292. [Google Scholar] [CrossRef]
- Biggerstaff, J.P.; Le Puil, M.; Weidow, B.L.; Leblanc-Gridley, J.; Jennings, E.; Busch-Harris, J.; Sublette, K.L.; White, D.C.; Alberte, R.S. A novel and in situ technique for the quantitative detection of MTBE and benzene degrading bacteria in contaminated matrices. J. Microbiol. Methods 2007, 68, 437–441. [Google Scholar] [CrossRef]
- Busch-Harris, J.; Sublette, K.; Roberts, K.P.; Landrum, C.; Peacock, A.D.; Davis, G.; Ogles, D.; Holmes, W.E.; Harris, D.; Ota, C.; et al. Bio-Traps coupled with molecular biological methods and stable isotope probing demonstrate the in situ biodegradation potential of MTBE and TBA in gasoline-contaminated aquifers. Ground Water Monit. Remediat. 2008, 28, 47–62. [Google Scholar] [CrossRef]
- Dettmer, K.; Engewald, W. Adsorbent materials commonly used in air analysis for adsorptive enrichment and thermal desorption of volatile organic compounds. Anal. Bioanal. Chem. 2002, 373, 490–500. [Google Scholar] [CrossRef]
- Zhao, D.; Pignatello, J.J. Model-aided characterization of Tenax®-TA for aromatic compound uptake from water. Environ. Toxicol. Chem. 2004, 23, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Kharey, G.; Scheffer, G.; Gieg, L.M. Combined use of diagnostic fumarate addition metabolites and genes provides evidence for anaerobic hydrocarbon biodegradation in contaminated groundwater. Microorganisms 2020, 8, 1532. [Google Scholar] [CrossRef] [PubMed]
- Alberta Environment and Parks. Alberta Tier 1 Soil and Groundwater Remediation Guidelines: Land Policy 2019 No. 1; Government of Alberta: Edmonton, Alberta, 2019; pp. 50–59. ISBN 978-1-4601-2695-0. [Google Scholar]
- Eastcott, L.; Shiu, W.Y.; Mackay, D. Environmentally relevant physical-chemical properties of hydrocarbons: A review of data and development of simple correlations. Oil Chem. Pollut. 1988, 4, 191–216. [Google Scholar] [CrossRef]
- Heath, J.S.; Koblis, K.; Sager, S.L. Review of chemical, physical, and toxicologic properties of components of total petroleum hydrocarbons. J. Soil Contam. 1993, 2, 1–25. [Google Scholar] [CrossRef]
- Toth, C.R.A. Characterizing and Accelerating Methanogenic Hydrocarbon Biodegradation. PhD Thesis, University of Calgary, Calgary, AB, Canada, 2017. [Google Scholar]
- Berdugo-Clavijo, C.; Sen, A.; Seyyedi, M.; Quintero, H.; O’Neil, B.; Gieg, L.M. High temperature utilization of PAM and HPAM by microbial communities enriched from oilfield produced water and activated sludge. AMB Express 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Voordouw, G.; Menon, P.; Pinnock, T.; Sharma, M.; Shen, Y.; Venturelli, A.; Voordouw, J.; Sexton, A. Use of homogeneously-sized carbon steel ball bearings to study microbially-influenced corrosion in oil field samples. Front. Microbiol. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Lovely, D.R.; Phillips, E.J.P. Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl. Environ. Microbiol. 1987, 53, 1536–1540. [Google Scholar] [CrossRef]
- Toth, C.; Gieg, L.M. Time course-dependent methanogenic crude oil biodegradation: Dynamics of fumarate addition metabolites, biodegradative genes, and microbial community composition. Front. Microbiol. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, Z.; Beyenal, H. Chapter nine: Protocols and procedures. In Fundamentals of Biofilm Research, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017; p. 612. [Google Scholar]
