The HrpG/HrpX Regulon of Xanthomonads—An Insight to the Complexity of Regulation of Virulence Traits in Phytopathogenic Bacteria
Abstract
1. Introduction
2. Virulence and Occurrence of Secretion Systems in Xanthomonas spp.
3. HrpG and HrpX Are Key Virulence Regulators in Xanthomonas spp.
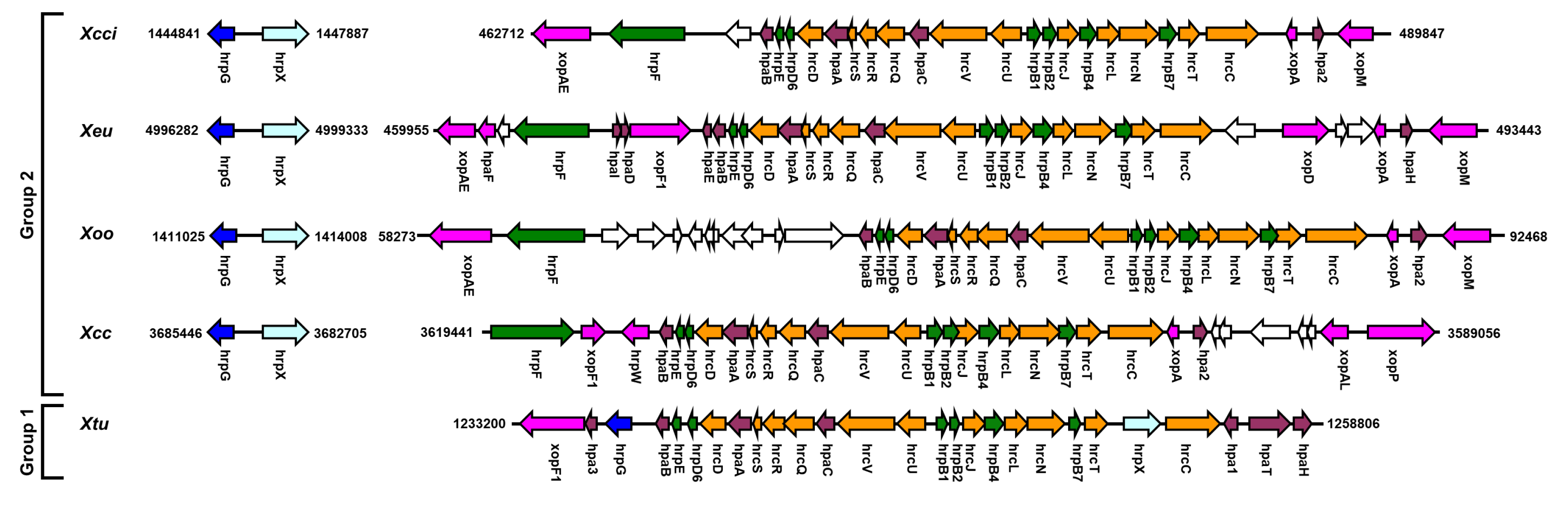
4. Genomic Organization of hrpG and hrpX
5. Downstream Targets and Regulatory Pathways Controlled by HrpG and HrpX
6. Metabolic Regulation of the HrpG/HrpX Regulon
7. Influence of Iron and Other Metals on HrpG/HrpX Regulation
8. Species-Specific Control of the HrpG/HrpX Regulon by Diffusible Signal Factor
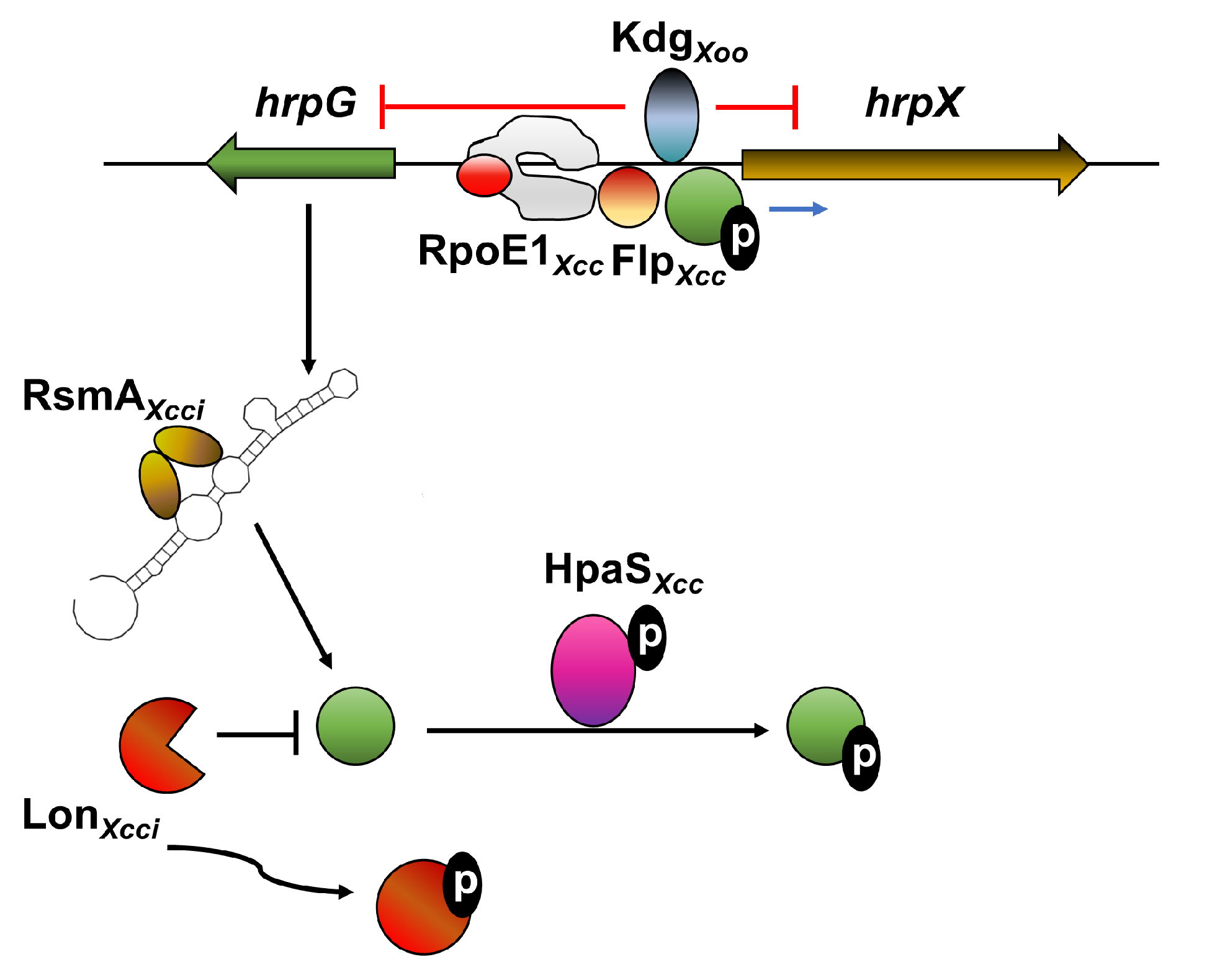
9. Transcriptional and Post-Transcriptional Regulation of hrpG and hrpX
10. Concluding Remarks
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- An, S.Q.; Potnis, N.; Dow, M.; Vorhölter, F.J.; He, Y.Q.; Becker, A.; Teper, D.; Li, Y.; Wang, N.; Bleris, L.; et al. Mechanistic insights into host adaptation, virulence and epidemiology of the phytopathogen Xanthomonas. FEMS Microbiol. Rev. 2019, 44, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Timilsina, S.; Potnis, N.; Newberry, E.A.; Liyanapathiranage, P.; Iruegas-Bocardo, F.; White, F.F.; Goss, E.M.; Jones, J.B. Xanthomonas diversity, virulence and plant-pathogen interactions. Nat. Rev. Microbiol. 2020, 18, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Büttner, D.; Bonas, U. Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol. Rev. 2010, 34, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Souza, D.P.; Oka, G.U.; Alvarez-Martinez, C.E.; Bisson-Filho, A.W.; Dunger, G.; Hobeika, L.; Cavalcante, N.S.; Alegria, M.C.; Barbosa, L.R.S.; Salinas, R.K.; et al. Bacterial killing via a type IV secretion system. Nat. Commun. 2015, 6, 6453. [Google Scholar] [CrossRef]
- Bayer-Santos, E.; Lima, L.D.P.; de Ceseti, L.M.; Ratagami, C.Y.; de Santana, E.S.; da Silva, A.M.; Farah, C.S.; Alvarez-Martinez, C.E. Xanthomonas citri T6SS mediates resistance to Dictyostelium predation and is regulated by an ECF σ factor and cognate Ser/Thr kinase. Environ. Microbiol. 2018, 20, 1562–1575. [Google Scholar] [CrossRef]
- Ray, S.K.; Rajeshwari, R.; Sonti, R.V. Mutants of Xanthomonas oryzae pv. oryzae deficient in general secretory pathway are virulence deficient and unable to secrete xylanase. Mol. Plant. Microbe Interact. 2000, 13, 394–401. [Google Scholar] [CrossRef]
- Szczesny, R.; Jordan, M.; Schramm, C.; Schulz, S.; Cogez, V.; Bonas, U.; Büttner, D. Functional characterization of the Xcs and Xps type II secretion systems from the plant pathogenic bacterium Xanthomonas campestris pv vesicatoria. New Phytol. 2010, 187, 983–1002. [Google Scholar] [CrossRef]
- Yan, Q.; Wang, N. High-throughput screening and analysis of genes of Xanthomonas citri subsp. citri involved in citrus canker symptom development. Mol. Plant. Microbe Interact. 2011, 25, 69–84. [Google Scholar] [CrossRef]
- Solé, M.; Scheibner, F.; Hoffmeister, A.-K.; Hartmann, N.; Hause, G.; Rother, A.; Jordan, M.; Lautier, M.; Arlat, M.; Büttner, D. Xanthomonas campestris pv. vesicatoria Secretes Proteases and Xylanases via the Xps Type II Secretion System and Outer Membrane Vesicles. J. Bacteriol. 2015, 197, 2879–2893. [Google Scholar] [CrossRef]
- Rajeshwari, R.; Jha, G.; Sonti, R.V. Role of an In Planta-Expressed Xylanase of Xanthomonas oryzae pv. oryzae in Promoting Virulence on Rice. Mol. Plant. Microbe Interact. 2005, 18, 830–837. [Google Scholar] [CrossRef]
- Tamir-Ariel, D.; Rosenberg, T.; Navon, N.; Burdman, S. A secreted lipolytic enzyme from Xanthomonas campestris pv. vesicatoria is expressed in planta and contributes to its virulence. Mol. Plant. Pathol. 2012, 13, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Rong, W.; He, C. Two Xanthomonas Extracellular Polygalacturonases, PghAxc and PghBxc, Are Regulated by Type III Secretion Regulators HrpX and HrpG and Are Required for Virulence. Mol. Plant. Microbe Interact. 2008, 21, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Jha, G.; Rajeshwari, R.; Sonti, R.V. Functional interplay between two Xanthomonas oryzae pv,. oryzae secretion systems in modulating virulence on rice. Mol. Plant. Microbe Interact. 2007, 20, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Tayi, L.; Maku, R.; Patel, H.K.; Sonti, R.V. Action of Multiple Cell Wall–Degrading Enzymes Is Required for Elicitation of Innate Immune Responses During Xanthomonas oryzae pv. oryzae Infection in Rice. Mol. Plant. Microbe Interact. 2016, 29, 599–608. [Google Scholar] [CrossRef]
