Relationship between the Oral and Vaginal Microbiota of South African Adolescents with High Prevalence of Bacterial Vaginosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Sample Collection
2.3. STI and BV Testing
2.4. Amplification and Sequencing of the V4 Region of the 16S rRNA Gene
2.5. Bioinformatics Analysis of the 16S rRNA Gene Sequencing Data
2.6. Statistical Analysis
2.7. Data Availability
3. Results
3.1. Cohort Characteristics and Vaginal Microbiota
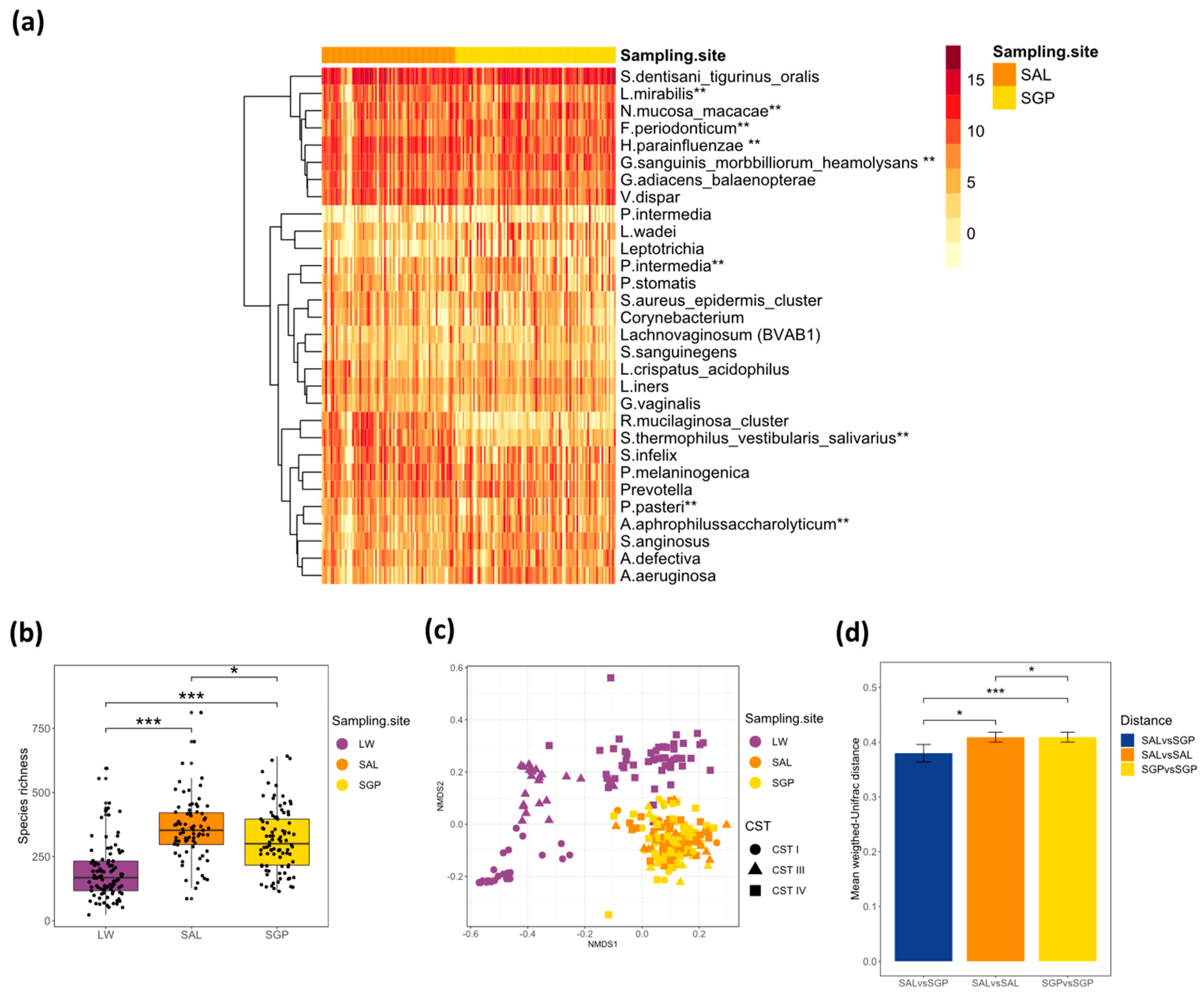
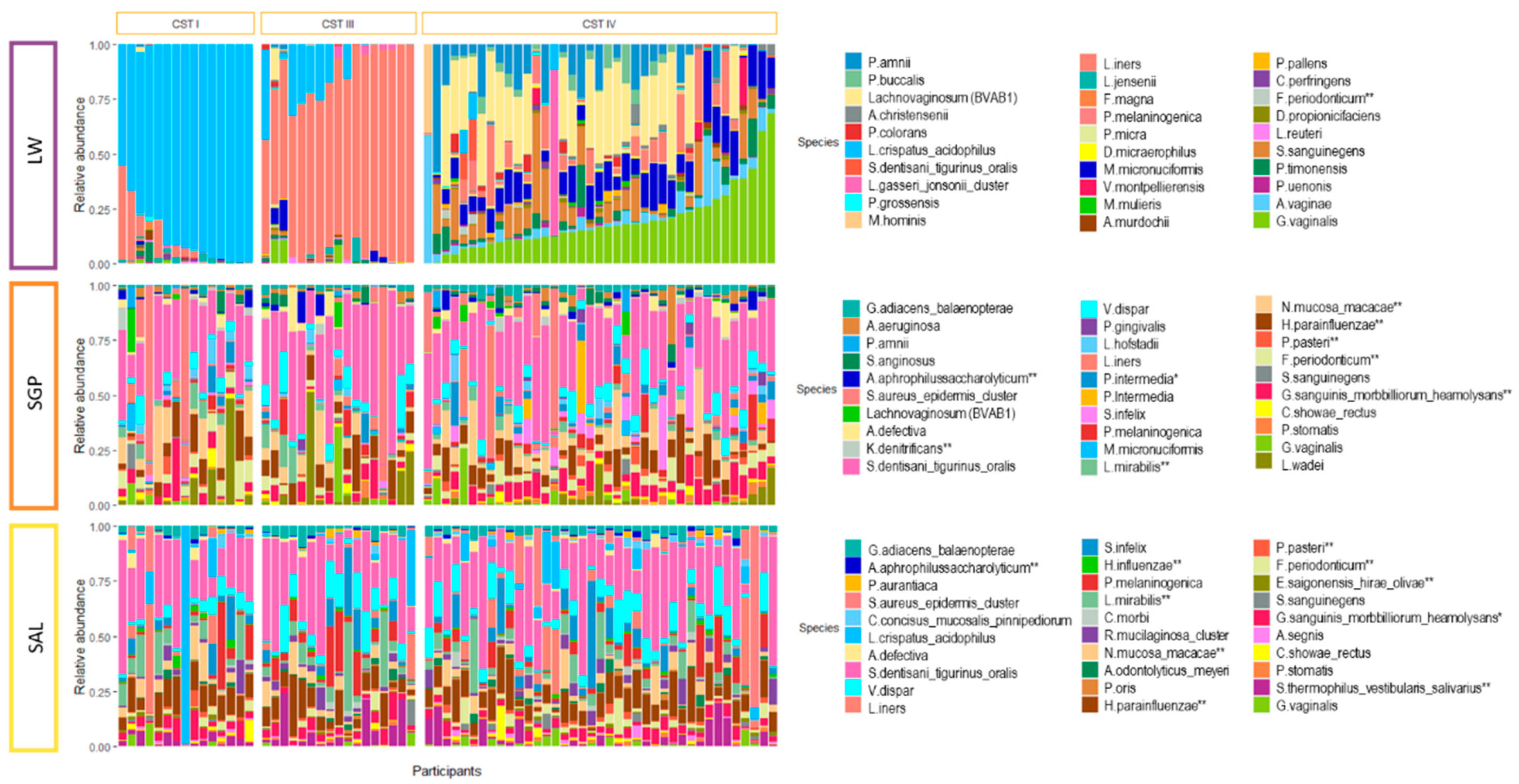
3.2. Salivary and Supragingival Oral Microbiota in South African Adolescents
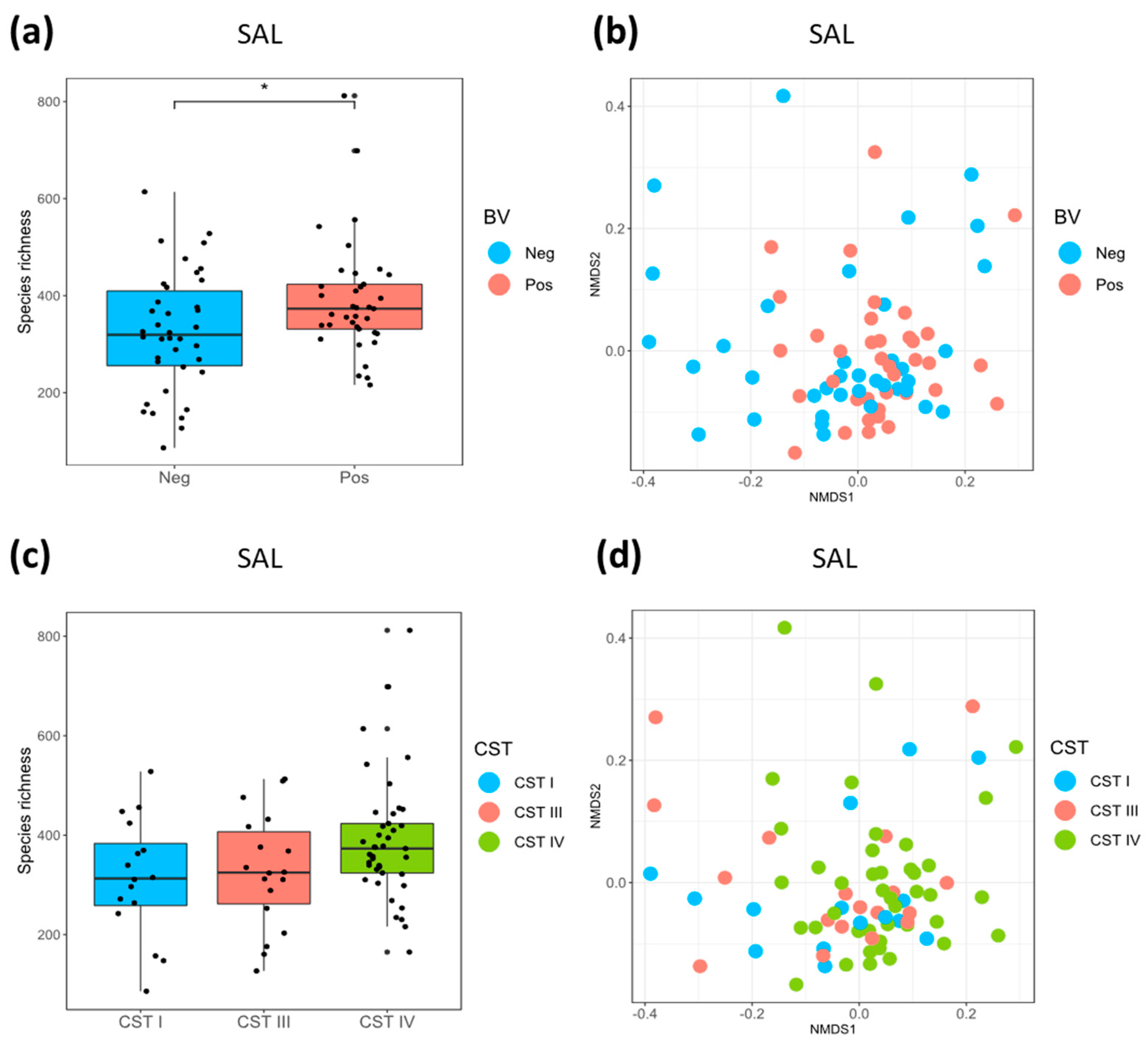
3.3. Relationship between the Oral and Vaginal Microbiota
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zaura, E.; Keijser, B.J.F.; Huse, S.M.; Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Bihan, M.; Methe, B.A. Analyses of the stability and core taxonomic memberships of the human microbiome. PLoS ONE 2013, 8, e63139. [Google Scholar] [CrossRef] [PubMed]
- Bik, E.M.; Long, C.D.; Armitage, G.C.; Loomer, P.; Emerson, J.; Mongodin, E.F.; Nelson, K.E.; Gill, S.R.; Fraser-Liggett, C.M.; Relman, D.A. Bacterial diversity in the oral cavity of 10 healthy individuals. ISME J. 2010, 4, 962–974. [Google Scholar] [CrossRef]
- Long, J.; Cai, Q.; Steinwandel, M.; Hargreaves, M.K.; Bordenstein, S.R.; Blot, W.J.; Zheng, W.; Shu, X.O. Association of oral microbiome with type 2 diabetes risk. J. Periodontal Res. 2017, 52, 636–643. [Google Scholar] [CrossRef]
- Michaud, D.S.; Izard, J. Microbiota, oral microbiome and pancreatic cancer. Cancer J. 2014, 20, 203–206. [Google Scholar] [CrossRef]
- Kistler, J.O.; Arirachakaran, P.; Poovorawan, Y.; Dahlen, G.; Wade, W.G. The oral microbiome in human immunodeficiency virus (HIV)-positive individuals. J. Med. Microbiol. 2015, 64, 1094–1101. [Google Scholar] [CrossRef]
- Lerner, A.; Aminov, R.; Matthias, T. Dysbiosis May Trigger Autoimmune Diseases via Inappropriate Post-Translational Modification of Host Proteins. Front. Microbiol. 2016, 7, 84. [Google Scholar] [CrossRef]
- Hayashi, C.; Gudino, C.V.; Gibson, F.C., 3rd; Genco, C.A. Review: Pathogen-induced inflammation at sites distant from oral infection: Bacterial persistence and induction of cell-specific innate immune inflammatory pathways. Mol. Oral Microbiol. 2010, 25, 305–316. [Google Scholar] [CrossRef]
