First Demonstration of Clinical Fusarium Strains Causing Cross-Kingdom Infections from Humans to Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
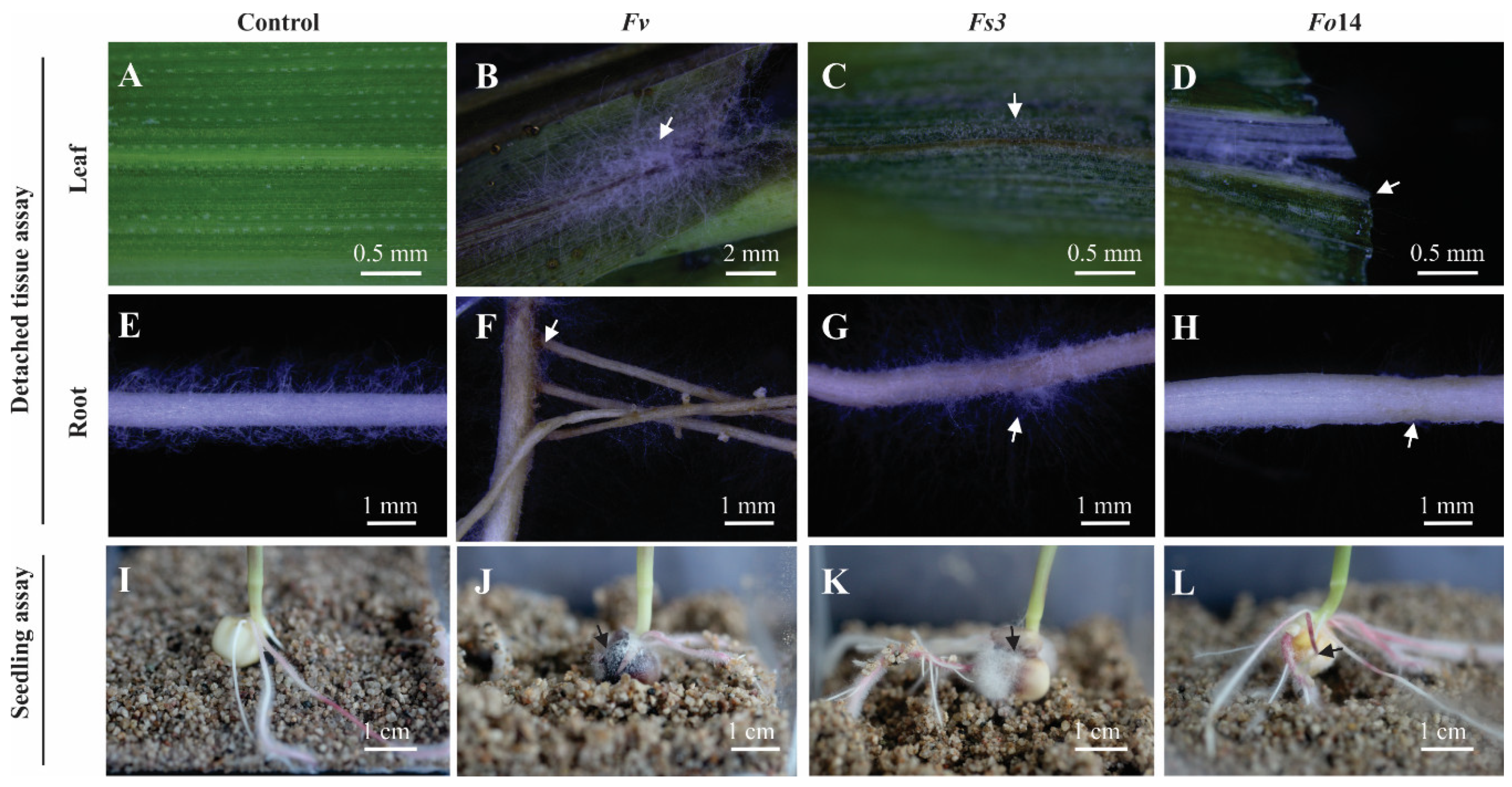
2.2. Detached in-vitro Tissue Assay
2.2.1. Surface Disinfection of Seeds
2.2.2. Conidia Suspensions and Inoculation of Tissue by Fusarium
2.3. In vitro Seedling Assay
2.3.1. Surface Disinfection of Seeds
2.3.2. Inoculation of Maize and Arabidopsis Seeds by Fusarium
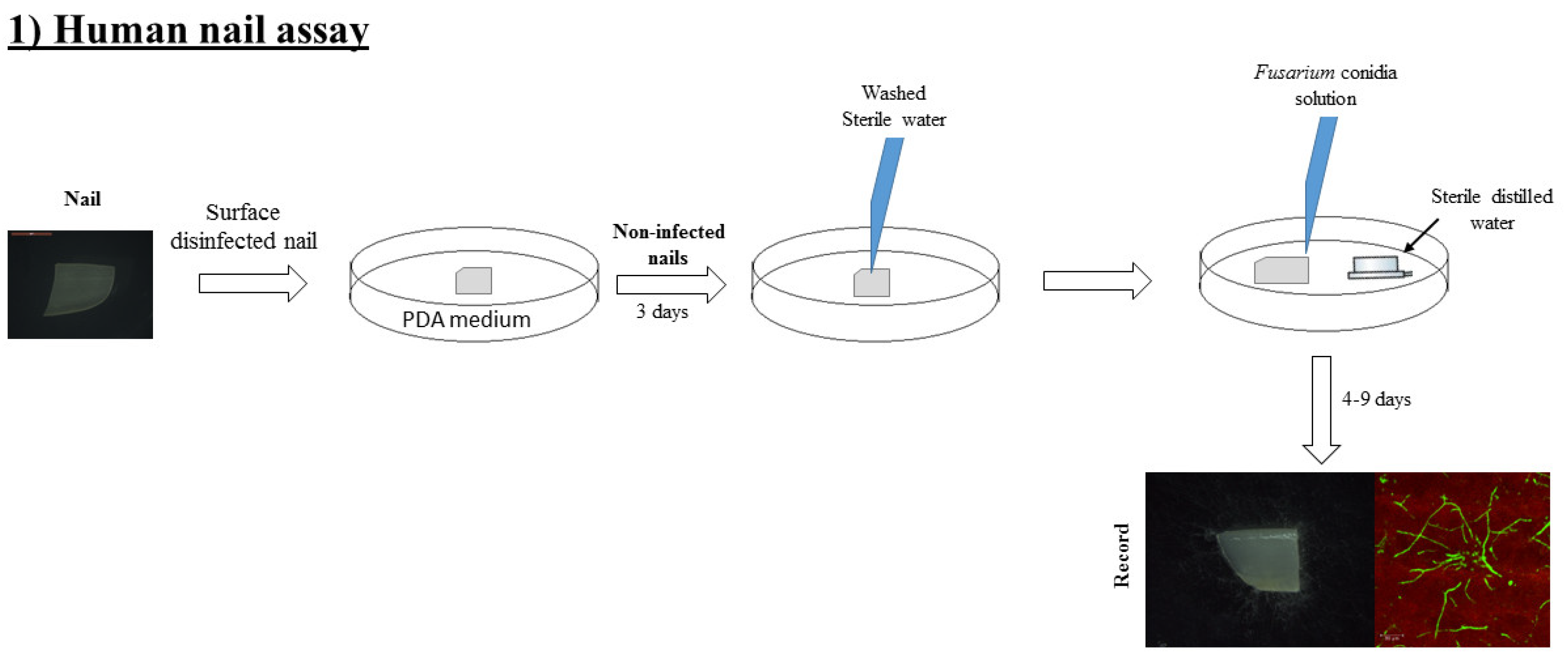
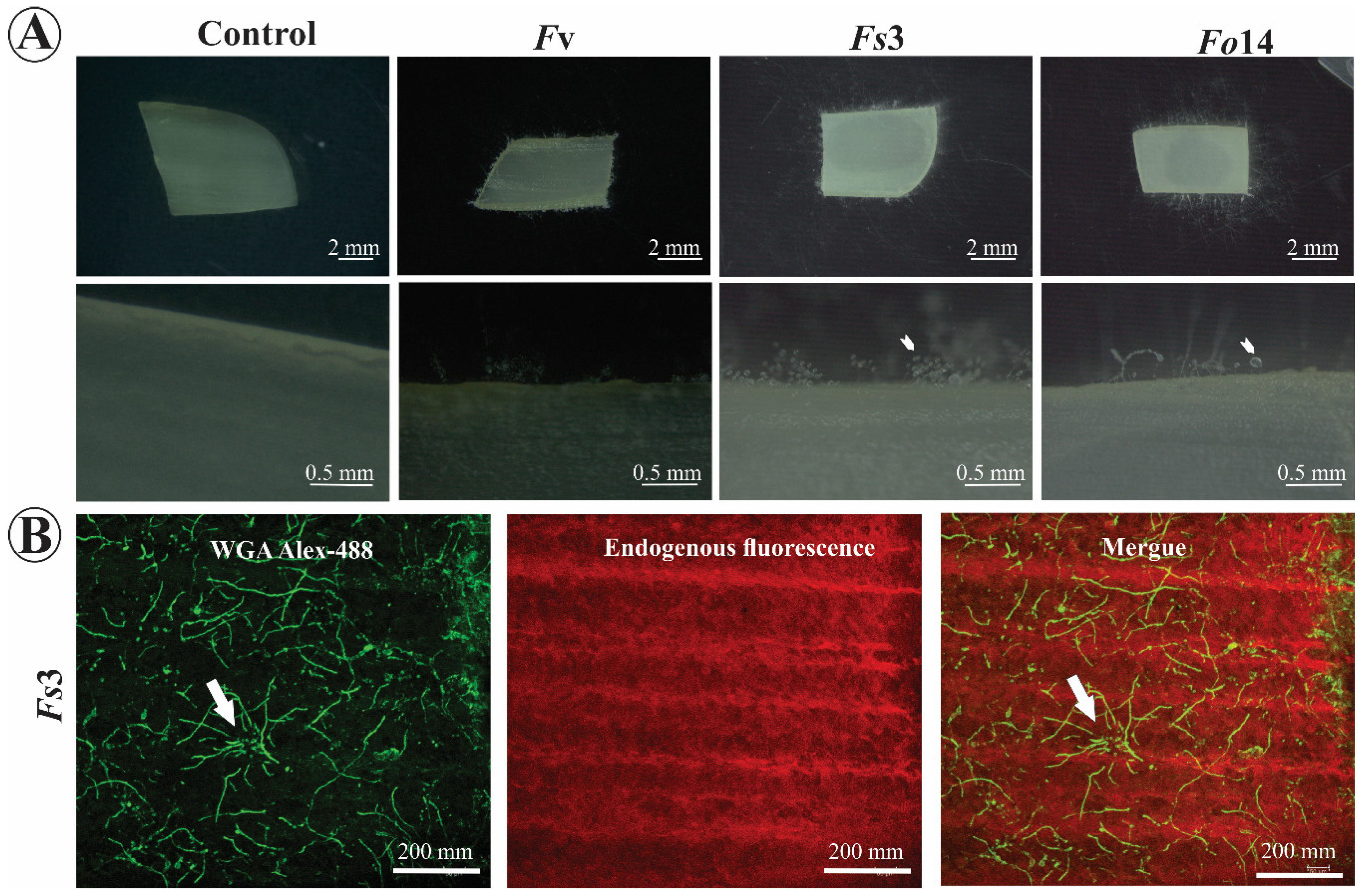
2.4. Human Onychomycosis Assay
2.5. Fusarium Detection by Confocal Microscopy
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Streicker, D.G.; Turmelle, A.S.; Vonhof, M.J.; Kuzmin, I.V.; McCracken, G.F.; Rupprecht, C.E. Host Phylogeny Constrains Cross-Species Emergence and Establishment of Rabies Virus in Bats. Science 2010, 329, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Egbuta, M.A.; Mwanza, M.; Babalola, O.O. Health Risks Associated with Exposure to Filamentous Fungi. Int. J. Env. Res. Public Health 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Marques, G. Fusarium, an Entomopathogen-A Myth or Reality? Pathogens 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Oliveira, I.; Raimundo, F.; Torres, L.; Marques, G. Soil Chemical Properties Barely Perturb the Abundance of Entomopathogenic Fusarium oxysporum: A Case Study Using a Generalized Linear Mixed Model for Microbial Pathogen Occurrence Count Data. Pathogens 2018, 7. [Google Scholar] [CrossRef]
- Sharma, L.; Goncalves, F.; Oliveira, I.; Torres, L.; Marques, G. Insect-associated fungi from naturally mycosed vine mealybug Planococcus ficus (Signoret) (Hemiptera: Pseudococcidae). Biocontrol Sci. Technol. 2018, 28, 122–141. [Google Scholar] [CrossRef]