- Shen, Y.; Chern, M.; Silva, F.G.; Ronald, P. Isolation of a Xanthomonas oryzae pv. oryzae Flagellar Operon Region and Molecular Characterization of flhF. Mol. Plant. Microbe Interact. 2001, 14, 204–213. [Google Scholar] [CrossRef]
- Malamud, F.; Torres, P.S.; Roeschlin, R.; Rigano, L.A.; Enrique, R.; Bonomi, H.R.; Castagnaro, A.P.; Marano, M.R.; Vojnov, A.A. The Xanthomonas axonopodis pv. citri flagellum is required for mature biofilm and canker development. Microbiology 2011, 157, 819–829. [Google Scholar] [CrossRef]
- Verma, R.K.; Samal, B.; Chatterjee, S. Xanthomonas oryzae pv. oryzae chemotaxis components and chemoreceptor Mcp2 are involved in the sensing of constituents of xylem sap and contribute to the regulation of virulence-associated functions and entry into rice. Mol. Plant. Pathol. 2018, 19, 2397–2415. [Google Scholar] [CrossRef]
- Dey, S.; Chakravarty, A.; Biswas, P.G.; De Guzman, R.N. The type III secretion system needle, tip, and translocon. Protein Sci. 2019, 28, 1582–1593. [Google Scholar] [CrossRef]
- Tampakaki, A.P.; Skandalis, N.; Gazi, A.D.; Bastaki, M.N.; Panagiotis, F.S.; Charova, S.N.; Kokkinidis, M.; Panopoulos, N.J. Playing the “Harp”: Evolution of Our Understanding of hrp/hrc Genes. Annu. Rev. Phytopathol. 2010, 48, 347–370. [Google Scholar] [CrossRef]
- Abby, S.S.; Rocha, E.P.C. The Non-Flagellar Type III Secretion System Evolved from the Bacterial Flagellum and Diversified into Host-Cell Adapted Systems. PLoS Genet. 2012, 8, e1002983. [Google Scholar] [CrossRef]
- White, F.F.; Potnis, N.; Jones, J.B.; Koebnik, R. The type III effectors of Xanthomonas. Mol. Plant. Pathol. 2009, 10, 749–766. [Google Scholar] [CrossRef] [PubMed]
- Koebnik, R.; Krüger, A.; Thieme, F.; Urban, A.; Bonas, U. Specific binding of the Xanthomonas campestris pv. vesicatoria AraC-type transcriptional activator HrpX to plant-inducible promoter boxes. J. Bacteriol. 2006, 188, 7652–7660. [Google Scholar] [CrossRef] [PubMed]
- Triplett, L.R.; Verdier, V.; Campillo, T.; Van Malderghem, C.; Cleenwerck, I.; Maes, M.; Deblais, L.; Corral, R.; Koita, O.; Cottyn, B.; et al. Characterization of a novel clade of Xanthomonas isolated from rice leaves in Mali and proposal of Xanthomonas maliensis sp. nov. Antonie Leeuwenhoek 2015, 107, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.M.; Pesce, C.; Lefeuvre, P.; Koebnik, R. Comparative genomics of a cannabis pathogen reveals insight into the evolution of pathogenicity in Xanthomonas. Front. Plant. Sci. 2015, 6, 431. [Google Scholar] [CrossRef] [PubMed]
- Garita-Cambronero, J.; Palacio-Bielsa, A.; López, M.M.; Cubero, J. Comparative Genomic and Phenotypic Characterization of Pathogenic and Non-Pathogenic Strains of Xanthomonas arboricola Reveals Insights into the Infection Process of Bacterial Spot Disease of Stone Fruits. PLoS ONE 2016, 11, e0161977. [Google Scholar] [CrossRef]
- Merda, D.; Briand, M.; Bosis, E.; Rousseau, C.; Portier, P.; Barret, M.; Jacques, M.; Saux, M.F.-L. Ancestral acquisitions, gene flow and multiple evolutionary trajectories of the type three secretion system and effectors in Xanthomonas plant pathogens. Mol. Ecol. 2017, 26, 5939–5952. [Google Scholar] [CrossRef]
- Pesce, C.; Jacobs, J.M.; Berthelot, E.; Perret, M.; Vancheva, T.; Bragard, C.; Koebnik, R. Comparative Genomics Identifies a Novel Conserved Protein, HpaT, in Proteobacterial Type III Secretion Systems that Do Not Possess the Putative Translocon Protein HrpF. Front. Microbiol. 2017, 8, 1177. [Google Scholar] [CrossRef]