- Zarco, M.F.; Vess, T.J.; Ginsburg, G.S. The oral microbiome in health and disease and the potential impact on personalized dental medicine. Oral Dis. 2012, 18, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Costalonga, M.; Herzberg, M.C. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol. Lett. 2014, 162, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Moss, K.; Beck, J.D.; Hefti, A.; Offenbacher, S. Persistently high levels of periodontal pathogens associated with preterm pregnancy outcome. J. Periodontol. 2007, 78, 833–841. [Google Scholar] [CrossRef]
- Albandar, J.M.; Rams, T.E. Global epidemiology of periodontal diseases: An overview. Periodontology 2000 2002, 29, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. (Qassim) 2017, 11, 72–80. [Google Scholar]
- Chikte, U.; Pontes, C.C.; Karangwa, I.; Kimmie-Dhansay, F.; Erasmus, R.T.; Kengne, A.P.; Matsha, T.E. Periodontal Disease Status among Adults from South Africa-Prevalence and Effect of Smoking. Int. J. Environ. Res. Public Health 2019, 16, 3662. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Bouchard, P.; Cagetti, M.G.; Campus, G.; Carra, M.-C.; Cocco, F.; Nibali, L.; Hujoel, P.; Laine, M.L.; Lingstrom, P.; et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: Consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017, 44 (Suppl. 1), S39–S51. [Google Scholar] [CrossRef]
- Albandar, J.M. Global risk factors and risk indicators for periodontal diseases. Periodontology 2000 2002, 29, 177–206. [Google Scholar] [CrossRef]
- Pretorius, C.; Jagatt, A.; Lamont, R.F. The relationship between periodontal disease, bacterial vaginosis and preterm birth. J. Perinat. Med. 2007, 35, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.M.; Parry, S.; Stamilio, D.M.; Odibo, A.O.; Cahill, A.G.; Strauss, J.F., 3rd; Macones, G.A. The interaction effect of bacterial vaginosis and periodontal disease on the risk of preterm delivery. Am. J. Perinatol. 2012, 29, 347–352. [Google Scholar] [CrossRef]
- Oittinen, J.; Kurki, T.; Kekki, M.; Kuusisto, M.; Pussinen, P.; Vilkuna-Rautiainen, T.; Nieminen, A.; Asikainen, S.; Paavonen, J. Periodontal disease and bacterial vaginosis increase the risk for adverse pregnancy outcome. Infect. Dis. Obstet. Gynecol. 2005, 13, 213–216. [Google Scholar] [CrossRef]