- Sitaraman, R. Pseudomonas spp. as models for plant-microbe interactions. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Ma, L.J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium Pathogenomics. Annu. Rev. Microbiol 2013, 67, 399–416. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2007; Volume 2, pp. 1–369. [Google Scholar]
- Al-Hatmi, A.M.S.; Hagen, F.; Menken, S.B.J.; Meis, J.F.; de Hoog, G.S. Global molecular epidemiology and genetic diversity of Fusarium, a significant emerging group of human opportunists from 1958 to 2015. Emerg. Microbes. Infec. 2016, 5. [Google Scholar] [CrossRef]
- Hayashida, M.Z.; Seque, C.A.; Enokihara, M.; Porro, A.M. Disseminated fusariosis with cutaneous involvement in hematologic malignancies: Report of six cases with high mortality rate. Bras Derm. 2018, 93, 726–729. [Google Scholar] [CrossRef]
- Sun, S.T.; Lui, Q.X.; Han, L.; Ma, Q.F.; He, S.Y.; Li, X.H.; Zhang, H.M.; Zhang, J.J.; Liu, X.H.; Wang, L.Y. Identification and Characterization of Fusarium proliferatum, a New Species of Fungi that Cause Fungal Keratitis. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Nucci, M.; Anaissie, E. Fusarium infections in immunocompromised patients. Clin. Microbiol. Rev. 2007, 20, 695–704. [Google Scholar] [CrossRef]
- Vanzinni Zago, V.; Manzano-Gayosso, P.; Hernández-Hernández, F. Queratomicosis en un centro de atención oftalmológica en la Ciudad de México. Rev. Iberoam. Micol. 2010, 27, 57–61. [Google Scholar] [CrossRef]
- Monod, M.; Mehul, B. Recent Findings in Onychomycosis and Their Application for Appropriate Treatment. J. Fungi. 2019, 5. [Google Scholar] [CrossRef]
- Gai, X.; Dong, H.; Wang, S.; Liu, B.; Zhang, Z.; Li, X.; Gao, Z. Infection cycle of maize stalk rot and ear rot caused by Fusarium verticillioides. PLoS ONE 2018, 13, e0201588. [Google Scholar] [CrossRef] [PubMed]
- Okello, P.N.; Petrović, K.; Kontz, B.; Mathew, F.M. Eight Species of Fusarium Cause Root Rot of Corn (Zea mays) in South Dakota. Plant Health Prog. 2019, 20, 38–43. [Google Scholar] [CrossRef]