- Kamoun, S.; Kado, C.I. A plant-inducible gene of Xanthomonas campestris pv. campestris encodes an exocellular component required for growth in the host and hypersensitivity on nonhosts. J. Bacteriol. 1990, 172, 5165–5172. [Google Scholar] [CrossRef]
- Kamdar, H.V.; Kamoun, S.; Kado, C.I. Restoration of pathogenicity of avirulent Xanthomonas oryzae pv. oryzae and X. campestris pathovars by reciprocal complementation with the hrpXo and hrpXc genes and identification of HrpX function by sequence analyses. J. Bacteriol. 1993, 175, 2017–2025. [Google Scholar] [CrossRef][Green Version]
- Wengelnik, K.; Bonas, U. HrpXv, an AraC-type regulator, activates expression of five of the six loci in the hrp cluster of Xanthomonas campestris pv. vesicatoria. J. Bacteriol. 1996, 178, 3462–3469. [Google Scholar] [CrossRef]
- Martin, R.G.; Rosner, J.L. The AraC transcriptional activators. Curr. Opin. Microbiol. 2001, 4, 132–137. [Google Scholar] [CrossRef]
- Escolar, L.; Van Den Ackerveken, G.; Pieplow, S.; Rossier, O.; Bonas, U. Type III secretion and in planta recognition of the Xanthomonas avirulence proteins AvrBs1 and AvrBsT. Mol. Plant. Pathol. 2001, 2, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Furutani, A.; Takaoka, M.; Sanada, H.; Noguchi, Y.; Oku, T.; Tsuno, K.; Ochiai, H.; Tsuge, S. Identification of Novel Type III Secretion Effectors in Xanthomonas oryzae pv. oryzae. Mol. Plant. Microbe Interact. 2009, 22, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Jiang, B.-L.; Xu, R.-Q.; Huang, J.-D.; Wei, H.-Y.; Jiang, G.-F.; Chen, W.-J.; Liu, J.; Ge, Y.-Y.; Li, G.-H.; et al. Identification of six type III effector genes with the PIP box in Xanthomonas campestris pv. campestris and five of them contribute individually to full pathogenicity. Mol. Plant. Microbe Interact. 2009, 22, 1401–1411. [Google Scholar] [CrossRef] [PubMed]
- Wengelnik, K.; Van den Ackerveken, G.; Bonas, U. HrpG, a key hrp regulatory protein of Xanthomonas campestris pv. vesicatoria is homologous to two-component response regulators. Mol. Plant. Microbe Interact. 1996, 9, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Noel, L.; Thieme, F.; Nennstiel, D.; Bonas, U. cDNA-AFLP analysis unravels a genome-wide hrpG-regulon in the plant pathogen Xanthomonas campestris pv. vesicatoria. Mol. Microbiol. 2001, 41, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Figueiredo, F.; Jones, J.; Wang, N. HrpG and HrpX play global roles in coordinating different virulence traits of Xanthomonas axonopodis pv. citri. Mol. Plant. Microbe Interact. 2011, 24, 649–661. [Google Scholar] [CrossRef]
- Ficarra, F.A.; Garofalo, C.G.; Gottig, N.; Ottado, J. The Amino Acid Arginine 210 of the Response Regulator HrpG of Xanthomonas citri subsp. citri Is Required for HrpG Function in Virulence. PLoS ONE 2015, 10, e0125516. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, J.; Qian, W.; Deng, C. Analysis of HrpG regulons and HrpG-interacting proteins by ChIP-seq and affinity proteomics in Xanthomonas campestris. Mol. Plant. Pathol. 2020, 21, 388–400. [Google Scholar] [CrossRef]
- Itou, H.; Tanaka, I. The OmpR-Family of Proteins: Insight into the Tertiary Structure and Functions of Two-Component Regulator Proteins. J. Biochem. 2001, 129, 343–350. [Google Scholar] [CrossRef]
- Andrade, M.O.; Farah, C.S.; Wang, N. The Post-transcriptional Regulator rsmA/csrA Activates T3SS by Stabilizing the 5′ UTR of hrpG, the Master Regulator of hrp/hrc Genes, in Xanthomonas. PLoS Pathog. 2014, 10, e1003945. [Google Scholar] [CrossRef] [PubMed]
- Wengelnik, K.; Rossier, O.; Bonas, U. Mutations in the Regulatory Gene hrpG of Xanthomonas campestris pv. vesicatoria Result in Constitutive Expression of All hrp Genes. J. Bacteriol. 1999, 181, 6828–6831. [Google Scholar] [CrossRef] [PubMed]
- Genin, S.; Gough, C.L.; Zischek, C.; Boucher, C.A. Evidence that the hrpB gene encodes a positive regulator of pathogenicity genes from Pseudomonas solanacearum. Mol. Microbiol. 1992, 6, 3065–3076. [Google Scholar] [CrossRef] [PubMed]