- Harrison, M.S.; Goldenberg, R.L. Global burden of prematurity. Semin. Fetal Neonatal Med. 2016, 21, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Oza, S.; Hogan, D.; Perin, J.; Rudan, I.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: An updated systematic analysis. Lancet 2015, 385, 430–440. [Google Scholar] [CrossRef]
- Kenyon, C.; Colebunders, R.; Crucitti, T. The global epidemiology of bacterial vaginosis: A systematic review. Am. J. Obstet. Gynecol. 2013, 209, 505–523. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Mendling, W.; Loening-Baucke, V.; Ladhoff, A.; Swidsinski, S.; Hale, L.P.; Lochs, H. Adherent biofilms in bacterial vaginosis. Obstet. Gynecol. 2005, 106, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Fredricks, D.N.; Fiedler, T.L.; Marrazzo, J.M. Molecular identification of bacteria associated with bacterial vaginosis. N. Engl. J. Med. 2005, 353, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4680–4687. [Google Scholar] [CrossRef]
- Zhou, X.; Hansmann, M.A.; Davis, C.C.; Suzuki1, H.; Brown, C.J.; Schutte, U.; Pierson, J.D.; Forney, L.J. The Vaginal Bacterial Communities of Japanese Women Resemble Those of Women in Other Racial Groups. FEMS Immunol. Med. Microbiol. 2011, 58, 1–19. [Google Scholar] [CrossRef]
- Anahtar, M.N.; Byrne, E.H.; Doherty, K.E.; Bowman, B.A.; Yamamoto, H.S.; Soumillon, M.; Padavattan, N.; Ismail, N.; Moodley, A.; Sabatini, M.E.; et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 2015, 42, 965–976. [Google Scholar] [CrossRef]
- Lennard, K.; Dabee, S.; Barnabas, S.L.; Havyarimana, E.; Blakney, A.; Jaumdally, S.Z.; Botha, G.; Mkhize, N.N.; Bekker, L.G.; Lewis, D.A.; et al. Microbial composition predicts genital tract inflammation and persistent bacterial vaginosis in South African adolescent females. Infect. Immun. 2018, 86. [Google Scholar] [CrossRef]
- Fettweis, J.M.; Serrano, M.G.; Sheth, N.U.; Mayer, C.M.; Glascock, A.L.; Brooks, J.P.; Jefferson, K.K.; Buck, G.A. Species-level classification of the vaginal microbiome. BMC Genom. 2012, 13 (Suppl. 8), S17. [Google Scholar] [CrossRef]
- Srinivasan, U.; Misra, D.; Marazita, M.L.; Foxman, B. Vaginal and Oral Microbes, Host Genotype and Preterm Birth. Med. Hypotheses 2009, 6, 963–975. [Google Scholar] [CrossRef]
