Mycobacteriosis in Aquatic Invertebrates: A Review of Its Emergence
Abstract
1. Introduction
2. The Immune System of Aquatic Invertebrates and Mycobacteriosis
3. Mycobacteriosis in Molluscs
3.1. The Phylum Mollusca
3.2. The Importance of Molluscs for the Global Economy as a Food Source
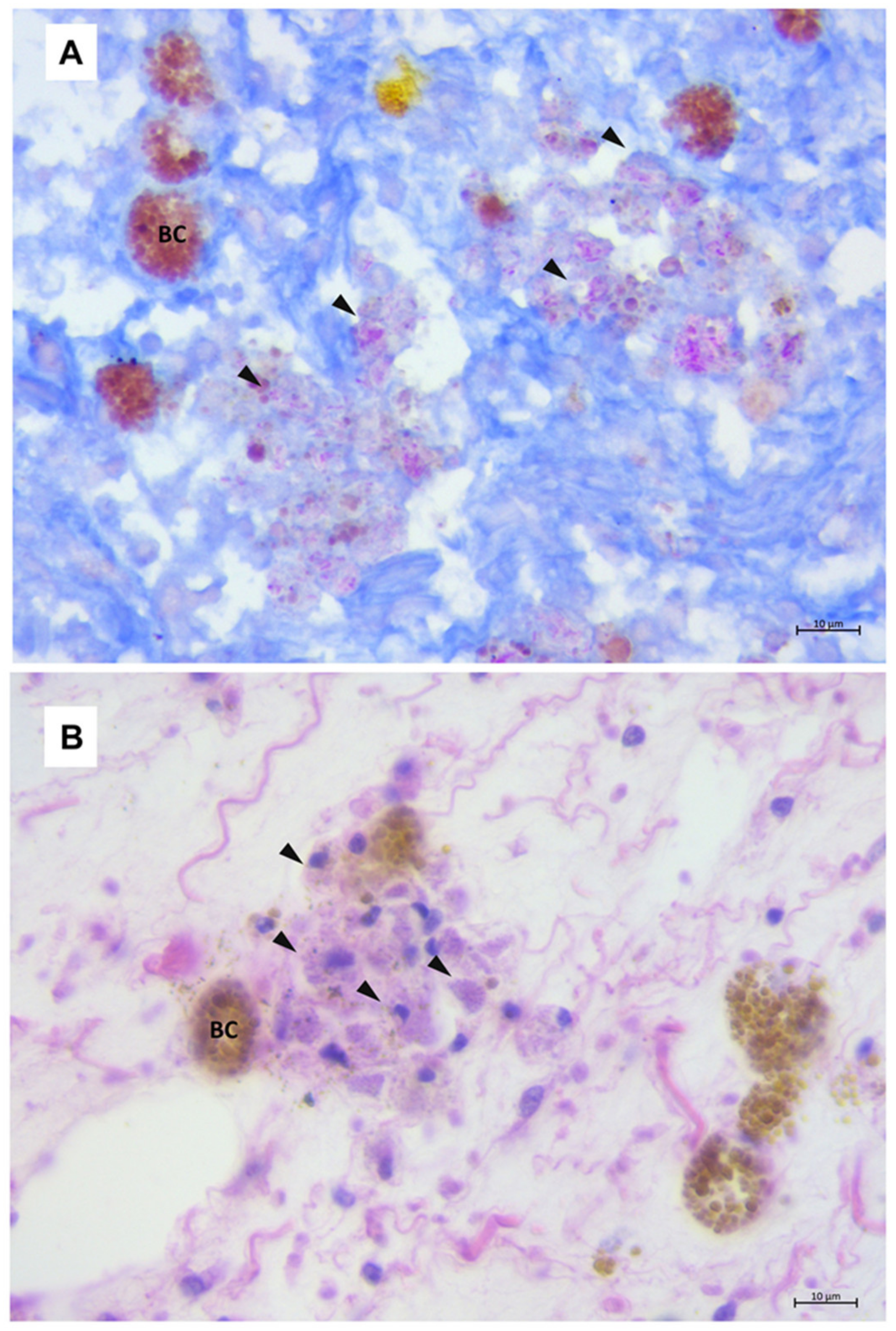
3.3. Mycobacteriosis in Gastropods
3.4. Mycobacteriosis in Bivalves
4. Mycobacteriosis in Crustaceans
4.1. Crustaceans and Their Importance for the Global Economy as a Food Source
4.2. Mycobacteriosis in Crustaceans of the Class Branchiopoda
4.3. Mycobacteriosis in Edible Crustaceans
5. Mycobacteriosis in Cnidarians, Echinoderms and Sponges
6. Diagnosis of Mycobacteriosis
7. Zoonotic Considerations
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tortoli, E. The taxonomy of the genus Mycobacterium. In Nontuberculous Mycobacteria (NTM); Velayati, A.A., Farnia, P., Eds.; Academic Press: London, UK, 2019; pp. 1–10. [Google Scholar]
- Lehmann, K.B.; Neuman, R. Atlas und Grundriss der Bakteriologie und Lehrbuch der Speziellen Bakteriologischen Disagnostik; Ulan Press: Munich, Germany, 1896. [Google Scholar]
- Fedrizzi, T.; Meehan, C.J.; Grottola, A.; Giacobazzi, E.; Fregni Serpini, G.; Tagliazucchi, S.; Fabio, A.; Bettua, C.; Bertorelli, R.; De Sanctis, V.; et al. Genomic characterization of nontuberculous mycobacteria. Sci. Rep. 2017, 7, 45258. [Google Scholar] [CrossRef]
- Carella, F.; Aceto, S.; Pollaro, F.; Miccio, A.; Iaria, C.; Carrasco, P.P.; Prado, P.; De Vico, G. A mycobacterial disease is associated with the silent mass mortality of the pen shell Pinna nobilis along the Tyrrhenian coastline of Italy. Sci. Rep. 2019, 9, 2725. [Google Scholar] [CrossRef] [PubMed]
- Davidovich, N.; Pretto, T.; Blum, S.E.; Baider, Z.; Grossman, R.; Kaidar-Shwartz, H.; Dveyrin, Z.; Rorman, E. Mycobacterium gordonae infecting redclaw crayfish Cherax quadricarinatus. Dis. Aquat. Organ. 2019, 135, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Kazda, J.; Falkinham, J.O.; Pavlik, I.; Hruska, K. The Ecology of Mycobacteria: Impact on Animal’s and Human’s Health; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Falkinham, J.O., 3rd. Epidemiology of infection by nontuberculous mycobacteria. Clin. Microbiol. Rev. 1996, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Vaerewijck, M.J.M.; Huys, G.; Palomino, J.C.; Swings, J.; Portaels, F. Mycobacteria in drinking water distribution systems: Ecology and significance for human health. FEMS Microbiol. Rev. 2005, 29, 911–934. [Google Scholar] [CrossRef] [PubMed]
- Pavlik, I.; Falkinham, J.O. The occurrence of pathogenic and potentially pathogenic mycobacteria in animals and the role of the environment in the spread of infection. In The Ecology of Mycobacteria: Impact on Animal’s and Human’s Health; Kazda, J., Pavlik, I., Falkinham, J.O., Hruska, K., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 199–281. [Google Scholar]
- Zanoni, R.G.; Florio, D.; Fioravanti, M.L.; Rossi, M.; Prearo, M. Occurrence of Mycobacterium spp. in ornamental fish in Italy. J. Fish. Dis. 2008, 31, 433–441. [Google Scholar] [CrossRef]