- Brito, B.; Marenda, M.; Barberis, P.; Boucher, C.; Genin, S. prhJ and hrpG, two new components of the plant signal-dependent regulatory cascade controlled by PrhA in Ralstonia solanacearum. Mol. Microbiol. 1999, 31, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, L.; Schell, M.A. Elucidation of the Regulon and cis-Acting Regulatory Element of HrpB, the AraC-Type Regulator of a Plant Pathogen-Like Type III Secretion System in Burkholderia pseudomallei. J. Bacteriol. 2011, 193, 1991–2001. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, M.; Yan, J.; Yang, L.; Yang, Y.; Guan, W.; Walcott, R.; Zhao, T. Involvement of hrpX and hrpG in the Virulence of Acidovorax citrulli Strain Aac5, Causal Agent of Bacterial Fruit Blotch in Cucurbits. Front. Microbiol. 2018, 9, 507. [Google Scholar] [CrossRef]
- Wichmann, F.; Vorhölter, F.-J.; Hersemann, L.; Widmer, F.; Blom, J.; Niehaus, K.; Reinhard, S.; Conradin, C.; Kölliker, R. The noncanonical type III secretion system of Xanthomonas translucens pv. graminis is essential for forage grass infection. Mol. Plant. Pathol. 2013, 14, 576–588. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Zou, L.-F.; Xue, X.-B.; Cai, L.-L.; Ma, W.-X.; Xiong, L.; Ji, Z.-Y.; Chen, G.-Y. HrcT Is a Key Component of the Type III Secretion System in Xanthomonas spp. and Also Regulates the Expression of the Key hrp Transcriptional Activator HrpX. Appl. Environ. Microbiol. 2014, 80, 3908–3919. [Google Scholar] [CrossRef][Green Version]
- Kogenaru, S.; Qing, Y.; Guo, Y.; Wang, N. RNA-seq and microarray complement each other in transcriptome profiling. BMC Genom. 2012, 13, 629. [Google Scholar] [CrossRef]
- Roux, B.; Bolot, S.; Guy, E.; Denancé, N.; Lautier, M.; Jardinaud, M.-F.; Fischer-Le Saux, M.; Portier, P.; Jacques, M.-A.; Gagnevin, L.; et al. Genomics and transcriptomics of Xanthomonas campestris species challenge the concept of core type III effectome. BMC Genom. 2015, 16, 975. [Google Scholar] [CrossRef]
- Perez-Quintero, A.L.; Szurek, B. A Decade Decoded: Spies and Hackers in the History of TAL Effectors Research. Annu. Rev. Phytopathol. 2019, 57, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Bole, J.; Rongqi, X.; Xianzhen, L.; Hongyu, W.; Faan, B.; Xi, H.; Yongqiang, H.; Jiliang, T. Construction and characterization of a hrpG mutant rendering constitutive expression of hrp genes in Xanthomonas campestris pv. campestris. Prog. Nat. Sci. 2006, 16, 480–485. [Google Scholar] [CrossRef]
- Wei, K.; Tang, D.-J.; He, Y.-Q.; Feng, J.-X.; Jiang, B.-L.; Lu, G.-T.; Chen, B.; Tang, J.-L. hpaR, a Putative marR Family Transcriptional Regulator, Is Positively Controlled by HrpG and HrpX and Involved in the Pathogenesis, Hypersensitive Response, and Extracellular Protease Production of Xanthomonas campestris pathovar campestris. J. Bacteriol. 2007, 189, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gómez, L.; Boller, T. Flagellin perception: A paradigm for innate immunity. Trends Plant. Sci. 2002, 7, 251–256. [Google Scholar] [CrossRef]
- Deochand, D.K.; Grove, A. MarR family transcription factors: Dynamic variations on a common scaffold. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 595–613. [Google Scholar] [CrossRef] [PubMed]
- Grove, A. Regulation of Metabolic Pathways by MarR Family Transcription Factors. Comput. Struct. Biotechnol. J. 2017, 15, 366–371. [Google Scholar] [CrossRef]
- Pan, Y.; Liang, F.; Li, R.-J.; Qian, W. MarR-Family Transcription Factor HpaR Controls Expression of the vgrR—vgrS Operon of Xanthomonas campestris pv. campestris. Mol. Plant. Microbe Interact. 2018, 31, 299–310. [Google Scholar] [CrossRef]
- Zhang, S.-S.; He, Y.-Q.; Xu, L.-M.; Chen, B.-W.; Jiang, B.-L.; Liao, J.; Cao, J.-R.; Liu, D.; Huang, Y.-Q.; Liang, X.-X.; et al. A putative colRXC1049–colSXC1050 two-component signal transduction system in Xanthomonas campestris positively regulates hrpC and hrpE operons and is involved in virulence, the hypersensitive response and tolerance to various stresses. Res. Microbiol. 2008, 159, 569–578. [Google Scholar] [CrossRef]
- Yan, Q.; Wang, N. The ColR/ColS Two-Component System Plays Multiple Roles in the Pathogenicity of the Citrus Canker Pathogen Xanthomonas citri subsp. citri. J. Bacteriol. 2011, 193, 1590–1599. [Google Scholar] [CrossRef]