- Zabor, E.C.; Klebanoff, M.; Yu, K.; Zhang, J.; Nansel, T.; Andrews, W.; Schwebke, J.; Jeffcoat, M. Association between periodontal disease, bacterial vaginosis and sexual risk behaviours. J. Clin. Periodontol. 2010, 37, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Persson, R.; Hitti, J.; Verhelst, R.; Vaneechoutte, M.; Persson, R.; Hirschi, R.; Weibel, M.; Rothen, M.; Temmerman, M.; Paul, K.; et al. The vaginal microflora in relation to gingivitis. BMC Infect. Dis. 2009, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Konstantinus, I.N.; Balle, C.; Jaumdally, S.Z.; Galmieldien, H.; Pidwell, T.; Masson, L.; Tanko, R.F.; Happel, A.-U.; Sinkala, M.; Myer, L.; et al. Impact of hormonal contraceptives on cervical Th17 phenotype and function in adolescents: Results from a randomized cross-over study comparing long-acting injectable norethisterone oenanthate (NET-EN), combined oral contraceptive pills and combined contraceptive vaginal rings. Clin. Infect. Dis. 2019, ciz1063. [Google Scholar] [CrossRef]
- Lewis, D.A.; Muller, E.; Steele, L.; Sternberg, M.; Radebe, F.; Lyall, M.; Ballard, R.C.; Paz-Bailey, G. Prevalence and associations of genital ulcer and urethral pathogens in men presenting with genital ulcer syndrome to primary health care clinics in South Africa. Sex. Transm. Dis. 2012, 39, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.M.; Hilt, E.E.; Rosenfeld, A.B.; Zilliox, M.J.; Thomas-White, K.; Fok, C.; Kliethermes, S.; Schreckenberger, P.C.; Brubaker, L.; Gai, X.; et al. The female urinary microbiome: A comparison of women with and without urgency urinary incontinence. mBio 2014, 5, e01283-14. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality control tool for high throughput sequence data. Babraham Bioinform. 2018, 3–5. [Google Scholar]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yu, W.-H.; Izard, J.; Baranova, O.V.; Lakshmanan, A.; Dewhirst, F.E. The Human Oral Microbiome Database: A web accessible resource for investigating oral microbe taxonomic and genomic information. Database (Oxford) 2010, 2010, baq013. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree: Computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K. Cluster Analysis Basics and Extensions. R package Version 2.0.6. 2017. Available online: https://cran.r-project.org/web/packages/cluster/cluster.pdfhttps://cran.r-project.org/web/packages/cluster/cluster.pdf (accessed on 18 June 2020).