- Astrofsky, K.M.; Schrenzel, M.D.; Bullis, R.A.; Smolowitz, R.M.; Fox, J.G. Diagnosis and management of atypical Mycobacterium spp. infections in established laboratory zebrafish (Brachydanio rerio) facilities. Comp. Med. 2000, 50, 666–672. [Google Scholar]
- Davidovich, N.; Pretto, T.; Sharon, G.; Zilberg, D.; Blum, S.E.; Baider, Z.; Edery, N.; Morick, D.; Grossman, R.; Shwartz, H.K.; et al. Cutaneous appearance of mycobacteriosis caused by Mycobacterium marinum, affecting gilthead seabream (Sparus aurata) cultured in recirculating aquaculture systems. Aquaculture 2020, 528, 735507. [Google Scholar] [CrossRef]
- Brock, J.A.; Nakagawa, L.K.; Shimojo, R.J. Infection of a cultured freshwater prawn, Macrobrachium rosenbergii de Man (Crustacea: Decapoda), by Mycobacterium spp., Runyon Group II. J. Fish. Dis. 1986, 9, 319–324. [Google Scholar] [CrossRef]
- Lightner, D.V.; Redman, R.M. A probable Mycobacterium sp. infection of the marine shrimp Penaeus vannamei (Crustacea: Decapoda). J. Fish. Dis. 1986, 9, 357–359. [Google Scholar] [CrossRef]
- Grimm, C.; Huntsberger, C.; Markey, K.; Inglis, S.; Smolowitz, R. Identification of a Mycobacterium sp. as the causative agent of orange nodular lesions in the Atlantic sea scallop Placopecten magellanicus. Dis. Aquat. Organ. 2016, 118, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Carella, F.; Antuofermo, E.; Farina, S.; Salati, F.; Mandas, D.; Prado, P.; Panarese, R.; Marino, F.; Fiocchi, E.; Pretto, T.; et al. In the wake of the ongoing mass mortality events: Co-occurrence of Mycobacterium, Haplosporidium and other pathogens in Pinna nobilis collected in Italy and Spain (Mediterranean Sea). Front. Mar. Sci. 2020, 7, 48. [Google Scholar] [CrossRef]
- Prado, P.; Catanese, G.; Jofre, A.G.; Andree, K.B.; Garcia March, J.R.; Cabanes, P.; Carella, F.; Garcia-March, J.R.; Tena, J.; Roque, A.; et al. Presence of Vibrio mediterranei associated to major mortality in stabled individuals of Pinna nobilis L. Aquaculture 2020, 519, 734899. [Google Scholar] [CrossRef]
- Söderhäll, K.; Smith, V.J. The prophenoloxidase activating system: The biochemistry of its activation and role in arthropod cellular immunity, with special reference to crustaceans. In Immunity in Invertebrates; Brehélin, M., Ed.; Springer: Berlin/Heidelberg, Germany, 1986; pp. 208–223. [Google Scholar]
- Abbas, M.N.; Kausar, S.; Sun, Y.-X.; Sun, Y.; Wang, L.; Qian, C.; Wei, G.-Q.; Zhu, B.-J.; Liu, C.-L. Molecular cloning, expression, and characterization of E2F Transcription Factor 4 from Antheraea pernyi. Bull. Entomol. Res. 2017, 107, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, N.A.; Rowley, A.F.; Fitzgerald, S.W.; Rhodes, C.P. Invertebrate immunity: Basic concepts and recent advances. Int. Rev. Cytol. 1985, 97, 183–350. [Google Scholar]
- Kausar, S.; Abbas, M.N.; Qian, C.; Zhu, B.; Gao, J.; Sun, Y.; Wang, L.; Wei, G.; Liu, C. Role of Antheraea pernyi serpin 12 in prophenoloxidase activation and immune responses. Arch. Insect Biochem. Phys. 2018, 97, e21435. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Man Lun, C.; Majeske, A.J.; Sacchi, S.; Schrankel, C.S.; Courtney Smith, L. Invertebrate immune diversity. Dev. Comp. Immun. 2011, 35, 959–974. [Google Scholar] [CrossRef]