- Baimatova, N.; Derbissalin, M.; Kabulov, A.; Kenessov, B. Adsorption of benzene, toluene, ethylbenzene and o-xylene by carbon-based adsorbents. Eurasian Chem. Technol. J. 2016, 18, 123–131. [Google Scholar] [CrossRef]
- Weelink, S.A.B.; van Eekert, M.H.A.; Stams, A.J.M. Degradation of BTEX by anaerobic bacteria: Physiology and application. Rev. Environ. Biotechnol. 2010, 9, 359–385. [Google Scholar] [CrossRef]
- Su, J.-J.; Kafkewitz, D. Utilization of toluene and xylenes by a nitrate-reducing strain of Pseudomonas maltophilia under low oxygen and anoxic conditions. FEMS Microbiol. Ecol. 1994, 15, 249–258. [Google Scholar]
- Symons, G.E.; Buswell, A.M. The methane fermentation of carbohydrates. J. Am. Chem. Soc. 1933, 55, 2028–2036. [Google Scholar] [CrossRef]
- Headley, J.V.; Goudey, S.; Birkholz, D.; Linton, L.R.; Dickson, L.C. Toxicity screening of benzene, toluene, ethylbenzene and xylene (BTEX) hydrocarbons in groundwater at sour-gas plants. Can. Water Resour. J. 2001, 26, 345–358. [Google Scholar] [CrossRef]
- Kuntasal, Ö.O.; Karman, D.; Wang, D.; Tuncel, S.G.; Tuncel, G. Determination of volatile organic compounds in different microenvironments by multibed adsorption and short-path thermal desorption followed by gas chromatographic–mass spectrometric analysis. J. Chromatogr. A 2005, 1099, 43–54. [Google Scholar] [CrossRef]
- Bansode, R.R.; Losso, J.N.; Marshall, W.E.; Rao, R.M.; Portier, R.J. Adsorption of volatile organic compounds by pecan shell- and almond shell-based granular activated carbons. Bioresour. Technol. 2003, 90, 175–184. [Google Scholar] [CrossRef]
- Lim, S.T.; Kim, J.H.; Lee, C.Y.; Koo, S.; Jerng, D.W.; Wongwises, S.; Ahn, H.S. Mesoporous graphene adsorbents for the removal of toluene and xylene at various concentrations and its reusability. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Sheshdeh, R.K.; Khosravi Nikou, M.R.; Badii, K.; Mohammadzadeh, S. Evaluation of adsorption kinetics and equilibrium for the removal of benzene by modified diatomite. Chem. Eng. Technol. 2013, 36, 1713–1720. [Google Scholar] [CrossRef]
- Sheshdeh, R.K.; Abbasizadeh, S.; Nikou, M.R.K.; Badii, K.; Sharafi, M.S. Liquid phase adsorption kinetics and equilibrium of toluene by novel modified-diatomite. J. Environ. Health Sci. Eng. 2014, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Aivalioti, M.; Vamvasakis, I.; Gidarakos, E. BTEX and MTBE adsorption onto raw and thermally modified diatomite. J. Hazard. Mater. 2010, 178, 136–143. [Google Scholar] [CrossRef]
- Demeyer, A.; Nkana, J.C.V.; Verloo, M.G. Characteristics of wood ash and influence on soil properties and nutrient uptake: An overview. Bioresour. Technol. 2001, 77, 287–295. [Google Scholar] [CrossRef]
- Manariotis, I.D.; Fotopoulou, K.N.; Karapanagioti, H.K. Preparation and characterization of biochar sorbents produced from malt spent rootlets. Ind. Eng. Chem. Res. 2015, 54, 9577–9584. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Lai, C.-W.; Hsien, K.-J. Characterization and adsorption properties of diatomaceous earth modified by hydrofluoric acid etching. J. Colloid Interface Sci. 2006, 297, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Benkacem, T.; Hamdi, B.; Chamayou, A.; Balard, H.; Calvet, R. Physicochemical characterization of a diatomaceous upon an acid treatment: A focus on surface properties by inverse gas chromatography. Powder Technol. 2016, 294, 498–507. [Google Scholar] [CrossRef]
- Wang, J.Y.; De Belie, N.; Verstraete, W. Diatomaceous earth as a protective vehicle for bacteria applied for self-healing concrete. J. Ind. Microbiol. Biotechnol. 2012, 39, 567–577. [Google Scholar] [CrossRef]