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Simpson, G.L.; Stevens, M.H.; Wagner, H. Vegan: Community Ecology Package. R. Package Version 2.4-1. 2016. Available online: https://cran.r-project.org/web/packages/vegan/index.htmlhttps://cran.r-project.org/web/packages/vegan/index.html (accessed on 18 June 2020).
- Gaujoux, R. Generating Heatmaps for Nonnegative Matrix Factorization. 2014. Available online: http://nmf.r-forge.r-project.org/vignettes/heatmaps.pdf (accessed on 18 June 2020).
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Gosmann, C.; Anahtar, M.N.; Handley, S.A.; Farcasanu, M.; Abu-Ali, G.; Bowman, B.A.; Padavattan, N.; Desai, C.; Droit, L.; Moodley, A.; et al. Lactobacillus-Deficient Cervicovaginal Bacterial Communities Are Associated with Increased HIV Acquisition in Young South African Women. Immunity 2017, 46, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Usin, M.M.; Menso, J.; Rodríguez, V.I.; González, A.; Tabares, S.; Parodi, R.; Sembaj, A. Association between maternal periodontitis and preterm and/or low birth weight infants in normal pregnancies. J. Matern. Fetal Neonatal Med. 2016, 29, 115–119. [Google Scholar] [CrossRef]
- Nasidze, I.; Li, J.; Quinque, D.; Tang, K.; Stoneking, M. Global diversity in the human salivary microbiome. Genome Res. 2009, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Quinque, D.; Horz, H.; Li, M.; Rzhetskaya, M.; Raff, J.A.; Hayes, M.G.; Stoneking, M. Comparative analysis of the human saliva microbiome from different climate zones: Alaska, Germany and Africa. BMC Microbiol. 2014, 14, 316. [Google Scholar] [CrossRef]
- Zhou, X.; Brown, C.J.; Abdo, Z.; Davis, C.C.; Hansmann, M.A.; Joyce, P.; Foster, J.A.; Forney, L.J. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J. 2007, 1, 121–133. [Google Scholar] [CrossRef]
- Borgdorff, H.; van der Veer, C.; van Houdt, R.; Alberts, C.J.; de Vries, H.J.; Bruisten, S.M.; Snijder, M.B.; Prins, M.; Geerlings, S.E.; Schim van der Loeff, M.F.; et al. The association between ethnicity and vaginal microbiota composition in Amsterdam, the Netherlands. PLoS ONE 2017, 12, e0181135. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, D.A.; Chandiramani, M.; Lee, Y.S.; Kindinger, L.; Smith, A.; Angelopoulos, N.; Lehne, B.; Arulkumaran, S.; Brown, R.; Teoh, T.G.; et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.P.; Edwards, D.J.; Blithe, D.L.; Fettweis, J.M.; Serrano, M.G.; Sheth, N.U.; Strauss, J.F., 3rd; Buck, G.A.; Jefferson, K.K. Effects of combined oral contraceptives, depot medroxyprogesterone acetate and the levonorgestrel-releasing intrauterine system on the vaginal microbiome. Contraception 2017, 95, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Roxby, A.C.; Fredricks, D.N.; Odem-Davis, K.; Asbjornsdottir, K.; Masese, L.; Fiedler, T.L.; De Rosa, S.; Jaoko, W.; Kiarie, J.N.; Overbaugh, J.; et al. Changes in Vaginal Microbiota and Immune Mediators in HIV-1-Seronegative Kenyan Women Initiating Depot Medroxyprogesterone Acetate. J. Acquir. Immune Defic. Syndr. 2016, 71, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Achilles, S.L.; Austin, M.N.; Meyn, L.A.; Mhlanga, F.; Chirenje, Z.M.; Hillier, S.L. Impact of contraceptive initiation on vaginal microbiota. Am. J. Obstet. Gynecol. 2018. [Google Scholar] [CrossRef]
- Klinger, G.; Eick, S.; Klinger, G.; Pfister, W.; Gräser, T.; Moore, C.; Oettel, M. Influence of hormonal contraceptives on microbial flora of gingival sulcus. Contraception 1998, 57, 381–384. [Google Scholar] [CrossRef]
- Brusca, M.I.; Rosa, A.; Albaina, O.; Moragues, M.D.; Verdugo, F.; Pontón, J. The impact of oral contraceptives on women’s periodontal health and the subgingival occurrence of aggressive periodontopathogens and Candida species. Br. Dent. J. 2010, 209, 509. [Google Scholar] [CrossRef][Green Version]