- Cerenius, L.; Söderhäll, K. Variable immune molecules in invertebrates. J. Exp. Biol. 2013, 216, 4313–4319. [Google Scholar] [CrossRef]
- Abbas, M.N.; Kausar, S.; Cui, H. The biological role of peroxiredoxins in innate immune responses of aquatic invertebrates. Fish. Shellfish Immunol. 2019, 89, 91–97. [Google Scholar] [CrossRef]
- Herrin, B.R.; Coope, M.D. Alternative adaptive immunity in jawless vertebrates. J. Immunol. 2010, 185, 1367–1374. [Google Scholar] [CrossRef]
- Tripp, M.R. (Ed.) Biological Bulletin; The University of Chicago Press: Chicago, IL, USA, 1960; Volume 119, pp. 210–223. [Google Scholar]
- Smith, V.J.; Chisholm, J.R.S. Antimicrobial proteins in crustaceans. In Phylogenetic Perspectives on the Vertebrate Immune System; Beck, G., Sugumaran, M., Cooper, E.L., Eds.; Springer: Boston, MA, USA, 2001; pp. 95–112. [Google Scholar]
- De Vico, G.; Carella, F. Morphological features of the inflammatory response in molluscs. Res. Vet. Sci. 2012, 93, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Kausar, S.; Abbas, M.N.; Qian, C.; Zhu, B.; Sun, Y.; Sun, Y.; Wang, L.; Wei, G.; Maqsood, I.; Liu, C.-L. Serpin-14 negatively regulates prophenoloxidase activation and expression of antimicrobial peptides in Chinese oak silkworm Antheraea pernyi. Dev. Comp. Immunol. 2017, 76, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, S.; Muta, T.; Iwanaga, S. Clotting cascade and defense molecules found in the hemolymph of the horseshoe crab. In New Directions in Invertebrate Immunology; Söderhäll, K., Iwanaga, S., Vasta, G.R., Eds.; SOS Pubns, Online Book Depositor: Fair Haven, CT, USA, 1996; p. 283. [Google Scholar]
- Vargas-Albores, F.; Yepiz-Plascencia, G. Beta glucan binding protein and its role in shrimp immune response. Aquaculture 2000, 191, 13–21. [Google Scholar] [CrossRef]
- Abbas, M.N.; Kausar, S.; Sun, Y.-X.; Tian, J.W.; Zhu, B.-J.; Liu, C.-L. Suppressor of Cytokine Signaling 6 can enhance epidermal growth factor receptor signaling pathway in Bombyx mori (Dazao). Dev. Comp. Immnol. 2018, 81, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Gerdol, M. Immune-related genes in gastropods and bivalves: A comparative overview. Invertebr. Surv. J. 2017, 14, 103–118. [Google Scholar]
- Matozzo, V.; Pagano, M.; Spinelli, A.; Caicci, F.; Faggio, C. Pinna nobilis: A big bivalve with big haemocytes? Fish. Shellfish Immunol. 2016, 55, 529–534. [Google Scholar] [CrossRef]
- Parkhaev, P.Y. Origin and the early evolution of the phylum Mollusca. Paleontol. J. 2017, 51, 663–686. [Google Scholar] [CrossRef]
- Pyron, M.; Brown, K.M. Introduction to Mollusca and the class Gastropoda. In Thorp and Covich’s Freshwater Invertebrates; Thorp, J.H., Rogers, D.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 383–421. [Google Scholar]
- FAO. The State of Fisheries and Aquaculture in the World 2018; FAO: Rome, Italy, 2018; Available online: http://www.fao.org/state-of-fisheries-aquaculture (accessed on 24 February 2020).