- Baldwin, T.T.; Zitomer, N.C.; Mitchell, T.R.; Zimeri, A.M.; Bacon, C.W.; Riley, R.T.; Glenn, A.E. Maize Seedling Blight Induced by Fusarium verticillioides: Accumulation of Fumonisin B-1 in Leaves without Colonization of the Leaves. J. Agric. Food Chem. 2014, 62, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.T.; Voss, K.A.; Speer, M.; Stevens, V.L.; Waes, J.G.-V. Fumonisin inhibition of ceramide synthease: A possible risk factor for human nueral tube defects. In Sphingolipid Biology, 1st ed.; Hirabayashi, Y., Igarashi, Y., Merrill, A.H., Eds.; Springer: Tokyo, Japan, 2006; pp. 345–361. [Google Scholar]
- Antonissen, G.; Martel, A.; Pasmans, F.; Ducatelle, R.; Verbrugghe, E.; Vandenbroucke, V.; Li, S.J.; Haesebrouck, F.; Van Immerseel, F.; Croubels, S. The Impact of Fusarium Mycotoxins on Human and Animal Host Susceptibility to Infectious Diseases. Toxins 2014, 6, 430–452. [Google Scholar] [CrossRef]
- Al-Hatmi, A.M.S.; Meis, J.F.; de Hoog, G.S. Fusarium: Molecular Diversity and Intrinsic Drug Resistance. PLoS Pathog. 2016, 12. [Google Scholar] [CrossRef]
- Zhang, N.; O’Donnell, K.; Sutton, D.A.; Nalim, F.A.; Summerbell, R.C.; Padhye, A.A.; Geiser, D.M. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J. Clin. Microbiol. 2006, 44, 2186–2190. [Google Scholar] [CrossRef] [PubMed]
- de Hoog, G.S.; Guarro, J.; Gené, J.; Figueras, M.J. Atlas of Clinical Fungi, 2nd ed.; Universatat Rovira i Virgili: Reus, Spain, 2000; p. 1126. [Google Scholar]
- Leyva-Madrigal, K.Y.; Larralde-Corona, C.P.; Apodaca-Sanchez, M.A.; Quiroz-Figueroa, F.R.; Mexia-Bolanos, P.A.; Portillo-Valenzuela, S.; Ordaz-Ochoa, J.; Maldonado-Mendoza, I.E. Fusarium Species from the Fusarium fujikuroi Species Complex Involved in Mixed Infections of Maize in Northern Sinaloa, Mexico. J. Phytopathol. 2015, 163, 486–497. [Google Scholar] [CrossRef]