- Kazerooni, T.; Ghaffarpasand, F.; Rastegar, N.; Kazerooni, Y. Effect of levonorgestrel implants on the periodontium. Int. J. Gynecol. Obstet. 2008, 103, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Tilakaratne, A.; Soory, M.; Ranasinghe, A.W.; Corea, S.M.X.; Ekanayake, S.L.; De Silva, M. Effects of hormonal contraceptives on the periodontium, in a population of rural Sri-Lankan women. J. Clin. Periodontol. 2000, 27, 753–757. [Google Scholar] [CrossRef]
- Taichman, L.S.; Sohn, W.; Kolenic, G.; Sowers, M. Depot medroxyprogesterone acetate use and periodontal health in 15- to 44-year-old US females. J. Periodontol. 2012, 83, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Abd-Ali, E.H.; Shaker, N.T. The effect of oral contraceptive on the oral health with the evaluation of Salivary IgA and Streptococcus mutans in some Iraqi Women. Marietta Dly. 2013, 10, 52–63. [Google Scholar]
| BV Status | |||
|---|---|---|---|
| Negative (N = 48) (Nugent 0–3) | Positive (N = 46) (Nugent 7–10) | P Value | |
| Mean Age (Std. Deviation) | 16.9 (1.38) | 17.1 (1.36) | 0.368 |
| Mean BMI (Std. Deviation) | 25.9 (4.67) | 26.3 (5.55) | 0.710 |
| Previous Pregnancy † | 10.6% (N = 4) | 13.0% (N = 6) | 0.970 |
| Any bacterial STI | 43.8% (N = 21) | 47.8% (N = 22) | 0.850 |
| Chlamydia trachomatis | 33.3% (N = 16) | 37.0% (N = 17) | 0.879 |
| Neisseria gonorrhoeae | 10.4% (N = 5) | 15.2% (N = 7) | 0.698 |
| Trichomonas vaginalis | 6.3% (N = 3) | 6.5% (N = 3) | 1.000 |
| Mycoplasma genitalium | 4.2% (N = 2) | 4.3% (N = 2) | 1.000 |
| Herpes Simplex Virus 2 | 31.9% (N = 15) | 34.8% (N = 16) | 0.942 |
| Any medication past month | 20.8% (N = 10) | 10.6% (N = 5) | 0.300 |
| Any antibiotic use past month | 4.2% (N = 2) | 8.9% (N = 4) | 0.634 |
| Current hormonal contraception ‡ | 83.0% (N = 39) | 71.1% (N = 32) | 0.268 |
| Intra-vaginal practices § Wash vagina with water Douching | 4.4% (N = 2) 0.0% (N = 0) | 17.1% (N = 7) 0.4% (N = 1) | 0.080 0.477 |
| Community state type (CST) CST-I CST-III CST-IV | 47.9% (N = 23) 43.8% (N = 21) 8.3% (N = 4) | 0.0% (N = 0) 2.2% (N = 1) 97.8 (N = 45) | <0.001 |
| Median supragingival plaque (SGP) richness ¶ (IQR) | 277 (215-525) | 324 (240-522) | 0.294 |
| Median salivary (SAL) richness ¶ (IQR) | 319 (256-515) | 373 (331-585) | 0.030 |
| Vaginal samples included | 47 | 46 | |
| Saliva (SAL) samples included | 38 | 37 | |
| Supragingival plaque (SGP) samples included | 46 | 44 | |
| Phylum | Genus | Species | Mean Relative Abundance |
|---|---|---|---|
| Actinobacteria | Actinomyces | oris_naeslundii _viscosus | 2% |
| Corynebacterium | 2% | ||
| Gardnerella | 1% | ||
| Rothia | mucilaginosa_cluster | 1% | |
| Bacteroidetes | Porphyromonas | pasteri | 1% |
| Prevotella | intermedia | 2% | |
| Prevotella | melaninogenica | 3% | |
| Prevotella | oris | 1% | |
| Firmicutes | Abiotrophia | defective | 1% |
| Catonella | morbi | 0% | |
| Lactobacillus | iners | 3% | |
| Lactobacillus | crispatus | 3% | |
| Granulicatella | adiacens_balaenopterae | 2% | |
| Peptostreptococcus | stomatis | 1% | |
| Selenomonas | infelix | 4% | |
| Lachnovaginosum | genomospecies (BVAB1) | 1% | |
| Staphylococcus | 4% | ||
| Streptococcus | anginosus | 1% | |
| Streptococcus | dentisani_tigurinus_oralis | 29% | |
| Veillonella | Dispar | 5% | |
| Gemella | sanguinis_morbbilliorum_heamolysans | 4% | |
| Fusobacteria | Fusobacterium | periodonticum | 3% |
| Leptotrichia | wadei | 3% | |
| Proteobacteria | Aggregatibacter | aphrophilussaccharolyticum | 1% |
| Campylobacter | showae_rectus | 2% | |