- Kazda, J.; Pavlik, I.; Falkinham, J.O.; Hruska, K. (Eds.) The chronology of Mycobacteria and the development of mycobacterial ecology. In The Ecology of Mycobacteria: Impact on Animal’s and Human’s Health; Springer: Dordrecht, The Netherlands, 2009; pp. 1–11. [Google Scholar]
- Pan, C. Studies on the biological control of schistosome-bearing snails: A preliminary report on pathogenic microorganisms found in Australorbis glabratus. J. Parasit. 1956, 42, 33. [Google Scholar]
- Lattos, A.; Giantsis, I.A.; Karagiannis, D.; Michaelidis, B. First detection of the invasive haplosporidian and mycobacteria parasites hosting the endangered bivalve Pinna nobilis in Thermaikos Gulf, North Greece. Mar. Environ. Res. 2020, 155, 104889. [Google Scholar] [CrossRef]
- Lopez-Sanmartin, M.; Lopez Fernandez, J.R.; de la Herran, R.; Garcia March, J.R.; Navas, J.I. Evidence of mycobacterial presence in Pinna nobilis infected by Haplosporidium pinnae maintained under quarantine conditions. In Proceedings of the II Congreso de Juvenes Investigadores del Mar, 2018–2020, Malaga, Spain, 1–4 October 2019; pp. 1–3. [Google Scholar]
- Čižmek, H.; Čolić, B.; Gračan, R.; Grau, A.; Catanese, G. An emergency situation for pen shells in the Mediterranean: The Adriatic Sea, one of the last Pinna nobilis shelters, is now affected by a mass mortality event. J. Invertebr. Pathol. 2020, 173, 107388. [Google Scholar] [CrossRef]
- Michelson, E.H. An acid-fast pathogen of fresh-water snails. Am. J. Trop. Med. Hyg. 1961, 10, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Beran, V.; Matlova, L.; Dvorska, L.; Svastova, P.; Pavlik, I. Distribution of mycobacteria in clinically healthy ornamental fish and their aquarium environment. J. Fish. Dis. 2006, 29, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Marsollier, L.; Sévérin, T.; Aubry, J.; Merritt, R.W.; Saint André, J.-P.; Legras, P.; Manceau, A.-L.; Chauty, A.; Carbonnelle, B.; Cole, S.T. Aquatic snails, passive hosts of Mycobacterium ulcerans. Appl. Environ. Microbiol. 2004, 70, 10–6296. [Google Scholar] [CrossRef]
- Kotlowski, R.; Martin, A.; Ablordey, A.; Chemlal, K.; Fonteyne, P.-A.; Portaels, F. One-tube cell lysis and DNA extraction procedure for PCR-based detection of Mycobacterium ulcerans in aquatic insects, molluscs and fish. J. Med. Microbiol. 2004, 53, 927–933. [Google Scholar] [CrossRef]
- Bean-Knudsen, D.E.; Uhazy, L.S.; Wagner, J.E.; Young, B.M. Systemic infection of laboratory-reared Biomphalaria glabrata (Mollusca: Gastropoda) with an acid-fast bacillus. J. Invertebr. Pathol. 1988, 51, 291–293. [Google Scholar] [CrossRef]
- Hosty, T.S.; McDurmont, C.I. Isolation of acid-fast organisms from milk and oysters. Health Lab. Sci. 1975, 12, 16–19. [Google Scholar] [PubMed]
- Beecham, H.J.; Oldfield, E.C.; Lewis, D.E.; Buker, J.L. Mycobacterium marinum infection from shucking oysters. Lancet 1991, 337, 1487. [Google Scholar] [CrossRef]
- Aubry, A.; Chosidow, O.; Caumes, E.; Robert, J.; Cambau, E. Sixty-three cases of Mycobacterium marinum infection. Arch. Int. Med. 2002, 162, 1746. [Google Scholar] [CrossRef]
- Hyman, L.H. The Invertebrates. Mollusca I.; McGraw-Hill Book Company: New York, NY, USA, 1967. [Google Scholar]
- Sesen, R.; Yildirim, M.Z. A study on Turkish freshwater snails that have parasitological importance. Türk. Parazitol. Derg. 1993, 17, 136–147. [Google Scholar]
- Coudereau, C.; Besnard, A.; Robbe-Saule, M.; Bris, C.; Kempf, M.; Johnson, R.C.; Brou, T.Y.; Gnimavo, R.; Eyangoh, S.; Khater, F.; et al. Stable and local reservoirs of Mycobacterium ulcerans inferred from the nonrandom distribution of bacterial genotypes, Benin. Emerg. Inf. Dis. 2020, 26, 491–503. [Google Scholar] [CrossRef]