- Román, S.G. Caracterización de genotipos de maíz (Zea mays L.) a la infección de Fusarium verticillioides en diferentes fases del ciclo de vida de la planta y su correlación con marcadores moleculares de tipo SNPs; Instituto Politécnico Nacional: Guasave, México, 2017. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Figueroa-López, A.M.; Cordero-Ramírez, J.D.; Quiroz-Figueroa, F.R.; Maldonado-Mendoza, I.E. A high-throughput screening assay to identify bacterial antagonists against Fusarium verticillioides. J. Basic Microbiol. 2014, 54, S125–S133. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.H.; Yu, M.; Lai, E.M. Agrobacterium-mediated plant transformation: Biology and applications. Arab. Book 2017, 15, e0186. [Google Scholar] [CrossRef] [PubMed]
- van Baarlen, P.; van Belkum, A.; Summerbell, R.C.; Crous, P.W.; Thomma, B.P.H.J. Molecular mechanisms of pathogenicity: How do pathogenic microorganisms develop cross-kingdom host jumps? Fems. Microbiol. Rev. 2007, 31, 239–277. [Google Scholar] [CrossRef] [PubMed]
- Nurnberger, T.; Brunner, F.; Kemmerling, B.; Piater, L. Innate immunity in plants and animals: Striking similarities and obvious differences. Immunol. Rev. 2004, 198, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Sexton, A.C.; Howlett, B.J. Parallels in fungal pathogenesis on plant and animal hosts. Eukaryot. Cell 2006, 5, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Ortoneda, M.; Guarro, J.; Madrid, M.P.; Caracuel, Z.; Roncero, M.I.; Mayayo, E.; Di, P.A. Fusarium oxysporum as a multihost model for the genetic dissection of fungal virulence in plants and mammals. Infect. Immun. 2004, 72, 1760–1766. [Google Scholar] [CrossRef]
- Navarro-Velasco, G.Y.; Prados-Rosales, R.C.; Ortiz-Urquiza, A.; Quesada-Moraga, E.; Di Pietro, A. Galleria mellonella as model host for the trans-kingdom pathogen Fusarium oxysporum. Fungal Genet. Biol. 2011, 48, 1124–1129. [Google Scholar] [CrossRef]