- Subramoni, S.; Pandey, A.; Priya, M.R.V.; Patel, H.K.; Sonti, R.V. The ColRS system of Xanthomonas oryzae pv. oryzae is required for virulence and growth in iron-limiting conditions. Mol. Plant. Pathol. 2012, 13, 690–703. [Google Scholar] [CrossRef]
- Zhang, Y.; Callaway, E.; Jones, J.B.; Wilson, M. Visualisation of hrp gene expression in Xanthomonas euvesicatoria in the tomato phyllosphere. Eur. J. Plant. Pathol. 2009, 124, 379–390. [Google Scholar] [CrossRef]
- Li, R.F.; Lu, G.T.; Li, L.; Su, H.Z.; Feng, G.F.; Chen, Y.; He, Y.Q.; Jiang, B.L.; Tang, D.J.; Tang, J.L. Identification of a putative cognate sensor kinase for the two-component response regulator HrpG, a key regulator controlling the expression of the hrp genes in Xanthomonas campestris pv. campestris. Environ. Microbiol. 2014, 16, 2053–2071. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yan, Q.; Wang, N. Deciphering the regulon of a GntR family regulator via transcriptome and ChIP-exo analyses and its contribution to virulence in Xanthomonas citri. Mol. Plant. Pathol. 2017, 18, 249–262. [Google Scholar] [CrossRef] [PubMed]
- de Bernonville, T.D.; Noël, L.D.; Cristobal, M.S.; Danoun, S.; Becker, A.; Soreau, P.; Arlat, M.; Lauber, E. Transcriptional reprogramming and phenotypical changes associated with growth of Xanthomonas campestris pv. campestris in cabbage xylem sap. FEMS Microbiol. Ecol. 2014, 89, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Chatnaparat, T.; Prathuangwong, S.; Lindow, S.E. Global Pattern of Gene Expression of Xanthomonas axonopodis pv. glycines Within Soybean Leaves. Mol. Plant. Microbe Interact. 2016, 29, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, J.; Jia, H.; Sosso, D.; Li, T.; Frommer, W.B.; Yang, B.; White, F.F.; Wang, N.; Jones, J.B. Lateral organ boundaries 1 is a disease susceptibility gene for citrus bacterial canker disease. Proc. Natl. Acad. Sci. USA 2014, 111, E521–E529. [Google Scholar] [CrossRef]
- Zhou, X.; Hu, X.; Li, J.; Wang, N. A Novel Periplasmic Protein, VrpA, Contributes to Efficient Protein Secretion by the Type III Secretion System in Xanthomonas spp. Mol. Plant. Microbe Interact. 2015, 28, 143–153. [Google Scholar] [CrossRef][Green Version]
- Kim, S.; Cho, Y.-J.; Song, E.-S.; Lee, S.H.; Kim, J.-G.; Kang, L.-W. Time-resolved pathogenic gene expression analysis of the plant pathogen Xanthomonas oryzae pv. oryzae. BMC Genom. 2016, 17, 345. [Google Scholar] [CrossRef]
- Schulte, R. A Xanthomonas Pathogenicity Locus Is Induced by Sucrose and Sulfur-Containing Amino Acids. Plant. Cell 1992, 4, 79–86. [Google Scholar] [CrossRef]
- Tsuge, S.; Furutai, A.; Fukunaka, R.; Oku, T.; Tsuno, K.; Ochiai, H.; Inoue, Y.; Kaku, H.; Kubo, Y. Expression of Xanthomonas oryzae pv. oryzae hrp Genes in XOM2, a Novel Synthetic Medium. J. Gen. Plant. Pathol. 2002, 68, 363–371. [Google Scholar] [CrossRef]
- Jiang, G.F.; Le Jiang, B.; Yang, M.; Liu, S.; Liu, J.; Liang, X.X.; Bai, X.F.; Tang, D.J.; Lu, G.T.; He, Y.Q.; et al. Establishment of an inducing medium for type III effector secretion in Xanthomonas campestris pv. campestris. Braz. J. Microbiol. 2013, 44, 945–952. [Google Scholar] [CrossRef] [PubMed]
- An, S.-Q.; Lu, G.-T.; Su, H.-Z.; Li, R.-F.; He, Y.-Q.; Jiang, B.-L.; Tang, D.-J.; Tang, J.-L. Systematic Mutagenesis of All Predicted gntR Genes in Xanthomonas campestris pv. campestris Reveals a GntR Family Transcriptional Regulator Controlling Hypersensitive Response and Virulence. Mol. Plant. Microbe Interact. 2011, 24, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- Irving, S.E.; Choudhury, N.R.; Corrigan, R.M. The stringent response and physiological roles of (pp)pGpp in bacteria. Nat. Rev. Microbiol. 2020, 1–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Teper, D.; Xu, J.; Wang, N. Stringent response regulators (p)ppGpp and DksA positively regulate virulence and host adaptation of Xanthomonas citri. Mol. Plant. Pathol. 2019, 20, 1550–1565. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vazquez, P.; Dewey, C.N.; Kitten, N.; Ross, W.; Gourse, R.L. Genome-wide effects on Escherichia coli transcription from ppGpp binding to its two sites on RNA polymerase. Proc. Natl. Acad. Sci. USA 2019, 116, 8310–8319. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, Y.; Tsuge, S. The quantitative regulation of the hrp regulator HrpX is involved in sugar-source-dependent hrp gene expression in Xanthomonas oryzae pv. oryzae. FEMS Microbiol. Lett. 2016, 363, fnw071. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.M.; Ikawa, Y.; Tsuge, S. GamR, the LysR-type galactose metabolism regulator, regulates hrp gene expression via transcriptional activation of two key hrp regulators, HrpG and HrpX, in Xanthomonas oryzae pv. oryzae. Appl. Environ. Microbiol. 2016, 82, 3947–3958. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, Y.; Ohnishi, S.; Shoji, A.; Furutani, A.; Tsuge, S. Concomitant Regulation by a LacI-Type Transcriptional Repressor XylR on Genes Involved in Xylan and Xylose Metabolism and the Type III Secretion System in Rice Pathogen Xanthomonas oryzae pv. oryzae. Mol. Plant. Microbe Interact. 2018, 31, 605–613. [Google Scholar] [CrossRef]
- Soberón-Chávez, G.; Alcaraz, L.D.; Morales, E.; Ponce-Soto, G.Y.; Servín-González, L. The Transcriptional Regulators of the CRP Family Regulate Different Essential Bacterial Functions and Can Be Inherited Vertically and Horizontally. Front. Microbiol. 2017, 8, 959. [Google Scholar] [CrossRef]
- de Crecy-Lagard, V.; Glaser, P.; Lejeune, P.; Sismeiro, O.; Barber, C.E.; Daniels, M.J.; Danchin, A. A Xanthomonas campestris pv. campestris protein similar to catabolite activation factor is involved in regulation of phytopathogenicity. J. Bacteriol. 1990, 172, 5877–5883. [Google Scholar] [CrossRef]
- He, Y.-W.; Ng, A.Y.-J.; Xu, M.; Lin, K.; Wang, L.-H.; Dong, Y.-H.; Zhang, L.-H. Xanthomonas campestris cell-cell communication involves a putative nucleotide receptor protein Clp and a hierarchical signalling network. Mol. Microbiol. 2007, 64, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, G.; Zhang, Y.; Qian, G.; Liu, F. Dissecting the virulence-related functionality and cellular transcription mechanism of a conserved hypothetical protein in Xanthomonas oryzae pv. oryzae. Mol. Plant. Pathol. 2018, 19, 1859–1872. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Gao, J.; Chen, Q.; Ma, B.; Fang, Y.; Liu, X.; Chen, G.; Liu, J.-Z. Crp-Like Protein Plays Both Positive and Negative Roles in Regulating the Pathogenicity of Bacterial Pustule Pathogen Xanthomonas axonopodis pv. glycines. Phytopathol. 2019, 109, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Teper, D.; Zhang, Y.; Wang, N. TfmR, a novel TetR-family transcriptional regulator, modulates the virulence of Xanthomonas citri in response to fatty acids. Mol. Plant. Pathol. 2019, 20, 701–715. [Google Scholar] [CrossRef]
- Subramoni, S.; Sonti, R.V. Growth Deficiency of a Xanthomonas oryzae pv. oryzae fur Mutant in Rice Leaves Is Rescued by Ascorbic Acid Supplementation. Mol. Plant. Microbe Interact. 2005, 18, 644–651. [Google Scholar] [CrossRef][Green Version]
- Pandey, A.; Sonti, R.V. Role of the FeoB Protein and Siderophore in Promoting Virulence of Xanthomonas oryzae pv. oryzae on Rice. J. Bacteriol. 2010, 192, 3187–3203. [Google Scholar] [CrossRef]
- Rai, R.; Javvadi, S.; Chatterjee, S. Cell-cell signalling promotes ferric iron uptake in Xanthomonas oryzae pv. oryzicola that contribute to its virulence and growth inside rice. Mol. Microbiol. 2015, 96, 708–727. [Google Scholar] [CrossRef]
- Pandey, S.S.; Patnana, P.K.; Rai, R.; Chatterjee, S. Xanthoferrin, the α-hydroxycarboxylate-type siderophore of Xanthomonas campestris pv. campestris, is required for optimum virulence and growth inside cabbage. Mol. Plant. Pathol. 2017, 18, 949–962. [Google Scholar] [CrossRef]
- Javvadi, S.; Pandey, S.S.; Mishra, A.; Pradhan, B.B.; Chatterjee, S. Bacterial cyclic β-(1,2)-glucans sequester iron to protect against iron-induced toxicity. EMBO Rep. 2018, 19, 172–186. [Google Scholar] [CrossRef]