- De Freitas Tallarico, L.; Borrely, S.I.; Hamada, N.; Siqueira Grazeffe, V.; Pires Ohlweiler, F.; Okazaki, K.; Granatelli, A.T.; Pereira, I.W.; de Braganca Pereira, C.A.; Nakano, E. Developmental toxicity, acute toxicity and mutagenicity testing in freshwater snails Biomphalaria glabrata (Mollusca: Gastropoda) exposed to chromium and water samples. Ecotoxicol. Environ. Saf. 2014, 110, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.H.; Bartell, J.; Gandolfo, L.; Smole, S.C.; Costa, S.F.; Weiss, L.M.; Johnson, L.K.; Osterhout, G.; Herbst, L.H. Characterization of Mycobacterium montefiorense sp. nov., a novel pathogenic mycobacterium from moray eels that is related to Mycobacterium triplex. J. Clin. Microbiol. 2003, 41, 2147–2152. [Google Scholar] [CrossRef]
- Vanhook, A.M.; Patel, N.H. Crustaceans. Curr. Biol. 2008, 18, 547–550. [Google Scholar] [CrossRef]
- Getchell, R.G. Bacterial shell disease in crustaceans: A review. J. Shellfish Res. 1989, 8, 1–6. [Google Scholar]
- Tubiash, H.S.; Sizemore, R.K.; Colwell, R.R. Bacterial flora of the hemolymph of the blue crab, Callinectes sapidus: Most probable numbers. App. Environ. Microbiol. 1975, 29, 388–392. [Google Scholar] [CrossRef]
- Wang, W. Bacterial diseases of crabs: A review. J. Invertebr. Pathol. 2011, 106, 18–26. [Google Scholar] [CrossRef]
- Vincent, A.G.; Breland, V.M.; Lotz, J.M. Experimental infection of Pacific white shrimp Litopenaeus vannamei with necrotizing hepto-pancreatitis (NHP) bacterium by per os exposure. Dis. Aquat. Org. 2004, 61, 227–233. [Google Scholar] [CrossRef]
- Eddy, F.; Powell, A.; Gregory, S.; Nunan, L.M.; Lightner, D.V.; Dyson, P.J.; Rowley, A.F.; Shields, R.J. A novel bacterial disease of the European shore crab, Carcinus maenas—Molecular pathology and epidemiology. Microbiology 2007, 153, 2839–2849. [Google Scholar] [CrossRef]
- Mansson, T. Mycobacteria from aquaria. Br. Med. J. 1970, 3, 46. [Google Scholar] [CrossRef]
- Soeffing, K. Verhaltensökologie der Libelle Leucorrhinia rubicunda L. unter Besonderer Berücksichtingung NahrungsöKologischer Aspekte. Dissertation, University of Hamburg, Hamburg, Germany, 1990; p. 148. [Google Scholar]
- Asem, A.; Rastegar-Pouyani, N.; De Los Ríos-Escalante, P. The genus Artemia leach, 1819 (Crustacea: Branchiopoda). I. True and false taxonomical descriptions. Lat. Am. J. Aquat. Res. 2010, 38, 501–506. [Google Scholar]
- LeBlanc, J.; Webster, D.; Tyrrell, G.J.; Chiu, I. Mycobacterium marinum infection from sea monkeys. Can. J. Inf. Dis. Med. Microbiol. 2012, 23, e106–e108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mohney, L.L.; Poulos, B.T.; Brooker, J.H.; Cage, G.D.; Lightner, D.V. Isolation and identification of Mycobacterium peregrinum from the Pacific white shrimp Penaeus vannamei. J. Aquat. Anim. Health 1998, 10, 83–88. [Google Scholar] [CrossRef]
- Pedrosa, V.F.; Wasielesky Júnior, W.; Klosterhoff, M.C.; Romano, L.A.; de Lara, G.R. Micobacteriose Em Camarão Branco Do Pacífico, Litopenaeus vannamei. Bol. Instit. Pesca 2017, 43, 291–296. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Saad El-deen, A.G.; Elkamel, A.A.; Mohamed, A.M. Mycobacteriosis in fresh water crayfish (Procambarus clarkii). In Proceedings of the 14th Science Congress, Faculty of Veterinary Medicine, Assiut, Egypt, 30 November–2 December 2010; 2010. [Google Scholar]
- Ahmed, W.A.; Al-gburi, N.M.; Abbas, M. Incidence of Mycobacteria spp. in shrimp in Iraq. MRVSA 2014, 3, 24–32. [Google Scholar]
- Jernigan, J.A.; Farr, B.M. Incubation period and sources of exposure for cutaneous Mycobacterium marinum infection: Case report and review of the literature. Clin. Infect. Dis. 2000, 31, 439–443. [Google Scholar] [CrossRef]
- Lee, S.J.; Hong, S.K.; Park, S.S.; Kim, E.-C. First Korean case of Mycobacterium arupense tenosynovitis. Ann. Lab. Med. 2014, 34, 321. [Google Scholar] [CrossRef]
- Kent, P.T.; Kubica, G.P. Public Health Mycobacteriology. A Guide for the Level III Laboratory; US Department of Health and Human Services, Centers for Disease Control: Atlanta, GA, USA, 1985; pp. 96–103. [Google Scholar]
- Boero, F.; Bouillon, J.; Piraino, S. The role of cnidaria in evolution and ecology. Ital. J. Zool. 2005, 72, 65–71. [Google Scholar] [CrossRef]