- Segorbe, D.; Di Pietro, A.; Perez-Nadales, E.; Turra, D. Three Fusarium oxysporum mitogen-activated protein kinases (MAPKs) have distinct and complementary roles in stress adaptation and cross-kingdom pathogenicity. Mol. Plant Pathol. 2017, 18, 912–924. [Google Scholar] [CrossRef]
- Abdallah, M.F.; De Boevre, M.; Landschoot, S.; De Saeger, S.; Haesaert, G.; Audenaert, K. Fungal Endophytes Control Fusarium graminearum and Reduce Trichothecenes and Zearalenone in Maize. Toxins 2018, 10. [Google Scholar] [CrossRef]
- Ausubel, F.M. Are innate immune signaling pathways in plants and animals conserved? Nat. Immunol. 2005, 6, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Neville, B.A.; Forster, S.C.; Lawley, T.D. Commensal Koch’s postulates: Establishing causation in human microbiota research. Curr. Opin. Microbiol. 2018, 42, 47–52. [Google Scholar] [CrossRef] [PubMed]

| Date | Lab ID | Symptomatology | Gender/Age | Patient Location | Occupation | Species |
|---|---|---|---|---|---|---|
| 10/01/2013 | 21564 | Ocular trauma | F/75 | Tuzamapan, Puebla | Housewife | Fusariumsolani |
| 19/02/2013 | 21791 | DM 10 years, cornea trauma | M/73 | Zacatecas | Farmer | Fusariumdimerum |
| 20/02/2013 | 21797 | Ocular trauma | M/28 | Puebla | Farmer | Fusariumsolani |
| 06/03/2013 | 21890 | Ocular trauma | F/32 | Quintana Roo | Housewife | Fusariumsolani |
| 08/04/2013 | 22083 | Ocular trauma | M/76 | Mexico city | Worker | Fusariumsolani |
| 18/06/2013 | 22503 | Ocular trauma | M/7 | Veracruz, Ver | Student | Fusariumsolani |
| 19/08/2013 | 22869 | Insidious, pain+++, immune ring | F/41 | La trinitaria Chiapas | Housewife | Fusariumdimerum |
| 23/12/2013 | 23544 | Insidious, pain++ | M/47 | AltamiraTamaulipas | Worker | Fusariumsolani |
| 17/02/2014 | 23813 | Ocular trauma | M/41 | Mexico city | Builder | Fusariumsolani |
| 20/08/2014 | 24810 | Ocular trauma | M/49 | Durango Durango | Farmer | Fusariumsolani |