- Wilson, B.H.; Smith, G.B.; Rees, J.F. Biotransformations of selected alkylbenzenes and halogenated aliphatic hydrocarbons in methanogenic aquifer material: A microcosm study. Environ. Sci. Technol. 1986, 20, 997–1002. [Google Scholar] [CrossRef]
- Edwards, E.A.; Grbić-Galić, D. Complete mineralization of benzene by aquifer microorganisms under strictly anaerobic conditions. Appl. Environ. Microbiol. 1992, 58, 2663–2666. [Google Scholar] [CrossRef]
- Nales, M.; Butler, B.J.; Edwards, E.A. Anaerobic benzene biodegradation: A microcosm survey. Bioremediat. J. 1998, 2, 125–144. [Google Scholar] [CrossRef]
- Korpi, A.; Pasanen, A.-L.; Pasanen, P. Volatile compounds originating from mixed microbial cultures on building materials under various humidity conditions. Appl. Environ. Microbiol. 1998, 64, 2914–2919. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Nguyen, N.-L.; Weon, H.-Y.; Yang, D.-C. Sediminibacterium ginsengisoli sp. nov., isolated from soil of a ginseng field, and emended descriptions of the genus Sediminibacterium and of Sediminibacterium salmoneum. Int. J. Syst. Evol. Microbiol. 2013, 63, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Berdugo-Clavijo, C.; Gieg, L.M. Conversion of crude oil to methane by a microbial consortium enriched from oil reservoir production waters. Front. Microbiol. 2014, 5, 1–10. [Google Scholar] [CrossRef]
- Jeon, C.O.; Park, W.; Padmanabhan, P.; DeRito, C.; Snape, J.R.; Madsen, E.L. Discovery of a bacterium, with distinctive dioxygenase, that is responsible for in situ biodegradation in contaminated sediment. Proc. Natl. Acad. Sci. USA 2003, 100, 13591–13596. [Google Scholar] [CrossRef] [PubMed]
- Mattes, T.E.; Alexander, A.K.; Richardson, P.M.; Munk, A.C.; Han, C.S.; Stothard, P.; Coleman, N.V. The genome of Polaromonas sp. strain JS666: Insights into the evolution of a hydrocarbon- and xenobiotic-degrading bacterium, and features of relevance to biotechnology. Appl. Environ. Microbiol. 2008, 74, 6405–6416. [Google Scholar] [CrossRef] [PubMed]
- Finneran, K.T.; Johnsen, C.V.; Lovely, D.R. Rhodoferax ferrireducens sp. nov., a psychrotolerant, facultatively anaerobic bacterium that oxidizes acetate with the reduction of Fe (III). Int. J. Syst. Evol. Microbiol. 2003, 53, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Aburto, A.; Peimbert, M. Degradation of a benzene–toluene mixture by hydrocarbon-adapted bacterial communities. Ann. Microbiol. 2011, 61, 553–562. [Google Scholar] [CrossRef]
- Golby, S.; Ceri, H.; Gieg, L.M.; Chatterjee, I.; Marques, L.L.; Turner, R.J. Evaluation of microbial biofilm communities from an Alberta oil sands tailings pond. FEMS Microbiol. Ecol. 2012, 79, 240–250. [Google Scholar] [CrossRef]
- McIlroy, S.J.; Nielsen, P.H. The Family Saprospiraceae. In The Prokaryotes: Other Major Lineages of Bacteria and the Archaea; Rosenberg, E., Ed.; Springer: Berlin, Germany, 2014; pp. 863–889. [Google Scholar]
- Sperfeld, M.; Diekert, G.; Studenik, S. Anaerobic aromatic compound degradation in Sulfuritalea hydrogenivorans sk43H. FEMS Microbiol. Ecol. 2018, 95, 1–9. [Google Scholar] [CrossRef]
- Beller, H.R.; Spormann, A.M. Substrate range of benzylsuccinate synthase from Azoarcus sp. strain T. FEMS Microbiol. Lett. 1999, 178, 147–153. [Google Scholar] [CrossRef]
- Achong, G.R.; Rodriguez, A.M.; Spormann, A.M. Benzylsuccinate synthase of Azoarcus sp. strain T: Cloning, sequencing, transcriptional organization, and its role in anaerobic toluene and m-xylene mineralization. J. Bacteriol. 2001, 183, 6763–6770. [Google Scholar] [CrossRef] [PubMed]