- Smith, S.; Taylor, G.D.; Fanning, E.A. Chronic cutaneous Mycobacterium haemophilum infection acquired from coral injury. Clin. Infect. Dis. 2003, 37, e100–e101. [Google Scholar] [CrossRef]
- De La Torre, C.; Vega, A.; Carracedo, A.; Toribio, J. Identification of Mycobacterium marinum in sea-urchin granulomas. Br. J. Dermatol. 2001, 145, 114–116. [Google Scholar] [CrossRef]
- López Zabala, I.; Poggio Cano, D.; García-Elvira, R.; Asunción Márquez, J. Mycobacterium marinum osteomyelitis of the first metatarsal. Eur. J. Orthop. Surg. Trauma 2012, 22 (Suppl. S1), 225–228. [Google Scholar] [CrossRef]
- Schefflein, J.; Umans, H.; Ellenbogen, D.; Abadi, M. Sea urchin spine arthritis in the foot. Skelet. Radiol. 2012, 41, 1327–1331. [Google Scholar] [CrossRef] [PubMed]
- Vargas, C.R.; Kanwar, A.; Dousa, K.M.; Skalweit, M.J.; Rowe, D.; Gatherwright, J. Mycobacterial tenosynovitis after sea urchin spine injury in an immunocompromised patient. Open Infect. Dis. 2018, 5, ofy285. [Google Scholar] [CrossRef] [PubMed]
- Padgitt, P.J.; Moshier, S.E. Mycobacterium poriferae sp. nov., a scotochromogenic, rapidly growing species isolated from a marine sponge. Int. J. Syst. Bacteriol. 1987, 37, 186–191. [Google Scholar] [CrossRef]
- Smith, L.C.; Arizza, V.; Barela Hudgell, M.A.; Barone, G.; Bodnar, A.G.; Buckley, K.M.; Cunsolo, V.; Dheilly, N.M.; Franchi, N.; Fugmann, S.D.; et al. Echinodermata: The complex immune system in echinoderms. In Advances in Comparative Immunology; Cooper, E.L., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 409–501. [Google Scholar]
- Gaté, J.; Cuilleret, P.; Chanial, G.; Bouquin, H. Lésions papulo-nécrotiques à réaction histologique tuberculoïde dues à l’inclusion d’épines d’oursins. Bull. Soc. Fr. Dermatol. Syphiligr. 1936, 43, 937–938. [Google Scholar]
- Rocha, G.; Fraga, S. Sea urchin granuloma of the skin. Arch. Dermatol. 1962, 85, 406. [Google Scholar] [CrossRef] [PubMed]
- Moynahan, E.J.; Montgomery, P.R. Echinoderm granuloma: A skin lesion resulting from injury by the spines of sea-urchins inhabiting temperate waters. A new mycobacterial infection. Br. J. Clin. Pract. 1968, 22, 265–269. [Google Scholar]
- Baden, H.P. Injuries from sea urchins. Clin. Dermatol. 1987, 5, 112–117. [Google Scholar] [CrossRef]
- Wörheide, G.; Dohrmann, M.; Erpenbeck, D.; Larroux, C.; Maldonado, M.; Voigt, O.; Borchiellini, C., II; Lavrov, D.V. Deep phylogeny and evolution of sponges (phylum Porifera). Adv. Mar. Biol. 2012, 61, 1–78. [Google Scholar]
- Gaino, E.; Manconi, R.; Pronzato, R. Organizational plasticity as a successful conservative tactics in sponges. Anim. Biol. 1995, 4, 31–43. [Google Scholar]
- Sun, W.; Dai, S.; Jiang, S.; Wang, G.; Liu, G.; Wu, H.; Li, X. Culture-dependent and culture-independent diversity of Actinobacteria associated with the marine sponge Hymeniacidon perleve from the South China Sea. Antonie van Leeuwenhoek 2010, 98, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Gauthier, M.E.A.; Degnan, B.M.; Ng, Y.K.; Hewavitharana, A.K.; Shaw, P.N.; Fuerst, J.A. Diversity of Mycobacterium species from marine sponges and their sensitivity to antagonism by sponge-derived rifamycin-synthesizing actinobacterium in the genus Salinispora. FEMS Microbiol. Lett. 2010, 313, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Tortoli, E.; Bartoloni, A.; Bozzetta, E.; Burrini, C.; Lacchini, C.; Mantella, A.; Penati, V.; Simonetti, M.T.; Ghittino, C. Identification of the newly described Mycobacterium poriferae from tuberculous lesions of snakehead fish (Channa striatus). Comp. Immunol. Microbiol. Infect. Dis. 1996, 19, 25–29. [Google Scholar] [CrossRef]
- Alderman, D.J.; Feist, S.W.; Polglase, J.L. Possible nocardiosis of crayfish, Austropotamobius pallipes. J. Fish Dis. 1986, 9, 345–347. [Google Scholar] [CrossRef]