| 30/01/2015 | 25704 | Insidious, pain | F/34 | Acapulco Guerrero | Housewife | Fusariumsolani |
| 27/04/2015 | 26256 | Ocular trauma | F/30 | Mexico City | Housewife | Fusariumdimerum |
| 18/08/2016 | 28615 | Ocular trauma | M/62 | Puebla | Farmer | Fusariumoxysporum |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meza-Menchaca, T.; Singh, R.K.; Quiroz-Chávez, J.; García-Pérez, L.M.; Rodríguez-Mora, N.; Soto-Luna, M.; Gastélum-Contreras, G.; Vanzzini-Zago, V.; Sharma, L.; Quiroz-Figueroa, F.R. First Demonstration of Clinical Fusarium Strains Causing Cross-Kingdom Infections from Humans to Plants. Microorganisms 2020, 8, 947. https://doi.org/10.3390/microorganisms8060947
Meza-Menchaca T, Singh RK, Quiroz-Chávez J, García-Pérez LM, Rodríguez-Mora N, Soto-Luna M, Gastélum-Contreras G, Vanzzini-Zago V, Sharma L, Quiroz-Figueroa FR. First Demonstration of Clinical Fusarium Strains Causing Cross-Kingdom Infections from Humans to Plants. Microorganisms. 2020; 8(6):947. https://doi.org/10.3390/microorganisms8060947
Chicago/Turabian StyleMeza-Menchaca, Thuluz, Rupesh Kumar Singh, Jesús Quiroz-Chávez, Luz María García-Pérez, Norma Rodríguez-Mora, Manuel Soto-Luna, Guadalupe Gastélum-Contreras, Virginia Vanzzini-Zago, Lav Sharma, and Francisco Roberto Quiroz-Figueroa. 2020. "First Demonstration of Clinical Fusarium Strains Causing Cross-Kingdom Infections from Humans to Plants" Microorganisms 8, no. 6: 947. https://doi.org/10.3390/microorganisms8060947
APA StyleMeza-Menchaca, T., Singh, R. K., Quiroz-Chávez, J., García-Pérez, L. M., Rodríguez-Mora, N., Soto-Luna, M., Gastélum-Contreras, G., Vanzzini-Zago, V., Sharma, L., & Quiroz-Figueroa, F. R. (2020). First Demonstration of Clinical Fusarium Strains Causing Cross-Kingdom Infections from Humans to Plants. Microorganisms, 8(6), 947. https://doi.org/10.3390/microorganisms8060947