- Junghare, M.; Schink, B. Desulfoprunum benzoelyticum gen. nov., sp. nov., a Gram-stain-negative, benzoate-degrading, sulfate-reducing bacterium isolated from a wastewater treatment plant. Int. J. Syst. Evol. Microbiol. 2015, 65, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Geesink, P.; Wegner, C.E.; Probst, A.J.; Herrmann, M.; Dam, H.T.; Kaster, A.K.; Küsel, K. Genome-inferred spatio-temporal resolution of an uncultivated Roizmanbacterium reveals its ecological preferences in groundwater. Environ. Microbiol. 2020, 22, 726–737. [Google Scholar] [CrossRef] [PubMed]

| Electron Acceptor | Matrix | Amendment | Treatment |
|---|---|---|---|
| One of: 313 mM O2, 10 mM NO3−, 30 mM Fe(OH)3, 10 mM SO42−, or none added | Tenax-TA | 5.6 μmoles benzene + 4.7 μmoles toluene | Live |
| Heat killed | |||
| Unamended | Live | ||
| Heat killed | |||
| No Tenax-TA | 5.6 μmoles benzene + 4.7 μmoles toluene | Live | |
| Heat killed | |||
| Unamended | Live | ||
| Heat killed |
| Electron Acceptor (Oxidized/Reduced) | Stoichiometric Equation | Source |
|---|---|---|
| O2/CO2 | C6H6 + 7.5O2 → 6CO2 + 3H2O | [47] |
| C7H8 + 9O2 → 7CO2 + 4H2O | [48] | |
| NO3−/N2 | C6H6 + 6NO3− → 6HCO3− + 3N2 | [13] |
| C7H8 + 7.2NO3− + 7.2H+ → 7CO2 + 7.6H2O + 3.6N2 | [48] | |
| Fe3+/Fe2+ | C6H6 + 18H2O + 30Fe3+ → 6HCO3− + 30Fe2+ + 36H+ | [13] |
| C6H6 + 21H2O + 36Fe3+ → 7HCO3− + 36Fe2+ + 43H+ | [7] | |
| SO42−/H2S | C6H6 + 3.75SO42− + 3H2O → 3.75HS− + 6HCO3− + 2.25H+ | [13] |
| C7H8 + 4.5SO42− + 3H2O → 2.25H2S + 2.25HS− + 7HCO3− + 0.25H+ | [8] | |
| CO2/CH4 | C6H6 + 4.5H2O → 3.75CH4 + 2.25CO2 | [49] |
| C7H8 + 5H2O → 4.5CH4 + 2.5CO2 | [49] |
| Redox Condition | Chemical Monitored | Total Hydrocarbons Consumed (μmoles) | Degradation Rate (μmoles/Day) | Predicted Yield or Consumption (μmoles) | Actual Yield or Consumption (μmoles) | p-Value | |
|---|---|---|---|---|---|---|---|
| + Tenax-TA | − Tenax-TA | ||||||
| Aerobic | CO2 | 31.0 | 0.4 | 199.9 | 97.9 ± 0.7 | 55.0 ± 2.0 | ** |
| Nitrate reducing | NO3− | 18.8 | 0.2 | 135.4 | 91.8 ± 5.5 | 113.2 ± 4.6 | * |
| Iron(III) reducing | Fe2+ | 14.1 | 0.2 | 507.6 | 35.9 ± 1.7 | 267.0 ± 68.4 | ns |
| Sulfate reducing | SO42− | 9.4 | 0.1 | 42.3 | 32.4 ± 7.2 | 38.3 ± 0.6 | ns |
| No EA added | CH4 | 14.1 | 0.2 | 63.5 | 0.0 | 0.0 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, N.M.; Toth, C.R.A.; Collins, V.; Mussone, P.; Gieg, L.M. The Effect of an Adsorbent Matrix on Recovery of Microorganisms from Hydrocarbon-Contaminated Groundwater. Microorganisms 2021, 9, 90. https://doi.org/10.3390/microorganisms9010090
Taylor NM, Toth CRA, Collins V, Mussone P, Gieg LM. The Effect of an Adsorbent Matrix on Recovery of Microorganisms from Hydrocarbon-Contaminated Groundwater. Microorganisms. 2021; 9(1):90. https://doi.org/10.3390/microorganisms9010090
Chicago/Turabian StyleTaylor, Nicole M., Courtney R. A. Toth, Victoria Collins, Paolo Mussone, and Lisa M. Gieg. 2021. "The Effect of an Adsorbent Matrix on Recovery of Microorganisms from Hydrocarbon-Contaminated Groundwater" Microorganisms 9, no. 1: 90. https://doi.org/10.3390/microorganisms9010090
APA StyleTaylor, N. M., Toth, C. R. A., Collins, V., Mussone, P., & Gieg, L. M. (2021). The Effect of an Adsorbent Matrix on Recovery of Microorganisms from Hydrocarbon-Contaminated Groundwater. Microorganisms, 9(1), 90. https://doi.org/10.3390/microorganisms9010090