| Kingella | dentrificans | 1% | |
| Haemophilus | parainfluenzae | 8% | |
| Haemophilus | influenzae | 1% | |
| Lautropia | mirabilis | 4% | |
| Neisseria | mucosa_macacae | 5% |
| A. | Vaginal Community State Type (CST) | ||||
| Phylum | Genus | Species | CST IV vs. CST-I Log2 Fold Change | P Value (Adjusted p-Value) | |
| SGP (N = 69) | Proteobacteria | Moraxella | NA | −25.73 | 2.98 × 10−28 (8.31 × 10−26) |
| Proteobacteria | Methylobacterium | NA | −6.051 | 1.15 × 10−6 (0.0002) | |
| Proteobacteria | Rhizobium | leguminosarum | −5.063 | 0.0002 (0.0142) | |
| Bacteroidetes | Prevotella | intermedia | −3.209 | 0.0006 (0.0306) | |
| Proteobacteria | Delftia | acidovorans | −2.710 | 0.0005 (0.0306) | |
| Bacteroidetes | Porphyromonas | endodontalis | −2.201 | 0.0009 (0.0414) | |
| Firmicutes | Lactobacillus | iners | 1.671 | 0.0017 (0.0606) | |
| Tenericutes | Ureaplasma | NA | 4.145 | 0.0017 (0.0606) | |
| Proteobacteria | NA | NA | −3.056 | 0.0034 (0.0868) | |
| Firmicutes | Oribacterium | NA | 2.301 | 0.0030 (0.0868) | |
| Firmicutes | Blautia | NA | −4.027 | 0.0032 (0.0868) | |
| SAL (N = 57) | Proteobacteria | Pseudomonas | aeruginosa | −3.716 | 0.0002 (0.0444) |
| Bacteroidetes | Odoribacter | NA | −3.338 | 0.0008 (0.0817) | |
| Firmicutes | Megasphaera | NA | −3.259 | 0.0011 (0.0817) | |
| Proteobacteria | Moraxella | NA | −3.142 | 0.0017 (0.0920) | |
| Bacteroidetes | Prevotella | intermedia | −3.003 | 0.0027 (0.0976) | |
| Actinobacteria | Mobiluncus | mulieris | −3.015 | 0.0026 (0.0976) | |
| B. | Vaginal Community State Type (CST) | ||||
| Phylum | Genus | Species | CST IV vs. CST III Log2 Fold Change | P Value (Adjusted p-Value) | |
| SGP (N = 68) | Bacteroidetes | Prevotella | intermedia | −3.471 | 0.0005 (0.0089) |
| Fusobacteria | Leptotrichia | wadei | 3.522 | 0.0004 (0.0287) | |
| Proteobacteria | Delftia | acidovorans | −3.700 | 0.0002 (0.0287) | |
| Bacteroidetes | Prevotella | NA | −3.464 | 0.0005 (0.0287) | |
| Firmicutes | Blautia | NA | −3.189 | 0.0014 (0.0287) | |
| Firmicutes | Clostridium | saccharogumia | −2.918 | 0.0035 (0.0641) | |
| Proteobacteria | Brachymonas | denitrificans | 2.903 | 0.0037 (0.0997) | |
| Firmicutes | Oribacterium | NA | 2.963 | 0.0030 (0.0997) | |
| Proteobacteria | Morganella | NA | −3.000 | 0.0027 (0.0997) | |
| SAL (N = 59) | Firmicutes | Staphylococcus | aureus_epidermis_cluster | −3.811 | 0.0001 (0.0259) |
| Bacteroidetes | Prevotella | amnii | −3.556 | 0.0004 (0.0273) | |
| Fusobacteria | Sneathia | sanguinegens | −3.483 | 0.0005 (0.0273) | |
| Firmicutes | Anaerovorax | NA | −3.439 | 0.0006 (0.0273) | |
| Firmicutes | Megasphaera | micronuciformis | −3.195 | 0.0014 (0.0522) | |
| Proteobacteria | Pseudomonas | aeruginosa | −2.944 | 0.0032 (0.0758) | |
| Firmicutes | Lactobacillus | iners | −3.031 | 0.0024 (0.0758) | |
| Firmicutes | Dialister | micraerophilus | −2.944 | 0.0032 (0.0758) | |
| Bacteroidetes | Prevotella | tannerae | −2.822 | 0.0048 (0.0944) | |
| Bacteroidetes | Bacteroides | heparinolyticus | −2.791 | 0.0052 (0.0944) | |
| Firmicutes | Facklamia | NA | −2.773 | 0.0056 (0.0944) | |
| Bacteroidetes | Prevotella | melaninogenica | 2.698 | 0.0070 (0.0989) | |
| Firmicutes | Lactobacillus | gasseri_jonsonii_cluster | −2.678 | 0.0074 (0.0989) | |
| Actinobacteria | Gardnerella | vaginalis | −2.716 | 0.0066 (0.0989) | |
| C. | Vaginal Community State Type (CST) | ||||
| Phylum | Genus | Species | CST I vs. CST III Log2 Fold Change | P Value (Adjusted p-Value) | |
| SGP (N = 43) | Firmicutes | Staphylococcus | aureus_epidermis_cluster | −2.845 | 0.0005 (0.0886) |
| Bacteroidetes | Porphyromonas | bennonis | −3.2701 | 0.0060 (0.0991) | |
| Firmicutes | Shuttleoworthia | BVAB-1 | −2.201 | 0.0042 (0.0991) | |