- Pandey, S.S.; Patnana, P.K.; Padhi, Y.; Chatterjee, S. Low-iron conditions induces the hypersensitive reaction and pathogenicity hrp genes expression in Xanthomonas and is involved in modulation of hypersensitive response and virulence. Environ. Microbiol. Rep. 2018, 10, 522–531. [Google Scholar] [CrossRef]
- Pandey, S.S.; Patnana, P.K.; Lomada, S.K.; Tomar, A.; Chatterjee, S. Co-regulation of Iron Metabolism and Virulence Associated Functions by Iron and XibR, a Novel Iron Binding Transcription Factor, in the Plant Pathogen Xanthomonas. PLoS Pathog. 2016, 12, e1006019. [Google Scholar] [CrossRef] [PubMed]
- Escolar, L.; Pérez-Martín, J.; de Lorenzo, V. Opening the Iron Box: Transcriptional Metalloregulation by the Fur Protein. J. Bacteriol. 1999, 181, 6223–6229. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-L.; Tang, D.-J.; Liao, Q.; Li, X.-Q.; He, Y.-Q.; Feng, J.-X.; Jiang, B.-L.; Lu, G.-T.; Tang, J.-L. The Zur of Xanthomonas campestris Is Involved in Hypersensitive Response and Positively Regulates the Expression of the hrp Cluster Via hrpX But Not hrpG. Mol. Plant. Microbe Interact. 2009, 22, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, Y.; Yuan, Z.-H.; Zhang, H.; Peng, B.-Y.; Wang, F.-F.; Qian, W. Two-Component Signaling System VgrRS Directly Senses Extracytoplasmic and Intracellular Iron to Control Bacterial Adaptation under Iron Depleted Stress. PLoS Pathog. 2016, 12, e1006133. [Google Scholar] [CrossRef]
- Dow, J.M. Diffusible signal factor-dependent quorum sensing in pathogenic bacteria and its exploitation for disease control. J. Appl. Microbiol. 2017, 122, 2–11. [Google Scholar] [CrossRef]
- Ryan, R.P.; An, S.; Allan, J.H.; McCarthy, Y.; Dow, J.M. The DSF Family of Cell–Cell Signals: An Expanding Class of Bacterial Virulence Regulators. PLoS Pathog. 2015, 11, e1004986. [Google Scholar] [CrossRef]
- He, Y.-W.; Zhang, L.-H. Quorum sensing and virulence regulation in Xanthomonas campestris. FEMS Microbiol. Rev. 2008, 32, 842–857. [Google Scholar] [CrossRef]
- Rai, R.; Ranjan, M.; Pradhan, B.B.; Chatterjee, S. Atypical regulation of virulence-associated functions by a diffusible signal factor in Xanthomonas oryzae pv. oryzae. Mol. Plant. Microbe Interact. 2012, 25, 789–801. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Li, J.-L.; Wang, N. Diffusible signal factor-mediated quorum sensing plays a central role in coordinating gene expression of Xanthomonas citri subsp. citri. Mol. Plant. Microbe Interact. 2012, 25, 165–179. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Zhang, Y.; Wang, N. Diffusible signal factor (DSF)-mediated quorum sensing modulates expression of diverse traits in Xanthomonas citri and responses of citrus plants to promote disease. BMC Genom. 2019, 20, 55. [Google Scholar] [CrossRef]
- Jiang, B.-L.; Jiang, G.-F.; Liu, W.; Yang, L.-C.; Yang, L.-Y.; Wang, L.; Hang, X.-H.; Tang, J.-L. RpfC regulates the expression of the key regulator hrpX of the hrp/T3SS system in Xanthomonas campestris pv. campestris. BMC Microbiol. 2018, 18, 103. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Li, R.-F.; Zhang, D.-P.; Tang, J.-L.; Lu, G.-T. HpaP, a novel regulatory protein with ATPase and phosphatase activity, contributes to full virulence in Xanthomonas campestris pv. campestris. Environ. Microbiol. 2018, 20, 1389–1404. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Rashidul, I.M.; Hirata, H.; Tsuyumu, S. KdgR, an IClR family transcriptional regulator, inhibits virulence mainly by repression of hrp genes in Xanthomonas oryzae pv. oryzae. J. Bacteriol. 2011, 193, 6674–6682. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-Y.; Yang, L.-C.; Gan, Y.-L.; Wang, L.; Zhao, W.-Z.; He, Y.-Q.; Jiang, W.; Jiang, B.-L.; Tang, J.-L. Systematic Functional Analysis of Sigma (σ) Factors in the Phytopathogen Xanthomonas campestris Reveals Novel Roles in the Regulation of Virulence and Viability. Front. Microbiol. 2018, 9, 1749. [Google Scholar] [CrossRef] [PubMed]
- Leng, M.; Lu, Z.; Qin, Z.; Qi, Y.; Lu, G.; Tang, J. Flp, a Fis-like protein, contributes to the regulation of type III secretion and virulence processes in the phytopathogen Xanthomonas campestris pv. campestris. Mol. Plant. Pathol. 2019, 1119–1133. [Google Scholar] [CrossRef]