- Plikaytis, B.B.; Plikaytis, B.D.; Yakrus, M.A.; Butler, W.R.; Woodley, C.L.; Silcox, V.A.; Shinnick, T.M. Differentiation of slowly growing Mycobacterium species, including Mycobacterium tuberculosis, by gene amplification and restriction fragment length polymorphism analysis. J. Clin. Microbiol. 1992, 30, 1815–1822. [Google Scholar] [CrossRef]
- Deepa, P.; Therese, K.L.; Madhavan, H.N. Application of PCR-based restriction fragment length polymorphism (RFLP) for the identification of mycobacterial isolates. Indian J. Med. Res. 2005, 121, 694–700. [Google Scholar]
- Roth, A.; Fischer, M.; Hamid, M.E.; Michalke, S.; Ludwig, W.; Mauch, H. Differentiation of phylogenetically related slowly growing mycobacteria based on 16S-23S rRNA gene internal transcribed spacer sequences. J. Clin. Microbiol. 1998, 36, 139–147. [Google Scholar] [CrossRef]
- Lappayawichit, P.; Rienthong, S.; Rienthong, D.; Chuchottaworn, C.; Chaiprasert, A.; Panbangred, W.; Saringcarinkul, H.; Palittapongarnpim, P. Differentiation of Mycobacterium species by restriction enzyme analysis of amplified 16S–23S ribosomal DNA spacer sequences. Tuberc. Lung Dis. 1996, 77, 257–263. [Google Scholar] [CrossRef]
- Harvell, C.D.; Mitchell, C.E.; Ward, J.R.; Altizer, S.; Dobson, A.P.; Ostfeld, R.S.; Samuel, M.D. Climate warming and disease risks for terrestrial and marine biota. Science 2002, 296, 2158–2162. [Google Scholar] [CrossRef]
- Brito-Morales, I.; Schoeman, D.S.; García Molinos, J.; Burrows, M.T.; Klein, C.J.; Arafeh-Dalmau, N.; Kaschner, K.; Garilao, C.; Kesner-Reyes, K.; Richardson, A.J. Climate velocity reveals increasing exposure of deep-ocean biodiversity to future warming. Nat. Clim. Chang. 2020, 10, 576–581. [Google Scholar] [CrossRef]
- Guschina, I.A.; Harwood, J.L. Mechanisms of temperature adaptation in poikilotherms. FEBS Lett. 2006, 580, 5477–5483. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Shin, J.H. Identification of nontuberculous mycobacteria using multilocous sequence analysis of 16S rRNA, hsp65, and rpoB. J. Clin. Lab. Anal. 2017, 32, e221. [Google Scholar] [CrossRef] [PubMed]

| Animal Species | Mycobacterium Species | Infected Tissue | Geographical Location | Reference |
|---|---|---|---|---|
| Pen shell (Pinna nobilis) | M. sherrisi (M. triplex), Mycobacterium sp. | Connective tissue surrounding the digestive gland, mantle interstitium, and kidney | Italy, Spain, Greece and Croatia | [4,16,17,40,41,42] |
| Atlantic sea scallop (Placopecten magellanicus) | Mycobacterium sp. | Adductor muscle, digestive gland, kidney, gills and mantle | Massachusetts to Maryland, USA | [15] |
| Australorbis glabratus | Mycobacterium sp. | Rectal ridge, kidney, gut, mantle, foot and gonad | nr | [39] |
| Two-ridge rams-horn (Helisoma anceps) | Mycobacterium sp. | Surface epithelium, connective tissue surrounding the digestive gland, kidney, rectal ridge and foot | USA | [43] |
| Great ramshorn (Planorbarius corneus) | M. chelonae, M. fortuitum | nr | Czech Republic | [44] |
| Planorbis planorbis, Pomacea canaliculata | M. ulcerans, M. gordonae, M. szulgai | Digestive tract | Daloa region of Ivory Coast | [45] |
| Bulinus senegalensis | M. ulcerans | nr | Benin | [46] |
| Marsh snail (Biomphalaria glabrata) | Mycobacterium sp. | Integument, pneumostome, base of the tentacle, digestive and genital epithelia | USA | [47] |
| Eastern oyster (Crassostrea virginica) | M. scrofulaceum, M. gordonae, M. terrae complex, M. parafortuitum complex | nr | Alabama, USA | [48] |
| Oysters (Crassostrea sp.) | M. marinum | nr | USA, France | [49,50] |
| Animal Species | Mycobacterium Species | Infected Tissue | Geographical Location | Reference |
|---|---|---|---|---|
| Redclaw crayfish (Cherax quadricarinatus) | M. gordonae | Hepatopancreas, gills, testis | Israel | [5] |
| Giant freshwater prawn (Macrobrachium rosenbergii) | Mycobacterium sp. | Hepatopancreas, heart, antennal gland, loose connective tissue of the gnathothorax, gills | USA | [13] |
| Whiteleg shrimp (Litopenaeus vannamei) | Mycobacterium sp., M. peregrinum, M. marinum | Mandibular organ and hepatopancreas; carapace and heart; carapace and muscle | USA, Brazil | [14,66,67] |