| Firmicutes | Clostridium | butyricum | −4.755 | 0.0014 (0.0991) | |
| Firmicutes | Moryella | indoligenes | −4.095 | 0.0054 (0.0991) | |
| Proteobacteria | Kingella | denitrificans | −2.201 | 0.0038 (0.0991) | |
| Firmicutes | Streptococcus | thermophilus_vestibularis_salivarius | 1.684 | 0.0066 (0.0991) | |
| Bacteroidetes | Bacteroides | fragilis | −4.210 | 0.0024 (0.0991) | |
| Firmicutes | Dialister | propionicifaciens | −2.856 | 0.0062 (0.0991) | |
| Firmicutes | Megasphaera | micronuciformis | −2.160 | 0.0034 (0.0991) | |
| Proteobacteria | Morganella | NA | −3.839 | 0.0031 (0.0991) | |
| Proteobacteria | Herbaspirillum | NA | 4.047 | 0.0046 (0.0991) | |
| SAL (N = 34) | - | - | - | NA | NA |
| D. | BV Status | ||||
| Phylum | Genus | Species | Neg vs. Pos Log2 Fold change | P Value (Adjusted p-Value) | |
| SGP (N = 90) | Proteobacteria | Delftia | acidovorans | 2.437 | 4.65 × 10−5 (0.0066) |
| Proteobacteria | Methylobacterium | NA | 3.893 | 5.39 × 10−5 (0.0066) | |
| Bacteroidetes | [Prevotella] | NA | 1.756 | 0.0007 (0.0556) | |
| Bacteroidetes | Prevotella | intermedia | 1.758 | 0.0023 (0.0618) | |
| Firmicutes | Staphylococcus | aureus_epidermis_cluster | −1.734 | 0.0012 (0.0618) | |
| Fusobacteria | Leptotrichia | wadei | −2.043 | 0.0019 (0.0618) | |
| Firmicutes | Lactobacillus | crispatus_acidophilus | −1.487 | 0.0015 (0.0618) | |
| Firmicutes | Oribacterium | NA | −2.208 | 0.0015 (0.0618) | |
| Bacteroidetes | Chryseobacterium | NA | −2.648 | 0.0022 (0.0618) | |
| Tenericutes | Ureaplasma | NA | −3.654 | 0.0025 (0.0618) | |
| Proteobacteria | NA | NA | 2.494 | 0.0036 (0.0762) | |
| Firmicutes | Blautia | NA | 3.409 | 0.0037 (0.0762) | |
| Firmicutes | Ruminococcus | bromii | 3.036 | 0.0041 (0.0762) | |
| Proteobacteria | Neisseria | oralis | −2.362 | 0.0052 (0.0888) | |
| Proteobacteria | Rhizobium | leguminosarum | 3.086 | 0.0054 (0.0888) | |
| Actinobacteria | Corynebacterium | durum | −1.530 | 0.0064 (0.0989) | |
| SAL (N = 75) | Fusobacterium | Sneathia | sanguinegens | 1.757 | 0.00015 (0.0417) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balle, C.; Esra, R.; Havyarimana, E.; Jaumdally, S.Z.; Lennard, K.; Konstantinus, I.N.; Barnabas, S.L.; Happel, A.-U.; Gill, K.; Pidwell, T.; et al. Relationship between the Oral and Vaginal Microbiota of South African Adolescents with High Prevalence of Bacterial Vaginosis. Microorganisms 2020, 8, 1004. https://doi.org/10.3390/microorganisms8071004
Balle C, Esra R, Havyarimana E, Jaumdally SZ, Lennard K, Konstantinus IN, Barnabas SL, Happel A-U, Gill K, Pidwell T, et al. Relationship between the Oral and Vaginal Microbiota of South African Adolescents with High Prevalence of Bacterial Vaginosis. Microorganisms. 2020; 8(7):1004. https://doi.org/10.3390/microorganisms8071004
Chicago/Turabian StyleBalle, Christina, Rachel Esra, Enock Havyarimana, Shameem Z. Jaumdally, Katie Lennard, Iyaloo N. Konstantinus, Shaun L. Barnabas, Anna-Ursula Happel, Katherine Gill, Tanya Pidwell, and et al. 2020. "Relationship between the Oral and Vaginal Microbiota of South African Adolescents with High Prevalence of Bacterial Vaginosis" Microorganisms 8, no. 7: 1004. https://doi.org/10.3390/microorganisms8071004
APA StyleBalle, C., Esra, R., Havyarimana, E., Jaumdally, S. Z., Lennard, K., Konstantinus, I. N., Barnabas, S. L., Happel, A.-U., Gill, K., Pidwell, T., Lingappa, J. R., Gamieldien, H., Bekker, L.-G., Passmore, J.-A. S., & Jaspan, H. B. (2020). Relationship between the Oral and Vaginal Microbiota of South African Adolescents with High Prevalence of Bacterial Vaginosis. Microorganisms, 8(7), 1004. https://doi.org/10.3390/microorganisms8071004