- Feng, J.-X.; Song, Z.-Z.; Duan, C.-J.; Zhao, S.; Wu, Y.-Q.; Wang, C.; Dow, J.M.; Tang, J.-L. The xrvA gene of Xanthomonas oryzae pv. oryzae, encoding an H-NS-like protein, regulates virulence in rice. Microbiology 2009, 155, 3033–3044. [Google Scholar] [CrossRef] [PubMed]
- Kametani-Ikawa, Y.; Tsuge, S.; Furutani, A.; Ochiai, H. An H-NS-like protein involved in the negative regulation of hrp genes in Xanthomonas oryzae pv. oryzae. FEMS Microbiol. Lett. 2011, 319, 58–64. [Google Scholar] [CrossRef]
- Liu, Y.; Long, J.; Shen, D.; Song, C. Xanthomonas oryzae pv. oryzae requires H-NS-family protein XrvC to regulate virulence during rice infection. FEMS Microbiol. Lett. 2016, 363, fnw067. [Google Scholar] [CrossRef][Green Version]
- Storz, G.; Papenfort, K. Global Regulation by CsrA and Its RNA Antagonists. In Regulating with RNA in Bacteria and Archaea; American Society of Microbiology: Washington, DC, USA, 2019; pp. 341–354. [Google Scholar]
- Chao, N.-X.; Wei, K.; Chen, Q.; Meng, Q.-L.; Tang, D.-J.; He, Y.-Q.; Lu, G.-T.; Jiang, B.-L.; Liang, X.-X.; Feng, J.-X.; et al. The rsmA -like Gene rsmA Xcc of Xanthomonas campestris pv. campestris Is Involved in the Control of Various Cellular Processes, Including Pathogenesis. Mol. Plant. Microbe Interact. 2008, 21, 411–423. [Google Scholar] [CrossRef]
- Zhu, P.-L.; Zhao, S.; Tang, J.-L.; Feng, J.-X. The rsmA-like gene rsmAXoo of Xanthomonas oryzae pv. oryzae regulates bacterial virulence and production of diffusible signal factor. Mol. Plant. Pathol. 2011, 12, 227–237. [Google Scholar] [CrossRef]
- Pourciau, C.; Lai, Y.-J.; Gorelik, M.; Babitzke, P.; Romeo, T. Diverse Mechanisms and Circuitry for Global Regulation by the RNA-Binding Protein CsrA. Front. Microbiol. 2020, 11, 601352. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, X.; Jia, Y.; Liang, Y.; He, Y.; Lu, T.; Zhu, C.; Han, B.; An, S.; Tang, J. Genome-wide screen and functional analysis in Xanthomonas reveal a large number of mRNA-derived sRNAs, including the novel RsmA-sequester RsmU. Mol. Plant. Pathol. 2020, 21, 1573–1590. [Google Scholar] [CrossRef] [PubMed]
- Cenens, W.; Andrade, M.O.; Llontop, E.; Alvarez-Martinez, C.E.; Sgro, G.G.; Farah, C.S. Bactericidal type IV secretion system homeostasis in Xanthomonas citri. PLoS Pathog. 2020, 16, e1008561. [Google Scholar] [CrossRef] [PubMed]
- Alegria, M.C.; Docena, C.; Khater, L.; Ramos, C.H.I.; da Silva, A.C.R.; Farah, C.S. New Protein-Protein Interactions Identified for the Regulatory and Structural Components and Substrates of the Type III Secretion System of the Phytopathogen Xanthomonas axonopodis pathovar citri. J. Bacteriol. 2004, 186, 6186–6197. [Google Scholar] [CrossRef]
- Zhou, X.; Teper, D.; Andrade, M.O.; Zhang, T.; Chen, S.; Song, W.-Y.; Wang, N. A Phosphorylation Switch on Lon Protease Regulates Bacterial Type III Secretion System in Host. MBio 2018, 9, e02146-17. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teper, D.; Pandey, S.S.; Wang, N. The HrpG/HrpX Regulon of Xanthomonads—An Insight to the Complexity of Regulation of Virulence Traits in Phytopathogenic Bacteria. Microorganisms 2021, 9, 187. https://doi.org/10.3390/microorganisms9010187
Teper D, Pandey SS, Wang N. The HrpG/HrpX Regulon of Xanthomonads—An Insight to the Complexity of Regulation of Virulence Traits in Phytopathogenic Bacteria. Microorganisms. 2021; 9(1):187. https://doi.org/10.3390/microorganisms9010187
Chicago/Turabian StyleTeper, Doron, Sheo Shankar Pandey, and Nian Wang. 2021. "The HrpG/HrpX Regulon of Xanthomonads—An Insight to the Complexity of Regulation of Virulence Traits in Phytopathogenic Bacteria" Microorganisms 9, no. 1: 187. https://doi.org/10.3390/microorganisms9010187
APA StyleTeper, D., Pandey, S. S., & Wang, N. (2021). The HrpG/HrpX Regulon of Xanthomonads—An Insight to the Complexity of Regulation of Virulence Traits in Phytopathogenic Bacteria. Microorganisms, 9(1), 187. https://doi.org/10.3390/microorganisms9010187