| Brine shrimp (Artemia salina) | Mycobacterium sp. | nr | Czech Republic | [44] |
| Common water flea (Daphnia sp.) | M. marinum | nr | Sweden | [62] |
| Ceriodaphnia reticulata | M. fortuitum, M. chelonae, M. flavescens | nr | Germany | [63] |
| Sea-monkey (Artemia nyos) | M. marinum | nr | Pennsylvania, USA | [65] |
| Red swamp crayfish (Procambarus clarkii) | M. fortuitum | Hepatopancreas | Ibrahimiyah Canal, Egypt | [68] |
| Shrimp | Mycobacterium sp. | nr | Iraq | [69] |
| Crab | M. marinum | nr | South Carolina coast, USA | [70] |
| Crab | M. arupense | nr | Republic of Korea | [71] |
| Animal Species | Mycobacterium Species | Infected Tissue | Geographical Location | Reference |
|---|---|---|---|---|
| Coral | M. haemophilum | nr | Thailand | [74] |
| Sea urchin (Paracentrotus lividus) | M. marinum | nr | Spain | [75] |
| Sea urchin | M. marinum | nr | Spain | [76] |
| Sea urchin | M. marinum | nr | USA | [77] |
| Sea urchin | M. chelonae | nr | Hawaii, USA | [78] |
| Sponge | M. poriferae | nr | USA | [79] |
| Animal Species | Mycobacterium Species | Human Injury | Country | Reference |
|---|---|---|---|---|
| Oysters (Crassostrea sp.) | M. marinum | A swollen left hand with six non-draining nodular lesions along the ulnar palm. | USA | [49] |
| Oysters (Crassostrea sp.) | M. marinum | Ulcer on the hand. | France | [50] |
| Common water fleas (Daphnia sp.) | M. marinum | Aquarium-borne infection on the lower arm of the owner. | Sweden | [62] |
| Sea-monkey (Artemia nyos) | M. marinum | Five nodular lesions on the right hand and forearm. One at the base of the nail of the right third digit, one in the region of the third metacarpophalangeal joint and three along the right forearm. | Pennsylvania, USA | [65] |
| Coral | M. haemophilum | A chronic cutaneous lesion in an immunologically normal patient after a coral injury, which implicated coral. | Thailand | [74] |
| Sea urchin (Paracentrotus lividus) | M. marinum | In a study of 50 biopsy specimens from 35 patients diagnosed as having sea urchin granuloma. Half of these patients were involved in fishing activities, and 50% of these were divers involved in commercial harvesting of sea urchins. | Spain | [75] |
| Sea urchin | M. marinum | Osteomyelitis of the first metatarsal bone, after accidental puncture injury by a sea urchin requiring surgical treatment in a non-immunosuppressed patient. | Spain | [76] |
| Sea urchin | M. marinum | Arthritis in the interphalangeal joint of the hallux while snorkelling in Japan. | Fukuoka, Japan | [77] |
| Sea urchin | M. chelonae | Multiple small, raised nodules over the volar index finger and thumb extending to the palm and resulting in tenosynovitis due to penetrating injury by a sea urchin to the hand. | Hawaii, USA | [78] |
| Crab | M. marinum | Granulomatous inflammation on the right fourth finger while fishing. | South Carolina coast, USA | [70] |
| Crab | M. arupense | Tenosynovitis in a patient with a history of puncture injury to the finger. | Republic of Korea | [71] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davidovich, N.; Morick, D.; Carella, F. Mycobacteriosis in Aquatic Invertebrates: A Review of Its Emergence. Microorganisms 2020, 8, 1249. https://doi.org/10.3390/microorganisms8081249
Davidovich N, Morick D, Carella F. Mycobacteriosis in Aquatic Invertebrates: A Review of Its Emergence. Microorganisms. 2020; 8(8):1249. https://doi.org/10.3390/microorganisms8081249
Chicago/Turabian StyleDavidovich, Nadav, Danny Morick, and Francesca Carella. 2020. "Mycobacteriosis in Aquatic Invertebrates: A Review of Its Emergence" Microorganisms 8, no. 8: 1249. https://doi.org/10.3390/microorganisms8081249
APA StyleDavidovich, N., Morick, D., & Carella, F. (2020). Mycobacteriosis in Aquatic Invertebrates: A Review of Its Emergence. Microorganisms, 8(8), 1249. https://doi.org/10.3390/microorganisms8081249







